Introduction
Thyroid cancer (TC) is the most common endocrine
malignancy and its incidence has increased rapidly in recent years
worldwide (1). Due to the absence of
obvious symptoms in the early stages of TC, ~30% of patients are
diagnosed at a late stage with lymph node metastases or invasion of
the surrounding tissues and organs (2). Furthermore, 10–20% of patients exhibit
distant metastases and recurrence, decreasing the 10-year survival
rate to 40% (3–5). In addition, selected TC cases are not
sensitive to radio-iodine treatment, and the subsequent curative
effect is poor (6). Therefore, it is
of great importance to understand the molecular mechanisms
underlying TC in order to develop and improve current therapeutic
approaches.
MicroRNAs (miRNAs) are a class of short endogenous
non-coding molecules (~22 nucleotides in length) that play key
roles in oncogenesis and cancer progression by regulating various
biological functions (7–9). Accumulating evidence has suggested that
aberrant miRNA expression contributes to multiple physiological and
pathological processes during TC (10–12). For
example, Boufraqech et al (13) indicated that miR-30a was
downregulated in TC, decreasing cellular invasion and migration by
regulating lysyl oxidase. In addition, miR-338-3p was revealed to
suppress cell proliferation, clonogenicity and metastasis in TC by
targeting RAC-γ serine/threonine-protein kinase (14). miR-215 was reported to inhibit TC
cell proliferation, migration and invasion via the AKT/glycogen
synthase kinase-3β/Snail signaling pathway by targeting brefeldin
A-inhibited guanine nucleotide-exchange protein 1 (15). Furthermore, Guan et al
(16) indicated that the
downregulation of miR-218-2 promoted the invasion and progression
of TC by regulating platelet-derived growth factor receptor α and
1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase γ-1. The
aim of the present study was to systematically investigate the
precise role of miR-3690 in TC and elucidate the underlying
mechanism, in order to determine the value of miR-3690 as a
biomarker for TC.
Materials and methods
Clinical specimens and expression
datasets
Clinical tissue samples were collected from 4 male
and 4 female patients (mean age, 35±8 years) who underwent total
thyroidectomy (cancerous samples were collected from unilateral
glandular lobe, and non-cancerous samples were collected from
unilateral glandular lobe on the other side) and who were
histopathologically diagnosed with TC between January 2016 and
January 2017 at Guangzhou First People's Hospital (Guangzhou,
China). The samples were immediately cryopreserved in liquid
nitrogen and stored at −80°C until further use. In order to
investigate the expression of miR-3690 in association with TC, the
miR-3690 expression data of 59 patients were downloaded from The
Cancer Genome Atlas (TCGA; http://cancergenome.nih.gov/) database and
subsequently analyzed.
Cell culture
The human thyroid follicular cell line Nthy-ori 3–1,
and various human TC cell lines, including TPC-1, SW1736, FRO, K1
and 8505C, were purchased from the National Rodent Laboratory
Animal Resource. The TC cell lines were authenticated by STR
profiling, and were cultured in RPMI-1640 medium (Invitrogen;
Thermo Fisher Scientific, Inc.) containing 10% FBS (Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 0.1 mg/ml streptomycin.
Nthy-ori 3-1 cells were cultured in F-12K medium (Genom Biotech
Pvt., Ltd.) containing 10% FBS, 100 U/ml penicillin and 0.1 mg/ml
streptomycin, and all cells were cultured in a humidified
atmosphere at 37°C (5% Co2).
Plasmids, short interfering RNA
(siRNA) and transfection
Synthetic miR-3690 (cat. no. miR10018119-1-5),
miR-3690-inhibitor (cat. no. miR20018119-1-5), miR-3690-mutant
(mut, 5′-GCUUGGACCCAGCGUAGACAAAG-3′) and relative negative control
miRNAs (vector: Cat. no. miR1N0000001-1-5, NC: Cat. no.
miR2N0000001-1-5) were synthesized by Guangzhou RiboBio Co., Ltd.
Cells transfections with 50 nM miRNAs were performed using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions.
Silencing DKK3 siRNAs (cat. no. HSH007526) were
synthesized and purified by GeneCopoeia, Inc., and transfections
were performed using Lipofectamine® 2000 transfection
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. All cells were harvested for subsequent
experimentation 48 h post-transfection.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNA from the human clinical tissues and
cultured cells was extracted using TRIzol® reagent
according to the manufacturer's recommendations (Thermo Fisher
Scientific, Inc.). Reverse transcription and detection of mRNA
(Cyclin E and c-myc) was performed using BlazeTaq™ SYBR®
Green qPCR mix 2.0 (GeneCopoeia, Inc.). RT-qPCR was performed to
detect miR-3690 expression using the All-in-One™ miRNA qRT-PCR
Detection kit 2.0 (GeneCopoeia, Inc, Guangzhou). Thermocycling
conditions were as follows: At 95°C for 30 sec, followed by 40
cycles of amplification at 95°C for 5 sec, at 59°C for 30 sec and
at 72°C for 30 sec. The following PCR primers were synthesized by
GeneCopoeia, Inc.: miR-3690 (cat. no. HmiRQP1976), cyclin E (cat.
no. HQP021819) and MYC (cat. no. HQP011597). U6 small nuclear RNA
(cat. no. HmiRQP9001) and GAPDH (cat. no. HQP006940) were used as
endogenous controls, and mRNA quantification was performed using
the 2−ΔΔCq method (17).
MTT and anchorage-independent growth
assays
In order to determine the cell proliferation rate,
an MTT assay (Sigma-Aldrich; Merck KGaA) was used according to the
manufacturer's protocol. The reaction was stopped by 200 µl DMSO;
the relative optical density was determined at a wavelength of 490
nm, and the results are expressed as the mean ± standard
deviation.
For the anchorage-independent growth assays,
transfected K1 cells (1×103 cell per well) were seeded
into 2 ml complete medium with 0.3% soft agar (Invitrogen; Thermo
Fisher Scientific, Inc.) and overlaid onto 2 ml preset 1.5% agar in
the same medium. The cells were cultured at 37°C with 5%
CO2 for 2 weeks, the colonies were then stained with
0.05% crystal violet for 10 min at room temperature, and colonies
sized >0.1 mm were counted under a microscope (Motic AE30
inverted fluorescence microscope; Microscope Systems Limited;
magnification, ×40).
Bioinformatics
The potential target genes of miR-3690 were
predicted using Target Scan Human (version 7.1; http://www.targetscan.org/vert_71). Luciferase
reporter assays. Based on the binding sites of DDK-3 and
miR-3690, which were predicted using TargetScan, wild-type DDK-3
sequences were designed and cloned into the pGL3 luciferase
reporter vector (Promega Corporation). Cells were then
co-transfected with the vectors and miR-3690, miR-3690-in or
miR-3690-mut, using Lipofectamine® 2000 transfection
reagent. Following a 48-h incubation, luciferase signals were
measured using the Dual luciferase reporter gene assay kit
(BioVision, Inc.) according to the manufacturer's protocol. Firefly
luciferase activity was normalized to that of Renilla
luciferase.
Western blotting
Transcfeted TC cells were lysed in protein RIPA
buffer (cat. no. P0013; Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. The protein concentration
was determined using a bicinchoninic acid protein assay. Proteins
(40 µg) were separated via 10% SDS-PAGE and subsequently
transferred onto PVDF membranes (EMD Millipore). Membranes were
blocked with 5% bovine serum albumin (BSA) for 1 h at room
temperature, and incubated with primary antibodies against: DKK3
(1:1,000; cat. no. SAB2701251; Sigma-Aldrich; Merck KGaA), cyclin E
(1:1,000; cat. no. 20808; Cell Signaling Technology, Inc.) and
c-myc (1:1,000; cat. no. 18583; Cell Signaling Technology, Inc.)
overnight at 4°C, as previously described (17). To control sample loading, the
membranes were stripped and re-probed with an anti-α-tubulin
antibody (1:500; cat. no. SAB5600206; Sigma-Aldrich; Merck KGaA).
The membranes were then probed with a peroxidase-conjugated
secondary antibody goat-anti-rabbit IgG (1:5,000; ZDR5306 or
ZDR5307; Zhongshan Golden Bridge Biotechnology, Inc.), and the
protein bands were quantified using Quantity One software version
4.6 (Bio-Rad Laboratories, Inc.).
Bromodeoxyuridine (BrdU) incorporation
analysis
To assess cell proliferation, cells were cultured
for 24 h following transfection. The medium was then supplemented
with 10 h BrdU and the cells were incubated for a further 6 h. The
labeled cells were thoroughly rinsed with PBS and incubated with an
anti-BrdU antibody (1:500; cat no. 61273; Upstate Biotechnology,
Inc.) for 1 h at 37°C, according to the manufacturer's protocol.
Images were captured using a laser scanning microscope
(magnification, ×100; Axioskop 2 plus; Carl Zeiss AG).
Cell cycle distribution analysis
Following miRNA-transfection for 48 h, the cells
were harvested, washed in ice-cold PBS, and fixed with pre-chilled
75% ethanol at 4°C overnight. The cells were stained using a
propidium iodide/RNase staining buffer (BD Biosciences) at room
temperature for 30 min in the dark, and the cell cycle distribution
was analyzed using a FACSCalibur™ flow cytometer (BD Biosciences)
according to the manufacturer's instructions.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc.). The data were analyzed using a paired Student's
t-test for pair-wise comparisons or one-way analysis of variance
followed by a post hoc Tukey test for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Upregulation of miR-3690 in TC
clinical tissues and cell lines
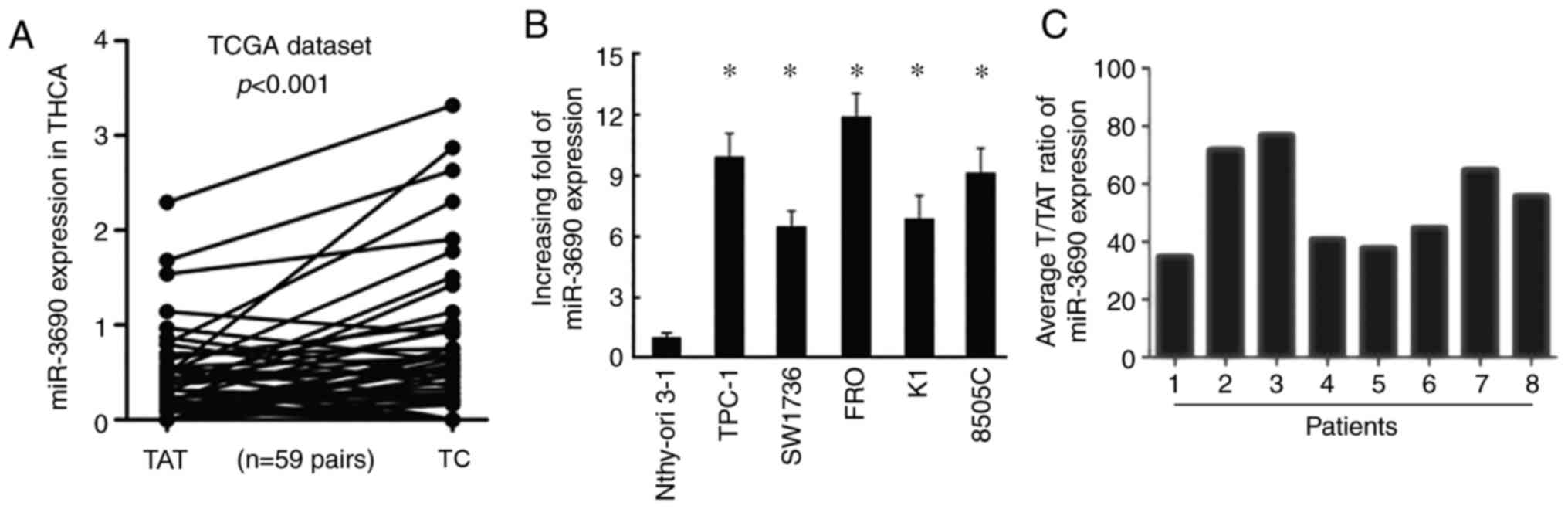
Based on the expression data downloaded from TCGA,
the expression level of miR-3690 was found to be higher in TC
tissues compared with that in adjacent non-tumor tissues (Fig. 1A). Consistently, miR-3690 expression
was also significantly increased in TC cell lines (TPC-1, SW1736,
FRO, K1 and 8505C) compared with that in human thyroid follicular
cells (Nthy-ori 3-1; Fig. 1B).
Furthermore, RT-qPCR analysis of samples from 8 patients with TC
revealed significantly increased levels of miR-3690 in TC tissues
(T) compared with tumor-adjacent normal tissues (TAT; Fig. 1C).
miR-3690 promotes TC cell
proliferation, which is counteracted by miR-3690-in
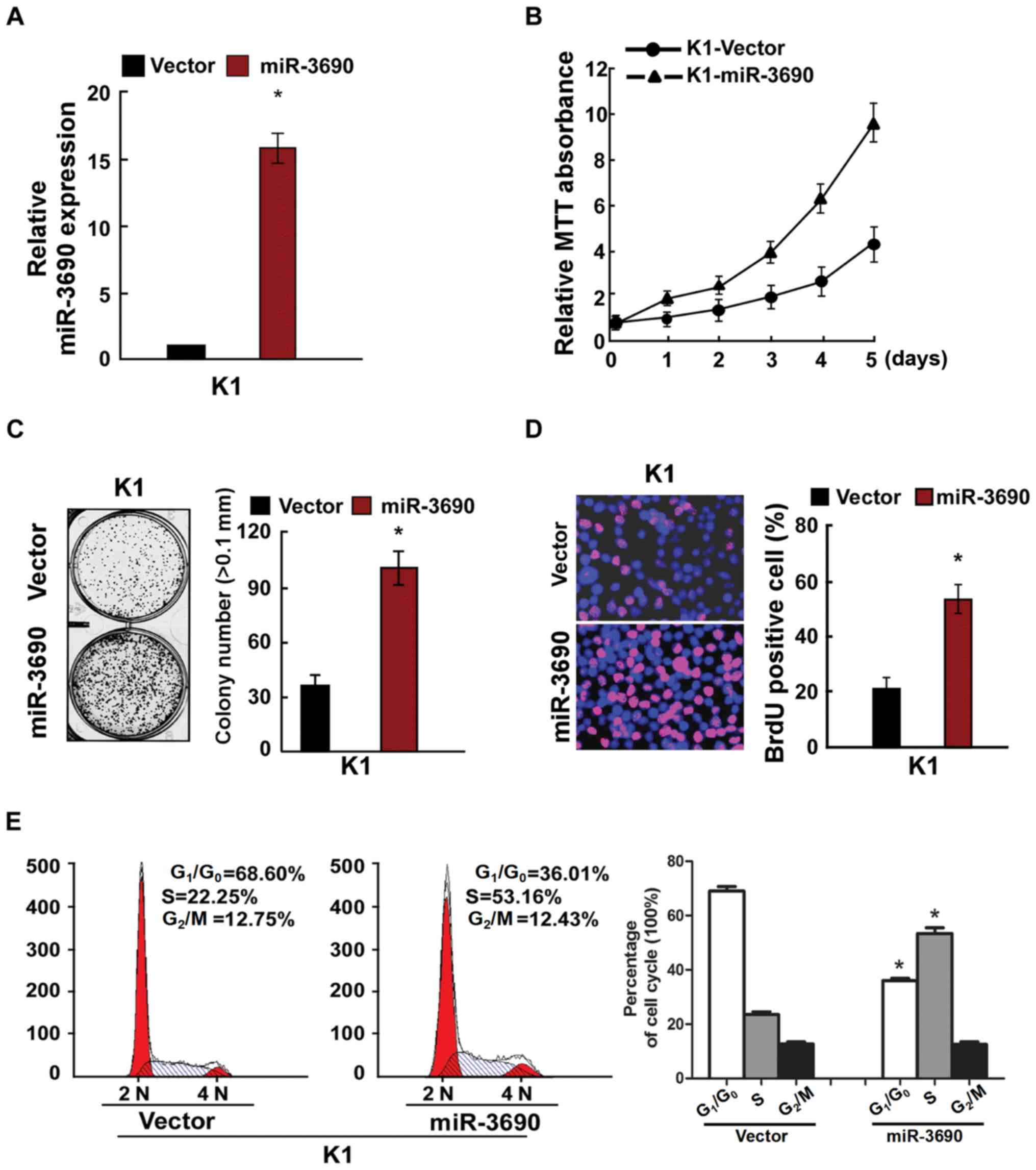
To determine the effects of miR-3690 on cellular
proliferation, TC cells were transfected with miR-3690 and assessed
using RT-qPCR, MTT and anchorage-independent growth assays, in
addition to BrdU labeling, immunofluorescence and cell cycle
analysis. Following transfection of K1 cells, the expression level
of miR-3690 was found to be significantly upregulated (Fig. 2A), and miR-3690 overexpression
facilitated cellular proliferation (Fig.
2B-D). Furthermore, miR-3690 overexpression significantly
decreased the number of cells in the G1/G0
phase, but increased the proportion of S phase cells, compared with
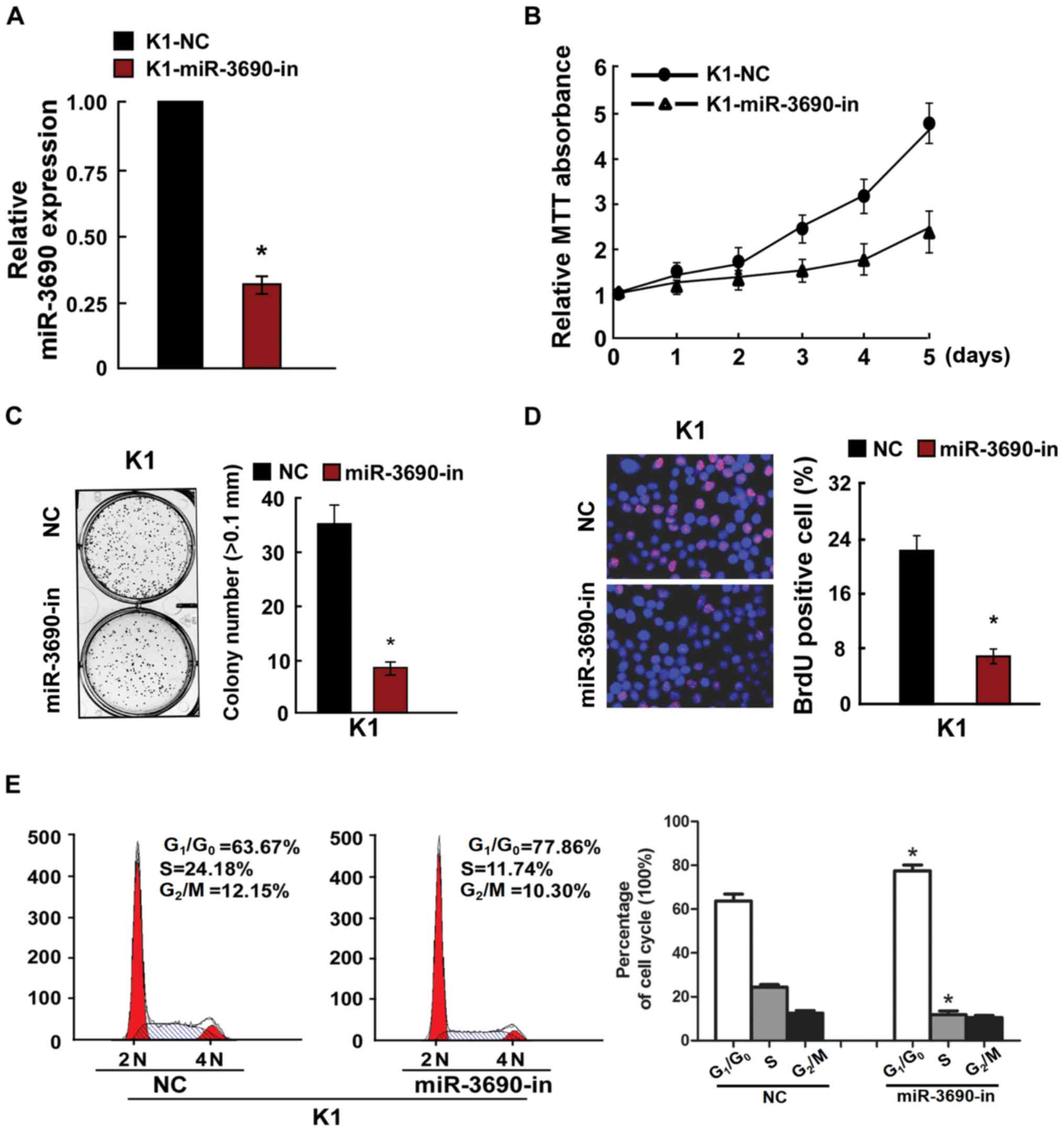
the control at 48 h post-transfection (Fig. 2E). In turn, miR-3690 expression was
successfully inhibited by miR-3690-in transfection in K1 cells
(Fig. 3A), which significantly
suppressed proliferation and cell cycle progression compared with
the control-transfected cells (Fig.
3B-E). Further to the effects of miR-3690 on TC cell
proliferation, the results of the MTT and anchorage-independent
growth assays revealed that the SW1736 cell line exhibited a lower
miR-3690 expression level compared with the K1 line, and thus was
selected for further experimentation. As presented in Fig. S1, the results reflected those
obtained from K1 cells.
miR-3690 regulates DKK3 via the 3′-UTR
and alters cell proliferation and the expression of cell
cycle-associated genes
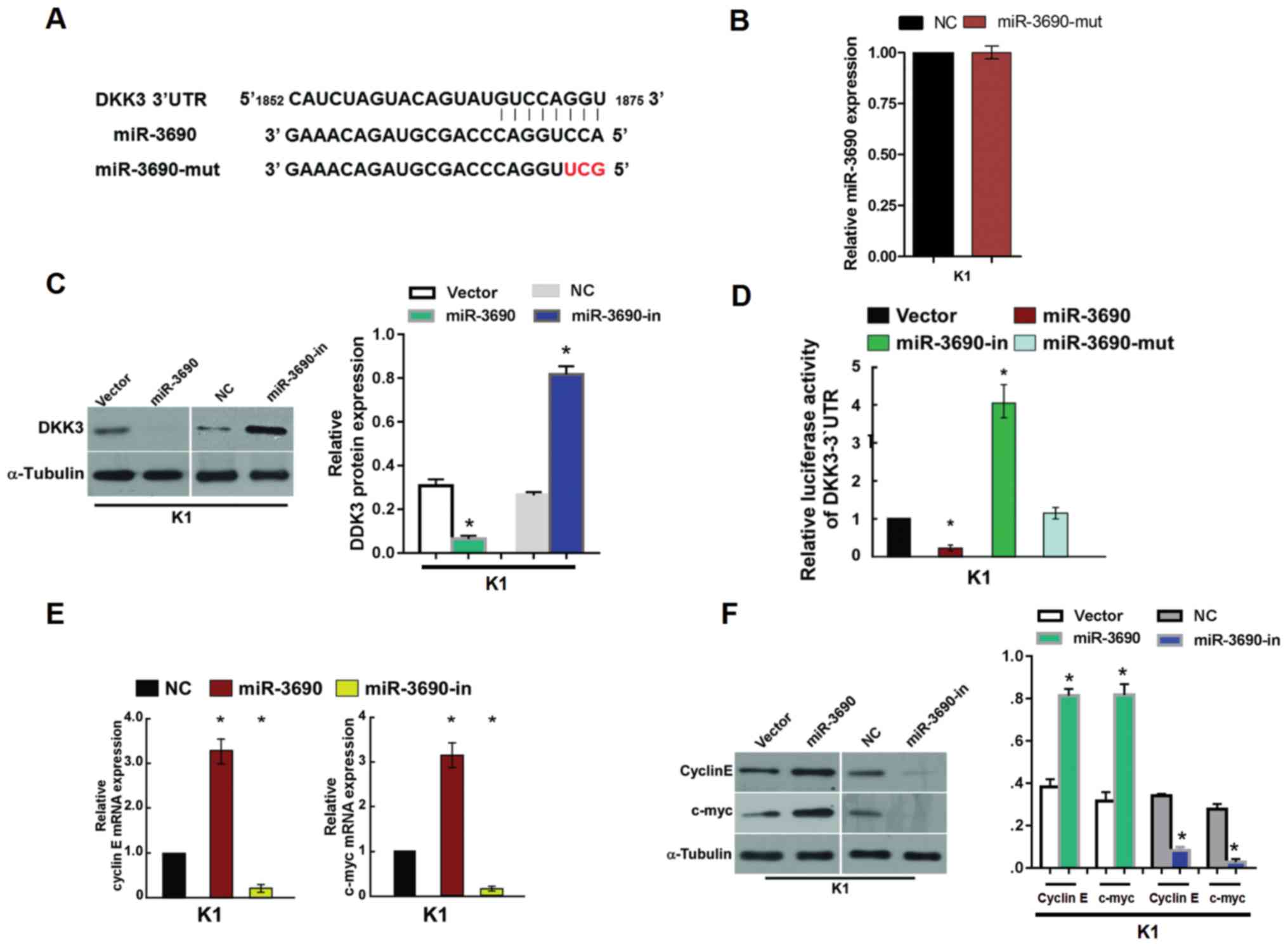
In order to identify the potential targets of
miR-3690, a bioinformatics analysis was conducted using TargetScan,
and revealed that DKK3 contains a putative binding site for
miR-3690 (Fig. 4A). The effect of
miR-3690-mut on TC cells was detected via RT-PCR analysis, the
result showed that no effects were observed following transfection
with miR-3690-mut compared with that in NC (Fig. 4B). To investigate the potential
regulatory effect of miR-3690 on DKK3, protein expression levels
were examined by western blot analysis in the present study.
miR-3690 overexpression significantly decreased the expression
levels of the DKK3 protein in K1 cells, while the levels were found
to be upregulated following miR-3690-in transfection (Fig. 4C). Furthermore, luciferase reported
assays indicated that miR-3690 significantly decreased the
luciferase activity of the wild-type 3′-UTR of DKK3, whereas
miR-3690-in transfection resulted in increased activity; no
suppressive effects were observed following transfection with
miR-3690-mut (Fig. 4D). These
findings indicate that DKK3 is a direct target of miR-3690.
As miR-3690 promoted cell proliferation and cell
cycle progression, regulatory genes associated with these processes
were detected. RT-qPCR and western blot analysis revealed elevated
mRNA and protein expression levels, respectively, of cyclin E and
c-myc in miR-3690-transfected cells, which was consistent with the
increased proportion of S phase and decreased proportion of
G1/G0 phase cells (Fig. 4E and F). By contrast, decreased
expression of cyclin E and c-myc was detected following
transfection with miR-3690-in, which was reflected by cell cycle
arrest in the G1/G0 phase (Fig. 4D and E).
DKK3 knockdown counteracts
miR-3690-in-associated proliferative arrest
As DKK3 was confirmed to be a direct target of
miR-3690, the effects of DKK3 downregulation on miR-3690-in-induced
proliferative arrest were investigated. Following treatment with
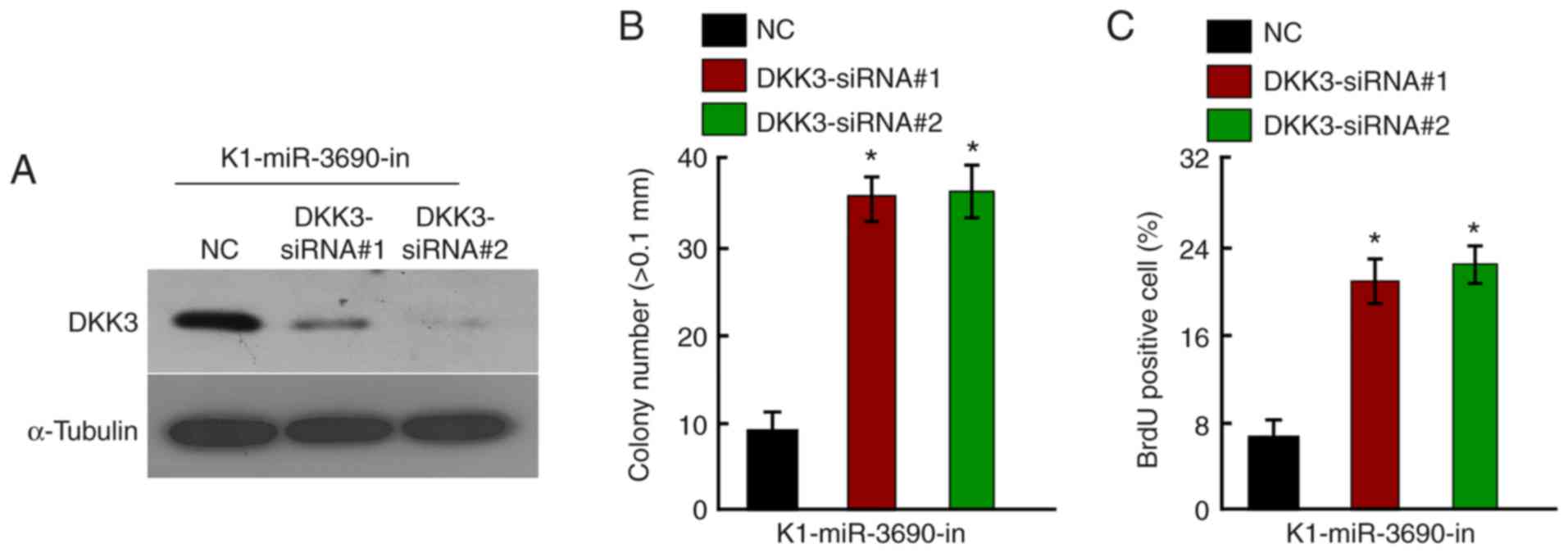
miR-3690-in, DKK3 was knocked down in K1 cells using specific
siRNAs; knockdown efficiency was confirmed by western blotting
(Fig. 5A). The growth-suppressive
effect of miR-3690-in was partially abrogated by DKK3-knockdown, as
demonstrated by anchorage-independent growth (Fig. 5B), BrdU labeling and
immunofluorescence assays (Fig. 5C).
These findings indicate that DKK3 was involved in the
miR-3690-in-induced suppression of cellular proliferation.
Discussion
Profiling studies have indicated that miRNAs play an
essential role in TC development by regulating their target genes.
miR-195 was found to suppress tumor growth and metastasis in TC by
targeting G1/S-specific cyclin D1 and fibroblast growth
factor 2 (18); additionally,
miR-577 was reported to inhibit the proliferation, migration and
invasive capacity of TC cells by targeting sphingosine kinase 2
(19). The findings of Ye et
al (20) indicated that
fibronectin 1 was a direct target of miR-139, which inhibits TC
progression. Furthermore, Sun et al (21) revealed that miR-144 suppressed TC
cell proliferation through WW domain-containing transcription
regulator protein 1. Our previous study revealed that miR-639
promoted cell proliferation and cell cycle progression in TC by
inhibiting cyclin-dependent kinase inhibitor 1 (22). Data from the present study provide
evidence that miR-3690 expression is upregulated in TC tissues from
patients and TC cell lines, and also suggest that miR-3690 promotes
cell proliferation and cell cycle progression by directly
suppressing DKK3.
Human DKK3 is a member of the Dickkopf family, which
perturbs the negative regulator Kremen and enhances Wnt signaling
via low-density lipoprotein receptor-related protein 6 (23). Previous studies have demonstrated
that DKK can act as a tumor promoter or suppressor in certain types
of cancer, thereby affecting tumor development and progression.
DDK3 has been reported to stimulate pancreatic ductal
adenocarcinoma growth and metastasis, as well as resistance to
chemotherapy, by activating NF-κB (24). In addition, overexpression of DKK3
reportedly contributes to tumor cell proliferation, invasion,
migration and survival in head and neck squamous cell carcinoma by
inducing PI3K-Akt signaling (25).
Furthermore, Xi et al (26)
observed that DKK3 was downregulated in prostatic cancer, and that
miR-95-3p contributed to the development of prostatic cancer by
repressing DKK3, thus activating the Wnt/β-catenin pathway. In
gastric cancer, DKK3 was shown to attenuate CD133-induced NK-cell
activation by inhibiting the Erk pathway and immunological synapse
formation (27). DKK3 was also found
to act as a biomarker and a therapeutic target by modulating Wnt
signaling in several types of human cancer (28). However, the role of DKK3 in TC
remains understudied. To the best of our knowledge, the present
study was the first to demonstrate that DKK3 was a downstream
target gene of miR-3690, and is considered to act as a tumor
suppressor in TC. Furthermore, DKK3 downregulation counteracted
miR-3690-in-associated proliferative arrest.
The present study revealed that miR-3690 promotes
cellular proliferation by regulating proliferation- and cell
cycle-associated genes, including cyclin E and c-myc. Cyclin E is a
key checkpoint protein for G1-to-S phase transition in the cell
cycle (29). C-myc is a known master
regulator of the cell cycle and plays a critical role in
carcinogenesis (30). In gastric
carcinoma, downregulated DKK3 (REIC) expression was closely
associated with aggressive phenotypes and poor prognosis (31). Ectopic DKK3 expression downregulated
β-catenin, cyclin D2 and E expression levels, as well as regulated
the cell cycle in gastric carcinoma (31). The results of the present study
revealed that downregulation of miR-3690 resulted in cell cycle
arrest at the G1/G0 phase in TC.
In conclusion, the present study demonstrated that
miR-3690 was upregulated and promoted cancer cell proliferation by
directly repressing DKK3. Downregulation of miR-3690 resulted in
cell cycle arrest at the G1/G0 phase by
inhibiting cyclin E and c-myc, which may affect the development of
TC. These findings indicate that miR-3690 may be of value as a
therapeutic target in TC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Guangzhou Science
and Technology Plan Project (grant no. 201805010003).
Availability of data and materials
The datasets generated or analyzed during the
present study are included in this published article.
Authors' contributions
FS and BX designed the present study. FS, XG, XD and
BX drafted the initial manuscript. FS, XG, XD and JF performed the
experiments. FS, XG, XD, JF, WC, LS and HX analyzed the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Guangzhou First People's Hospital (Guangzhou, China;
approval no. 185-01), and written informed consent was provided by
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao H, Huang T and Li H: Risk factors for
skip metastasis and lateral lymph node metastasis of papillary
thyroid cancer. Surgery. 166:55–60. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cabanillas ME, McFadden DG and Durante C:
Thyroid cancer. Lancet. 388:2783–2795. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim H, Devesa SS, Sosa JA, Check D and
Kitahara CM: Trends in thyroid cancer incidence and mortality in
the United States, 1974–2013. JAMA. 317:1338–1348. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kitahara CM and Sosa JA: The changing
incidence of thyroid cancer. Nat Rev Endocrinol. 12:646–653. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wagner M, Khoury H, Bennetts L, Willet J,
Lister J, Berto P, Ehreth J, Badia X, Grimaldi-Bensouda L and
Goetghebeur M: Appraising the value of lenvatinib for radio-iodine
refractory differentiated thyroid cancer (Rr-Dtc): A multi-country
study applying holistic multicriteria decision analysis (Mcda).
Value Health. 18:PA477–PA478. 2015. View Article : Google Scholar
|
|
7
|
Pei ZJ, Zhang ZG, Hu AX, Yang F and Gai Y:
miR-122-5p inhibits tumor cell proliferation and induces apoptosis
by targeting MYC in gastric cancer cells. Pharmazie. 72:344–347.
2017.PubMed/NCBI
|
|
8
|
Xu FF, Xie WF, Zha GQ, Chen HW and Deng L:
MiR-520f promotes cell aggressiveness by regulating fibroblast
growth factor 16 in hepatocellular carcinoma. Oncotarget.
8:109546–109558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang WL, Cao J, Xu B, Yang P, Shen F, Sun
Z, Li WL, Wang Q and Liu F: miR-892a regulated PPP2R2A expression
and promoted cell proliferation of human colorectal cancer cells.
Biomed Pharmacother. 72:119–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsai MM, Wang CS, Tsai CY, Huang CG, Lee
KF, Huang HW, Lin YH, Chi HC, Kuo LM, Lu PH and Lin KH: Circulating
microRNA-196a/b are novel biomarkers associated with metastatic
gastric cancer. Eur J Cancer. 64:137–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong Y, Wu W, Zou X, Liu F, Wei T and Zhu
J: MiR-26a inhibits thyroid cancer cell proliferation by targeting
ARPP19. Am J Cancer Res. 8:1030–1039. 2018.PubMed/NCBI
|
|
12
|
Sun J, Shi R, Zhao S, Li X, Lu S, Bu H, Ma
X and Su C: E2F8, a direct target of miR-144, promotes papillary
thyroid cancer progression via regulating cell cycle. J Exp Clin
Cancer Res. 36:402017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boufraqech M, Nilubol N, Zhang L, Gara SK,
Sadowski SM, Mehta A, He M, Davis S, Dreiling J, Copland JA, et al:
miR30a inhibits LOX expression and anaplastic thyroid cancer
progression. Cancer Res. 75:367–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sui GQ, Fei D, Guo F, Zhen X, Luo Q, Yin S
and Wang H: MicroRNA-338-3p inhibits thyroid cancer progression
through targeting AKT3. Am J Cancer Res. 7:1177–1187.
2017.PubMed/NCBI
|
|
15
|
Han J, Zhang M, Nie C, Jia J, Wang F, Yu
J, Bi W, Liu B, Sheng R, He G, et al: miR-215 suppresses papillary
thyroid cancer proliferation, migration, and invasion through the
AKT/GSK-3β/Snail signaling by targeting ARFGEF1. Cell Death Dis.
10:1952019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guan H, Wei G, Wu J, Fang D, Liao Z, Xiao
H, Li M and Li Y: Down-regulation of miR-218-2 and its host gene
SLIT3 cooperate to promote invasion and progression of thyroid
cancer. J Clin Endocrinol Metab. 98:E1334–E1344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin Y, Hong S, Yu S, Huang Y, Chen S, Liu
Y, Zhang Q, Li Y and Xiao H: MiR-195 inhibits tumor growth and
metastasis in papillary thyroid carcinoma cell lines by targeting
CCND1 and FGF2. Int J Endocrinol. 2017:61804252017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue KC, Hu DD, Zhao L, Li N and Shen HY:
MiR-577 inhibits papillary thyroid carcinoma cell proliferation,
migration and invasion by targeting SphK2. Eur Rev Med Pharmacol
Sci. 21:3794–3800. 2017.PubMed/NCBI
|
|
20
|
Ye Y, Zhuang J, Wang G, He S, Ni J and Xia
W: MicroRNA-139 targets fibronectin 1 to inhibit papillary thyroid
carcinoma progression. Oncol Lett. 14:7799–7806. 2017.PubMed/NCBI
|
|
21
|
Sun W, Lan X, Wang Z, Dong W, He L, Zhang
T, Zhang P and Zhang H: MicroRNA-144 inhibits proliferation by
targeting WW domain-containing transcription regulator protein 1 in
papillary thyroid cancer. Oncol Lett. 15:1007–1013. 2018.PubMed/NCBI
|
|
22
|
Lei ST, Shen F, Chen JW, Feng JH, Cai WS,
Shen L, Hu ZW and Xu B: MiR-639 promoted cell proliferation and
cell cycle in human thyroid cancer by suppressing CDKN1A
expression. Biomed Pharmacother. 84:1834–1840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferrari N, Ranftl R, Chicherova I, Slaven
ND, Moeendarbary E, Farrugia AJ, Lam M, Semiannikova M, Westergaard
MCW, Tchou J, et al: Dickkopf-3 links HSF1 and YAP/TAZ signalling
to control aggressive behaviours in cancer-associated fibroblasts.
Nat Commun. 10:1302019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou L, Husted H, Moore T, Lu M, Deng D,
Liu Y, Ramachandran V, Arumugam T, Niehrs C, Wang H, et al:
Suppression of stromal-derived Dickkopf-3 (DKK3) inhibits tumor
progression and prolongs survival in pancreatic ductal
adenocarcinoma. Sci Transl Med. 10:eaat34872018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Katase N, Nishimatsu SI, Yamauchi A,
Yamamura M, Terada K, Itadani M, Okada N, Hassan NMM, Nagatsuka H,
Ikeda T, et al: DKK3 overexpression increases the malignant
properties of head and neck squamous cell carcinoma cells. Oncol
Res. 26:45–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xi M, Cheng L, Hua W, Zhou YL, Gao QL,
Yang JX and Qi SY: MicroRNA-95-3p promoted the development of
prostatic cancer via regulating DKK3 and activating Wnt/β-catenin
pathway. Eur Rev Med Pharmacol Sci. 23:1002–1011. 2019.PubMed/NCBI
|
|
27
|
Xia P and Xu XY: DKK3 attenuates the
cytotoxic effect of natural killer cells on CD133 +
gastric cancer cells. Mol Carcinog. 56:1712–1721. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hamzehzadeh L, Caraglia M, Atkin SL and
Sahebkar A: Dickkopf homolog 3 (DKK3): A candidate for detection
and treatment of cancers? J Cell Physiol. 233:4595–4605. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jing L, Gong M, Lu X, Jiang Y, Li H and
Cheng W: LINC01127 promotes the development of ovarian tumors by
regulating the cell cycle. Am J Transl Res. 11:406–417.
2019.PubMed/NCBI
|
|
30
|
Elliott B, Millena AC, Matyunina L, Zhang
M, Zou J, Wang G, Zhang Q, Bowen N, Eaton V, Webb G, et al:
Essential role of JunD in cell proliferation is mediated via MYC
signaling in prostate cancer cells. Cancer Lett. 448:155–167. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu XY, Xia P, Yu M, Nie XC, Yang X, Xing
YN, Liu YP, Takano Y and Zheng HC: The roles of REIC gene and its
encoding product in gastric carcinoma. Cell Cycle. 11:1414–1431.
2012. View
Article : Google Scholar : PubMed/NCBI
|