Introduction
Glioblastoma (GBM) is one of the most malignant
types of brain tumor in adults, affecting every 10 in 100,000
individuals worldwide (1,2). The prognosis of GBM remains dismal due
to its location, aggressive biological behavior and intratumoral
heterogeneity (3,4). Despite the rapid development of surgery
combined with chemotherapy and/or radiotherapy as a treatment
strategy, the aggressiveness of GBM cells in invading surrounding
normal brain tissue is a major therapeutic challenge, resulting in
a median survival time of <15 months (5). Therefore, a more in-depth exploration
of the molecular mechanism underlying GBM progression may help to
develop promising treatment strategies.
MicroRNA (miRNA/miR) is an important type of
non-coding RNA that contributes to the epigenetic phenotype and
represents an endogenous form of RNA interference (6). The ubiquitous presence and essential
role of miRNA in the pathological processes of lung cancer
(7), osteosarcoma (8) and hepatocellular carcinoma (9) is well recognized; miRNAs have been
implicated in cell proliferation, apoptosis, invasion, colony
formation and migration through binding to the 3′-untranslated
region (UTR) region of the target gene (10,11).
Abnormal miRNA expression was discovered to serve a prognostic role
in the tumorigenesis of GBM; for example, upregulated miR-21
expression levels were associated with a poor GBM prognosis in one
study (12), while miR-128 and
miR-451 expression levels were identified to be significantly
downregulated in GBM tissues in other previous studies (13–15).
However, further studies on the relationship between miRNAs and GBM
are required, and as such, the rapid development of bioinformatics
technology may be an important resource for researchers to further
understand how miRNAs influence GBM progression (6,16).
The objective of this study was to investigate
whether miR-138-5p mediates the inhibitory effect in GBM
development as well as examine if the miR-138-5p suppresses the GBM
cell viability via targeting cyclin D3. The expression levels of
miR-138-5p were discovered to be significantly downregulated in GBM
tissues and cell lines. Subsequently, the functions of miR-138-5p
in GBM were investigated through a series of cellular and molecular
experiments. Most importantly, it was predicted that cyclin D3
(CCND3) was the target gene of miR-138-5p and this relationship was
verified using a dual luciferase reporter assay. In summary,
miR-138-5p was suggested to function as a tumor suppressor gene by
targeting CCND3 in GBM, thus leading to cell cycle arrest, and the
inhibition of tumor cell viability and colony formation, thereby
indicating that miR-138-5p may be a potential diagnostic and/or
therapeutic target for GBM.
Materials and methods
Bioinformatics analysis
The existing GEO chip data from the GEO database was
first analyzed using the ‘GBM miRNA expression’ as a search
condition. The miRNA expression profile dataset GSE103229 and
GSE13140 (17) were downloaded from
the Gene Expression Omnibus database (GEO; http://www.ncbi.nlm.nih.gov/geo). GSE103229 was used
for personal purpose while GSE13140 has reported the miRNA
expressions in the mice with or without IL-4 stimulation.
Expression profiling was analyzed using the Exiqon human V3
microRNA PCR panel I+II platform (Qiagen, Inc.) according to the
manufacturer's protocol. GEO2R analysis was performed on the
website provided directly by the GEO project (https://www.ncbi.nlm.nih.gov/geo/geo2r/).
To predict miR-138-5p target mRNA, TargetScan
version 7.2 (http://www.targetscan.org/vert_72) was used with
‘miR-138-5p’ as the key word to identify the candidate genes.
Patient studies
A total of 20 GBM tissues and adjacent normal
tissues were collected from patients who underwent surgical
resection between March 2018 and March 2019 at The First Affiliated
Hospital of Zhejiang Chinese Medical University (Hangzhou, China).
The mean age was 61±13 years old (range, 27–83 years; 16 males, 4
females) The key inclusion criteria included patients with newly
diagnosed GMB (histologically confirmed by biopsy or resection)
within 4 weeks of diagnosis and >18 years old. The present study
excluded patients unwilling to abide by the protocol as well as
being legally incapacitated. Patients who had either received
radiotherapy or chemotherapy prior to the surgical resection
procedure were also excluded. The present study was approved by the
Ethics Committee of The First Affiliated Hospital of Zhejiang
Chinese Medical University and all human tissue samples were
obtained following written informed consent. Fresh tissues were
immediately frozen in liquid nitrogen and stored at −80°C before
RNA extraction.
Cell lines and cell culture
Three human GBM cell lines, including U87, U251 and
U373 were purchased from the American Type Culture Collection
(ATCC), and the normal brain glial cell line HEB was purchased from
The Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. The U373 and U87 (ATCC® HTB-14™) cells lines
were authenticated by STR profiling and identified as GBM cells of
unknown origin. All cell lines were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.), supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), 100 µg/ml streptomycin and
100 U/ml penicillin. The cells were incubated at 37°C in a humid
incubator containing 5% CO2.
Cell transfection
In total, 1×105 of U87 and U251 cells
were seeded into 6-well plates and incubated at 37°C in a humid
incubator overnight until 50–60% confluence was reached. Then, the
cells were transiently transfected with 20 nM small interfering RNA
(siRNA/si) (Shanghai GenePharma Co., Ltd.) or 50 nM miRNA mimics
using Lipofectamine® 3000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The cells were collected for subsequent experimentation following
24 h of transfection at 37°C. The following siRNA sequences were
used: Si-CCND3 sense, 5′-GGAUCUUUGUGGCCAAGGATT-3′ and antisense,
5′-UCCUUGGCCACAAAGAUCCTT-3′; and si-negative control (NC) sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. Synthetic miRNA mimics targeting the
sequence of miR-138-5p (5′-AGCUGGUGUUGUGAAUCAGGCCG-3′) and miR-NC
(5′-ACUCUAUCUGCACGCUGACUU-3′) were produced by Invitrogen; Thermo
Fisher Scientific, Inc.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from GBM cells and tissues was extracted
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). Total RNA was reverse transcribed into cDNA
using High-Capacity cDNA Reverse Transcription kit with RNase
Inhibitor (Fermentas; Thermo Fisher Scientific, Inc.) and Maxima
SYBR green/ROX qPCR Master Mix was used (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturers' protocols. qPCR
was subsequently performed using a QuantStudio 6 Flex Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used for the qPCR: Initial
denaturation at 95°C for 10 min; followed by 40 cycles at 92°C for
15 sec and 60°C for 1 min. The following primer pairs were used for
the qPCR: GAPDH forward, 5′-ATTCCATGGCACCGTCAAGGCTGA-3′ and
reverse, 5′-TTCTCCATGGTGGTGAAGACGCCA-3′; miR-138-5p forward,
5′-TGCAATGGGTTTGGCGTAGAAC-3′ and reverse,
5′-CCAGTGCCGCAGGGTAGGT-3′; CCND3 forward, 5′-TACCCGCCATCCATGATCG
and reverse, 5′-AGGCAGTCCACTTCAGTGC; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. U6
was used as the loading control for miRNA expression levels, while
GAPDH was used as the loading control for mRNA expression levels.
Relative quantification of gene expressions were calculated with
the 2−ΔΔCq method (18).
Cell viability assay
A Cell Counting Kit-8 (CCK-8) assay (Beyotime
Institute of Biotechnology) was used to investigate the cell
viability according to the manufacturer's protocol. Briefly,
following transfection, 5×103 cells/well were seeded in
96-wells plates and cultured at 37°C for 0, 24, 48, 72 and 96 h.
Subsequently, 10 µl CCK-8 reagent was added/well and further
incubated at 37°C for 2 h in the dark. The optical density value
was measured using a microplate reader at a wavelength of 450 nm to
determine the cell viability.
Colony formation assay
Following transfection, 1×103 U87 and
U251 cells/well were plated into 6-well plates and incubated at
37°C for 14 days. A colony was defined as a clump of cells that
could be clearly distinguished from another clone after staining.
Then, the formed cell colonies were fixed with 10% formaldehyde for
20 min at room temperature and stained at 37°C with 0.1% Coomassie
Brilliant Blue R250 for 2 min. Cell colonies were visualized using
a camera and counted.
Plasmid construction and dual
luciferase reporter gene assay
The CCND3 3′-UTR wild-type (wt) sequence was
amplified using PCR and cloned into the restrictive site between
XhoI and Bg1II in the firefly luciferase reporter
vector repGL3 (Promega Corporation). DNA sequencing was performed
to verify the cDNA sequence. The following primers were used for
the cDNA amplification: CCND3-3′-UTR-wt-upstream,
5′-CCCTGGAGAGGCCCTCTGGA-3′ and CCND3-3′UTR-wt-downstream,
5′-TTCCAAGAAGCCAAAGCCA-3′. Subsequently, the QuickMutation™ Plus
Site Mutation kit (Beyotime Institute of Biotechnology) was used to
mutate the miR-138-5p putative binding site in the
3′-UTR-containing vector.
For the dual luciferase reporter gene assay,
5×103 cells/well were seeded into 96-wells plates and
transfected with wt or mutated (mut) reporter vector or empty
plasmid (p-con) for 48 h at 37°C using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
concentration of plasmids used above was 1 µg/µl. The firefly
luciferase activity was measured using a dual luciferase reporter
assay system (Beyotime Institute of Biotechnology). The mean of the
results from cells co-transfected with p-con and miR-138-5p NC was
set as 100 and the frefly luciferase activity was calculated as
mean ± standard deviation following normalization to Renilla
luciferase activity.
Flow cytometric analysis of the cell
cycle
Flow cytometric analysis was performed using a Cell
Cycle Analysis kit (Beyotime Institute of Biotechnology). Briefly,
10×105 cells were washed with PBS twice, collected by
trypsinization and fixed with 70% ethanol overnight at 4°C.
Subsequently, 100 µl propidium iodide staining buffer was added to
the cells following the resuspension in ice-cold PBS containing 50
µg/ml of RNase. After incubation for 30 min at 37°C in the dark,
the cell cycle distribution was analyzed using a BD FACSLyric™ flow
cytometer (BD Biosciences) with a laser beam at 488 nm. The date
were analyzed by FlowJo version 7.6 Software (BD Biosciences).
Western blotting
Total protein was extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology). Total protein
was quantified using a bicinchoninic acid assay kit (Beyotime
Institute of Biotechnology) and 20 µg protein/lane was separated
via 10% SDS-PAGE. The separated proteins were subsequently
transferred onto polyvinylidene fluoride membranes (MilliporeSigma)
and blocked with 5% non-fat milk in TBS-Tween-20 (TBST; 0.1%
Tween-20) at room temperature for 1 h. The membranes were then
incubated at 4°C overnight with the following primary antibodies:
Anti-CCND3 (1:1,000; cat. no. ab28283; Abcam) and anti-GAPDH
(1:1,000; cat. no. ab181602; Abcam). Following the primary antibody
incubation, the membranes were rinsed five times with TBST and
incubated with the secondary antibodies: Horseradish,
peroxidase-conjugated goat anti-rabbit secondary antibody
(1:10,000; cat. no. ab205718; Abcam) and horseradish
peroxidase-conjugated goat anti-mouse secondary antibody (1:10,000;
cat. no. ab205719; Abcam) for 1 h at room temperature. Protein
bands were visualized using an enhanced chemiluminescence (Thermo
Fisher Scientific, Inc.) reagent and densitometric analysis was
performed using ImageJ version 1.50d software (National Institutes
of Health).
Transwell assay
Transwell insert chambers with an 8-µm pore size
membrane (Costar; Corning, Inc.) as well as chambers coated with
Matrigel (BD Biosciences) were used for Transwell assay. A total of
3×104 cells were seeded in the upper chamber,
maintaining in the medium without serum. Medium containing 20% FBS
(Gibco; Thermo Fisher Scientific, Inc.) was added to the lower
chamber as chemoattractant. Subsequently, the cells were incubated
at 37°C for 24 h. Non-invading cells were removed using cotton
swabs while the cells migrated to the bottom of the membrane were
fixed with cold methanol and stained with 0.1% crystal violet at
37°C for 20 min. The stained cells were captured under a light
microscope in five random fields with 100× magnification, and the
average number of migratory cells was calculated. Data analysis was
performed using GraphPad Prism 7.0 software (GraphPad Software,
Inc.).
Statistical analysis
All experiments were repeated in triplicate.
Statistical analysis was performed using SPSS 19.0 software (IBM
Corp.) and data are presented as the mean ± SD. The normality
assumption of data distribution was assessed using a
Kolmogorov-Smirnov test. For normally distributed data, a paired or
unpaired Student's t-test was used for two groups depending on
whether the data were paired. A one-way ANOVA followed by a Tukey's
post hoc test was used for multiple group comparisons. The
Kruskal-Wallis test followed by a Dunn's post hoc test was used for
non-parametric statistical analysis. As the data analyzed was
non-parametric, correlation analysis was performed using Spearman's
correlation coefficient. P<0.05 was considered to indicate a
statistically significant difference.
Results
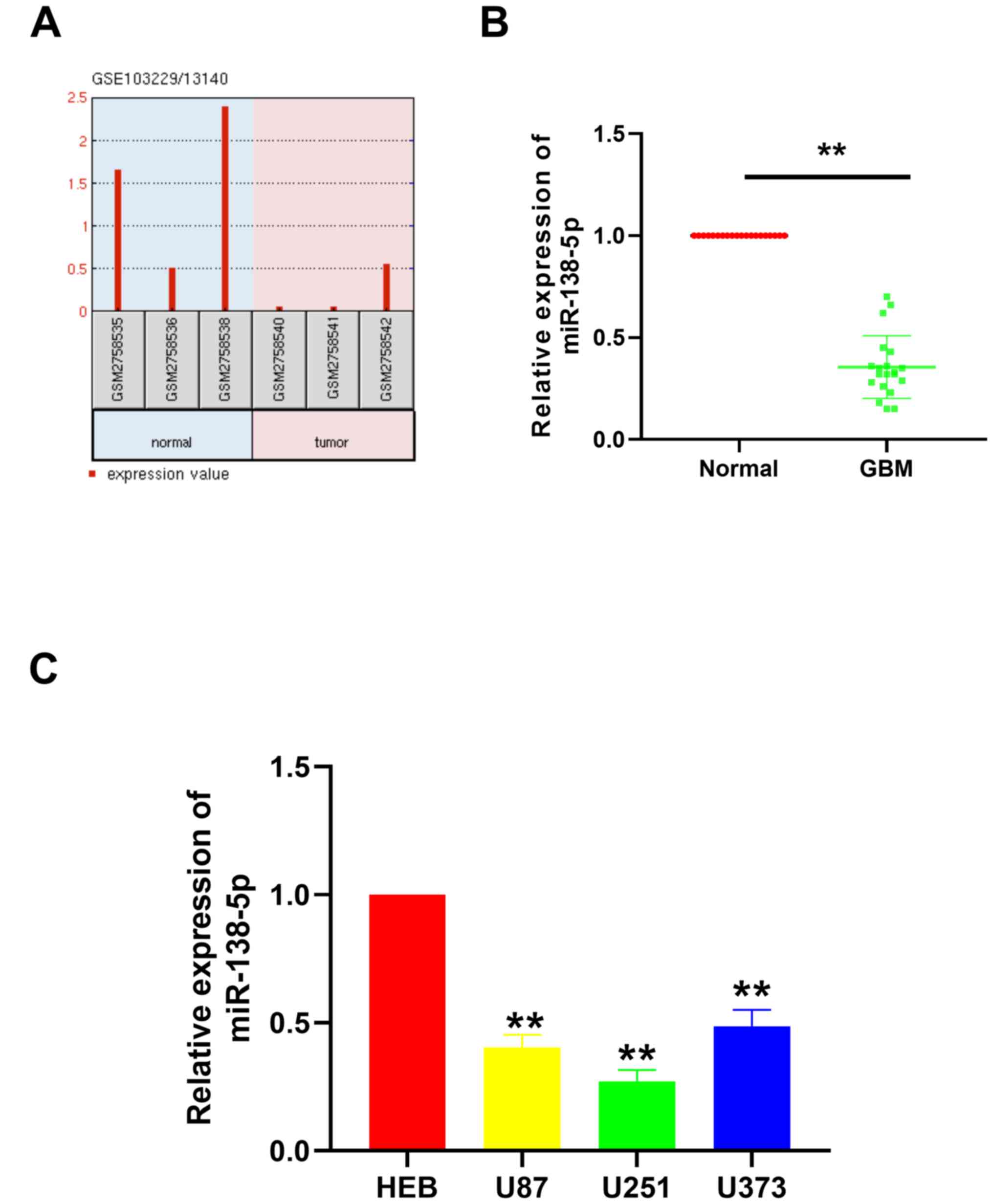
Expression levels of miR-138-5p are
downregulated in GBM tissues and cell lines
The existing GEO chip data from the GEO database was
first analyzed using the ‘GBM miRNA expression’ as a search
condition. Two databases were identified: GSE103229 and GSE13140.
After analyzing the data in the two data sets, the expression level
of miRNA in glioma and the expression in normal tissues adjacent to
the tumor were compared, and a data set with lower miRNA expression
level was obtained. Finally, we took the intersection of the
results obtained in the two databases, and obtained four miRNAs,
namely miR-124-3p, miR-129-5p, miR-138-5p and miR-338-3p. As the
role of miR-124-3p, miR-129-5p and miR-338-3p in GBM has been
previously reported, the role of miR-138-5p in GBM was further
investigated in the present study. miR-138-5p expression levels in
GBM tissues were discovered to be notably downregulated compared
with the normal tissues (Fig. 1A).
To validate this result, RT-qPCR was performed to analyze the
miR-138-5p expression levels in 20 paired GBM tissues and adjacent
normal tissues collected from The First Affiliated Hospital of
Zhejiang Chinese Medical University. Similar to the results
obtained from the GEO datasets, miR-138-5p expression levels were
significantly downregulated in the GBM tissues compared with the
normal tissues (Fig. 1B). In
addition, miR-138-5p expression levels were investigated in
different GBM cell lines, including U87, U251 and U373. The results
illustrated that miR-138-5p expression levels were also
significantly downregulated in the GBM cell lines compared with the
normal brain glial cell line, HEB (Fig.
1C). These results suggested that miR-138-5p expression levels
may be significantly downregulated in GBM tissues and cells.
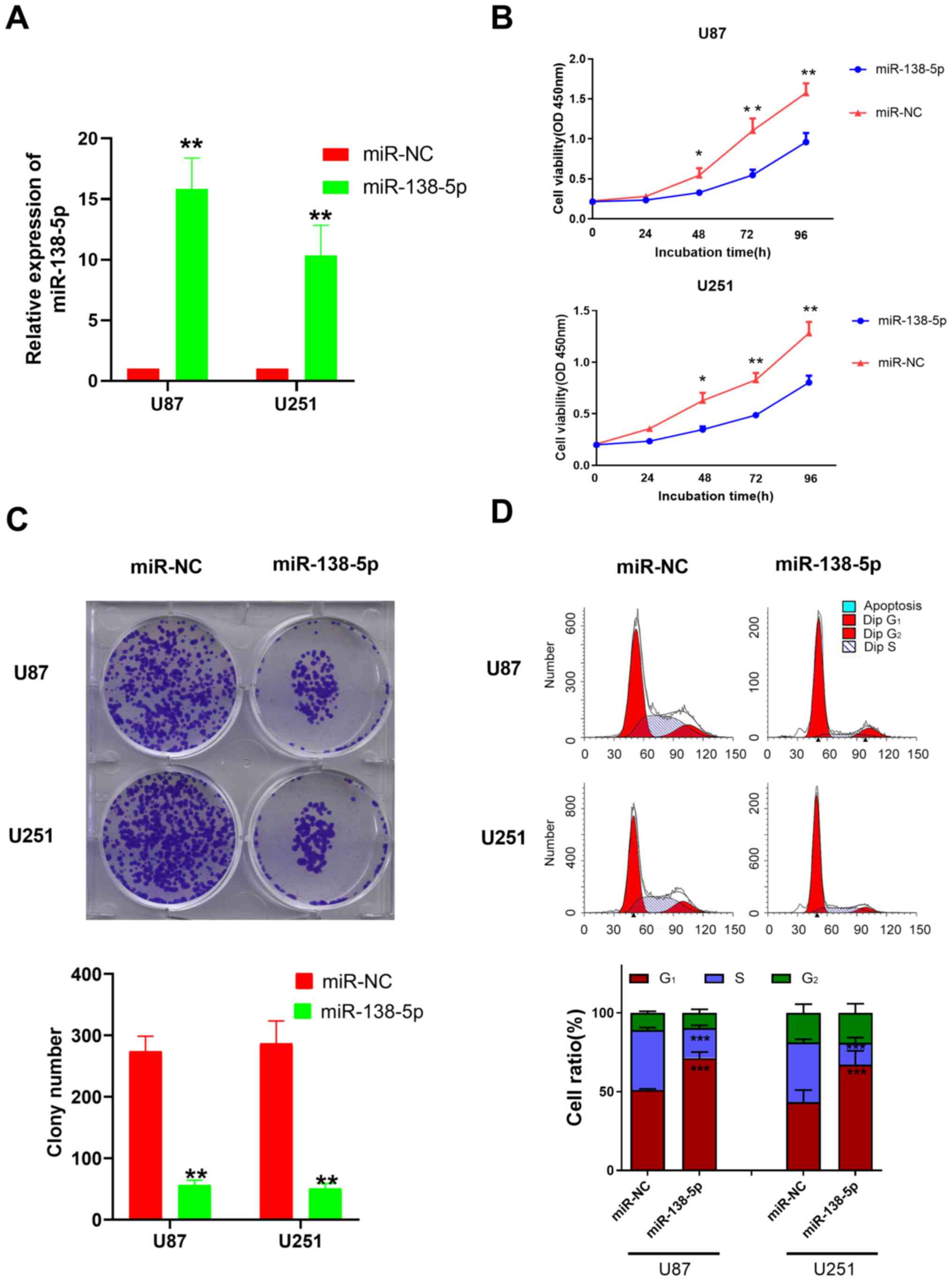
miR-138-5p inhibits the viability,
colony formation and invasion of GBM cells
To investigate the biological function of miR-138-5p
in GBM, miR-138-5p mimics and a miR-NC were transfected into U87
and U251 cells. The transfection efficiency was evaluated using
RT-qPCR; the miR-138-5p mimic-transfected cells had significantly
upregulated expression levels of miR-138-5p compared with the
miR-NC-transfected cells (Fig.
2A).
Following the successful transfection, a CCK-8 assay
was used to evaluate the effect of miR-138-5p upregulation on cell
viability. The results identified that the cell viability of U87
and U251 cells transfected with the miR-138-5p mimic was
significantly decreased compared with the miR-NC-transfected cells
following 48–96 h of incubation (Fig.
2B). The colony formation assay revealed that the number of
colonies formed in cells transfected with the miR-138-5p mimics was
significantly decreased compared with the miR-NC group in both cell
lines (Fig. 2C), suggesting a
suppressive effect of miR-138-5p on GBM colony formation. The
results from the Transwell assay also revealed that the invasive
ability of the miR-138-5p mimic group was significantly decreased
compared with the miR-NC group in both cell lines (Fig. S1). These data indicated that
miR-138-5p may serve as a tumor suppressor and inhibit the
viability, colony formation and invasion of GBM cells.
It is well known that CCND3 is one of the highly
conserved cyclin family members that serves a critical role in the
cell cycle (19,20). As a result, flow cytometric analysis
was performed to determine whether miR-138-5p impacted the GBM cell
cycle. The results revealed that compared with the miR-NC, a
significant increase was observed in the number of cells arrested
at the G0/G1 phase in the cells transfected
with the miR-138-5p mimics, while significantly fewer cells were
located in the S phase (Fig.
2D).
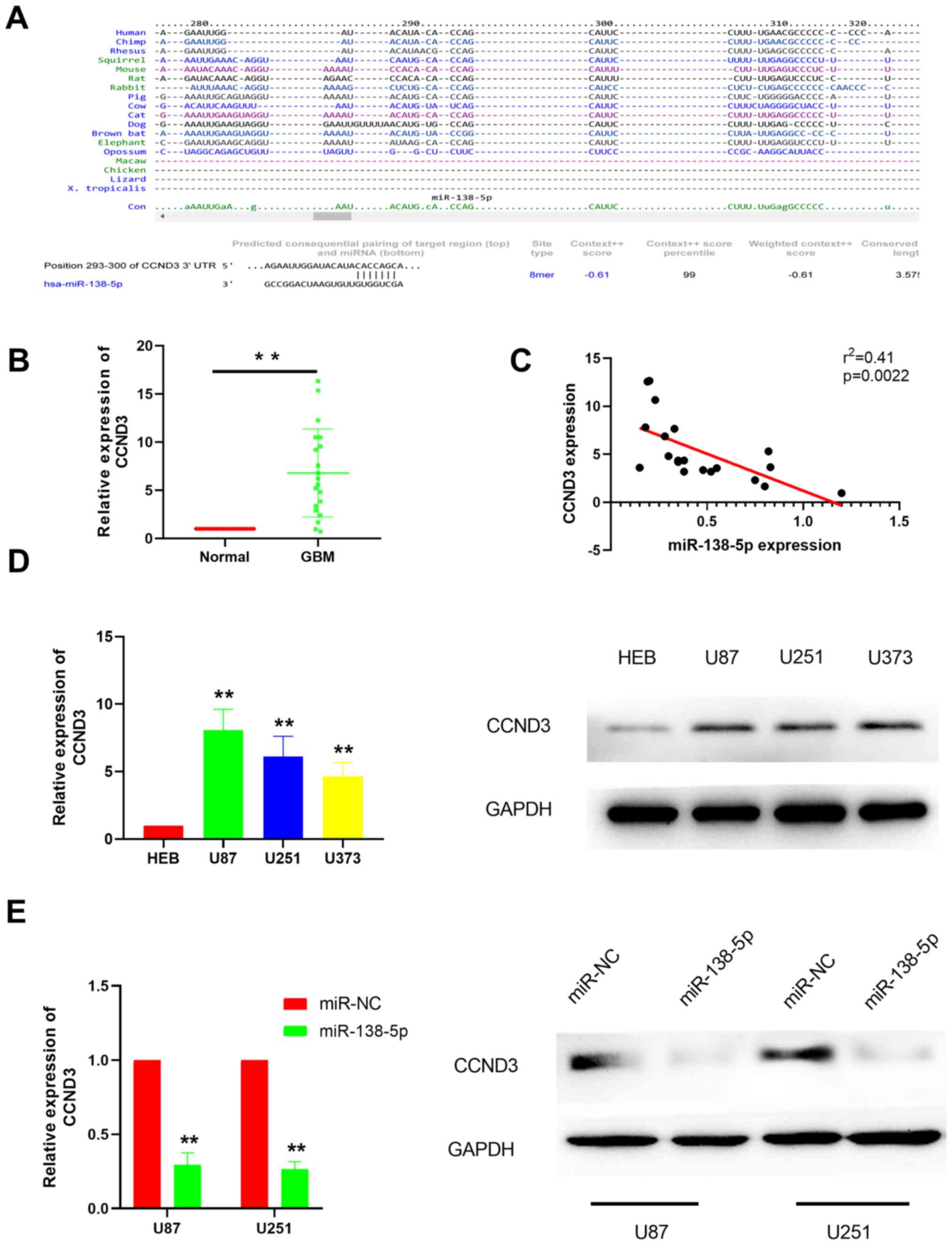
CCND3 expression levels are
upregulated in GBM tissues and CCND3 is a direct target of
miR-138-5p
It is well established that miRNAs bind to the
3′-UTR of target mRNAs to suppress their expression in order to
participate in various physiological activities (21–23). To
identify miR-138-5p target mRNA, the TargetScan database was used
to predict the potential target genes, which revealed CCND3 as a
potential miR-138-5p target (Fig.
3A). For further validation, RT-qPCR was performed to
investigate CCND3 expression levels in GBM tissues. The results
discovered that CCND3 expression levels were significantly
upregulated in GBM tissues compared with the adjacent normal
tissues (Fig. 3B), suggesting a
negative correlation between CCND3 and miR-138-5p expression
levels, which was subsequently confirmed by correlation analysis
(Fig. 3C). As expected, RT-qPCR and
western blotting analysis demonstrated that CCND3 expression levels
were also markedly upregulated in various GBM cell lines compared
with the normal brain glial HEB cells (Fig. 3D). To further confirm that CCND3 was
a target gene of miR-138-5p in GBM, miR-138-5p mimics were used to
overexpress miR-138-5p and then CCND3 mRNA and protein expression
levels were analyzed in U87 and U251 cells. The results revealed
that CCND3 mRNA and protein expression levels were both markedly
downregulated in the cells transfected with the miR-138-5p mimics
compared with the miR-NC-transfected cells (Fig. 3E).
A dual luciferase reporter gene assay was performed
to validate the direct interaction between miR-138-5p and CCND3.
The supposed 3′-UTR binding site of CCND3 was cloned into the
luciferase reporter vector as a wt version [plasmid (p)-CCND3-wt],
while a mut type fragment of the 3′-UTR was also constructed as a
mutant version (p-CCND3-mut). The cells were co-transfected with
miR-138-5p mimics and luciferase reporter plasmids containing the
wt or mut type of CCND3 3′-UTR. The results identified a
significantly reduced luciferase activity in the U87 and U251 cells
co-transfected with the p-CCND3-wt and miR-138-5p mimic compared
with the p-CCND3-mut (Fig. 4A).
Taken together, these results suggested that miR-138-5p may target
CCND3 in GBM cells.
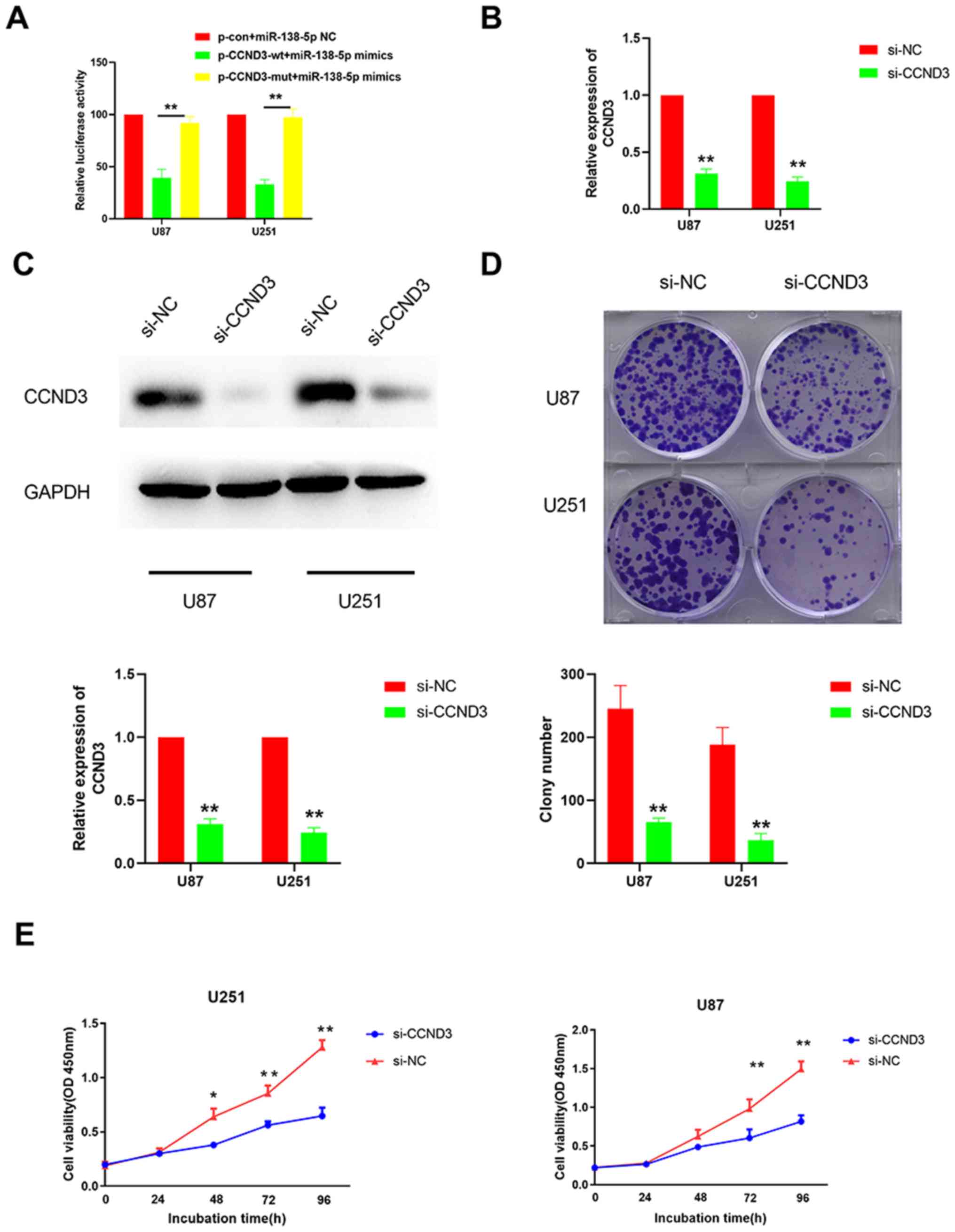 | Figure 4.Knockdown of CCND3 inhibits the
colony forming ability and viability of glioblastoma cells. (A)
Dual luciferase reporter gene assay was performed to determine
whether miR-138-5p directly targeted CCND3 in cells co-transfected
with p-CCND3-wt or p-CCND3-mut and either miR-138-5p mimics. The
mean of the results from cells transfected with miR-138-5p NC and
p-con was set as 100. **P<0.01. The silencing effect of CCND3
knockdown using siRNA was analyzed using (B) reverse
transcription-quantitative PCR and (C) western blotting in U87 and
U251 cell lines transfected with si-CCND3 or si-NC. (D) Colony
forming ability of U87 and U251 cells transfected with si-CCND3 or
si-NC was analyzed. (E) Cell viability of U87 and U251 cells
transfected with si-CCND3 or si-NC was analyzed using a Cell
Counting Kit-8 assay. *P<0.05, **P<0.01 vs. si-NC. A one-way
ANOVA followed by a Tukey's test post hoc was used in parts (A and
E) while an unpaired Student's t-test was used in parts (B-D)
CCND3, cyclin D3; miR, microRNA; wt, wild-type; mut, mutant; p,
plasmid; con, control; si/siRNA, small interfering RNA; NC,
negative control; OD, optical density. |
CCND3 knockdown inhibits the colony
formation ability and viability of GBM cells
To determine whether CCND3 was responsible for the
tumor suppressive role of miR-138-5p in GBM cells, CCND3 was
knocked down in U87 and U251 cells using siRNA. The successful
transfection efficiency was confirmed using RT-qPCR and western
blotting (Fig. 4B and C).
Subsequently, colony formation and CCK-8 assays were performed,
which identified that the colony forming ability and cell viability
were significantly decreased when U87 and U251 cells were
transfected with si-CCND3 compared with si-NC (Fig. 4D and E). These data indicated that
CCND3-knockdown inhibits the colony formation ability and viability
of GBM cells.
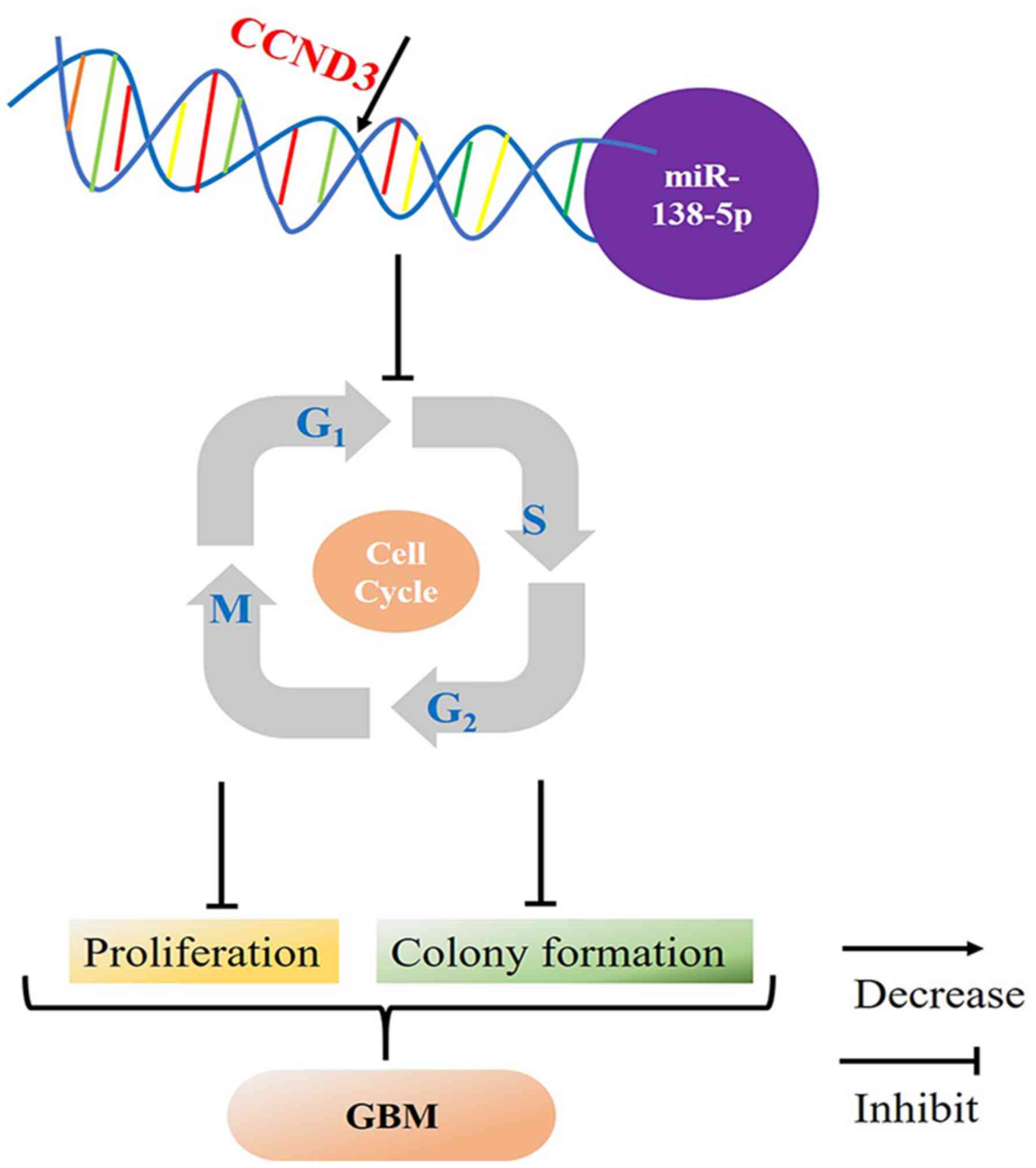
Taken together, the present study revealed that
CCND3 expression levels were negatively regulated by miR-138-5p in
GBM, which indicated that decreased miR-138-5p expression levels
may promote CCND3 and the consequent progression from the
G0/G1 phase to the S phase, resulting in
increased cell proliferation and colony formation in GBM (Fig. 5).
Discussion
As the most common malignant type of brain tumor in
adults, GBM affecting every 10 in 100,000 individuals worldwide
(1,2). With a median survival of only 15 months
(24), GBM remains a devastating
threat despite the significant progress in treatment strategies
(25). Treatment resistance in GBM
is mainly due to the aggressive biological behavior of the cancer
cells, which may proliferate and easily invade into the normal
tissues (24,26). Therefore, there remains an urgent
requirement to improve the understanding of how to suppress the
growth of GBM cells. Over the past decade, miRNAs have emerged as
novel regulatory molecules involved in tumor progression through
targeting certain oncogenes and/or tumor suppressors (27). For example, miR-138-5p was suggested
to be a tumor suppressor in bladder (28), non-small cell lung (29), colorectal (30) and pancreatic cancer (31). However, to the best of our knowledge,
the function and mechanism of miR-138-5p in GBM remains
unknown.
In the present study, miR-138-5p expression levels
were significantly downregulated in GBM tissues compared with the
normal tissues in the GEO datasets. RT-qPCR was also performed
using both GBM tissues from our hospital and different GBM cell
lines to determine whether miR-138-5p functioned as a tumor
suppressor in GBM. Consistent with the findings from the GEO
analysis, a significant downregulation of miR-138-5p expression
levels was observed in the GBM tissues and cell lines.
Subsequently, in vitro experiments were used to investigate
the effect of miR-138-5p on GBM cell viability, colony formation
and invasion. The results revealed that the overexpression of
miR-138-5p inhibited GBM cell viability, colony formation and the
invasive ability, which suggested that miR-138-5p may be an GBM
suppressor gene.
The underlying mechanism of miR-138-5p inhibition in
GBM progression was also investigated. As previously mentioned,
miRNAs contribute to the epigenetic phenotype through binding to
the 3′-UTR of specific target mRNAs (7,21,23). As
a result, bioinformatics analysis and dual luciferase reporter gene
assays were performed in the present study to determine the
miR-138-5p target gene. Although numerous genes have been reported
to be the binding targets of miR-138-5p, including BIRC5
(32), GRP124 (33) and Sirtuin1 (34), the present study identified CCND3 as
the direct target of miR-138-5p.
Considering the cell cycle regulation function of
CCND3 (20,35), flow cytometric analysis and
subsequent CCND3 silencing using siRNA were conducted to confirm
that CCND3 was an oncogene highly expressed in GBM cells, and to
further investigate its ability to enhance cell cycle transition
from the G0/G1 phase to the S phase, thus
leading to GBM progression. CCND3 is one of the three well-known
members of the D cyclin family that serves a critical role in the
mammalian cell cycle machinery (36,37).
Once activated, CCND3 binds to activate CDKs, which then
phosphorylate a series of proteins to release E2F transcription
factors that promote the progression from the
G0/G1 phase to the S phase (38–40).
Previous studies have reported that CCND3 expression levels were
upregulated in breast cancer (39,41) and
osteosarcoma (42). The upregulated
expression levels of CCND3 were associated with a poor prognosis in
gastric cancer via accelerating cell cycle function (43). As a result, high expression levels of
CCND3 are usually considered a biomarker for cancer phenotype and
disease progression in liver and prostate cancer (39,44). The
present study revealed that CCND3 expression levels were negatively
regulated by miR-138-5p in GBM, which indicated that decreased
miR-138-5p expression levels may promote CCND3 and the consequent
progression from the G0/G1 phase to the S
phase, resulting in increased cell proliferation and colony
formation in GBM. However, further studies are required to
investigate the exact mechanism of how CCND3 impacts the cell cycle
machinery in GBM.
After the first miRNA gene was discovered in 1993
(45), the non-coding RNA has
attracted significant attention to determine how the
post-transcriptional regulator participates in diverse
physiological and pathological processes (46). An increasing number of miRNAs have
been reported to serve crucial roles in tumor development, making
these small molecules attractive tools and targets for tumor
diagnosis and novel therapeutic approaches (22,47).
Numerous miRNAs, including miR-16 (clinical trial gov identifier,
NCT02369198) and miR-34 (clinical trial gov identifier,
NCT01829971) activators have reached clinical trial stages for lung
and liver cancer treatment respectively, enabling miRNA
therapeutics to hopefully become a reality. miR-138-5p is a
well-known miRNA involved in breast, ovarian and lung cancer
progression processes through its demonstrated ability to affect
apoptosis (48), and the sirtuin-1
(49) and p53 (50) signaling pathways. In the present
study, CCND3, a novel target of miR-138-5p was reported, providing
an enhanced understanding of GBM development, as well as enriching
the current understanding of miRNAs.
In conclusion, the results of the present study
demonstrated that miR-138-5p expression levels were significantly
downregulated in GBM tissues and cell lines, suggesting that
miR-138-5p may be a tumor suppressor in GBM. The upregulated
expression levels of miR-138-5p significantly inhibited the
viability and colony forming ability of GBM cells. In addition,
CCND3 was identified as a direct and functional binding target of
miR-138-5p, which may have contributed to cell cycle arrest and the
inhibition of tumor cell proliferation when miR-138-5p was
overexpressed.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW, CW, YL, CY and XLL performed the experiments; XZ
performed the statistical analysis; and XL designed the study,
analyzed the data and drafted the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Zhejiang Chinese
Medical University (Hangzhou, China) and all human tissue samples
were obtained following written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Adamson C, Kanu OO, Mehta AI, Di C, Lin N,
Mattox AK and Bigner DD: Glioblastoma multiforme: A review of where
we have been and where we are going. Expert Opin Investig Drugs.
18:1061–1083. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Tellingen O, Yetkin-Arik B, de Gooijer
MC, Wesseling P, Wurdinger T and de Vries HE: Overcoming the
blood-brain tumor barrier for effective glioblastoma treatment.
Drug Resist Updat. 19:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Furnari FB, Cloughesy TF, Cavenee WK and
Mischel PS: Heterogeneity of epidermal growth factor receptor
signalling networks in glioblastoma. Nat Rev Cancer. 15:302–310.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ricklefs F, Mineo M, Rooj AK, Nakano I,
Charest A, Weissleder R, Breakefield XO, Chiocca EA, Godlewski J
and Bronisz A: Extracellular vesicles from high-grade glioma
exchange diverse pro-oncogenic signals that maintain intratumoral
heterogeneity. Cancer Res. 76:2876–2881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nikaki A, Piperi C and Papavassiliou AG:
Role of microRNAs in gliomagenesis: Targeting miRNAs in
glioblastoma multiforme therapy. Expert Opin Investig Drugs.
21:1475–1488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang Y, Zang A, Li J, Jia Y, Li X, Zhang
L, Huo R, Yang J, Feng J, Ge K, et al: MicroRNA-383 is a tumor
suppressor and potential prognostic biomarker in human non-small
cell lung caner. Biomed Pharmacother. 83:1175–1181. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo J, Wu Q, Peng X and Yu B: miR-509-5p
inhibits the proliferation and invasion of osteosarcoma by
targeting TRIB2. Biomed Res Int. 2019:25230322019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rashad NM, El-Shal AS, Shalaby SM and
Mohamed SY: Serum miRNA-27a and miRNA-18b as potential predictive
biomarkers of hepatitis C virus-associated hepatocellular
carcinoma. Mol Cell Biochem. 447:125–136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim VN: MicroRNA biogenesis: Coordinated
cropping and dicing. Nat Rev Mol Cell Biol. 6:376–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wuchty S, Arjona D, Li A, Kotliarov Y,
Walling J, Ahn S, Zhang A, Maric D, Anolik R, Zenklusen JC and Fine
HA: Prediction of associations between microRNAs and gene
expression in glioma biology. PLoS One. 6:e146812011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui JG, Zhao Y, Sethi P, Li YY, Mahta A,
Culicchia F and Lukiw WJ: Micro-RNA-128 (miRNA-128) down-regulation
in glioblastoma targets ARP5 (ANGPTL6), Bmi-1 and E2F-3a, key
regulators of brain cell proliferation. J Neurooncol. 98:297–304.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Godlewski J, Bronisz A, Nowicki MO,
Chiocca EA and Lawler S: microRNA-451: A conditional switch
controlling glioma cell proliferation and migration. Cell Cycle.
9:2742–2748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nan Y, Han L, Zhang A, Wang G, Jia Z, Yang
Y, Yue X, Pu P, Zhong Y and Kang C: miRNA-451 plays a role as tumor
suppressor in human glioma cells. Brain Res. 1359:14–21. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahir BK, Ozer H, Engelhard HH and Lakka
SS: MicroRNAs in glioblastoma pathogenesis and therapy: A
comprehensive review. Crit Rev Oncol Hematol. 120:22–33. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Helming L, Tomasello E, Kyriakides TR,
Martinez FO, Takai T, Gordon S and Vivier E: Essential role of
DAP12 signaling in macrophage programming into a fusion-competent
state. Sci Signal. 1:ra112008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Nicolay BN, Chick JM, Gao X, Geng
Y, Ren H, Gao H, Yang G, Williams JA, Suski JM, et al: The
metabolic function of cyclin D3-CDK6 kinase in cancer cell
survival. Nature. 546:426–430. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diehl JA: Cyclin D3: To translate or not
to translate. Cell Cycle. 15:3018–3019. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang F, Xu C, Reece EA, Li X, Wu Y, Harman
C, Yu J, Dong D, Wang C, Yang P, et al: Protein kinase C-alpha
suppresses autophagy and induces neural tube defects via miR-129-2
in diabetic pregnancy. Nat Commun. 8:151822017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alifieris C and Trafalis DT: Glioblastoma
multiforme: Pathogenesis and treatment. Pharmacol Ther. 152:63–82.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jansen M, Yip S and Louis DN: Molecular
pathology in adult gliomas: Diagnostic, prognostic, and predictive
markers. Lancet Neurol. 9:717–726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Omuro A and DeAngelis LM: Glioblastoma and
other malignant gliomas: A clinical review. JAMA. 310:1842–1850.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu KP, Zhang CL, Ma XL, Hu JP, Cai T and
Zhang L: Analyzing the interactions of mRNAs and ncRNAs to predict
competing endogenous RNA networks in osteosarcoma chemo-resistance.
Mol Ther. 27:518–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang R, Liu M, Liang H, Guo S, Guo X, Yuan
M, Lian H, Yan X, Zhang S, Chen X, et al: miR-138-5p contributes to
cell proliferation and invasion by targeting Survivin in bladder
cancer cells. Mol Cancer. 15:822016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao Y, Fan X, Li W, Ping W, Deng Y and Fu
X: miR-138-5p reverses gefitinib resistance in non-small cell lung
cancer cells via negatively regulating G protein-coupled receptor
124. Biochem Biophys Res Commun. 446:179–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao L, Yu H, Yi S, Peng X, Su P, Xiao Z,
Liu R, Tang A, Li X, Liu F and Shen S: The tumor suppressor
miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget.
7:45370–45384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu C, Wang M, Li Z, Xiao J, Peng F, Guo X,
Deng Y, Jiang J and Sun C: MicroRNA-138-5p regulates pancreatic
cancer cell growth through targeting FOXC1. Cell Oncol (Dordr).
38:173–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yeh M, Oh CS, Yoo JY, Kaur B and Lee TJ:
Pivotal role of microRNA-138 in human cancers. Am J Cancer Res.
9:1118–1126. 2019.PubMed/NCBI
|
|
33
|
Wei J, Nduom EK, Kong LY, Hashimoto Y, Xu
S, Gabrusiewicz K, Ling X, Huang N, Qiao W, Zhou S, et al: miR-138
exerts anti-glioma efficacy by targeting immune checkpoints. Neuro
Oncol. 18:639–648. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ye Z, Fang B, Pan J, Zhang N, Huang J, Xie
C, Lou T and Cao Z: miR-138 suppresses the proliferation,
metastasis and autophagy of non-small cell lung cancer by targeting
Sirt1. Oncol Rep. 37:3244–3252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ren D, Liu F, Dong G, You M, Ji J, Huang
Y, Hou Y and Fan H: Activation of TLR7 increases CCND3 expression
via the downregulation of miR-15b in B cells of systemic lupus
erythematosus. Cell Mol Immunol. 13:764–775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sankaran VG, Orkin SH and Walkley CR: Rb
intrinsically promotes erythropoiesis by coupling cell cycle exit
with mitochondrial biogenesis. Genes Dev. 22:463–475. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sherr CJ and Roberts JM: Living with or
without cyclins and cyclin-dependent kinases. Genes Dev.
18:2699–2711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin J, Jinno S and Okayama H: Cdk6-cyclin
D3 complex evades inhibition by inhibitor proteins and uniquely
controls cell's proliferation competence. Oncogene. 20:2000–2009.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang GL, Shi X, Salisbury E, Sun Y,
Albrecht JH, Smith RG and Timchenko NA: Cyclin D3 maintains
growth-inhibitory activity of C/EBPalpha by stabilizing
C/EBPalpha-cdk2 and C/EBPalpha-Brm complexes. Mol Cell Biol.
26:2570–2582. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song S, Wu S, Wang Y, Wang Z, Ye C, Song
R, Song D and Ruan Y: 17β-estradiol inhibits human umbilical
vascular endothelial cell senescence by regulating autophagy via
p53. Exp Gerontol. 114:57–66. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xiang X, Huang J, Song S, Wang Y, Zeng Y,
Wu S and Ruan Y: 17β-estradiol inhibits H 2 O
2-induced senescence in HUVEC cells through upregulating
SIRT3 expression and promoting autophagy. Biogerontology.
21:549–557. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang J, Annala M, Ji P, Wang G, Zheng H,
Codgell D, Du X, Fang Z, Sun B, Nykter M, et al: Recurrent
LRP1-SNRNP25 and KCNMB4-CCND3 fusion genes promote tumor cell
motility in human osteosarcoma. J Hematol Oncol. 7:762014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shan YS, Hsu HP, Lai MD, Hung YH, Wang CY,
Yen MC and Chen YL: Cyclin D1 overexpression correlates with poor
tumor differentiation and prognosis in gastric cancer. Oncol Lett.
14:4517–4526. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim Y, Kim J, Jang SW and Ko J: The role
of sLZIP in cyclin D3-mediated negative regulation of androgen
receptor transactivation and its involvement in prostate cancer.
Oncogene. 34:226–236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun Q, Shi R, Wang X, Li D, Wu H and Ren
B: Overexpression of ZIC5 promotes proliferation in non-small cell
lung cancer. Biochem Biophys Res Commun. 479:502–509. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 9:8522017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bao XY and Cao J: miRNA-138-5p protects
the early diabetic retinopathy by regulating NOVA1. Eur Rev Med
Pharmacol Sci. 23:7749–7756. 2019.PubMed/NCBI
|
|
49
|
Guo S, Ma B, Jiang X, Li X and Jia Y:
Astragalus polysaccharides inhibits tumorigenesis and lipid
metabolism through miR-138-5p/SIRT1/SREBP1 pathway in prostate
cancer. Front Pharmacol. 11:5982020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li D, He C, Wang J, Wang Y, Bu J, Kong X
and Sun D: MicroRNA-138 inhibits cell growth, invasion, and EMT of
non-small cell lung cancer via SOX4/p53 feedback loop. Oncol Res.
26:385–400. 2018. View Article : Google Scholar : PubMed/NCBI
|