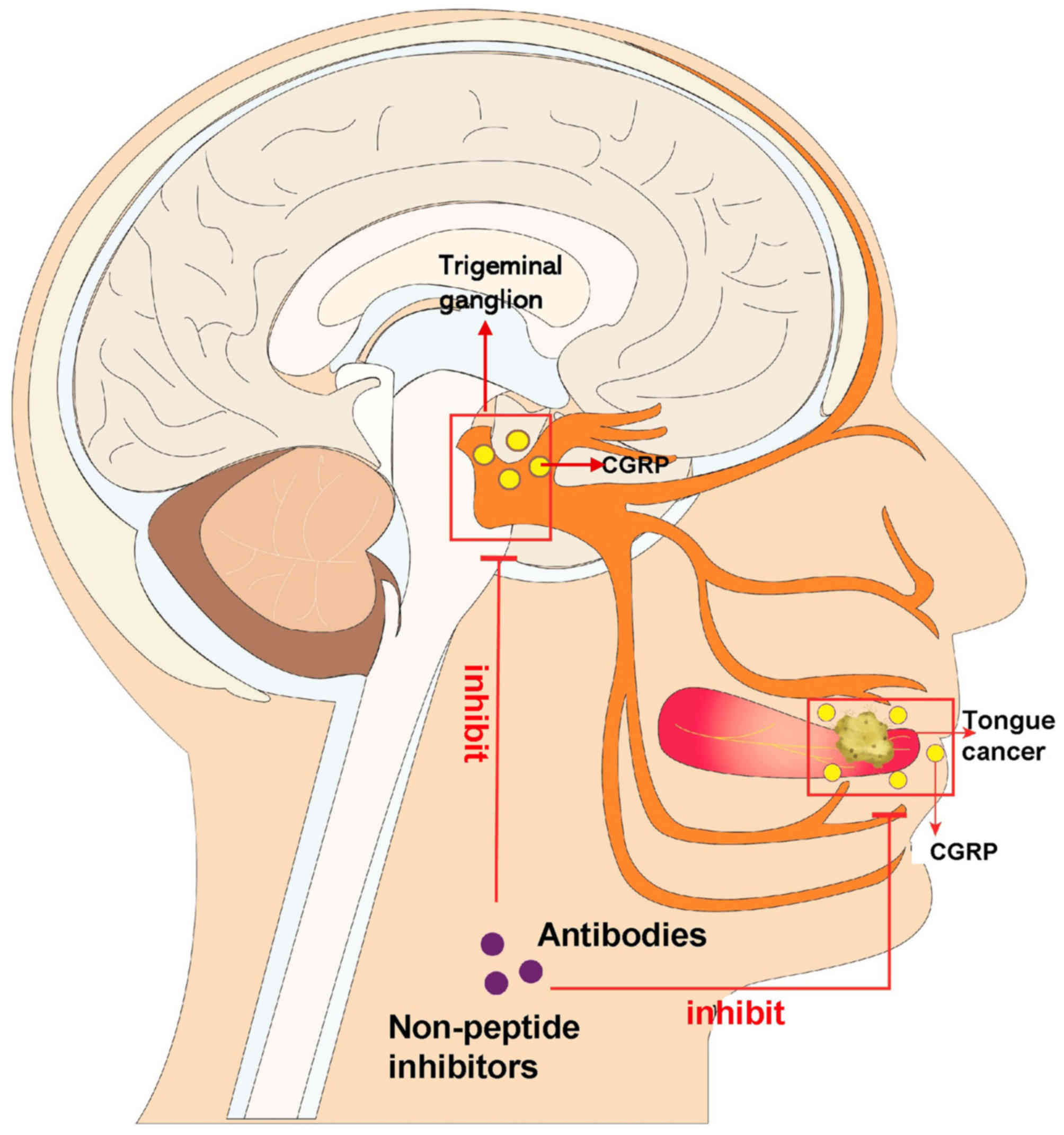
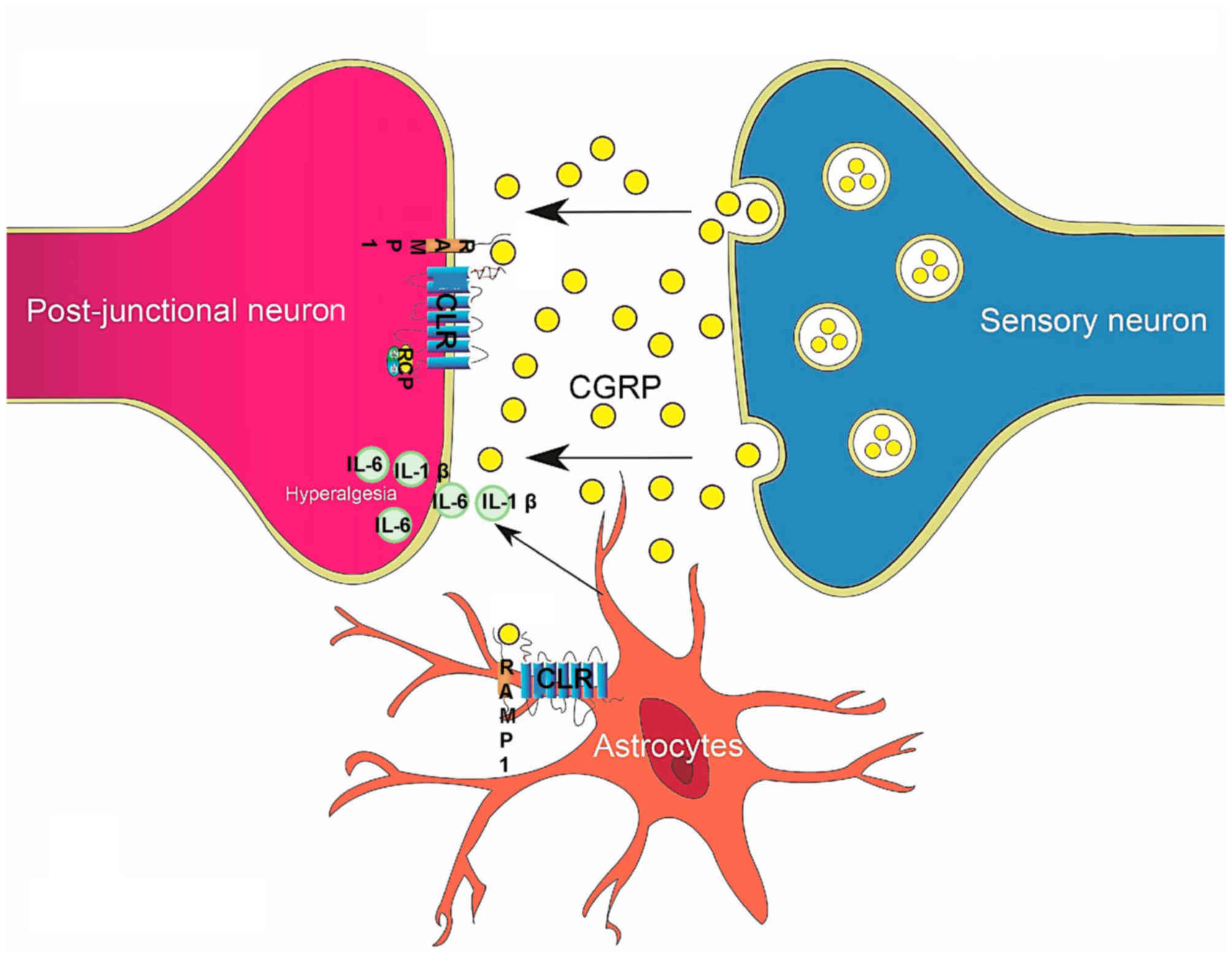
Similarly to normal organs, tumors establish their
own microenvironment to fulfill their dynamic growing demands. This
microenvironment may include the extracellular matrix, vessels,
lymphangions and, as an increasingly recognized compartment, nerves
(1). The role of vessels in tumor
microenvironments has been widely explored (2,3).
However, so for the curative effects of anti-angiogenesis
treatments have been proven dissatisfying, and more and more
studies find that the nerves play a pivotal role in various cancer
(4,5).
Oral cancer is a common malignant cancer type in the
head and neck region with over 50,000 new cases and 10,000 new
deaths every year in the U.S.A (6).
Oral squamous cell carcinoma (OSCC) constitutes 90% of cases of
oral cancer (6). OSCC is associated
with severe disease and treatment-associated morbidity, and is
frequently reported as having high rates of recurrence despite
advances in cancer treatment (7).
The oral cavity is innervated by cranial nerves with a high density
of sensory nerves, particularly the trigeminal nerve (8). Therefore, pain is the most frequent
complaint at the primary site (9),
and the prevalence and intensity of OSCC-associated pain are higher
than those in all other types of cancer (10,11). The
mechanisms involved in the OSCC-nerve microenvironment are likely
to differ from those of prostate, pancreatic and breast cancer,
which are predominantly innervated by autonomic nerves (4,12).
As the most abundant neuropeptide in the trigeminal
ganglion, the calcitonin gene-related peptide (CGRP) exerts a dual
effect on both cancer development and cancer-associated pain in
various types of cancer, such as osteosarcoma (13) and breast cancer (14). The present review discussed the
potential molecular mechanisms by which CGRP is implicated in the
development of OSCC and associated pain, suggesting that CGRP may
be a promising therapeutic target in OSCC (Fig. 1).
CGRP is a 37-amino acid peptide that has a
ubiquitous distribution, particularly in trigeminal ganglions (TGs)
(15). CGRP has two major isoforms:
α- and β-CGRP, which have similar structures and biological
activities, but are encoded by separate genes(CALCA for
α-CGRP and CALCB for β-CGRP) (16). In general, it is thought that α-CGRP
is the cardinal form occurring in the central and peripheral
nervous system, whereas β-CGRP, the only product of the CALCB gene,
acts mainly in the enteric nervous system (16). Along with the wide innervation of C
and Aδ fibers, CGRP exerts various physical effects, including the
regulation of energy metabolism, cardiovascular function and pain
conduction (17). CGRP has crucial
functions in pathological situations, including hypertension,
migraine, atherosclerosis and vessel remodeling, which has been
elaborated on in a previous review (17).
Pain is one of the most common factors affecting the
quality of life in patients with cancer and 75–95% of patients with
metastatic or advanced-stage cancer will experience considerable
amounts of cancer-induced pain (30). The incidence rate of cancer related
pain varies for different types of cancers. For instance, breast
adenocarcinoma is frequently characterized as being painful only
after it metastasizes to the bone (31). However, melanomas (even in the head
and neck) are typically not painful at the primary site or
metastatic sites (32). In OSCC,
pain is the most frequent complaint at the primary site (9), and the prevalence and intensity of
OSCC-associated pain are higher than in all other types of cancer
(10,11). In a prospective study, the
pretreatment pain in OSCC has been able to predict peripheral nerve
invasion (33), and was identified
as an independent predictor of survival in 2,340 patients with OSCC
in a retrospective study (10). The
dorsal root ganglion (DRG) and TG neurons are pseudounipolar, which
means that the axon bifurcates and sends projections to both the
dorsal horn of the spinal cord or brainstem, and to the periphery
(34). This unique morphology allows
the release of transmitters at central and peripheral sites, and
allows for bidirectional communication between the two terminals
(35). When peripheral noxious
stimuli (including high hydrogen, high temperature, capsaicin or
prostaglandin E2) act on peripheral nociceptors, CGRP serves a dual
role in afferent and efferent nerves (36). On one hand, CGRP is released in the
synapse of primary afferent nerves through anterograde axoplasmic
transport and acts on its receptors on the postsynaptic neurons in
the DRG and TG, thus transmitting algesia to pain centers. On the
other hand, it is also released from peripheral nerve terminals and
exerts paracrine effects on the surrounding tissues by retrograde
axoplasmic transport (36).
CGRP modulates pain in two major ways. A relatively
well-researched mechanism is that following the vasodilation effect
of CGRP, the accumulated proinflammatory factors irritate
peripheral nociceptors. In addition, CGRP may directly influence
peripheral and central sensitization, resulting in hyperalgesia and
allodynia (37) (Fig. 2). A recent study demonstrated that
CGRP induces differential regulation of cytokines, mostly
interleukin (IL)-1β and IL-6, from satellite glial cells in TG,
causing increased pain (37). In
most situations, these two effects often co-exist and mutually
promote each other (36).
The present review summarized the existent studies
on the role of CGRP in cancer-associated pain (Table SI). Most studies used in vivo
experiments to reveal that there are higher numbers of CGRP(+)
sensory nerves sprouting adjacent to an ectopic cancer and in the
DRG or TG than elsewhere, and mechanical allodynia and thermal
hyperalgesia exist in various types of tumor, such as gingival
cancer, metastatic bone cancer and breast cancer (35,38–45).
Animal models should be optimized to study the role of CGRP in
OSCC-associated pain. Nagamine et al (44) established a rat model of oral cancer
pain by inoculating cancer cells into the lower gingiva, and
instead of hind paw withdrawal, used head withdrawal as the
evaluation index; in addition, it was revealed that the mechanical
allodynia and thermal hyperalgesia in the ipsilateral maxillary and
mandibular nerve area were accompanied by nerves with upregulated
CGRP expression in the TG. Although this was an innovative
discovery, perhaps adding another assessment index, such as food
intake, may help to confirm this result.
Overall, CGRP exerts considerable roles in
cancer-associated pain, as well as in OSCC. The active sites of
CGRP are the peripheral nociceptors and central nerve endings in
the DRG or TG. However, the current in vivo models and
antagonists require further improvements.
Initially, high levels of CGRP were identified in
tumor tissues and serum of patients with medullary thyroid
carcinoma (46). Subsequent studies
emphasized the diagnostic value of CGRP in non-small cell lung
carcinoma (47,48), neuroendocrine tumors (49), prostate cancer (50,51) and
particularly medullary thyroid carcinoma (46,52).
Recently, Angenendt et al (53) analyzed the expression levels of the
gene encoding the CLR, CALCRL, in >1,500 patients with
well-characterized acute myeloid leukemia from five international
cohorts, revealing that increasing transcript levels of CALCRL were
associated with decreasing complete remission rates, 5-year overall
survival and event-free survival. Furthermore, CRISPR-Cas9-mediated
knockout of CALCRL markedly impaired colony formation in human
myeloid leukemia cell lines (53).
One way in which CGRP is able to influence tumor
behavior is secondary to its potent vasodilation effect. A previous
study on CGRP-knockout mice revealed that endogenous CGRP
facilitated tumor-associated angiogenesis and tumor growth in Lewis
lung carcinoma-bearing mice and that the administration of the CGRP
antagonist CGRP8-37 or denervation markedly suppressed tumor growth
and tumor-associated angiogenesis (54).
A number of studies have revealed that CGRP can
directly modulate development of various types of cancer, with a
large amount of literature reporting the expression of the CGRP
receptor complex in cancer cells (55–57).
CGRP was demonstrated to be able to modify the chemokinetic
abilities of a metastatic breast cancer cell line and increase the
expression of its receptors (58).
In addition, it has been demonstrated that CGRP increased the
invasive ability of prostate cancer PC-3 cells and an osteosarcoma
cell line (13,50). Recently, Dallmayer et al
(56) revealed that targeting the
CALCB/RAMP1 (the receptor complex of β-CGRP) axis inhibited the
growth of Ewing sarcoma cells. Despite the lack of studies on the
role of CGRP in OSCC, several studies have revealed a wide
distribution of CLR/RAMP1 in oral soft tissues, bones and dental
tissues (59). Therefore, the
present review discussed the mechanisms that CGRP may utilize in
cancer development.
It is well known that rapidly growing cancer cells
tend to utilize glycolysis during proliferation, regardless of
oxygen availability, which is known as the Warburg effect (60). Aerobic glycolysis is one of the
hallmarks of cancer, including OSCC (61). Various enzymes participating in
glycolysis are upregulated in OSCC and are associated with poor
prognosis (62–64). Rossetti et al (65) demonstrated that CGRP potently
antagonizes the effect of insulin in glycogen synthesis and
enhances glycolysis in muscles, which was attributed to the
activation of cyclic adenosine monophosphate (cAMP). However, a
recent study on insulin resistance revealed that with the
activation of transient receptor potential vanilloid-1
proteins/CGRP axis, glucose transporter 4 expression was
upregulated at the protein and mRNA levels, which ameliorated
insulin resistance (66). Despite
the disparity of certain results, these findings suggest that CGRP
may serve important roles in cell glycometabolism.
A pivotal signaling pathway in cell glycometabolism
in which CGRP participates is the AMP-activated protein kinase
(AMPK) signaling pathway (67). AMPK
senses the intracellular AMP/adenosine triphosphate (ATP) or
adenosine diphosphate/ATP levels, promoting metabolic pathways that
generate ATP, such as glycolysis and lipolysis, and inhibiting
those consuming ATP, such as glycogen and lipid synthesis (68). Danaher et al (67) suggested that CGRP activates AMPK
through phosphorylation of Thr172 in its α-subunit. As a
serine/threonine protein kinase that works as a sensor of cellular
energy, phosphorylated AMPK is able to directly phosphorylate
proteins or modulate gene transcription involved in glucose and
lipid metabolism, which increases the cell utilization of glucose
(68).
Lipid metabolism is also reprogrammed in different
types of cancer, but not in the same way for all. Multiple lines of
evidence suggest that CGRP may regulate lipid metabolism in
physiological and pathological conditions. Compared with
normal-weight females, the plasma CGRP concentration is increased
in obese females (body mass index >35 kg/m2)
(69). In pre-obese Zucker rats, the
fasting plasma CGRP levels were elevated in lean animals prior to
the appearance of body weight differences compared to control group
(70). Furthermore, CGRP-knockout
mice were observed to be protected from diet-induced obesity
compared with CGRP+/+ mice, without any obvious
attenuation of food intake (70).
Danaher et al (67) suggested
that levels of CGRP similar to those in the blood markedly
stimulated fatty acid β-oxidation and evoked concomitant
mobilization of muscle lipid via receptor-mediated activation of
muscle lipolysis in rodents. In OSCC, it was demonstrated that
fenofibrate, a particularly potent clinical lipid-lowering agent,
suppressed oral tumorigenesis and cancer development by
downregulating mTOR activity and activating the AMPK signaling
pathway (71). In another
retrospective study including 576 patients diagnosed with T1/2N0M0
OSCC without any weight loss prior to diagnosis, the
progression-free survival time was poorer in obese patients than in
those of normal weight (72). Future
studies should investigate whether CGRP may regulate lipid
metabolism in OSCC.
It is normal for the internalization of receptors to
serve as a physiological feedback mechanism to switch off the
initiated cellular signaling pathways. However, studies on certain
GPCR-like receptors, such as the thyroid-stimulating hormone (TSH)
receptor and the parathyroid hormone receptor, have demonstrated
that an internalized ligand-receptor compound is able to convey
sustained signals in endosomes (73,74). The
CLR/RAMP1 receptor complex is internalized together into an
endosome after combining with CGRP (75). Yarwood et al (76) revealed that endosomal CLR is able to
activate protein kinase C in the cytosol, as well as ERK in the
cytosol and nucleus. In addition, the authors developed a
cholestanol-conjugated antagonist (CGRP8-37-cholestanol) to prevent
sustained neuronal excitation caused by CGRP, which accumulates in
endosomes and is capable of inhibiting endosomal CLR/cAMP signaling
without affecting CLR/RAMP1 receptors at the cell surface. A
similar result was obtained in a study on substance P, which is
frequently regarded as coexisting with CGRP (77,78). A
number of studies agree that endosomal signaling is more efficient
compared with the traditional plasma membrane signaling and may
result in tumorigenesis (76,79).
Godbole et al (79)
demonstrated that the TSH receptor co-internalizes with TSH and
traffics retrogradely to the trans-Golgi network, where it
activates an endogenous pool of Gs proteins; this leads
to a delayed phase of local cAMP production and protein kinase A
activation at a critical position near the nucleus, which appears
to be required for efficient phosphorylation and gene transcription
of the cAMP response element-binding protein.
Overall, the aberrant internalization of receptors
contributes to tumorigenesis. CLR/RAMP1 may be sustained and
activated through an endosomal signaling pathway, which is an
unexploited but promising field in OSCC. Cholestanol-conjugated
antagonists may represent a novel approach for receptor antagonist
development.
In OSCC, an impaired and suppressed immune function
in both the whole human system and in the local tumor
microenvironment is associated with poor prognosis for patients
with OSCC (61). Future studies
should analyze how CGRP may regulate immune reactions in OSCC.
Clinical and pathological features confirm that
nerves serve an important role in OSCC, as well as in other types
of cancer. However, sensory nerves, rather than autonomic nerves,
appear to have a pivotal role in OSCC. CGRP is mostly expressed in
sensory neurons in the TG and DRG (94,95).
CGRP exerts a significant role in both cancer development and
cancer-associated pain. CGRP promotes cancer development through
metabolic reprogramming, anomalous receptor internalization or
inhibition of antitumor immune responses. In addition, CGRP may
augment pain by inducing mechanical allodynia peripherally and
centrally. Based on the current knowledge, the present review
suggested that CGRP may represent an important bridge between
cancer development and cancer-associated pain in OSCC, and this
hypothesis should be further investigated in future studies.
Not applicable.
The present review was supported by the National
Natural Science Foundation of China (grant nos. 8187100500 and
81702675)) and the Science and Technology Commission of Shanghai
Municipality (grant no. YDZX20173100004422).
Not applicable.
YZ, TJ and XW conceived and designed the study. CL
researched the literature. YZ wrote the manuscript. CL and XW
designed the figures. YZ, CL, XW and TJ revised and edited the
manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Senthebane DA, Rowe A, Thomford NE,
Shipanga H, Munro D, Mazeedi MAMA, Almazyadi HAM, Kallmeyer K,
Dandara C, Pepper MS, et al: The role of tumor microenvironment in
chemoresistance: To survive, keep your enemies closer. Int J Mol
Sci. 18:15862017. View Article : Google Scholar
|
|
2
|
Yadav L, Puri N, Rastogi V, Satpute P and
Sharma V: Tumour angiogenesis and angiogenic inhibitors: A review.
J Clin Diagn Res. 9:XE01–XE05. 2015.PubMed/NCBI
|
|
3
|
Boilly B, Faulkner S, Jobling P and
Hondermarck H: Nerve dependence: From regeneration to cancer.
Cancer Cell. 31:342–354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amit M, Takahashi H, Dragomir MP,
Lindemann A, Gleber-Netto FO, Pickering CR, Anfossi S, Osman AA,
Cai Y, Wang R, et al: Loss of p53 drives neuron reprogramming in
head and neck cancer. Nature. 578:449–454. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mauffrey P, Tchitchek N, Barroca V,
Bemelmans AP, Firlej V, Allory Y, Roméo PH and Magnon C:
Progenitors from the central nervous system drive neurogenesis in
cancer. Nature. 569:672–678. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rogers SN, Brown JS, Woolgar JA, Lowe D,
Magennis P, Shaw RJ, Sutton D, Errington D and Vaughan D: Survival
following primary surgery for oral cancer. Oral Oncol. 45:201–211.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mu L and Sanders I: Human tongue
neuroanatomy: Nerve supply and motor endplates. Clin Anat.
23:777–791. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bagan J, Sarrion G and Jimenez Y: Oral
cancer: Clinical features. Oral Oncol. 46:414–417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reyes-Gibby CC, Anderson KO, Merriman KW,
Todd KH, Shete SS and Hanna EY: Survival patterns in squamous cell
carcinoma of the head and neck: Pain as an independent prognostic
factor for survival. J Pain. 15:1015–1022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van den Beuken-van Everdingen MH, de Rijke
JM, Kessels AG, Schouten HC, van Kleef M and Patijn J: Prevalence
of pain in patients with cancer: A systematic review of the past 40
years. Ann Oncol. 18:1437–1449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zahalka AH and Frenette PS: Nerves in
cancer. Nat Rev Cancer. 20:143–157. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Drissi H, Lieberherr M, Hott M, Marie PJ
and Lasmoles F: Calcitonin gene-related peptide (CGRP) increases
intracellular free Ca2+ concentrations but not cyclic AMP formation
in CGRP receptor-positive osteosarcoma cells (OHS-4). Cytokine.
11:200–207. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Papantoniou V, Tsiouris S, Sotiropoulou M,
Valsamaki P, Koutsikos J, Ptohis N, Dimitrakakis C, Sotiropoulou E,
Melissinou M, Nakopoulou L, et al: The potential role of calcitonin
gene-related peptide (CGRP) in breast carcinogenesis and its
correlation with 99mTc-(V)DMSA scintimammography. Am J Clin Oncol.
30:420–427. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eftekhari S, Salvatore CA, Calamari A,
Kane SA, Tajti J and Edvinsson L: Differential distribution of
calcitonin gene-related peptide and its receptor components in the
human trigeminal ganglion. Neuroscience. 169:683–696. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brain SD and Grant AD: Vascular actions of
calcitonin gene-related peptide and adrenomedullin. Physiol Rev.
84:903–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Russell FA, King R, Smillie SJ, Kodji X
and Brain SD: Calcitonin gene-related peptide: Physiology and
pathophysiology. Physiol Rev. 94:1099–1142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosenfeld MG, Mermod JJ, Amara SG, Swanson
LW, Sawchenko PE, Rivier J, Vale WW and Evans RM: Production of a
novel neuropeptide encoded by the calcitonin gene via
tissue-specific RNA processing. Nature. 304:129–135. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McLatchie LM, Fraser NJ, Main MJ, Wise A,
Brown J, Thompson N, Solari R, Lee MG and Foord SM: RAMPs regulate
the transport and ligand specificity of the
calcitonin-receptor-like receptor. Nature. 393:333–339. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luebke AE, Dahl GP, Roos BA and Dickerson
IM: Identification of a protein that confers calcitonin
gene-related peptide responsiveness to oocytes by using a cystic
fibrosis transmembrane conductance regulator assay. Proc Natl Acad
Sci USA. 93:3455–3460. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Negro A and Martelletti P: Gepants for the
treatment of migraine. Expert Opin Investig Drugs. 28:555–567.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edvinsson L, Haanes KA, Warfvinge K and
Krause DN: CGRP as the target of new migraine therapies-successful
translation from bench to clinic. Nat Rev Neurol. 14:338–350. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferrari MD, Diener HC, Ning X, Galic M,
Cohen JM, Yang R, Mueller M, Ahn AH, Schwartz YC, Grozinski-Wolff
M, et al: Fremanezumab versus placebo for migraine prevention in
patients with documented failure to up to four migraine preventive
medication classes (FOCUS): A randomised, double-blind,
placebo-controlled, phase 3b trial. Lancet. 394:1030–1040. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goadsby PJ, Dodick DW, Leone M, Bardos JN,
Oakes TM, Millen BA, Zhou C, Dowsett SA, Aurora SK, Ahn AH, et al:
Trial of galcanezumab in prevention of episodic cluster headache. N
Engl J Med. 381:132–141. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reuter U, Goadsby PJ, Lanteri-Minet M, Wen
S, Hours-Zesiger P, Ferrari MD and Klatt J: Efficacy and
tolerability of erenumab in patients with episodic migraine in whom
two-to-four previous preventive treatments were unsuccessful: A
randomised, double-blind, placebo-controlled, phase 3b study.
Lancet. 392:2280–2287. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dodick DW, Lipton RB, Ailani J, Lu K,
Finnegan M, Trugman JM and Szegedi A: Ubrogepant for the treatment
of migraine. N Engl J Med. 381:2230–2241. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lipton RB, Dodick DW, Ailani J, Lu K,
Finnegan M, Szegedi A and Trugman JM: Effect of ubrogepant vs
placebo on pain and the most bothersome associated symptom in the
acute treatment of migraine: The ACHIEVE II randomized clinical
trial. JAMA. 322:1887–1898. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lipton RB, Croop R, Stock EG, Stock DA,
Morris BA, Frost M, Dubowchik GM, Conway CM, Coric V and Goadsby
PJ: Rimegepant, an oral calcitonin gene-related peptide receptor
antagonist, for migraine. N Engl J Med. 381:142–149. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Croop R, Goadsby PJ, Stock DA, Conway CM,
Forshaw M, Stock EG, Coric V and Lipton RB: Efficacy, safety, and
tolerability of rimegepant orally disintegrating tablet for the
acute treatment of migraine: A randomised, phase 3, double-blind,
placebo-controlled trial. Lancet. 394:737–745. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Portenoy RK, Payne D and Jacobsen P:
Breakthrough pain: Characteristics and impact in patients with
cancer pain. Pain. 81:129–134. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Truscott BM: Carcinoma of the breast; an
analysis of the symptoms, factors affecting prognosis, results of
treatment and recurrences in 1211 cases treated at the middlesex
hospital. Br J Cancer. 1:129–145. 1947. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Slagelse C, Munch T, Glazer C, Greene K,
Finnerup NB, Kashani-Sabet M, Leong SP, Petersen KL and Rowbotham
MC: Natural history of pain associated with melanoma surgery. Pain
Rep. 3:e6892018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yeh CF, Li WY, Chu PY, Kao SY, Chen YW,
Lee TL, Hsu YB, Yang CC and Tai SK: Pretreatment pain predicts
perineural invasion in oral squamous cell carcinoma: A prospective
study. Oral Oncol. 61:115–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brain SD: Sensory neuropeptides: Their
role in inflammation and wound healing. Immunopharmacology.
37:133–152. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abdelaziz DM, Stone LS and Komarova SV:
Localized experimental bone metastasis drives osteolysis and
sensory hypersensitivity at distant non-tumor-bearing sites. Breast
Cancer Res Treat. 153:9–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Auer J, Reeh PW and Fischer MJ:
Acid-induced CGRP release from the stomach does not depend on TRPV1
or ASIC3. Neurogastroenterol Motil. 22:680–687. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Afroz S, Arakaki R, Iwasa T, Oshima M,
Hosoki M, Inoue M, Baba O, Okayama Y and Matsuka Y: CGRP induces
differential regulation of cytokines from satellite glial cells in
trigeminal ganglia and orofacial nociception. Int J Mol Sci.
20:7112019. View Article : Google Scholar
|
|
38
|
Hansen RR, Vacca V, Pitcher T, Clark AK
and Malcangio M: Role of extracellular calcitonin gene-related
peptide in spinal cord mechanisms of cancer-induced bone pain.
Pain. 157:666–676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Niiyama Y, Kawamata T, Yamamoto J, Omote K
and Namiki A: Bone cancer increases transient receptor potential
vanilloid subfamily 1 expression within distinct subpopulations of
dorsal root ganglion neurons. Neuroscience. 148:560–572. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jimenez-Andrade JM, Bloom AP, Stake JI,
Mantyh WG, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA and
Mantyh PW: Pathological sprouting of adult nociceptors in chronic
prostate cancer-induced bone pain. J Neurosci. 30:14649–14656.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bloom AP, Jimenez-Andrade JM, Taylor RN,
Castañeda-Corral G, Kaczmarska MJ, Freeman KT, Coughlin KA,
Ghilardi JR, Kuskowski MA and Mantyh PW: Breast cancer-induced bone
remodeling, skeletal pain, and sprouting of sensory nerve fibers. J
Pain. 12:698–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wakabayashi H, Wakisaka S, Hiraga T, Hata
K, Nishimura R, Tominaga M and Yoneda T: Decreased sensory nerve
excitation and bone pain associated with mouse Lewis lung cancer in
TRPV1-deficient mice. J Bone Miner Metab. 36:274–285. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wacnik PW, Baker CM, Herron MJ, Kren BT,
Blazar BR, Wilcox GL, Hordinsky MK, Beitz AJ and Ericson ME:
Tumor-induced mechanical hyperalgesia involves CGRP receptors and
altered innervation and vascularization of DsRed2 fluorescent
hindpaw tumors. Pain. 115:95–106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nagamine K, Ozaki N, Shinoda M, Asai H,
Nishiguchi H, Mitsudo K, Tohnai I, Ueda M and Sugiura Y: Mechanical
allodynia and thermal hyperalgesia induced by experimental squamous
cell carcinoma of the lower gingiva in rats. J Pain. 7:659–670.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Levy MJ, Classey JD, Maneesri S, Meeran K,
Powell M and Goadsby PJ: The association between calcitonin
gene-related peptide (CGRP), substance P and headache in pituitary
tumours. Pituitary. 7:67–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dottorini ME, Assi A, Sironi M, Sangalli
G, Spreafico G and Colombo L: Multivariate analysis of patients
with medullary thyroid carcinoma. Prognostic significance and
impact on treatment of clinical and pathologic variables. Cancer.
77:1556–1565. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Goadsby PJ, Edvinsson L and Ekman R:
Release of vasoactive peptides in the extracerebral circulation of
humans and the cat during activation of the trigeminovascular
system. Ann Neurol. 23:193–196. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kelley MJ, Snider RH, Becker KL and
Johnson BE: Small cell lung carcinoma cell lines express mRNA for
calcitonin and alpha- and beta-calcitonin gene related peptides.
Cancer Lett. 81:19–25. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Takami H, Shikata J, Horie H, Horiuchi J,
Sakurai H and Ito K: Radioimmunoassay of plasma calcitonin
gene-related peptide (CGRP) levels in patients with endocrine
tumor. Gan To Kagaku Ryoho. 16:2219–2225. 1989.PubMed/NCBI
|
|
50
|
Nagakawa O, Ogasawara M, Fujii H, Murakami
K, Murata J, Fuse H and Saiki I: Effect of prostatic neuropeptides
on invasion and migration of PC-3 prostate cancer cells. Cancer
Lett. 133:27–33. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nagakawa O, Ogasawara M, Murata J, Fuse H
and Saiki I: Effect of prostatic neuropeptides on migration of
prostate cancer cell lines. Int J Urol. 8:65–70. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wimalawansa SJ: CGRP radioreceptor assay:
A new diagnostic tool for medullary thyroid carcinoma. J Bone Miner
Res. 8:467–473. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Angenendt L, Bormann E, Pabst C, Alla V,
Görlich D, Braun L, Dohlich K, Schwöppe C, Bohlander SK, Arteaga
MF, et al: The neuropeptide receptor calcitonin receptor-like
(CALCRL) is a potential therapeutic target in acute myeloid
leukemia. Leukemia. 33:2830–2841. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Toda M, Suzuki T, Hosono K, Hayashi I,
Hashiba S, Onuma Y, Amano H, Kurihara Y, Kurihara H, Okamoto H, et
al: Neuronal system-dependent facilitation of tumor angiogenesis
and tumor growth by calcitonin gene-related peptide. Proc Natl Acad
Sci USA. 105:13550–13555. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ostrovskaya A, Hick C, Hutchinson DS,
Stringer BW, Wookey PJ, Wootten D, Sexton PM and Furness SGB:
Expression and activity of the calcitonin receptor family in a
sample of primary human high-grade gliomas. BMC Cancer. 19:1572019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dallmayer M, Li J, Ohmura S, Alba Rubio R,
Baldauf MC, Hölting TLB, Musa J, Knott MML, Stein S, Cidre-Aranaz
F, et al: Targeting the CALCB/RAMP1 axis inhibits growth of Ewing
sarcoma. Cell Death Dis. 10:1162019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Höppener JW, Steenbergh PH, Slebos RJ,
Visser A, Lips CJ, Jansz HS, Bechet JM, Lenoir GM, Born W,
Haller-Brem S, et al: Expression of the second
calcitonin/calcitonin gene-related peptide gene in Ewing sarcoma
cell lines. J Clin Endocrinol Metab. 64:809–817. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gutierrez S and Boada MD:
Neuropeptide-induced modulation of carcinogenesis in a metastatic
breast cancer cell line (MDA-MB-231LUC+). Cancer Cell
Int. 18:2162018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fristad I, Vandevska-Radunovic V, Fjeld K,
Wimalawansa SJ and Hals Kvinnsland I: NK1, NK2, NK3 and CGRP1
receptors identified in rat oral soft tissues, and in bone and
dental hard tissue cells. Cell Tissue Res. 311:383–391. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sasahira T and Kirita T: Hallmarks of
cancer-related newly prognostic factors of oral squamous cell
carcinoma. Int J Mol Sci. 19:24132018. View Article : Google Scholar
|
|
62
|
Kurihara-Shimomura M, Sasahira T,
Nakashima C, Kuniyasu H, Shimomura H and Kirita T: The multifarious
functions of pyruvate kinase M2 in oral cancer cells. Int J Mol
Sci. 19:29072018. View Article : Google Scholar
|
|
63
|
Lu H, Li X, Luo Z, Liu J and Fan Z:
Cetuximab reverses the Warburg effect by inhibiting HIF-1-regulated
LDH-A. Mol Cancer Ther. 12:2187–2199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhai X, Yang Y, Wan J, Zhu R and Wu Y:
Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and
increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol
Rep. 30:2983–2991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rossetti L, Farrace S, Choi SB, Giaccari
A, Sloan L, Frontoni S and Katz MS: Multiple metabolic effects of
CGRP in conscious rats: Role of glycogen synthase and
phosphorylase. Am J Physiol. 264:E1–E10. 1993.PubMed/NCBI
|
|
66
|
Tang W and Fan Y: SIRT6 as a potential
target for treating insulin resistance. Life Sci. 231:1165582019.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Danaher RN, Loomes KM, Leonard BL, Whiting
L, Hay DL, Xu LY, Kraegen EW, Phillips AR and Cooper GJ: Evidence
that alpha-calcitonin gene-related peptide is a neurohormone that
controls systemic lipid availability and utilization.
Endocrinology. 149:154–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Faubert B, Vincent EE, Poffenberger MC and
Jones RG: The AMP-activated protein kinase (AMPK) and cancer: Many
faces of a metabolic regulator. Cancer Lett. 356:165–170. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zelissen PM, Koppeschaar HP, Lips CJ and
Hackeng WH: Calcitonin gene-related peptide in human obesity.
Peptides. 12:861–863. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gram DX, Hansen AJ, Wilken M, Elm T,
Svendsen O, Carr RD, Ahrén B and Brand CL: Plasma calcitonin
gene-related peptide is increased prior to obesity, and sensory
nerve desensitization by capsaicin improves oral glucose tolerance
in obese Zucker rats. Eur J Endocrinol. 153:963–969. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jan CI, Tsai MH, Chiu CF, Huang YP, Liu CJ
and Chang NW: Fenofibrate suppresses oral tumorigenesis via
reprogramming metabolic processes: Potential drug repurposing for
oral cancer. Int J Biol Sci. 12:786–798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hu Q, Peng J, Chen X, Li H, Song M, Cheng
B and Wu T: Obesity and genes related to lipid metabolism predict
poor survival in oral squamous cell carcinoma. Oral Oncol.
89:14–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Eichel K, Jullié D and von Zastrow M:
β-Arrestin drives MAP kinase signalling from clathrin-coated
structures after GPCR dissociation. Nat Cell Biol. 18:303–310.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Calebiro D, Nikolaev VO, Gagliani MC, de
Filippis T, Dees C, Tacchetti C, Persani L and Lohse MJ: Persistent
cAMP-signals triggered by internalized G-protein-coupled receptors.
PLoS Biol. 7:e10001722009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kuwasako K, Shimekake Y, Masuda M,
Nakahara K, Yoshida T, Kitaura M, Kitamura K, Eto T and Sakata T:
Visualization of the calcitonin receptor-like receptor and its
receptor activity-modifying proteins during internalization and
recycling. J Biol Chem. 275:29602–29609. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yarwood RE, Imlach WL, Lieu T, Veldhuis
NA, Jensen DD, Klein Herenbrink C, Aurelio L, Cai Z, Christie MJ,
Poole DP, et al: Endosomal signaling of the receptor for calcitonin
gene-related peptide mediates pain transmission. Proc Natl Acad Sci
USA. 114:12309–12314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mantyh PW, DeMaster E, Malhotra A,
Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR,
Maggio JE, et al: Receptor endocytosis and dendrite reshaping in
spinal neurons after somatosensory stimulation. Science.
268:1629–1632. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Steinhoff MS, von Mentzer B, Geppetti P,
Pothoulakis C and Bunnett NW: Tachykinins and their receptors:
Contributions to physiological control and the mechanisms of
disease. Physiol Rev. 94:265–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Godbole A, Lyga S, Lohse MJ and Calebiro
D: Publisher correction: Internalized TSH receptors en route to the
TGN induce local Gs-protein signaling and gene transcription. Nat
Commun. 9:54592018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Steinman L: Elaborate interactions between
the immune and nervous systems. Nat Immunol. 5:575–581. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sternberg EM: Neural regulation of innate
immunity: A coordinated nonspecific host response to pathogens. Nat
Rev Immunol. 6:318–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Antúnez C, Torres MJ, López S,
Rodriguez-Pena R, Blanca M, Mayorga C and Santamaría-Babi LF:
Calcitonin gene-related peptide modulates interleukin-13 in
circulating cutaneous lymphocyte-associated antigen-positive T
cells in patients with atopic dermatitis. Br J Dermatol.
161:547–553. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xing L, Guo J and Wang X: Induction and
expression of beta-calcitonin gene-related peptide in rat T
lymphocytes and its significance. J Immunol. 165:4359–4366. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bracci-Laudiero L, Aloe L, Buanne P, Finn
A, Stenfors C, Vigneti E, Theodorsson E and Lundeberg T: NGF
modulates CGRP synthesis in human B-lymphocytes: A possible
anti-inflammatory action of NGF? J Neuroimmunol. 123:58–65. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Bracci-Laudiero L, Aloe L, Caroleo MC,
Buanne P, Costa N, Starace G and Lundeberg T: Endogenous NGF
regulates CGRP expression in human monocytes, and affects HLA-DR
and CD86 expression and IL-10 production. Blood. 106:3507–3514.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ding W, Stohl LL, Wagner JA and Granstein
RD: Calcitonin gene-related peptide biases Langerhans cells toward
Th2-type immunity. J Immunol. 181:6020–6026. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Harzenetter MD, Novotny AR, Gais P, Molina
CA, Altmayr F and Holzmann B: Negative regulation of TLR responses
by the neuropeptide CGRP is mediated by the transcriptional
repressor ICER. J Immunol. 179:607–615. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang F, Millet I, Bottomly K and Vignery
A: Calcitonin gene-related peptide inhibits interleukin 2
production by murine T lymphocytes. J Biol Chem. 267:21052–21057.
1992.PubMed/NCBI
|
|
90
|
Kawamura N, Tamura H, Obana S, Wenner M,
Ishikawa T, Nakata A and Yamamoto H: Differential effects of
neuropeptides on cytokine production by mouse helper T cell
subsets. Neuroimmunomodulation. 5:9–15. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Hosoi J, Murphy GF, Egan CL, Lerner EA,
Grabbe S, Asahina A and Granstein RD: Regulation of langerhans cell
function by nerves containing calcitonin gene-related peptide.
Nature. 363:159–163. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Carucci JA, Ignatius R, Wei Y, Cypess AM,
Schaer DA, Pope M, Steinman RM and Mojsov S: Calcitonin
gene-related peptide decreases expression of HLA-DR and CD86 by
human dendritic cells and dampens dendritic cell-driven T
cell-proliferative responses via the type I calcitonin gene-related
peptide receptor. J Immunol. 164:3494–3499. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Holzmann B: Modulation of immune responses
by the neuropeptide CGRP. Amino Acids. 45:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Nguyen MQ, Wu Y, Bonilla LS, von Buchholtz
LJ and Ryba NJP: Diversity amongst trigeminal neurons revealed by
high throughput single cell sequencing. PLoS One. 12:e01855432017.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zeisel A, Hochgerner H, Lönnerberg P,
Johnsson A, Memic F, vander Zwan J, Häring M, Braun E, Borm LE, La
Manno G, et al: Molecular architecture of the mouse nervous system.
Cell. 174:999–1014 e22. 2018. View Article : Google Scholar : PubMed/NCBI
|