In the past few decades, there has been a rapid
development in tumor immunotherapy, which has been recognized as a
key strategy to control the progression of malignant tumors
(1). Among these immunotherapies,
immune checkpoint inhibitors (ICIs) (2), chimeric antigen receptor T cells
(3) and bispecific antibodies
(4) are the most promising
immunotherapeutic strategies. ICI therapy is one of the most
well-studied immunotherapies (2,5).
Inhibitors block the transmission of inhibitory signals, stimulate
the activation of cytotoxic T lymphocytes (CTL) and induce the
antitumor effects of T lymphocytes. Two ICIs have been
investigated, including cytotoxic T lymphocyte antigen 4 (CTLA-4)
(6) and programmed cell death 1/cell
death 1 ligand, (PD-1/PD-L1) (7).
However, the response rate of anti-PD-1/PD-L1 monoclonal antibodies
(mAbs) and anti-CTLA-4mAb in patients with cancer overall is poor
(8,9). Most patients show primary or acquired
resistance to these ICIs (10,11). As
a member of the ICI family, LAG-3 is an inhibitory molecule with
multiple biological effects on the function of T cells (12). LAG-3 is highly expressed in various
types of tumor infiltrating lymphocytes (TILs) and participates in
the immune escape mechanism of tumors (12). Therefore, LAG-3 could be used as an
indicator of tumor prognosis and a target of tumor therapy. The
present review primarily describes the research progress of LAG-3
in immunotherapy.
LAG-3 is a member of the immunoglobulin superfamily,
which was identified in 1990 (13).
LAG-3, also known as CD223, has a molecular weight of 70kDa and is
located on human chromosome 12 (12p13) (14). The LAG-3 gene is located in the same
region as the CD4 gene, and there is a certain homology between the
two (15); however, they
share<20% homology at the protein level (13). Similar to CD4, LAG-3 binds to major
histocompatibility complex-II (MHC-II) on antigen presenting cells
(APCs), but with a much stronger affinity (13). The LAG-3 gene contains 8 exons, and
the corresponding cDNA encodes a membrane protein, with 498 amino
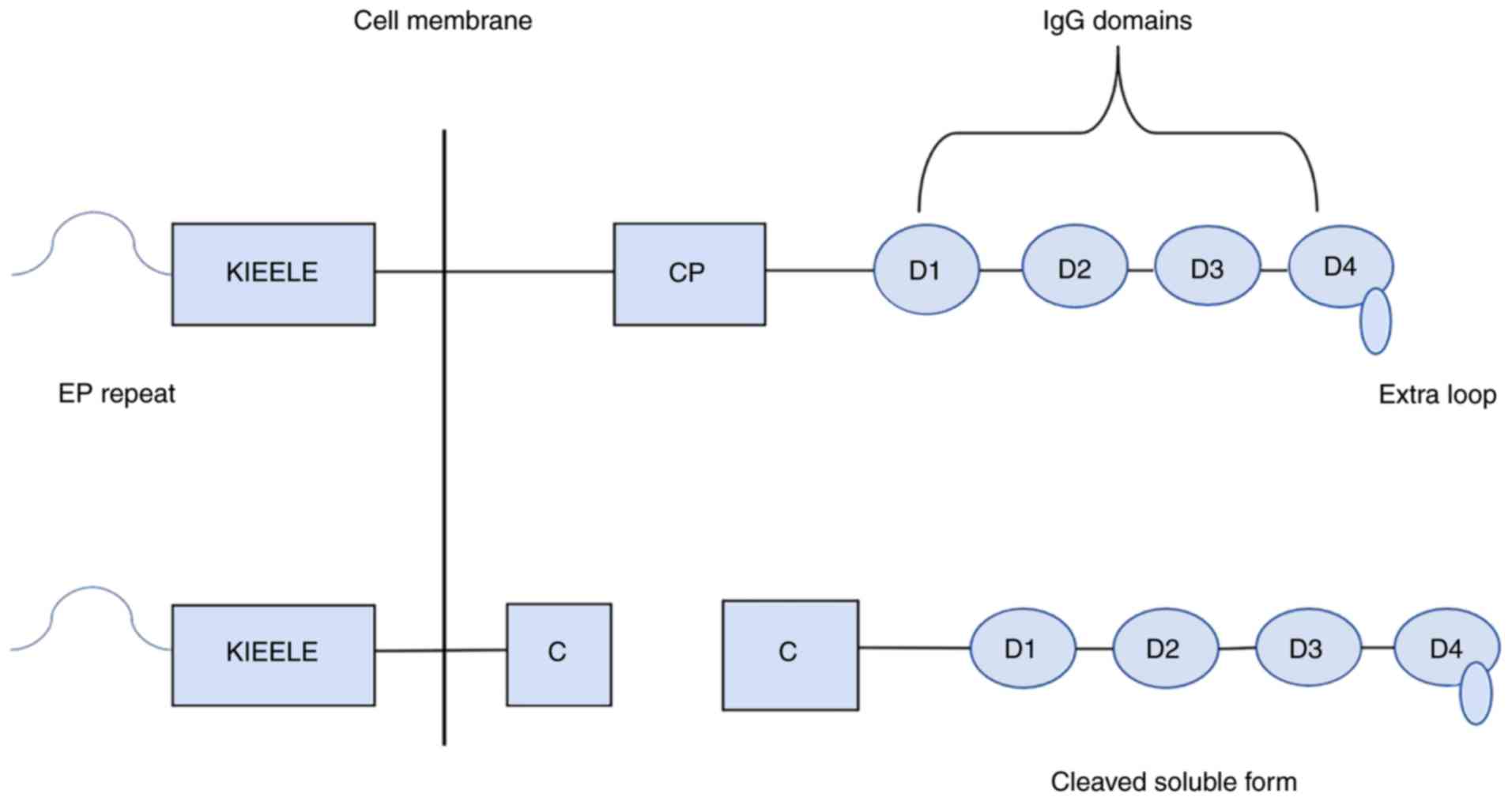
acids (16). LAG-3 is composed of an
extracellular region, a transmembrane region and an intracellular
region (17). The membrane protein
contains four extracellular immunoglobulin superfamily domains: One
V region and three C2 regions (16,17). The
V region is special, as there is an extra ring in the middle of the
domain and also contains an abnormal in-chain disulfide bridge
(18). The cytoplasmic region of
LAG-3 is composed of three parts: A serine phosphorylation site, a
‘KIEELE’ motif and a glutamate-proline dipeptide repeat sequence
(EP sequence), among which the ‘KIEELE’ motif is a highly conserved
sequence that has not been found in other proteins and is involved
in intracellular signal transduction of the LAG-3 molecule
(17,19) (Fig.
1). It was initially hypothesized that the EP sequence was the
key basis for the downstream signal of LAG-3. However, the result
of mutation analysis found that this was not the case. The ‘KIEELE’
motif in the LAG-3 molecule is conserved (17), and molecules that lack this domain
are unable to exert an inhibitory effect on T cells, suggesting
that this motif may be associated with the downstream inhibitory
signal of LAG-3 (17). However, the
mechanism of downstream signal transduction is still unclear. Under
the action of the membrane-penetrating metalloproteinases
disintegrin and metalloproteinase domain-containing protein (ADAM)
10 and ADAM17, the LAG-3 molecule on the cell membrane splits into
two parts at the junction peptide: Soluble LAG-3(sLAG-3) and a
transmembrane-cytoplasm molecule (20). sLAG-3 has been found to induce
maturation of dendritic cells and target tumor cells (21). Previous studies have discovered five
primary ligands of LAG-3. In a quantitative cell adhesion
experiment, COS-7 cells transfected with LAG-3 and DaudiB
lymphocytes labeled with 51Chromium were co-cultured
together, and it was found, for the first time, that a garland was
formed between the two cells, which was specifically dependent on
LAG-3 and MHC-II molecules (14,22).
Anti-LAG-3 or HLA-DR Ab have been found to destroy the formation of
this garland, confirming that the ligand of LAG-3 is the MHC II
protein and combines with a conservative extension ring in the
LAG-3 D1 domain (14,22). LAG-3, once combined with MHC-II,
transmits inhibitory signals via the cytoplasmic domain; however,
the signal transduction mechanism is still unclear (17). Galectin-3 (Gal-3), a 31-kDa
galactose-binding lectin that regulates T cell activation and
immunoprecipitation, was found to interact with LAG-3 and inhibits
the secretion of interferon-γ by CD8+T cells in
vitro, indicating that Gal-3 is also an LAG-3 ligand (23). Gal-3 can be expressed on the surface
of different cancers, such as lung cancer (24), prostate cancer (25), colorectal cancer (26), breast cancer (27) and so on. Thus it exerting its
regulatory function on CD8+T cells via multiple
mechanisms (28). LSECtin has also
been proposed to be a LAG-3 ligand. LSECtin binds to the four
glycosylated sites on LAG-3 and is a member of the DC-SIGN family
of molecules (28). LSECtin is
expressed in the liver and in melanoma tumor cells, suggesting a
mechanism by which LAG-3 can regulate CD8+T cells and
natural killer (NK) cell function in these environments (29). α-synuclein (α-syn) fibrils is a
protein aggregation, which exists in the substantia nigra of the
brain in patients with paralysis tremor and is a member of the Syn
fibrils family (28–30). α-syn was found to bind to LAG-3,
which leads to the intercellular delivery of pathological α-syn
fibrils, while blocking their combination with a LAG-3 Ab could
significantly reduce the toxicity and the intercellular delivery of
pathological α-syn fibrils, suggesting that α-syn fibrils were also
a LAG-3 ligand (31).
Fibrinogen-like protein 1(FGL1) was recently identified as the
primary ligand of LAG-3, and a genome-scale reporter array
identified that the FGL1 protein was tightly bound to the LAG-3
receptor (32). Functional detection
of the FGL1 protein revealed that upon binding to the LAG-3
receptor on the surface of T cells, T cell proliferation was
inhibited and immune activity was also affected (33). Blocking the interaction between FGL1
and LAG-3 could enhance the antitumor effect of T lymphocytes,
which has important significance in the research of tumor
immunotherapy (32). LAG-3 is
expressed on activated CD4+ (34) and CD8+T cells (35), regulatory T cells (Tregs) (36), a subpopulation of NK cells (37), B cells (38) and plasmacytoid dendritic cells
(39). As an inhibitory molecule
expressed on the surface of lymphocytes, LAG-3 is involved in
regulating the proliferation and activation of T lymphocytes and
effector T lymphocytes, and plays a specific role in maintaining
environmental stability in vivo (36). The process of T cell inactivation and
death are present both in cancer and chronic infection (40). As a co-inhibitory receptor of PD-1,
LAG-3 is highly expressed in chronic virus infection and various
tumors (41). The high expression of
LAG-3 is also associated with autoimmune diseases, tumors and
chronic toxic infectious diseases (18,42–44).
Immunosuppressive receptor molecules play an
important role in the maintenance of immune homeostasis. When T
lymphocytes are activated to a certain extent, immunosuppressive
molecules, such as LAG-3, CTLA-4 and PD-1 are expressed to maintain
the immune response in a stable state (45). The molecule LAG-3 blocks the signal
transduction pathway of T cell activation; however, the
intracellular segment of the LAG-3 molecule produces
immunosuppressive signals, which have been found to regulate
CD4+T cell activity (46). LAG-3 regulates the immune response of
T cells in three ways: Firstly, it directly inhibits the
proliferation and activation of T cells via negative regulation of
T cells. Secondly, it can promote the inhibitory function of Tregs,
and the T cell response can then be indirectly inhibited. Thirdly,
it can prevent T cell activation by regulating the function of APCs
(47). A LAG-3 knockout mouse model
showed increased numbers of CD4+T and CD8+T
cells in the compared with wild-type mice; however, there were no
significant changes in the ratio of CD4+T and
CD8+T cells (48).
Additionally, studies also found that LAG-3mAbs or LAG-3 knockout
cells did not significantly alter the apoptosis of T cells.
However, the proportion of S phase cells increased significantly,
suggesting that the inhibitory effect of LAG-3 on T cell
proliferation may be by controlling the cell cycle rather than
through apoptosis (49). Therefore,
LAG-3 plays a key role in regulating immune homeostasis. Under
normal physiological conditions, naive CD8+T cells are
expressed at low levels (48). LAG-3
expression was significantly increased when tumor antigen cells
were stimulated (50). LAG-3 has
also been shown to bind to tumor infiltrating lymphocytes (TILs) in
multiple solid tumors, and TILs are highly expressed in human solid
tumors (51–53). LAG-3 negatively regulates TILs and
anti-LAG-3 Ab can enhance antitumor immunity (54). Woo et al (55) found that LAG-3 and PD-1 were
expressed in CD4+ and CD8+TIL in mouse cancer
models and can enhance the cellular immune response of
CD8+T cells by co-inhibiting LAG-3 and PD-1.
Surprisingly, despite the high affinity, only a handful of residues
located in the D1 loop of LAG-3 are involved in MHC-II and LAG-3
binding, in contrast to the extensive molecular interaction between
MHC-II and CD4 (22). In 2006,
Casati et al (56) found that
sLAG-3 binds to MHC-II and mediates APC activation, thereby
activating and promoting the production and proliferation of
CD8+T lymphocytes. This indicated that sLAG-3 could
compete with LAG-3 molecules to bind MHC-II and thus inhibiting
LAG-3 from exerting a negative regulatory role on the proliferation
and activation of T cells. Blocking with an inhibitor or knockdown
of the LAG-3 gene has been found to accelerate the progression of
autoimmune diseases in several animal models of autoimmune disease
(17). The progression of diabetes
was found to accelerate in mice lacking the LAG-3 gene in an obese
diabetic mouse model (57). The
infiltration of CD4+T and CD8+T cells around
the islet cells in the mice increased significantly, accelerated
the destruction of islet cells and all of the mice eventually
developed diabetes (57). LAG-3 was
also found to be involved in immune homeostasis and autoimmune
diseases with other immunosuppressive molecules, such as Tim-3,
TIGIT (58).
Typically, LAG-3 is expressed on the surface of
activated T and NK cells to maintain immune response homeostasis
and prevent the occurrence of immune overreaction or autoimmune
diseases (22). However, in the
tumor microenvironment (TME) or chronic infectious diseases, the
continuous activation of T cells leads to co-expression of
immunosuppressive molecules, such as LAG-3, PD-1, T-cell
immunoglobulin-and mucin domain-containing (Tim)-3, TIGIT and
CD160, resulting in the incapacitant and even apoptosis of T cells,
which is the ‘deceptive mechanism’ by which tumors and chronic
infectious diseases escape from being killed by the immune system
(59). On the surface of activated
CD4+and CD8+T cells, LAG-3 is physically
integrated with CD3 (60) and then
specifically binds to the CD3-TCR complex after TCR participation
acting as a co-receptor for negative signals (61).
LAG-3 expression in the TME has also been associated
with increased tumor mutational burden. For example, cancers with
high microsatellite instability (MSIhi), which includes
a subset of patients with colorectal cancer, exhibited higher
somatic mutations and higher levels of immunogenic neoantigens. The
TME of these MSIhi tumors are characterized by increased
expression of multiple IRs including LAG-3, PD-1, PD-L1, CTLA4 and
indoleamine 2,3-dioxygenase (IDO) compared with in microsatellite
stable tumors (79). These data may
suggest an association between tumor mutational burden, antitumor
immune response and the co-expression of LAG-3 and other IRs. In
fact, the presence of LAG-3 and other co-expressed IRs in the TME
may explain why MSIhi tumors are not naturally
eliminated, despite a hostile immune microenvironment (79).
ICIs have innovated the treatment of cancer, and the
combination of LAG-3 and PD-1 drugs has been effective (54). The corresponding clinical trials of
LAG-3 and its effects on tumors have been performed in the USA,
Australia, etc. (54,80). Until 2019, one LAG-3 fusion protein
and 11 LAG-3 inhibitors were in clinical trials or are being used
as anticancer therapies (Table I). A
phase I clinical trial using LAG-3 as an immunotarget for cancer is
under way, including a single center trial for hematological
malignancies (clinical trial no. NCT02061761). In addition, LAG-3
specific Abs have been used in the combination of anti-PD-L1 and
anti-LAG-3 therapy for solid tumors (clinical trial no.
NCT01968109). However, there are still numerous problems to be
solved in the investigation of LAG-3. Firstly, the biological
function of LAG-3 binding to ligands is still unclear, and the
specific mechanism of its negative regulation of T cell function is
also still unknown. Therefore, further investigation is required.
Secondly, there might be other potential ligands of LAG-3 that
could be used as biological markers for the diagnosis and
evaluation of prognosis. Thirdly, whether LAG-3 can play an
antitumor role by regulating NK cells or B cells remains to be
further investigated. Fourthly, how the molecular mechanism of
different ICIs plays a co-role is not clear, and it is still
important to identify the optimal IC combination. Lastly, whether
modern advanced biological technology can be used to optimize the
molecular structure of LAG-3 inhibitors and reduce production costs
and to ensure improvements in a clinical setting remains to be
determined. Taken together, LAG-3 has the potential to target
molecules involved in biological functions and play a therapeutic
role. There are numerous unknown links in the specific mechanism,
and more depth understanding is required, such as ensuring LAG-3
inhibitors can be applied to clinical patients earlier for more
beneficial effects, such as improved survival. The immune
checkpoints of LAG-3 play crucial roles in cancer development and
may be used in future clinical practice for cancer therapy. Along
with the progress of additional and further observations, a broad
variety of scientific questions will emerge and require to be
addressed by scientists and physicians.
Not applicable.
This study was supported by grants from the National
Nature Science Foundation of China (grant no. 81800283).
Not applicable.
CS and JZ conceived and designed the review. CS
drafted the manuscript. XL and JZ critically revised the article
for intellectual content. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Galluzzi L, Vacchelli E, Bravo-San Pedro
JM, Buqué A, Senovilla L, Baracco EE, Bloy N, Castoldi F, Abastado
JP, Agostinis P, et al: Classification of current anticancer
immunotherapies. Oncotarget. 5:12472–12508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Shao C, Shi Y and Han W: Lessons
learned from the blockade of immune checkpoints in cancer
immunotherapy. J Hematol Oncol. 11:312018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fang Z, Han H and Liang L: Progression in
chimeric antigen receptor T (CAR-T) cell therapy of solid tumors: A
review. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 35:944–948. 2019.(In
Chinese). PubMed/NCBI
|
|
4
|
Krishnamurthy A and Jimeno A: Bispecific
antibodies for cancer therapy: A review. Pharmacol Ther.
185:122–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sadreddini S, Baradaran B, Aghebati-Maleki
A, Sadreddini S, Shanehbandi D, Fotouhi A and Aghebati-Maleki L:
Immune checkpoint blockade opens a new way to cancer immunotherapy.
J Cell Physiol. 234:8541–8549. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao Y, Yang W, Huang Y, Cui R, Li X and
Li B: Evolving roles for targeting CTLA-4 in cancer immunotherapy.
Cell Physiol Biochem. 47:721–734. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weinmann SC and Pisetsky DS: Mechanisms of
immune-related adverse events during the treatment of cancer with
immune checkpoint inhibitors. Rheumatology (Oxford). 58 (Suppl
7):vii59–vii67. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shergold AL, Millar R and Nibbs RJB:
Understanding and overcoming the resistance of cancer to PD-1/PD-L1
blockade. Pharmacol Res. 145:1042582019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Yuan R, Song W, Sun J, Liu D and
Li Z: PD-1, PD-L1 (B7-H1) and tumor-site immune modulation therapy:
The historical perspective. J Hematol Oncol. 10:342017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao X and Subramanian S: Intrinsic
resistance of solid tumors to immune checkpoint blockade therapy.
Cancer Res. 77:817–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burugu S, Gao D, Leung S, Chia SK and
Nielsen TO: LAG-3+ tumor infiltrating lymphocytes in breast cancer:
Clinical correlates and association with PD-1/PD-L1+ tumors. Ann
Oncol. 28:2977–2984. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Triebel F, Jitsukawa S, Baixeras E,
Roman-Roman S, Genevee C, Viegas-Pequignot E and Hercend T: LAG-3,
a novel lymphocyte activation gene closely related to CD4. J Exp
Med. 171:1393–1405. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huard B, Prigent P, Tournier M, Bruniquel
D and Triebel F: CD4/major histocompatibility complex class II
interaction analyzed with CD4- and lymphocyte activation gene-3
(LAG-3)-Ig fusion proteins. Eur J Immunol. 25:2718–2721. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dijkstra JM, Somamoto T, Moore L, Hordvik
I, Ototake M and Fischer U: Identification and characterization of
a second CD4-like gene in teleost fish. Mol Immunol. 43:410–419.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sierro S, Romero P and Speiser DE: The
CD4-like molecule LAG-3, biology and therapeutic applications.
Expert Opin Ther Targets. 15:91–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Workman CJ, Dugger KJ and Vignali DA:
Cutting edge: Molecular analysis of the negative regulatory
function of lymphocyte activation gene-3. J Immunol. 169:5392–5395.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldberg MV and Drake CG: LAG-3 in cancer
immunotherapy. Curr Top Microbiol Immunol. 344:269–278.
2011.PubMed/NCBI
|
|
19
|
Iouzalen N, Andreae S, Hannier S and
Triebel F: LAP, a lymphocyte activation gene-3 (LAG-3)-associated
protein that binds to a repeated EP motif in the intracellular
region of LAG-3, may participate in the down-regulation of the
CD3/TCR activation pathway. Eur J Immunol. 31:2885–2891. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li N, Wang Y, Forbes K, Vignali KM, Heale
BS, Saftig P, Hartmann D, Black RA, Rossi JJ, Blobel CP, et al:
Metalloproteases regulate T-cell proliferation and effector
function via LAG-3. EMBO J. 26:494–504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Demeure CE, Wolfers J, Martin-Garcia N,
Gaulard P and Triebel F: T Lymphocytes infiltrating various tumour
types express the MHC class II ligand lymphocyte activation gene-3
(LAG-3): Role of LAG-3/MHC class II interactions in cell-cell
contacts. Eur J Cancer. 37:1709–1718. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huard B, Mastrangeli R, Prigent P,
Bruniquel D, Donini S, El-Tayar N, Maigret B, Dréano M and Triebel
F: Characterization of the major histocompatibility complex class
II binding site on LAG-3 protein. Proc Natl Acad Sci USA.
94:5744–5749. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kouo T, Huang L, Pucsek AB, Cao M, Solt S,
Armstrong T and Jaffee E: Galectin-3 shapes antitumor immune
responses by suppressing CD8+ T cells via LAG-3 and
inhibiting expansion of plasmacytoid dendritic cells. Cancer
Immunol Res. 3:412–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Driscoll L, Linehan R, Liang YH, Joyce
H, Oglesby I and Clynes M: Galectin-3 expression alters adhesion,
motility and invasion in a lung cell line (DLKP), in vitro.
Anticancer Res. 22:3117–3125. 2002.PubMed/NCBI
|
|
25
|
Califice S, Castronovo V, Bracke M and van
den Brûle F: Dual activities of galectin-3 in human prostate
cancer: Tumor suppression of nuclear galectin-3 vs. tumor promotion
of cytoplasmic galectin-3. Oncogene. 23:7527–7536. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang C, Zhou X, Ma L, Zhuang Y, Wei Y,
Zhang L, Jin S, Liang W, Shen X, Li C, et al: Galectin-3 may serve
as a marker for poor prognosis in colorectal cancer: A
meta-analysis. Pathol Res Pract. 215:1526122019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boutas I, Potiris A, Brenner W, Lebrecht
A, Hasenburg A, Kalantaridou S and Schmidt M: The expression of
galectin-3 in breast cancer and its association with
chemoresistance: A systematic review of the literature. Arch
Gynecol Obstet. 300:1113–1120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dumic J, Dabelic S and Flogel M:
Galectin-3: An open-ended story. Biochim Biophys Acta.
1760:616–635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu F, Liu J, Liu D, Liu B, Wang M, Hu Z,
Du X, Tang L and He F: LSECtin expressed on melanoma cells promotes
tumor progression by inhibiting antitumor T-cell responses. Cancer
Res. 74:3418–3428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Wang H, Wang M, Liu B, Xu H, Xu F,
Zhao D, Hu B, Zhao N, Wang J, et al: Involvement of LSECtin in the
hepatic natural killer cell response. Biochem Biophys Res Commun.
476:49–55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mao X, Ou MT, Karuppagounder SS, Kam TI,
Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin JH, et al:
Pathological α-synuclein transmission initiated by binding
lymphocyte-activation gene 3. Science. 353:aah33742016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Sanmamed MF, Datar I, Su TT, Ji L,
Sun J, Chen L, Chen Y, Zhu G, Yin W, et al: Fibrinogen-like protein
1 is a major immune inhibitory ligand of LAG-3. Cell. 176:334–347
e312. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Visan I: New ligand for LAG-3. Nat
Immunol. 20:1112019. View Article : Google Scholar
|
|
34
|
Durham NM, Nirschl CJ, Jackson CM, et al:
Lymphocyte activation gene 3 (LAG-3) modulates the ability of CD4
T-cells to be suppressed in vivo. PLoS One. 9:e1090802014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pena J, Jones NG, Bousheri S, Bangsberg DR
and Cao H: Lymphocyte activation gene-3 expression defines a
discrete subset of HIV-specific CD8+ T cells that is associated
with lower viral load. AIDS Res Hum Retroviruses. 30:535–541. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang CT, Workman CJ, Flies D, Pan X,
Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, et
al: Role of LAG-3 in regulatory T cells. Immunity. 21:503–513.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huard B, Tournier M and Triebel F: LAG-3
does not define a specific mode of natural killing in human.
Immunol Lett. 61:109–112. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kisielow M, Kisielow J, Capoferri-Sollami
G and Karjalainen K: Expression of lymphocyte activation gene 3
(LAG-3) on B cells is induced by T cells. Eur J Immunol.
35:2081–2088. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Workman CJ, Wang Y, El Kasmi KC, Pardoll
DM, Murray PJ, Drake CG and Vignali DA: LAG-3 regulates
plasmacytoid dendritic cell homeostasis. J Immunol. 182:1885–1891.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wherry EJ, Ha SJ, Kaech SM, Haining WN,
Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL and Ahmed
R: Molecular signature of CD8+ T cell exhaustion during chronic
viral infection. Immunity. 27:670–684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fourcade J, Sun Z, Pagliano O, Guillaume
P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V and
Zarour HM: CD8(+) T cells specific for tumor antigens can be
rendered dysfunctional by the tumor microenvironment through
upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res.
72:887–896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brown KE, Freeman GJ, Wherry EJ and Sharpe
AH: Role of PD-1 in regulating acute infections. Curr Opin Immunol.
22:397–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vieyra-Lobato MR, Vela-Ojeda J,
Montiel-Cervantes L, Lopez-Santiago R and Moreno-Lafont MC:
Description of CD8(+) regulatory T lymphocytes and their specific
intervention in graft-versus-host and infectious diseases,
autoimmunity, and cancer. J Immunol Res. 2018:37587132018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Haanen JB, Thienen H and Blank CU:
Toxicity patterns with immunomodulating antibodies and their
combinations. Semi Oncol. 42:423–428. 2015. View Article : Google Scholar
|
|
45
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huard B, Tournier M, Hercend T, Triebel F
and Faure F: Lymphocyte-activation gene 3/major histocompatibility
complex class II interaction modulates the antigenic response of
CD4+ T lymphocytes. Eur J Immunol. 24:3216–3221. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Joller N and Kuchroo VK: Tim-3, Lag-3, and
TIGIT. Curr Top Microbiol Immunol. 410:127–156. 2017.PubMed/NCBI
|
|
48
|
Grosso JF, Kelleher CC, Harris TJ, Maris
CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC,
et al: LAG-3 regulates CD8+ T cell accumulation and effector
function in murine self- and tumor-tolerance systems. J Clin
Invest. 117:3383–3392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Workman CJ and Vignali DA: Negative
regulation of T cell homeostasis by lymphocyte activation gene-3
(CD223). J Immunol. 174:688–695. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Baitsch L, Legat A, Barba L, Fuertes
Marraco SA, Rivals JP, Baumgaertner P, Christiansen-Jucht C,
Bouzourene H, Rimoldi D, Pircher H, et al: Extended co-expression
of inhibitory receptors by human CD8 T-cells depending on
differentiation, antigen-specificity and anatomical localization.
PLoS One. 7:e308522012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Baitsch L, Baumgaertner P, Devêvre E,
Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke
B, Romero P, et al: Exhaustion of tumor-specific CD8(+) T cells in
metastases from melanoma patients. J Clin Invest. 121:2350–2360.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
McLane LM, Abdel-Hakeem MS and Wherry EJ:
CD8 T cell exhaustion during chronic viral infection and cancer.
Annu Rev Immunol. 37:457–495. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Andrews LP, Marciscano AE, Drake CG and
Vignali DA: LAG3 (CD223) as a cancer immunotherapy target. Immunol
Rev. 276:80–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
He Y, Rivard CJ, Rozeboom L, Yu H, Ellison
K, Kowalewski A, Zhou C and Hirsch FR: Lymphocyte-activation
gene-3, an important immune checkpoint in cancer. Cancer Sci.
107:1193–1197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Woo SR, Turnis ME, Goldberg MV, Bankoti J,
Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et
al: Immune inhibitory molecules LAG-3 and PD-1 synergistically
regulate T-cell function to promote tumoral immune escape. Cancer
Res. 72:917–927. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Casati C, Camisaschi C, Rini F, Arienti F,
Rivoltini L, Triebel F, Parmiani G and Castelli C: Soluble human
LAG-3 molecule amplifies the in vitro generation of type 1
tumor-specific immunity. Cancer Res. 66:4450–4460. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bettini M, Szymczak-Workman AL, Forbes K,
Castellaw AH, Selby M, Pan X, Drake CG, Korman AJ and Vignali DA:
Cutting edge: Accelerated autoimmune diabetes in the absence of
LAG-3. J Immunol. 187:3493–3498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Burugu S, Dancsok AR and Nielsen TO:
Emerging targets in cancer immunotherapy. Semin Cancer Biol.
52:39–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kuchroo VK, Anderson AC and Petrovas C:
Coinhibitory receptors and CD8 T cell exhaustion in chronic
infections. Curr Opin HIV AIDS. 9:439–445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hannier S and Triebel F: The MHC class II
ligand lymphocyte activation gene-3 is co-distributed with CD8 and
CD3-TCR molecules after their engagement by mAb or peptide-MHC
class I complexes. Int Immunol. 11:1745–1752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Triebel F: LAG-3: A regulator of T-cell
and DC responses and its use in therapeutic vaccination. Trends
Immunol. 24:619–622. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Villadolid J and Amin A: Immune checkpoint
inhibitors in clinical practice: Update on management of
immune-related toxicities. Transl Lung Cancer Res. 4:560–575.
2015.PubMed/NCBI
|
|
63
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhou G, Sprengers D, Boor PPC, Doukas M,
Schutz H, Mancham S, Pedroza-Gonzalez A, Polak WG, de Jonge J,
Gaspersz M, et al: Antibodies against immune checkpoint molecules
restore functions of tumor-infiltrating T cells in hepatocellular
carcinomas. Gastroenterology. 153:1107–1119.e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
He Y, Yu H, Rozeboom L, Rivard CJ, Ellison
K, Dziadziuszko R, Suda K, Ren S, Wu C, Hou L, et al: LAG-3 protein
expression in non-small cell lung cancer and its relationship with
PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol.
12:814–823. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Matsuzaki J, Gnjatic S, Mhawech-Fauceglia
P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant
P, Old LJ and Odunsi K: Tumor-infiltrating NY-ESO-1-specific CD8+ T
cells are negatively regulated by LAG-3 and PD-1 in human ovarian
cancer. Proc Natl Acad Sci USA. 107:7875–7880. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ma QY, Huang DY, Zhang HJ, Wang S and Chen
XF: Function and regulation of LAG3 on
CD4+CD25− T cells in non-small cell lung
cancer. Exp Cell Res. 360:358–364. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hald SM, Rakaee M, Martinez I, Richardsen
E, Al-Saad S, Paulsen EE, Blix ES, Kilvaer T, Andersen S, Busund
LT, et al: LAG-3 in non-small-cell lung cancer: expression in
primary tumors and metastatic lymph nodes is associated with
improved survival. Clin Lung Cancer. 19:249–259.e2. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li FJ, Zhang Y, Jin GX, Yao L and Wu DQ:
Expression of LAG-3 is coincident with the impaired effector
function of HBV-specific CD8(+) T cell in HCC patients. Immunol
Lett. 150:116–122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Camisaschi C, Casati C, Rini F, Perego M,
De Filippo A, Triebel F, Parmiani G, Belli F, Rivoltini L and
Castelli C: LAG-3 expression defines a subset of
CD4(+)CD25(high)Foxp3(+) regulatory T cells that are expanded at
tumor sites. J Immunol. 184:6545–6551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chen J and Chen Z: The effect of immune
microenvironment on the progression and prognosis of colorectal
cancer. Med Oncol. 31:822014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gagliani N, Magnani CF, Huber S, Gianolini
ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini
F, et al: Coexpression of CD49b and LAG-3 identifies human and
mouse T regulatory type 1 cells. Nat Med. 19:739–746. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen BJ, Dashnamoorthy R, Galera P,
Makarenko V, Chang H, Ghosh S and Evens AM: The immune checkpoint
molecules PD-1, PD-L1, TIM-3 and LAG-3 in diffuse large B-cell
lymphoma. Oncotarget. 10:2030–2040. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wierz M, Pierson S, Guyonnet L, Viry E,
Lequeux A, Oudin A, Niclou SP, Ollert M, Berchem G, Janji B, et al:
Dual PD1/LAG3 immune checkpoint blockade limits tumor development
in a murine model of chronic lymphocytic leukemia. Blood.
131:1617–1621. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Huang RY, Eppolito C, Lele S, Shrikant P,
Matsuzaki J and Odunsi K: LAG3 and PD1 co-inhibitory molecules
collaborate to limit CD8+ T cell signaling and dampen
antitumor immunity in a murine ovarian cancer model. Oncotarget.
6:27359–27377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Baumeister SH, Freeman GJ, Dranoff G and
Sharpe AH: Coinhibitory pathways in immunotherapy for cancer. Annu
Rev Immunol. 34:539–573. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li B, Chan HL and Chen P: Immune
checkpoint inhibitors: Basics and challenges. Curr Med Chem.
26:3009–3025. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Akinleye A and Rasool Z: Immune checkpoint
inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol.
12:922019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Llosa NJ, Cruise M, Tam A, Wicks EC,
Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS,
et al: The vigorous immune microenvironment of microsatellite
instable colon cancer is balanced by multiple counter-inhibitory
checkpoints. Cancer Discov. 5:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Vilgelm AE, Johnson DB and Richmond A:
Combinatorial approach to cancer immunotherapy: Strength in
numbers. J Leukoc Biol. 100:275–290. 2016. View Article : Google Scholar : PubMed/NCBI
|