Renal cell carcinoma (RCC) originates from the renal
parenchyma and is the most common subtype of kidney cancer
(1). According to 2018 GLOBOCAN
data, RCC accounts for up to 2.4% of all cancer cases, with an
estimated 338,000 patients diagnosed globally each year (2). It has been shown that 25–30% of
affected patients have metastatic disease and therefore poor
survival outcomes (3). Surgery
remains the most effective treatment for both localized and locally
advanced RCC, with those patients with RCC who receive surgical
excision showing a good prognosis. However, the total recurrence
rate after surgical resection is high, estimated at 35% (4).
Although there is now a greater understanding of the
occurrence and development of RCC based on previous research, the
clinical prognosis of patients with regard to the biological
behavioral characteristics of RCC is still unsatisfactory. In
recent years, the association between inflammation and tumors has
become the focus of cancer research. Inflammation is a fundamental
innate immune response to perturbed tissue homeostasis (5). Numerous studies have shown that
inflammatory molecules and pathways play an important role in the
development of cancer, such as breast cancer, pancreatic cancer,
colorectal cancer, colon cancer, rectal cancer, prostate cancer,
bladder cancer, lung cancer and ovarian cancer (6–8).
Inflammation is highly associated with RCC, and participates in the
development of RCC tumors, which are considered to be immunogenic
(9,10). The present review summarizes the main
inflammatory response features in RCC, focusing primarily on
immune-related molecules and immunotherapy to elucidate the
association between inflammation and RCC, and its role in the
treatment of RCC.
Inflammation is considered a hallmark of cancer
development. It is estimated that potential infections and
inflammatory responses contribute to 15–20% of cancer-associated
deaths globally (11). Evidence
shows that the inflammatory response plays a vital role in the
occurrence and development of tumors (12). Inflammatory bodies, cytokines,
chemokines, transcription factors and immune cells drive the
inflammatory tumor microenvironment (TME) through multiple
inflammation-associated pathways. It is now increasingly being
recognized that inflammation is inextricably linked to cancer
(5,13,14).
Growth factors alter endocytosis and receptor cycling in cancer
cells through several pathways, inhibition of negative feedback
mechanisms that attenuate growth and enhancement of receptor-like
tyrosine kinases to enrich proliferation-related downstream
signaling (15). These changes
increase genetic mutations and anti-apoptotic signaling, and
promote angiogenesis, thereby promoting cancer progression
(6,16,17).
Inflammation is the natural defense mechanism of the
body against microbial infection and other noxious stimuli, which
inevitably cause tissue damage. Inflammatory cells accumulate at
the site of injury, secrete a large amount of inflammatory
mediators, promote tissue breakdown and enhance the defense of the
host against potential pathogens (18,19).
Inflammation is divided into acute inflammation and chronic
inflammation. Acute inflammation contributes to cancer regression
(7,20), whereas chronic inflammation promotes
cancer progression (21). Currently,
details regarding the pathogenesis of cancer arising from
inflammation are accumulating at an exponential rate. There are two
different modes for the association between inflammation and
cancer, namely the intrinsic and extrinsic pathways (22). DNA damage, chromosomal instability
and epigenetic changes lead to aberrant gene expression, which is a
key feature of intrinsic pathways. Inflammatory signals caused by
infections and autoimmune diseases are associated with extrinsic
pathways. A variety of important transcription factors, including
nuclear factor-κB (NF-κB) and signal transducer and activator of
transcription 3 (STAT3) are activated in these two pathways and
drive the inflammatory cascade (23,24).
Inflammation and RCC are closely associated, and
numerous inflammatory pathways interact with RCC. Four of these
pathways that are particularly important include the Von
Hippel-Lindau (VHL), mechanistic target of rapamycin (mTOR), tumor
necrosis factor (TNF) and STAT pathways.
VHL is a gene that suppresses the development of RCC
and has the highest mutation rate among other genes involved in RCC
(25,26). The main function of VHL is to control
the oxygen-sensing mechanism of cells by regulating the
hypoxia-inducible factor (HIF) α subunits (−1α, −2α and −3α)
(27–29). HIF is a heterodimeric transcription
factor composed of two subunits, HIF-α and HIF-β (30). Although the β-subunit of HIF is
constitutively expressed, HIF-α protein is only expressed under
hypoxic conditions and when VHL is inactivated (31). VHL promotes the degradation of HIF-α
under normoxic conditions to keep the HIF-α subunit level low
(32). Under hypoxic conditions, VHL
is inactivated, leading to the accumulation of HIF-α, which binds
to HIF-β and forms heterodimers that activate target genes.
Therefore, the inactivation of VHL in RCC results in the
upregulation of HIF-α isoforms, hence an increase in HIF, thereby
activating the downstream carcinogenesis-related genes such as
those associated with angiogenesis [vascular endothelial growth
factor A (VEGFA) and platelet-derived growth factor β (PDGF-β)],
erythropoiesis (erythropoietin) and glycolysis (solute carrier
family 2 member 1). This causes tumor development processes such as
cell growth, survival (cyclin G2 and transforming growth factor-α
are increased) and migration [C-X-C motif chemokine receptor 4
(CXCR4) is increased] to be enhanced (33–35).
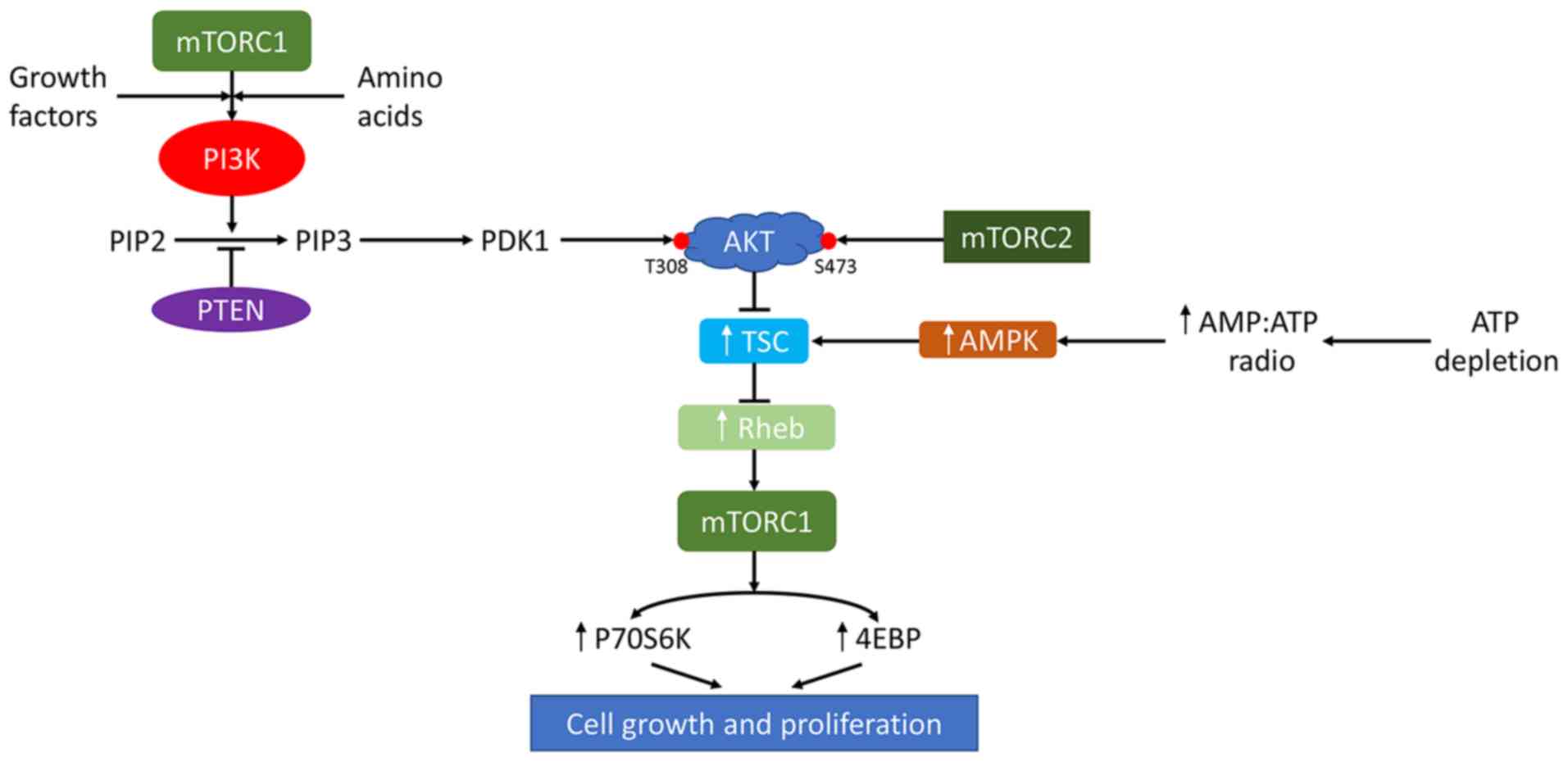
mTOR is a member of the protein kinase
phosphatidylinositol 3-kinase (PI3K)-associated kinase family,
which plays a key role in cell growth, cell proliferation, cell
motility, cell survival, protein synthesis, autophagy and
transcription (36). mTOR is
composed of two complexes, mTOR complex 1 (mTORC1) and mTORC2,
containing two different scaffolding proteins (37,38).
mTORC1 can be activated by growth factors and amino acids to
promote cell growth (e.g., increases in cell size and mass) and
cell proliferation. Activation of mTORC2 contributes to the
regulation of cell polarity and the actin cytoskeleton (39,40).
mTOR is closely associated with the incidence of RCC. AKT, a
molecule with an important role in the mTOR pathway, can be
activated by inflammation, which causes tumorigenesis through mTOR
signal transduction (41). Mutations
in associated genes and inflammation lead to an increase in the
incidence of metastatic RCC after an increase in mTOR activity
(Fig. 1). Functional deletion
mutations of the mTOR negative regulator PTEN, via the PI3K/AKT
pathway, occur in ~5% of patients with RCC (42,43).
Furthermore, the increased constitutive activity of mTORC1 promotes
the proliferation and invasiveness of metastatic RCC (44). This is since the increased activity
of mTORC1 promotes cell growth and proliferation (45). In addition, mTORC1 increases the
level of HIF-1α in cells, thereby activating the production of
proangiogenic factors such as VEGF, PDGF-α and TNF-α (46). In certain patients with RCC,
activation of mTORC1 is mediated by a phosphatase PTEN
loss-of-function mutation, which negatively regulates mTORC1 via
the upstream PI3K/AKT pathway (47).
Additionally, the occurrence of RCC in patients with tuberous
sclerosis is due to mutations in tuberous sclerosis complex
interfering with its negative regulation of mTORC1 activity
(48,49).
TNF is a multifunctional pro-inflammatory cytokine
secreted by macrophages and is the core driver of inflammatory
responses (50). TNF binds and
functions through its two different receptors, TNF receptor 1
(TNFR1) and TNFR2 (51). TNFR1 is
mainly expressed in endothelial cells in normal kidneys; it can
activate apoptotic signal kinase 1 and NF-κB leading to cell death
(52). TNFR2 is expressed primarily
on damaged endothelial cells and tubular epithelial cells (TECs),
which can activate endothelial/epithelial tyrosine kinase (Etk) and
transactivate VEGF receptor 2 (VEGFR2) to promote cell
proliferation (53). Aberrant
expression of TNFR2 on tumor cells has been found in human RCC
(54). The expression of TNFR2 in
RCC is associated with grade of malignancy (55). Al-Lamki et al (56) showed that TNF is an autocrine growth
factor that selectively promotes clear cell RCC (ccRCC) progression
via the TNFR2/Etk/VEGFR2 pathway (56). It was earlier reported that TNF-α
selectively activates the TNFR2 response, leading to activation of
epithelial cells and Etk, and apparent transactivation of VEGR-2
phosphorylation at Tyr(P)-1054-1059. This pathway promotes the
activation of NF-κB, which participates in tumor malignancy
(55). The activation of NF-κB
triggers transcription of anti-apoptotic proteins, including
apoptosis inhibitors [cellular inhibitor of apoptosis proteins
(c-IAPs)], cFLICE (procaspase-8) inhibitory protein (c-FLIP),
mitogen-activated protein kinase (MAPK)-specific phosphatase and
A20 (57). In addition,
myeloid-derived suppressor cells (MDSCs) contribute to tumor immune
evasion. Recent studies have shown that the generation,
accumulation and function of MDSCs depend on TNF-TNFR2 signaling
(58–60). Thus, the activation of TNFR2 can
promote the progression of RCC.
The STAT proteins are a family of cytoplasmic
transcription factors comprising seven members, STAT1, STAT2,
STAT3, STAT4, STAT5a, STAT5b and STAT6. Since cancer cells are more
dependent on the activity of these proteins than their normal
counterparts, STAT proteins are considered to be ideal targets for
anticancer therapy (61). STAT3 is a
potential transcription factor that mediates extracellular signals,
such as cytokines and growth factors, by interacting with cell
surface polypeptide receptors. Studies have shown that STAT3
promotes RCC occurrence and development (62–64).
STAT3 responds to extracellular stimuli and is activated after
tyrosine phosphorylation. Phosphorylated STAT3 dimerizes and
translocates to the nucleus where it then binds the
sequence-specific DNA elements, thereby activating transcription of
the target gene (65).
Cancer-associated inflammatory mediators, such as the interleukin
(IL)-6 and IL-10 cytokine families, recruit Janus kinase (JAK)
family members (JAK1, JAK2 and TYK2) to activate STAT3
phosphorylation after cross-phosphorylation. STAT3 forms homodimers
in the cytoplasm, which migrate to the nucleus to regulate gene
expression that leads to cancer (66). Numerous lines of evidence have
reported that STAT3 regulates genes that play important roles in
cell physiology, including the cell cycle, apoptosis, inflammatory
immunity, metabolism and angiogenesis (67–69).
Enhanced STAT3 activity can block the process of apoptosis and
induce the upregulation of Cyclin D1, c-Myc and Survivin
expression, resulting in abnormal cell proliferation (70). STAT has also been extensively studied
in the field of RCC. Studies have shown that activated STAT3 is a
potential regulator of HIF-1, which mediates VEGF expression in RCC
(71,72). The aforementioned findings show that
STAT affects not only gene expression through the JAK/STAT3
pathway, but also the expression of VEGF by regulating HIF-1. In
this way, it affects the occurrence and progression of renal
cancer.
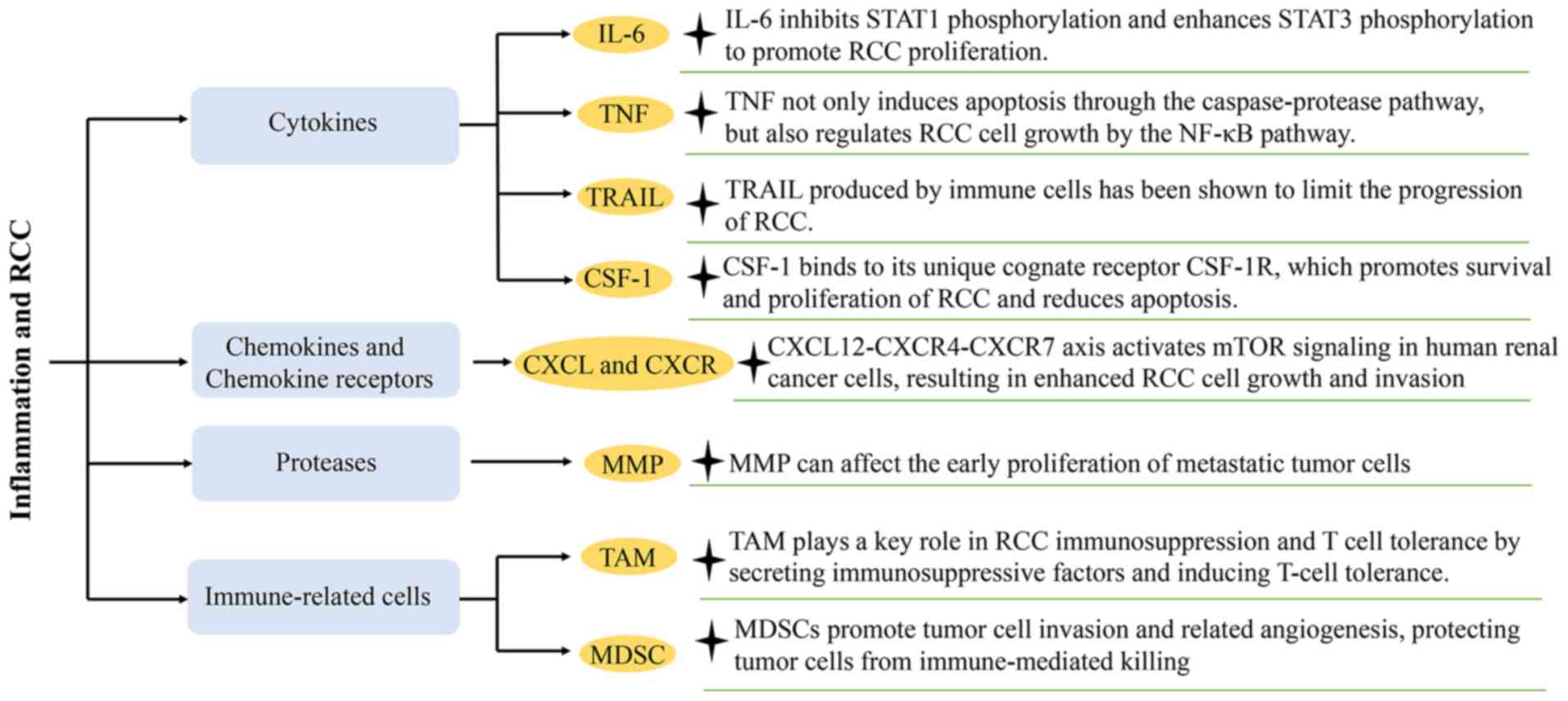
A variety of inflammatory factors and immune-related
cells are involved in the interactions between inflammation and
RCC, where they play an important role. Cytokines, chemokines and
other small inflammatory proteins from host cells coordinate
intracellular communication in the TME. Continuous crosstalk
between cells is critical for tumor growth, invasion, angiogenesis
and metastatic spread (9). The
present review focuses on the major contributors to
tumor-associated inflammation and local immune responses, including
cytokines and chemokine receptors, transcription factors and
immune-related cells (Fig. 2).
IL-6 is an inflammatory cytokine with multiple
biological effects; it is composed of 184 amino acids, with a
molecular weight of 21–28 kDa. IL-6 has a 4-helix bundle structure
consisting of 4 long α-helices (73–75). It
has been reported that enhancing the production of IL-6 stimulates
the expression of proinflammatory factors, such as IL-1, TNF-α,
interferons, bacterial endotoxin and lipopolysaccharide or viral
infection (76,77). Numerous studies have explored the
role of IL-6 in kidney functions. Renal cortical tissue and kidney
cancer tissue can produce IL-6 (78). IL-6 is expressed in most RCC cell
lines and in patient tissues, and it plays an important role in the
proliferation of RCC cells (78).
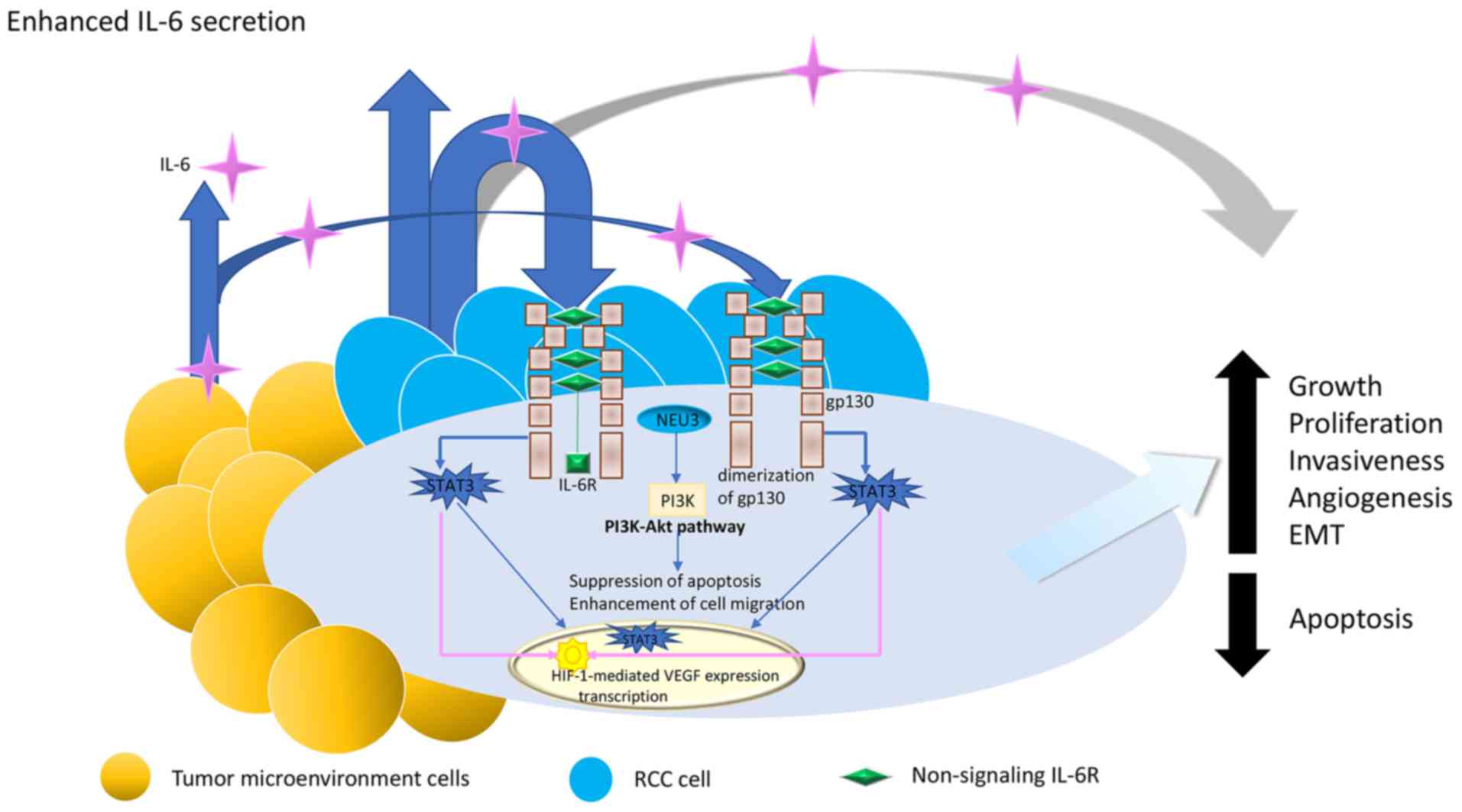
Mechanistic studies showed that IL-6 activates IL-6 receptor
(IL-6R) and glycoprotein 130 (gp130), causing phosphorylation of
the tyrosine kinases JAK1, JAK2 and TYK2. This results in
phosphorylation of STAT3 (79).
Pathophysiological conditions play an important role in the
regulation of IL-6. The transcriptional signal of IL-6 appears to
be activated only during immune stress, and the IL-6/soluble IL-6R
complex is usually upregulated in pathophysiological conditions
(80). Matsumoto et al
(81) indicated that tumor
endothelial cells upregulate the expression of gp130 and
downregulate the expression of membrane-bound IL-6R through the
IL-6/IL-6sR complex, leading to cell proliferation, inhibition of
apoptosis and enhancement of tumor development. IL-6 inhibits STAT1
phosphorylation, enhances STAT3 phosphorylation, promotes RCC
proliferation and blunts the antitumor effect of IFN (82). Meanwhile, studies have shown that
IL-6-activated plasma membrane-associated sialidase (NEU3) may also
contribute to the expression of malignant phenotypes in RCC
(83) (Fig. 3).
TNF is a potent pro-inflammatory cytokine that
mediates complex biological responses, including inflammation,
antiviral response, septic shock and apoptotic cell death (84). TNF can trigger apoptosis through the
caspase-protease pathway and the NF-κB pathway. In the NF-κB
pathway, TNF binds to TNFR to activate atypical protein kinase C
and phosphorylates inhibitor of nuclear factor-κB kinase subunit β
(IKKβ). Subsequently, the activated IKKβ phosphorylates inhibitor
of NF-κB (IκB), resulting in ubiquitin-mediated degradation of IκB.
NF-κB is released after IκB degradation and translocated into the
nucleus where it activates the transcription of a number of
anti-apoptotic genes, including the c-FLIP and c-IAP families
(85,86). It has been shown that TNF-α regulates
RCC cells growth by modulating the NF-κB-mediated anti-apoptotic
pathway (55). Harrison et al
(87) used an anti-TNF monoclonal
antibody, infliximab, in two phase II clinical trials in patients
with locally advanced and metastatic RCC. It was found that
targeting TNF may be a beneficial therapeutic approach for cancer
management. A previous study has reported that patients with high
serum inflammatory cytokine levels in RCC have a poor prognosis,
thus, TNF-α can be used as an independent prognostic indicator.
Normal TNF-α plasma levels are high predictors of good prognosis in
untreated patients (88).
TRAIL is a molecule belonging to the TNF
superfamily; it is an effective anticancer agent as it specifically
targets cancer cells while retaining normal cells, thereby inducing
apoptosis (89). TRAIL can bind to
death receptor DR4 and DR5, and assembles a death-inducing
signaling complex by recruiting FAS-associated protein associated
with death domain and caspase-8. Autocatalytic activation of
caspase-8 leads to caspase cascade activation, ultimately leading
to cell death (90). In cancer
treatment, normal cells highly express decoy receptors DcR1 and
DcR2, while cancer cells highly express death receptors DR4 and DR5
(89). TRAIL can bind to DR4 and DR5
and ultimately target cancer cell death (91). The complex formed by the binding of
TRAIL to the DcR does not activate the apoptotic signaling pathway.
Therefore, there is a weaker influence of TRAIL on the apoptosis of
normal cells than cancer cells. Furthermore, TRAIL produced by
immune cells has been shown to limit the progression of RCC
(92).
Chemokines are a subfamily of cytokines that contain
~50 different signaling proteins. Similar to other cytokines,
chemokines affect cell behavior through both autocrine and
paracrine modes (100). Chemokines
and their receptors are involved in the regulation of growth,
angiogenesis and metastasis of RCC (101). It has been shown that CXCR4 is an
ideal target for tumor diagnosis and treatment (102). The expression of CXCR4 in most
cases is associated with tumor-specific survival in ccRCC with VHL
mutations. A close association between VHL and CXCR4 has been
observed. The VHL disease tumor suppressor (pVHL) is a protein that
negatively regulates CXCR4. pVHL can target the degradation of HIF
under normoxic conditions to prevent CXCR4 expression. This process
is inhibited under hypoxic conditions (103–105).
A study by Ieranò et al (106) indicated that the CXCL12-CXCR4-CXCR7
axis activates mTOR signaling in human renal cancer cells,
resulting in enhanced RCC cell growth and invasion (106). Other studies have shown that the
CXCR4-CXCL12-CXCR7 axis also plays a key role in RCC. The activity
of CXCR4 is mainly γ-mediated; however, CXCR7 is considered to be
an atypical G protein-coupled receptor, as it does not cause
intracellular Ca2+ release when bound to a ligand
(107). Some studies have shown
that CXCR7 is a DcR that isolates extracellular CXCL12 or regulates
the CXCR4 signaling pathway by forming a CXCR7-CXCR4 heterodimer
(108,109). High expression of CXCR7 in human
cancer types such as bladder cancer, glioma, colorectal cancer,
ovarian cancer and breast cancer, and in tumor-associated blood
vessels, may be critical for tumor cell survival, adhesion and
growth (110–115). Overall, CXCR4 and CXCR7 are
potential molecules affecting the prognosis of RCC (116).
MMPs are members of the zinc-dependent endopeptidase
family; they regulate signaling pathways that control cell growth,
inflammation or angiogenesis. Hence, MMPs participate in molecular
communication between tumors and stroma, mediating
microenvironmental changes during tumor progression (117,118).
Compelling evidence from knockout mouse experiments has shown that
MMPs play an important role in acute and chronic inflammation
(119). Numerous studies have
demonstrated that MMPs can aggregate leukocytes to cause
tumor-related inflammation (120,121).
Studies have confirmed that MMPs affect the early proliferation of
metastatic tumor cells, while tissue inhibitors of MMP (TIMPs)
inhibit MMP activity to indirectly regulate tumor cell
proliferation (122,123). Among these MMPs, MMP-9 has the most
important function in tumors. MMP-9 exhibit higher expression in
malignant tumors than in benign or non-invasive tumors, and it also
exhibits high expression in RCC; it can denature type I, II and IV
collagen, enabling tumor cells to penetrate the basement membrane
(124,125). Additionally, membrane type 1 MMP
(MT1-MMP/MMP-14) is involved in tumor invasion; it is widely
expressed in most malignant tumors, and its overexpression enhances
cell invasion ability (126).
MT1-MMP not only degrades extracellular matrix molecules such as
type I, II and III collagen, vitronectin, laminin-1 and −5,
fibronectin and aggrecan (127),
but it also recruits pro-MMP-2 to the cell surface and causes the
activation of MMP-2 by cleaving the propeptide sequence (128). A study by Petrella and Brinckerhoff
(129) showed that MT1-MMP is the
main trigger of type I collagen degradation and the invasiveness of
VHL RCC cells expressing MT1-MMP or HIF-2α. In addition, in the
absence of VHL, the protein and gene levels of MT1-MMP are
significantly upregulated in RCC (130).
TAMs are derived from peripheral blood mononuclear
cells. Macrophages usually undergo M1 or M2 activation under
inflammatory stimuli (131). In
RCC, M1 cells produce inflammatory cytokines such as TNF-α, IL-6,
IL-12 and IL-23, while M2 cells produce anti-inflammatory cytokines
such as IL-10, thereby promoting RCC-related immune dysfunction
(132). Santoni et al
(133) showed that high TAM
infiltration in the RCC microenvironment promotes tumor progression
and metastasis by stimulating angiogenesis, tumor growth and cell
migration. RCC is a typical hemangioma in which VEGF is
significantly upregulated. VEGF, considered to be a chemokine of
TAM, supports tumor proliferation. TAM can self-produce VEGF, which
increases the accumulation of TAM in tumor sites (134). TAM plays a key role in RCC
immunosuppression and T-cell tolerance by secreting
immunosuppressive factors and inducing T-cell immunity without
response (135).
MDSCs are a group of heterogeneous cells derived
from the bone marrow, which are preferentially expanded in cancer
and have a significant ability to suppress immune cell responses;
they primarily inhibit T-cell proliferation and NK-cell activation,
and induce differentiation and proliferation of regulatory T cells
(136). MDSCs have the ability to
inhibit T-cell activation through upregulation of arginase-1 (Arg1)
and inducible nitric oxide synthase in monocytic MDSCs, and Arg1
and reactive oxygen species in granulocytic MDSCs (137). The TME affects the progression and
metastasis of solid tumors, which consist of tumor cells and other
primitive stromal cells (138,139).
MDSCs are the main components of the TME, and the increase in blood
volume is associated with a shorter patient survival time.
Mechanistic studies have shown that MDSCs promote tumor cell
survival, associated angiogenesis, invasion and metastasis
(140,141). MDSCs also protect tumor cells from
immune-mediated killing, establish a TME and interact with tumor
cells to promote epithelial-mesenchymal transition to support tumor
growth and metastasis (142). A
close association between MDSCs and clinical outcomes of cancer
patients has been established. MDSCs hold great promise as novel
biomarkers for tumor prognosis (143).
A number of inflammation-related factors, including
Th1-related factors (IFN-γ), Th1-related chemokines (monokine
induced by IFN-γ [MIG], IFN-γ inducible protein 10 [IP-10] and
IFN-γ-inducible T-cell A chemoattractant [I-TAC]), Th2-related
factors (IL-4) and Th2-associated chemokines (eotaxin and
macrophage-derived chemokine [MDC]) are elevated in tumor tissues
(138). A variety of inflammatory
factors are associated with recurrence in patients, for example,
MIG and IFNγ-mediated mononuclear factors (144).
Inflammation-related factors TNF-α, CXCR4 and C-C
chemokine receptor type 3 (CCR3) are associated with the prognosis
and staging of patients with RCC. For instance, TNF-α is an
independent prognostic indicator, and normal levels in plasma can
highly predict the good prognosis of untreated RCC patients
(88,145). In addition, CXCR4 has significant
prognostic value in univariate analysis, and its low expression
indicates a good prognosis (146).
The high expression of CCR3 in immunohistochemical analysis is
associated with the degree of malignancy of the tumor. Upregulation
of CCR3 and its ligands may promote tumor cell proliferation
(147). In another study, Kallakury
et al (148) showed that
increased expression of MMP2, MMP9, TIMP1 and TIMP2 in RCC
correlated with a poor prognosis. In summary, inflammation-related
factors may be predictive indicators of RCC clinical prognosis and
have a huge potential role in RCC therapy.
With in-depth basic research and a better
understanding of the mechanism of inflammation in RCC, new
anti-inflammation-related therapeutics and immunotherapy-related
agents may be developed. In recent years, simvastatin, all-trans
retinoic acid (ATRA), nivolumab and other immunotherapeutic agents
have played a role in the treatment of RCC (Table I).
Simvastatin is a cholesterol-lowering drug for the
prevention of cardiovascular disease, and is involved in tumor
growth, spread and endothelial function (149). A previous study has reported that
the extracellular signal-regulated kinase (ERK)1/2 signaling
pathway is a statin-dependent pro-apoptotic mediator (150). Knockdown of ERK can make RCC cells
sensitive to simvastatin-induced anticancer effects. Simvastatin
can inhibit the proliferation and migration of RCC cells by
inhibiting the phosphorylation of AKT, mTOR and ERK; it also
exhibits antitumor effects by inhibiting the IL-6-induced
phosphorylation of JAK2 and STAT3 (151).
ATRA is the active metabolite of vitamin A, involved
in cell proliferation, differentiation and apoptosis; its role is
mediated by nuclear flavonoid receptors, MAPK and
cAMP/cAMP-dependent protein kinase signaling pathways (152). ATRA reduces cell proliferation and
alters gene expression through nuclear receptor- and
non-receptor-mediated pathways, thereby accelerating cell
differentiation and apoptosis. In genomic action, the function of
ATRA is mediated by nuclear receptors, particularly retinoic acid
receptors (RARs) (α, β and γ). Nuclear RAR acts as a
retinoid-inducible transcription factor, and regulates cell cycle
arrest, cell differentiation and cell regulation through
heterodimer formation with retinoid X receptor (153). Among the RARs, RARβ is a tumor
suppressor that is expressed at low levels in a number of cancer
types, such as breast and prostate cancer, and whose expression is
regulated by ATRA (154). In the
non-genomic pathway, ATRA independently activates the transcription
of genes involved in the PI3K/AKT pathway in cells, reversing the
dysregulation of the PI3K/AKT pathway in most human cancer types.
In more detail, this process entails the activation of PI3K by ATRA
through G-protein coupled receptors and multiple receptor tyrosine
kinases. Activated PI3K catalyzes the production of
phosphatidylinositol-3,4,5-triphosphate to promote aggregation and
activation of AKT on the membrane (155).
Nivolumab is a programmed death 1 (PD-1) checkpoint
inhibitor that selectively blocks the interaction between PD-1 and
PD ligand (PD-L)1/PD-L2 expressed on activated T cells, thereby
preventing T-cell inactivation (156). Expression of PD-L1 as a remote
immunomodulator occurs in 20–50% of human cancer types. A variety
of cancer immunotherapies targeting the interaction between PD-L1
and PD-1 have been developed (157,158).
PD-L1 effectively inhibits the tumor-killing ability of T cells.
Once the PD-L1:PD-1 interaction is blocked, T cells can quickly
restore their effector function. PD-L1 expressed on tumor cells
binds to PD-1 on activated effector T cells, and phosphatase SHP-2
is recruited, resulting in inactivation of the PI3K signaling
cascade (159). It has been found
that PD-L1 or PD-1 inhibitors have a positive effect on the
treatment of cancer. Nivolumab exerts antitumor activity in
metastatic RCC (160). The
immunotherapeutic agent ipilimumab is a human cytotoxic
T-lymphocyte antigen-4 (CTLA-4) immune checkpoint inhibitor
antibody, which can prevent CD80 and CD86 ligands on
antigen-presenting cells from binding to the CTLA-4 receptor on
activated T cells, thereby preventing the downregulation of
antitumor T cell activity (161).
CTLA-4 plays a significant role in early immune response, primarily
occurring in lymphoid tissues, while PD-1, whose expression is
upregulated after T-cell activation in peripheral tissues, is more
involved in late immune response (162). The combined application of CTLA-4
and PD-1 blockers can synergistically activate the antitumor immune
response and increase the response rate of patients (163). Thus, a combination of nivolumab and
ipilimumab will effectively control tumor development with a good
safety profile (164).
Emerging clinical data reveals that immunotherapy
has great potential in the treatment of cancer. Associated studies
have shown that a series of gradual and repeated immune response
events, defined as the cancer-immune cycle, can effectively kill
cancer cells. In the first step of this process, tumor antigens are
captured by dendritic cells (DCs) for processing (165). Proinflammatory cytokines and
factors released by dying tumor cells can be used as a designated
immune signal to avoid induction of tumor tolerance antigens (step
1) (166,167). DCs then present the captured
antigen on the major histocompatibility complex I (MHCI) and MHCII
molecules to the T cells (step 2) (168). This initiates and activates the
effector T-cell response (step 3) (169). This is followed by the entry (step
4) and invasion (step 5) of the tumor bed by the activated effector
T cells (170), which then
recognize and bind to specific cancer cells (step 6) and kill them
(step 7) (171). Subsequently, the
tumor-associated antigens released from the dying cells amplify the
cycle of immune response and make it more widespread and repetitive
(172).
The entire immune cycle is enhanced through the
positive regulatory signal or suppressed via the negative
regulatory signal in the aforementioned steps during therapeutic
treatments. The first phase of treatment corresponds to
chemotherapy, radiotherapy and targeted therapy. These treatments
induce immune cell death by activating the immune system (173). The second phase corresponds to use
of cancer vaccines, which rely on tumor cell-associated antigens to
awaken the immune system against cancer (174). The third phase is mainly associated
with CTLA-4 inhibitors. CTLA-4 is a receptor found on the surface
of activated T-cells, which predominantly acts by competing with
CD28 receptors for binding to B7 ligands on antigen presenting
cells (APCs). In the process of T cell activation, CD28 receptors
on the T-cells bind to the B7 ligands on APCs and provide the
essential second activation signal for T-cell. However, CTLA-4
receptors can competitively bind to B7 ligands with higher
affinity, resulting in the lack of second activation signal. Lack
of the second activation signal in the presence of CTLA-4 receptors
would lead to anergy in T-cells (163,175).
The fourth phase involves the transport of T lymphocytes, and no
intervention is available at this stage. The fifth stage is
predominantly through anti-VEGF treatment to enhance T cell
transport and tumor bed infiltration. The transfer of activated T
cells from the lymph nodes into circulation and then to the tumor
requires a series of steps. VEGF promotes the formation of abnormal
tumor blood vessels, which can negatively affect the transmigration
of T-cells from lymph nodes to the tumor bed (176). In addition, the blockade of VEGF
can increase the expression of E-selectin on tumor vascular
endothelium and down-regulate the Fas ligand on vascular
endothelial cells, ultimately promoting the increased of T-cell
tumor infiltration (177). The
sixth phase involves CAR-T-cell therapy, which is to generate a
powerful immune-mediated anti-tumor response through the in
vitro manipulation of T-cells (178). This treatment is achieved through
the selection and expansion of tumor-infiltrating lymphocytes
(TILs), or through gene transfer of a synthetic TCR (sTCR) or a
chimeric antigen receptor (CAR) into T-cells (179). The seventh stage corresponds to
PD-1/PD-L1 inhibitor treatment (180). Immunotherapy has successfully been
applied to RCC in recent years (172).
In addition, it has been found that inhibiting the
inflammatory pathway is an effective approach to suppress the
progression of RCC. Thus agents, such as LY294002, that inhibit the
PI3K/AKT signaling cascade may benefit patients with RCC (181). Activation of the 15-lipoxygenase
2/15(S)-hydroxyeicosatetraenoic acid pathway increases the
metabolism of arachidonic acid in the RCC TME, compromising the
function of the recruited immune cells, thereby promoting local
immunosuppression and tumor escape (182).
Inflammation influences all aspects of tumor
occurrence and progression, as well as the tumor response to
treatment. In the early stages of tumor formation, inflammatory
cells play a pro-tumor development role, creating favorable
conditions for tumor growth (183).
Chemokines and cytokines provided by inflammatory cells affect the
entire tumor organ and regulate the growth, migration and
differentiation of cells in the TME. In the later stages of
tumorigenesis, tumor cells also switch inflammatory mechanisms,
such as the production of chemokines and MMP, favoring tumor
proliferation and metastasis (119,184).
However, aggregation of inflammatory cells may suppress tumor
growth (7).
Accumulating evidence indicates that the TME
harbors multiple inflammatory cells that regulate tumor cell
growth, proliferation, survival and migration (185). The aforementioned
inflammation-related pathways (the VHL, mTOR, TNF and STAT
pathways) can promote the occurrence and progression of RCC by
activating the inflammatory response. Pro-inflammatory cytokines
(IL-6, TNF and CSF-1) and chemokines (CXCL and CXCR) are involved
in tumor-related pathways and promote the proliferation of RCC
cells. When highly expressed, they confer a poor prognosis for RCC.
TRAIL expression has been recorded as a prognostic factor for
improved RCC-specific survival (186). As a key participant in the
molecular communication between tumors and stroma, MMP
overexpression enhances RCC cell invasion ability (187). TAM cells and MDSCs support the
growth and metastasis of RCC by suppressing the ability of immune
cell responses. In terms of the therapy, agents targeting
inflammation (simvastatin, ATRA, nivolumab, PI3K/AKT pathway
inhibitor and cancer immune cycle inhibitors) have shown great
promise in prolonging the survival time of RCC patients.
Understanding the nature of inflammation and RCC will reveal
important targets for developing treatments of RCC (9,188).
On the basis of recent technological advances and
in-depth research on the pathology and mechanism of RCC, new
findings and breakthroughs have been reported. The association
between inflammation and RCC phenotypes has become the new hotspot
of current cancer research (9,189,190).
Numerous studies have demonstrated that the expression of most
inflammation-related factors is associated with the poor prognosis
of RCC and that immune-infiltrating cell profiles can also predict
the prognosis of patients (72,92,191).
Research on immune-targeted treatments has become one of the
hotspots in basic and clinical research, providing hope for tumor
treatment. In the past 12 years, the medical treatment of RCC has
transitioned from non-specific immune methods to targeted therapy
for VEGF to new immunotherapeutic agents (192). The interaction between tumor immune
status and cancer-related systemic inflammation is essential for
the treatment of RCC. Therapies targeted at inflammatory pathways
are likely to be a new direction for RCC treatment.
Not applicable.
No funding was received.
Not applicable.
JS, KC and XZ conceived the present study. JS, KW
and ZX drafted the manuscript. CY, CW, QC, HYu, XM and ZC made
substantial contributions to the interpretation and analysis of the
data, drafting the study and revising it critically for important
intellectual content. KX and HYa were major contributors in the
revision of the manuscript. All authors read and approved the final
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Wiechno P, Kucharz J, Sadowska M,
Michalski W, Sikora-Kupis B, Jonska-Gmyrek J, Poniatowska G,
Nietupski K, Ossolinski K and Demkow T: Contemporary treatment of
metastatic renal cell carcinoma. Med Oncol. 35:1562018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hammers HJ, Plimack ER, Infante JR, Rini
BI, McDermott DF, Lewis LD, Voss MH, Sharma P, Pal SK, Razak ARA,
et al: Safety and efficacy of nivolumab in combination with
ipilimumab in metastatic renal cell carcinoma: The CheckMate 016
study. J Clin Oncol. 35:3851–3858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galdiero MR, Marone G and Mantovani A:
Cancer inflammation and cytokines. Cold Spring Harb Perspect Biol.
10:a0285302018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kay J, Thadhani E, Samson L and Engelward
B: Inflammation-induced DNA damage, mutations and cancer. DNA
Repair (Amst). 83:1026732019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Korniluk A, Koper O, Kemona H and
Dymicka-Piekarska V: From inflammation to cancer. Ir J Med Sci.
186:57–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ha H, Debnath B and Neamati N: Role of the
CXCL8-CXCR1/2 axis in cancer and inflammatory diseases.
Theranostics. 7:1543–1588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brighi N, Farolfi A, Conteduca V, Gurioli
G, Gargiulo S, Gallà V, Schepisi G, Lolli C, Casadei C and De
Giorgi U: The interplay between inflammation, anti-angiogenic
agents, and immune checkpoint inhibitors: Perspectives for renal
cell cancer treatment. Cancers (Basel). 11:19352019. View Article : Google Scholar
|
|
10
|
Nakamura K and Smyth MJ: Targeting
cancer-related inflammation in the era of immunotherapy. Immunol
Cell Biol. 95:325–332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ngabire D and Kim GD: Autophagy and
inflammatory response in the tumor microenvironment. Int J Mol Sci.
18:20162017. View Article : Google Scholar
|
|
13
|
Todoric J, Antonucci L and Karin M:
Targeting inflammation in cancer prevention and therapy. Cancer
Prev Res (Phila). 9:895–905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borroni EM, Savino B, Bonecchi R and
Locati M: Chemokines sound the alarmin: The role of atypical
chemokine in inflammation and cancer. Semin Immunol. 38:63–71.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin
C and Flavell RA: Inflammation-induced cancer: Crosstalk between
tumours, immune cells and microorganisms. Nat Rev Cancer.
13:759–771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marelli G, Sica A, Vannucci L and Allavena
P: Inflammation as target in cancer therapy. Curr Opin Pharmacol.
35:57–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greten FR and Grivennikov SI: Inflammation
and cancer: Triggers, mechanisms, and consequences. Immunity.
51:27–41. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Munn LL: Cancer and inflammation. Wiley
Interdiscip Rev Syst Biol Med. 9:2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murata M: Inflammation and cancer. Environ
Health Prev Med. 23:502018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Urban-Wojciuk Z, Khan MM, Oyler BL,
Fåhraeus R, Marek-Trzonkowska N, Nita-Lazar A, Hupp TR and Goodlett
DR: The role of TLRs in anti-cancer immunity and tumor rejection.
Front Immunol. 10:23882019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kunnumakkara AB, Sailo BL, Banik K, Harsha
C, Prasad S, Gupta SC, Bharti AC and Aggarwal BB: Chronic diseases,
inflammation, and spices: How are they linked? J Transl Med.
16:142018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kundu JK and Surh YJ: Emerging avenues
linking inflammation and cancer. Free Radic Biol Med. 52:2013–2037.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu YS, Han X and Liu XH: STAT3: A
potential drug target for tumor and inflammation. Curr Top Med
Chem. 19:1305–1317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martínez-Sáez O, Gajate Borau P,
Alonso-Gordoa T, Molina-Cerrillo J and Grande E: Targeting HIF-2 α
in clear cell renal cell carcinoma: A promising therapeutic
strategy. Crit Rev Oncol Hematol. 111:117–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tarade D and Ohh M: The HIF and other
quandaries in VHL disease. Oncogene. 37:139–147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chappell JC, Payne LB and Rathmell WK:
Hypoxia, angiogenesis, and metabolism in the hereditary kidney
cancers. J Clin Invest. 129:442–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duan C: Hypoxia-inducible factor 3
biology: Complexities and emerging themes. Am J Physiol Cell
Physiol. 310:C260–C269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choudhry H and Harris AL: Advances in
hypoxia-inducible factor biology. Cell Metab. 27:281–298. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Strowitzki MJ, Cummins EP and Taylor CT:
Protein hydroxylation by hypoxia-inducible factor (HIF)
hydroxylases: Unique or Ubiquitous? Cells. 8:3842019. View Article : Google Scholar
|
|
32
|
Yu Y, Yu Q and Zhang X: Allosteric
inhibition of HIF-2α as a novel therapy for clear cell renal cell
carcinoma. Drug Discov Today. 24:2332–2340. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schödel J, Grampp S, Maher ER, Moch H,
Ratcliffe PJ, Russo P and Mole DR: Hypoxia, hypoxia-inducible
transcription factors, and renal cancer. Eur Urol. 69:646–657.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qureshi AS and Ali S: Review: Warburg
effect and renal cancer caused by errs in fumarate hydratase
encoding gene. Pak J Pharm Sci. 32:743–749. 2019.PubMed/NCBI
|
|
35
|
Bao Y, Wang Z, Liu B, Lu X, Xiong Y, Shi
J, Li P, Chen J, Zhang Z, Chen M, et al: A feed-forward loop
between nuclear translocation of CXCR4 and HIF-1α promotes renal
cell carcinoma metastasis. Oncogene. 38:881–895. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mossmann D, Park S and Hall MN: mTOR
signalling and cellular metabolism are mutual determinants in
cancer. Nat Rev Cancer. 18:744–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hua H, Kong Q, Zhang H, Wang J, Luo T and
Jiang Y: Targeting mTOR for cancer therapy. J Hematol Oncol.
12:712019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Murugan AK: mTOR: Role in cancer,
metastasis and drug resistance. Semin Cancer Biol. 59:92–111. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Saxton RA and Sabatini DM: mTOR signaling
in growth, metabolism, and disease. Cell. 168:960–976. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim LC, Cook RS and Chen J: mTORC1 and
mTORC2 in cancer and the tumor microenvironment. Oncogene.
36:2191–2201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Song M, Bode AM, Dong Z and Lee MH: AKT as
a therapeutic target for cancer. Cancer Res. 79:1019–1031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lieberthal W and Levine JS: The role of
the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc
Nephrol. 20:2493–2502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Deng W, Han W, Fan T, Wang X, Cheng Z, Wan
B and Chen J: Scutellarin inhibits human renal cancer cell
proliferation and migration via upregulation of PTEN. Biomed
Pharmacother. 107:1505–1513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Figlin RA, Kaufmann I and Brechbiel J:
Targeting PI3K and mTORC2 in metastatic renal cell carcinoma: New
strategies for overcoming resistance to VEGFR and mTORC1
inhibitors. Int J Cancer. 133:788–796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ben-Sahra I and Manning BD: mTORC1
signaling and the metabolic control of cell growth. Curr Opin Cell
Biol. 45:72–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Duran I, Lambea J, Maroto P,
González-Larriba JL, Flores L, Granados-Principal S, Graupera M,
Sáez B, Vivancos A and Casanovas O: Resistance to targeted
therapies in renal cancer: The importance of changing the mechanism
of action. Target Oncol. 12:19–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Poletto V, Rosti V, Biggiogera M, Guerra
G, Moccia F and Porta C: The role of endothelial colony forming
cells in kidney cancer's pathogenesis, and in resistance to
anti-VEGFR agents and mTOR inhibitors: A speculative review. Crit
Rev Oncol Hematol. 132:89–99. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma WW and Jimeno A: Temsirolimus. Drugs
Today (Barc). 43:659–669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bedke J, Stühler V, Stenzl A and Brehmer
B: Immunotherapy for kidney cancer: Status quo and the future. Curr
Opin Urol. 28:8–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wajant H and Beilhack A: Targeting
regulatory T cells by addressing tumor necrosis factor and its
receptors in allogeneic hematopoietic cell transplantation and
cancer. Front Immunol. 10:20402019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Martínez-Reza I, Díaz L and García-Becerra
R: Preclinical and clinical aspects of TNF-α and its receptors
TNFR1 and TNFR2 in breast cancer. J Biomed Sci. 24:902017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ting AT and Bertrand MJM: More to life
than NF-κB in TNFR1 signaling. Trends Immunol. 37:535–545. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mehta AK, Gracias DT and Croft M: TNF
activity and T cells. Cytokine. 101:14–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang J and Al-Lamki RS: Tumor necrosis
factor receptor 2: Its contribution to acute cellular rejection and
clear cell renal carcinoma. Biomed Res Int. 2013:8213102013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qi H and Ohh M: The von Hippel-Lindau
tumor suppressor protein sensitizes renal cell carcinoma cells to
tumor necrosis factor-induced cytotoxicity by suppressing the
nuclear factor-kappaB-dependent antiapoptotic pathway. Cancer Res.
63:7076–7080. 2003.PubMed/NCBI
|
|
56
|
Al-Lamki RS, Sadler TJ, Wang J, Reid MJ,
Warren AY, Movassagh M, Lu W, Mills IG, Neal DE, Burge J, et al:
Tumor necrosis factor receptor expression and signaling in renal
cell carcinoma. Am J Pathol. 177:943–954. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Borghi A, Verstrepen L and Beyaert R:
TRAF2 multitasking in TNF receptor-induced signaling to NF-κB, MAP
kinases and cell death. Biochem Pharmacol. 116:1–10. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhao X, Rong L, Zhao X, Li X, Liu X, Deng
J, Wu H, Xu X, Erben U, Wu P, et al: TNF signaling drives
myeloid-derived suppressor cell accumulation. J Clin Invest.
122:4094–4104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sheng Y, Li F and Qin Z: TNF receptor 2
makes tumor necrosis factor a friend of tumors. Front Immunol.
9:11702018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang S, Yang X, Wang L and Zhang C:
Interplay between inflammatory tumor microenvironment and cancer
stem cells. Oncol Lett. 16:679–686. 2018.PubMed/NCBI
|
|
61
|
Trivedi S and Starz-Gaiano M: Drosophila
Jak/STAT signaling: Regulation and relevance in human cancer and
metastasis. Int J Mol Sci. 19:40562018. View Article : Google Scholar
|
|
62
|
Chen Y, Zhu Y, Sheng Y, Xiao J, Xiao Y,
Cheng N, Chai Y, Wu X, Zhang S and Xiang T: SIRT1 downregulated FGB
expression to inhibit RCC tumorigenesis by destabilizing STAT3. Exp
Cell Res. 382:1114662019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu Y, Wang JX, Nie ZY, Wen Y, Jia XJ,
Zhang LN, Duan HJ and Shi YH: Upregulation of ERp57 promotes clear
cell renal cell carcinoma progression by initiating a STAT3/ILF3
feedback loop. J Exp Clin Cancer Res. 38:4392019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wei X, Yu L and Li Y: PBX1 promotes the
cell proliferation via JAK2/STAT3 signaling in clear cell renal
carcinoma. Biochem Biophys Res Commun. 500:650–657. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Huynh J, Etemadi N, Hollande F, Ernst M
and Buchert M: The JAK/STAT3 axis: A comprehensive drug target for
solid malignancies. Semin Cancer Biol. 45:13–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Fathi N, Rashidi G, Khodadadi A, Shahi S
and Sharifi S: STAT3 and apoptosis challenges in cancer. Int J Biol
Macromol. 117:993–1001. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Guanizo AC, Fernando CD, Garama DJ and
Gough DJ: STAT3: A multifaceted oncoprotein. Growth Factors.
36:1–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Galoczova M, Coates P and Vojtesek B:
STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett.
23:122018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Huynh J, Chand A, Gough D and Ernst M:
Therapeutically exploiting STAT3 activity in cancer - using tissue
repair as a road map. Nat Rev Cancer. 19:82–96. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yeung YT, Aziz F, Guerrero-Castilla A and
Arguelles S: Signaling pathways in inflammation and
anti-inflammatory therapies. Curr Pharm Des. 24:1449–1484. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang Y, Fu D, Chen Y, Su J, Wang Y, Li X,
Zhai W, Niu Y, Yue D and Geng H: G3BP1 promotes tumor progression
and metastasis through IL-6/G3BP1/STAT3 signaling axis in renal
cell carcinomas. Cell Death Dis. 9:5012018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Taher MY, Davies DM and Maher J: The role
of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem Soc
Trans. 46:1449–1462. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kampan NC, Xiang SD, McNally OM, Stephens
AN, Quinn MA and Plebanski M: Immunotherapeutic interleukin-6 or
interleukin-6 receptor blockade in cancer: Challenges and
opportunities. Curr Med Chem. 25:4785–4806. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ray K, Ujvari B, Ramana V and Donald J:
Cross-talk between EGFR and IL-6 drives oncogenic signaling and
offers therapeutic opportunities in cancer. Cytokine Growth Factor
Rev. 41:18–27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jordan SC, Choi J, Kim I, Wu G, Toyoda M,
Shin B and Vo A: Interleukin-6, a cytokine critical to mediation of
inflammation, autoimmunity and allograft rejection: Therapeutic
implications of IL-6 receptor blockade. Transplantation. 101:32–44.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jones SA and Jenkins BJ: Recent insights
into targeting the IL-6 cytokine family in inflammatory diseases
and cancer. Nat Rev Immunol. 18:773–789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kamińska K, Czarnecka AM, Escudier B, Lian
F and Szczylik C: Interleukin-6 as an emerging regulator of renal
cell cancer. Urol Oncol. 33:476–485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Qi QR and Yang ZM: Regulation and function
of signal transducer and activator of transcription 3. World J Biol
Chem. 5:231–239. 2014.PubMed/NCBI
|
|
80
|
Jones SA, Horiuchi S, Topley N, Yamamoto N
and Fuller GM: The soluble interleukin 6 receptor: Mechanisms of
production and implications in disease. FASEB J. 15:43–58. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Matsumoto S, Hara T, Mitsuyama K, Yamamoto
M, Tsuruta O, Sata M, Scheller J, Rose-John S, Kado S and Takada T:
Essential roles of IL-6 trans-signaling in colonic epithelial
cells, induced by the IL-6/soluble-IL-6 receptor derived from
lamina propria macrophages, on the development of
colitis-associated premalignant cancer in a murine model. J
Immunol. 184:1543–1551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Oguro T, Ishibashi K, Sugino T, Hashimoto
K, Tomita S, Takahashi N, Yanagida T, Haga N, Aikawa K, Suzutani T,
et al: Humanised antihuman IL-6R antibody with interferon inhibits
renal cell carcinoma cell growth in vitro and in vivo through
suppressed SOCS3 expression. Eur J Cancer. 49:1715–1724. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ueno S, Saito S, Wada T, Yamaguchi K,
Satoh M, Arai Y and Miyagi T: Plasma membrane-associated sialidase
is up-regulated in renal cell carcinoma and promotes
interleukin-6-induced apoptosis suppression and cell motility. J
Biol Chem. 281:7756–7764. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Annibaldi A and Meier P: Checkpoints in
TNF-induced cell death: Implications in inflammation and cancer.
Trends Mol Med. 24:49–65. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Patel HJ and Patel BM: TNF-α and cancer
cachexia: Molecular insights and clinical implications. Life Sci.
170:56–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Josephs SF, Ichim TE, Prince SM, Kesari S,
Marincola FM, Escobedo AR and Jafri A: Unleashing endogenous
TNF-alpha as a cancer immunotherapeutic. J Transl Med. 16:2422018.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Harrison ML, Obermueller E, Maisey NR,
Hoare S, Edmonds K, Li NF, Chao D, Hall K, Lee C, Timotheadou E, et
al: Tumor necrosis factor alpha as a new target for renal cell
carcinoma: Two sequential phase II trials of infliximab at standard
and high dose. J Clin Oncol. 25:4542–4549. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Dosquet C, Coudert MC, Lepage E, Cabane J
and Richard F: Are angiogenic factors, cytokines, and soluble
adhesion molecules prognostic factors in patients with renal cell
carcinoma? Clin Cancer Res. 3:2451–2458. 1997.PubMed/NCBI
|
|
89
|
Wong SHM, Kong WY, Fang CM, Loh HS, Chuah
LH, Abdullah S and Ngai SC: The TRAIL to cancer therapy: Hindrances
and potential solutions. Crit Rev Oncol Hematol. 143:81–94. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
von Karstedt S, Montinaro A and Walczak H:
Exploring the TRAILs less travelled: TRAIL in cancer biology and
therapy. Nat Rev Cancer. 17:352–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yuan X, Gajan A, Chu Q, Xiong H, Wu K and
Wu GS: Developing TRAIL/TRAIL death receptor-based cancer
therapies. Cancer Metastasis Rev. 37:733–748. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Xu YM, Brooks AD, Wijeratne EM, Henrich
CJ, Tewary P, Sayers TJ and Gunatilaka AA: 17β-Hydroxywithanolides
as sensitizers of renal carcinoma cells to tumor necrosis factor-α
related apoptosis inducing ligand (TRAIL) mediated apoptosis:
Structure-activity relationships. J Med Chem. 60:3039–3051. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Cannarile MA, Weisser M, Jacob W, Jegg AM,
Ries CH and Rüttinger D: Colony-stimulating factor 1 receptor
(CSF1R) inhibitors in cancer therapy. J Immunother Cancer.
5:532017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Menke J, Kriegsmann J, Schimanski CC,
Schwartz MM, Schwarting A and Kelley VR: Autocrine CSF-1 and CSF-1
receptor coexpression promotes renal cell carcinoma growth. Cancer
Res. 72:187–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ngambenjawong C, Gustafson HH and Pun SH:
Progress in tumor-associated macrophage (TAM)-targeted
therapeutics. Adv Drug Deliv Rev. 114:206–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Cassetta L, Fragkogianni S, Sims AH,
Swierczak A, Forrester LM, Zhang H, Soong DYH, Cotechini T, Anur P,
Lin EY, et al: Human tumor-associated macrophage and monocyte
transcriptional landscapes reveal cancer-specific reprogramming,
biomarkers, and therapeutic targets. Cancer Cell. 35:588–602.e10.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Achkova D and Maher J: Role of the
colony-stimulating factor (CSF)/CSF-1 receptor axis in cancer.
Biochem Soc Trans. 44:333–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Peyraud F, Cousin S and Italiano A: CSF-1R
inhibitor development: Current clinical status. Curr Oncol Rep.
19:702017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li X, Qin Z, Xue J, Zhang J, Zheng Y, Xu
W, Xu T and Zou Q: Genetic variants in macrophage
colony-stimulating factor are associated with risk of renal cell
carcinoma in a Chinese population. Int J Biol Markers. 33:321–328.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Nagarsheth N, Wicha MS and Zou W:
Chemokines in the cancer microenvironment and their relevance in
cancer immunotherapy. Nat Rev Immunol. 17:559–572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Cimadamore A, Scarpelli M, Piva F, Massari
F, Gasparrini S, Doria A, Cheng L, Lopez-Beltran A and Montironi R:
Activity of chemokines in prostate and renal tumors and their
potential role as future therapeutic targets. Future Oncol.
13:1105–1114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhou W, Guo S, Liu M, Burow ME and Wang G:
Targeting CXCL12/CXCR4 Axis in Tumor Immunotherapy. Curr Med Chem.
26:3026–3041. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Si X, Ma J, Yu F, Zhao H, Huang H and Sun
YW: Clinicopathological and prognostic significance of CXCR4 high
expression in renal cell carcinoma: A meta-analysis and literature
review. Int J Surg. 71:12–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Micucci C, Matacchione G, Valli D, Orciari
S and Catalano A: HIF2α is involved in the expansion of
CXCR4-positive cancer stem-like cells in renal cell carcinoma. Br J
Cancer. 113:1178–1185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Elhence P: A Commentary on
‘Clinico-pathological and prognostic significance of CXCR4 high
expression in renal cell carcinoma: A meta-analysis and literature
review’. (Int J Surg 2019; 71: 12–18). Int J Surg. 72:214–215.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ieranò C, Santagata S, Napolitano M,
Guardia F, Grimaldi A, Antignani E, Botti G, Consales C, Riccio A,
Nanayakkara M, et al: CXCR4 and CXCR7 transduce through mTOR in
human renal cancer cells. Cell Death Dis. 5:e13102014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Adlere I, Caspar B, Arimont M, Dekkers S,
Visser K, Stuijt J, de Graaf C, Stocks M, Kellam B, Briddon S, et
al: Modulators of CXCR4 and CXCR7/ACKR3 function. Mol Pharmacol.
96:737–752. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Salazar N and Zabel BA: Support of tumor
endothelial cells by chemokine receptors. Front Immunol.
10:1472019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Morein D, Erlichman N and Ben-Baruch A:
Beyond cell motility: The expanding roles of chemokines and their
receptors in malignancy. Front Immunol. 11:9522020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Nazari A, Khorramdelazad H and Hassanshahi
G: Biolog-ical/pathological functions of the CXCL12/CXCR4/CXCR7
axes in the pathogenesis of bladder cancer. Int J Clin Oncol.
22:991–1000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Daniel SK, Seo YD and Pillarisetty VG: The
CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in
gastrointestinal malignancies. Semin Cancer Biol. 65:176–188. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Cabrero-de Las Heras S and
Martínez-Balibrea E: CXC family of chemokines as prognostic or
predictive biomarkers and possible drug targets in colorectal
cancer. World J Gastroenterol. 24:4738–4749. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Krikun G: The CXL12/CXCR4/CXCR7 axis in
female reproductive tract disease: Review. Am J Reprod Immunol.
80:e130282018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Cheng X, Wang H, Zhang X, Zhao S, Zhou Z,
Mu X, Zhao C and Teng W: The role of SDF-1/CXCR4/CXCR7 in neuronal
regeneration after cerebral ischemia. Front Neurosci. 11:5902017.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Al-Toub M, Almohawes M, Vishnubalaji R,
Alfayez M, Aldahmash A, Kassem M and Alajez NM: CXCR7 signaling
promotes breast cancer survival in response to mesenchymal stromal
stem cell-derived factors. Cell Death Discov. 5:872019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Floranović MP and Veličković LJ: Effect of
CXCL12 and its receptors on unpredictable renal cell carcinoma.
Clin Genitourin Cancer. 18:e337–e342. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Winer A, Adams S and Mignatti P: Matrix
metalloproteinase inhibitors in cancer therapy: turning past
failures into future successes. Mol Cancer Ther. 17:1147–1155.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Cui N, Hu M and Khalil RA: Biochemical and
biological attributes of matrix metalloproteinases. Prog Mol Biol
Transl Sci. 147:1–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Parks WC, Wilson CL and López-Boado YS:
Matrix metalloproteinases as modulators of inflammation and innate
immunity. Nat Rev Immunol. 4:617–629. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Fingleton B: Matrix metalloproteinases as
regulators of inflammatory processes. Biochim Biophys Acta Mol Cell
Res. 1864:2036–2042. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Peters F and Becker-Pauly C: Role of
meprin metalloproteases in metastasis and tumor microenvironment.
Cancer Metastasis Rev. 38:347–356. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Su CW, Lin CW, Yang WE and Yang SF: TIMP-3
as a therapeutic target for cancer. Ther Adv Med Oncol. Jul
16–2019.(Epub ahead of print). 1758835919864247, 2019. doi:
10.1177/1758835919864247. View Article : Google Scholar
|
|
123
|
Eckfeld C, Häußler D, Schoeps B, Hermann
CD and Krüger A: Functional disparities within the TIMP family in
cancer: Hints from molecular divergence. Cancer Metastasis Rev.
38:469–481. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Gong D, Zhang J, Chen Y, Xu Y, Ma J, Hu G,
Huang Y, Zheng J, Zhai W and Xue W: The m6A-suppressed
P2RX6 activation promotes renal cancer cells migration and invasion
through ATP-induced Ca(2+) influx modulating ERK1/2 phosphorylation
and MMP9 signaling pathway. J Exp Clin Cancer Res. 38:2332019.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Niu H, Li F, Wang Q, Ye Z, Chen Q and Lin
Y: High expression level of MMP9 is associated with poor prognosis
in patients with clear cell renal carcinoma. PeerJ. 6:e50502018.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Gonzalez-Molina J, Gramolelli S, Liao Z,
Carlson JW, Ojala PM and Lehti K: MMP14 in sarcoma: A regulator of
tumor microenvironment communication in connective tissues. Cells.
8:9912019. View Article : Google Scholar
|
|
127
|
Gifford V and Itoh Y: MT1-MMP-dependent
cell migration: Proteolytic and non-proteolytic mechanisms. Biochem
Soc Trans. 47:811–826. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Cepeda MA, Evered CL, Pelling JJH and
Damjanovski S: Inhibition of MT1-MMP proteolytic function and
ERK1/2 signalling influences cell migration and invasion through
changes in MMP-2 and MMP-9 levels. J Cell Commun Signal.
11:167–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Petrella BL and Brinckerhoff CE: Tumor
cell invasion of von Hippel Lindau renal cell carcinoma cells is
mediated by membrane type-1 matrix metalloproteinase. Mol Cancer.
5:662006. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Jiang B, Liu J and Lee MH: Targeting a
designer TIMP-1 to the cell surface for effective MT1-MMP
inhibition: A potential role for the prion protein in renal
carcinoma therapy. Molecules. 24:2552019. View Article : Google Scholar
|
|
131
|
Wu G, Ma Z, Cheng Y, Hu W, Deng C, Jiang
S, Li T, Chen F and Yang Y: Targeting Gas6/TAM in cancer cells and
tumor microenvironment. Mol Cancer. 17:202018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Kovaleva OV, Samoilova DV, Shitova MS and
Gratchev A: Tumor associated macrophages in kidney cancer. Anal
Cell Pathol (Amst). 2016:93075492016.PubMed/NCBI
|
|
133
|
Santoni M, Massari F, Amantini C, Nabissi
M, Maines F, Burattini L, Berardi R, Santoni G, Montironi R,
Tortora G and Cascinu S: Emerging role of tumor-associated
macrophages as therapeutic targets in patients with metastatic
renal cell carcinoma. Cancer Immunol Immunother. 62:1757–1768.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Toge H, Inagaki T, Kojimoto Y, Shinka T
and Hara I: Angiogenesis in renal cell carcinoma: The role of
tumor-associated macrophages. Int J Urol. 16:801–807. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Fu Q, Xu L, Wang Y, Jiang Q, Liu Z, Zhang
J, Zhou Q, Zeng H, Tong S, Wang T, et al: Tumor-associated
macrophage-derived interleukin-23 interlinks kidney cancer
glutamine addiction with immune evasion. Eur Urol. 75:752–763.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Tcyganov E, Mastio J, Chen E and
Gabrilovich DI: Plasticity of myeloid-derived suppressor cells in
cancer. Curr Opin Immunol. 51:76–82. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Sun L, Clavijo PE, Robbins Y, Patel P,
Friedman J, Greene S, Das R, Silvin C, Van Waes C, Horn LA, et al:
Inhibiting myeloid-derived suppressor cell trafficking enhances T
cell immunotherapy. JCI Insight. 4:e1268532019. View Article : Google Scholar
|
|
138
|
Zhang J, Shi Z, Xu X, Yu Z and Mi J: The
influence of microenvironment on tumor immunotherapy. FEBS J.
286:4160–4175. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Martinez M and Moon EK: CAR T cells for
solid tumors: New strategies for finding, infiltrating, and
surviving in the tumor microenvironment. Front Immunol. 10:1282019.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Fleming V, Hu X, Weber R, Nagibin V, Groth
C, Altevogt P, Utikal J and Umansky V: targeting myeloid-derived
suppressor cells to bypass tumor-induced immunosuppression. Front
Immunol. 9:3982018. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Hinshaw DC and Shevde LA: The tumor
microenvironment innately modulates cancer progression. Cancer Res.
79:4557–4566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Marvel D and Gabrilovich DI:
Myeloid-derived suppressor cells in the tumor microenvironment:
Expect the unexpected. J Clin Invest. 125:3356–3364. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Najjar YG, Rayman P, Jia X, Pavicic PG Jr,
Rini BI, Tannenbaum C, Ko J, Haywood S, Cohen P, Hamilton T, et al:
Myeloid-derived suppressor cell subset accumulation in renal cell
carcinoma parenchyma is associated with intratumoral expression of
IL1β, IL8, CXCL5, and Mip-1α. Clin Cancer Res. 23:2346–2355. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Kondo T, Nakazawa H, Ito F, Hashimoto Y,
Osaka Y, Futatsuyama K, Toma H and Tanabe K: Favorable prognosis of
renal cell carcinoma with increased expression of chemokines
associated with a Th1-type immune response. Cancer Sci. 97:780–786.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Jöhrer K, Zelle-Rieser C, Perathoner A,
Moser P, Hager M, Ramoner R, Gander H, Höltl L, Bartsch G, Greil R
and Thurnher M: Up-regulation of functional chemokine receptor CCR3
in human renal cell carcinoma. Clin Cancer Res. 11:2459–2465. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Yang JF, Shi SN, Xu WH, Qiu YH, Zheng JZ,
Yu K, Song XY, Li F, Wang Y, Wang R, et al: Screening,
identification and validation of CCND1 and PECAM1/CD31 for
predicting prognosis in renal cell carcinoma patients. Aging
(Albany NY). 11:12057–12079. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Wang C, Wang Y, Hong T, Cheng B, Gan S,
Chen L, Zhang J, Zuo L, Li J and Cui X: Blocking the autocrine
regulatory loop of Gankyrin/STAT3/CCL24/CCR3 impairs the
progression and pazopanib resistance of clear cell renal cell
carcinoma. Cell Death Dis. 11:1172020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Kallakury BV, Karikehalli S, Haholu A,
Sheehan CE, Azumi N and Ross JS: Increased expression of matrix
metalloproteinases 2 and 9 and tissue inhibitors of
metalloproteinases 1 and 2 correlate with poor prognostic variables
in renal cell carcinoma. Clin Cancer Res. 7:3113–3119.
2001.PubMed/NCBI
|
|
149
|
Woschek M, Kneip N, Jurida K, Marzi I and
Relja B: Simvastatin reduces cancerogenic potential of renal cancer
cells via geranylgeranyl pyrophosphate and mevalonate pathway. Nutr
Cancer. 68:420–427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Pang X, Si J, Xu S, Li Y and Liu J:
Simvastatin inhibits homocysteine-induced CRP generation via
interfering with the ROS-p38/ERK1/2 signal pathway in rat vascular
smooth muscle cells. Vascul Pharmacol. 88:42–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Fang Z, Tang Y, Fang J, Zhou Z, Xing Z,
Guo Z, Guo X, Wang W, Jiao W, Xu Z and Liu Z: Simvastatin inhibits
renal cancer cell growth and metastasis via AKT/mTOR, ERK and
JAK2/STAT3 pathway. PLoS One. 8:e628232013. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ni X, Hu G and Cai X: The success and the
challenge of all-trans retinoic acid in the treatment of cancer.
Crit Rev Food Sci Nutr. 59 (Sup 1):S71–S80. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Schultze E, Collares T, Lucas CG and
Seixas FK: Synergistic and additive effects of ATRA in combination
with different anti-tumor compounds. Chem Biol Interact. 285:69–75.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ren M, Pozzi S, Bistulfi G, Somenzi G,
Rossetti S and Sacchi N: Impaired retinoic acid (RA) signal leads
to RARbeta2 epigenetic silencing and RA resistance. Mol Cell Biol.
25:10591–10603. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Chu JH, Gao ZH and Qu XJ: Down-regulation
of sphingosine kinase 2 (SphK2) increases the effects of
all-trans-retinoic acid (ATRA) on colon cancer cells. Biomed
Pharmacother. 68:1089–1097. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Zarrabi K and Wu S: An evaluation of
nivolumab for the treatment of metastatic renal cell carcinoma.
Expert Opin Biol Ther. 18:695–705. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Zhang J, Dang F, Ren J and Wei W:
Biochemical aspects of PD-L1 regulation in cancer immunotherapy.
Trends Biochem Sci. 43:1014–1032. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Shi Y: Regulatory mechanisms of PD-L1
expression in cancer cells. Cancer Immunol Immunother.
67:1481–1489. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Ai L, Xu A and Xu J: Roles of PD-1/PD-L1
pathway: Signaling, cancer, and beyond. Adv Exp Med Biol.
1248:33–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Motzer RJ, Tannir NM, McDermott DF, Arén
Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P,
Porta C, George S, et al: Nivolumab plus ipilimumab versus
sunitinib in advanced renal-cell carcinoma. N Engl J Med.
378:1277–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Chambers CA, Kuhns MS, Egen JG and Allison
JP: CTLA-4-mediated inhibition in regulation of T cell responses:
Mechanisms and manipulation in tumor immunotherapy. Annu Rev
Immunol. 19:565–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Izzedine H, Mateus C, Boutros C, Robert C,
Rouvier P, Amoura Z and Mathian A: Renal effects of immune
checkpoint inhibitors. Nephrol Dial Transplant. 32:936–942.
2017.PubMed/NCBI
|
|
163
|
Rotte A: Combination of CTLA-4 and PD-1
blockers for treatment of cancer. J Exp Clin Cancer Res.
38:2552019. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Motzer RJ, Rini BI, McDermott DF, Arén
Frontera O, Hammers HJ, Carducci MA, Salman P, Escudier B,
Beuselinck B, Amin A, et al: Nivolumab plus ipilimumab versus
sunitinib in first-line treatment for advanced renal cell
carcinoma: Extended follow-up of efficacy and safety results from a
randomised, controlled, phase 3 trial. Lancet Oncol. 20:1370–1385.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Wculek SK, Cueto FJ, Mujal AM, Melero I,
Krummel MF and Sancho D: Dendritic cells in cancer immunology and
immunotherapy. Nat Rev Immunol. 20:7–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng
P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et
al: Blockade of B7-H1 improves myeloid dendritic cell-mediated
antitumor immunity. Nat Med. 9:562–567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Gottfried E, Kunz-Schughart LA, Ebner S,
Mueller-Klieser W, Hoves S, Andreesen R, Mackensen A and Kreutz M:
Tumor-derived lactic acid modulates dendritic cell activation and
antigen expression. Blood. 107:2013–2021. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Delamarre L and Mellman I: Harnessing
dendritic cells for immunotherapy. Semin Immunol. 23:2–11. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Farhood B, Najafi M and Mortezaee K:
CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J
Cell Physiol. 234:8509–8521. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Boissonnas A, Fetler L, Zeelenberg IS,
Hugues S and Amigorena S: In vivo imaging of cytotoxic T cell
infiltration and elimination of a solid tumor. J Exp Med.
204:345–356. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Dyck L and Mills KHG: Immune checkpoints
and their inhibition in cancer and infectious diseases. Eur J
Immunol. 47:765–779. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Motz GT and Coukos G: Deciphering and
reversing tumor immune suppression. Immunity. 39:61–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Zitvogel L, Galluzzi L, Smyth MJ and
Kroemer G: Mechanism of action of conventional and targeted
anticancer therapies: Reinstating immunosurveillance. Immunity.
39:74–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Palucka K and Banchereau J:
Dendritic-cell-based therapeutic cancer vaccines. Immunity.
39:38–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Xu W, Atkins MB and McDermott DF:
Checkpoint inhibitor immunotherapy in kidney cancer. Nat Rev Urol.
17:137–150. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Chen DS and Hurwitz H: Combinations of
Bevacizumab with cancer immunotherapy. Cancer J. 24:193–204. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Motz GT, Santoro SP, Wang LP, Garrabrant
T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F and Coukos
G: Tumor endothelium FasL establishes a selective immune barrier
promoting tolerance in tumors. Nat Med. 20:607–615. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Kochenderfer JN and Rosenberg SA: Treating
B-cell cancer with T cells expressing anti-CD19 chimeric antigen
receptors. Nat Rev Clin Oncol. 10:267–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Khalil DN, Smith EL, Brentjens RJ and
Wolchok JD: The future of cancer treatment: Immunomodulation, CARs
and combination immunotherapy. Nat Rev Clin Oncol. 13:273–290.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Sourbier C, Lindner V, Lang H, Agouni A,
Schordan E, Danilin S, Rothhut S, Jacqmin D, Helwig JJ and
Massfelder T: The phosphoinositide 3-kinase/Akt pathway: A new
target in human renal cell carcinoma therapy. Cancer Res.
66:5130–5142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Daurkin I, Eruslanov E, Stoffs T, Perrin
GQ, Algood C, Gilbert SM, Rosser CJ, Su LM, Vieweg J and Kusmartsev
S: Tumor-associated macrophages mediate immunosuppression in the
renal cancer microenvironment by activating the 15-lipoxygenase-2
pathway. Cancer Res. 71:6400–6409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Shinko D, Diakos CI, Clarke SJ and Charles
KA: Cancer-related systemic inflammation: The challenges and
therapeutic opportunities for personalized medicine. Clin Pharmacol
Ther. 102:599–610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Struyf S, Proost P, Vandercappellen J,
Dempe S, Noyens B, Nelissen S, Gouwy M, Locati M, Opdenakker G,
Dinsart C and Van Damme J: Synergistic up-regulation of MCP-2/CCL8
activity is counteracted by chemokine cleavage, limiting its
inflammatory and anti-tumoral effects. Eur J Immunol. 39:843–857.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Iyengar NM, Gucalp A, Dannenberg AJ and
Hudis CA: Obesity and cancer mechanisms: Tumor microenvironment and
inflammation. J Clin Oncol. 34:4270–4276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Voelkel-Johnson C: TRAIL-mediated
signaling in prostate, bladder and renal cancer. Nat Rev Urol.
8:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Chen YS, Hung TW, Su SC, Lin CL, Yang SF,
Lee CC, Yeh CF, Hsieh YH and Tsai JP: MTA2 as a potential biomarker
and its involvement in metastatic progression of human renal cancer
by miR-133b Targeting MMP-9. Cancers (Basel). 11:18512019.
View Article : Google Scholar
|
|
188
|
De Giorgi U, Procopio G, Giannarelli D,
Sabbatini R, Bearz A, Buti S, Basso U, Mitterer M, Ortega C, Bidoli
P, et al: Association of systemic inflammation index and body mass
index with survival in patients with renal cell cancer treated with
nivolumab. Clin Cancer Res. 25:3839–3846. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Qayyum T, McArdle PA, Lamb GW, Going JJ,
Orange C, Seywright M, Horgan PG, Oades G, Aitchison MA and Edwards
J: Prospective study of the role of inflammation in renal cancer.
Urol Int. 88:277–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Wen L, Guo L, Zhang W, Li Y, Jiang W, Di
X, Ma J, Feng L, Zhang K and Shou J: Cooperation between the
inflammation and coagulation systems promotes the survival of
circulating tumor cells in renal cell carcinoma patients. Front
Oncol. 9:5042019. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Sun KH, Sun GH, Wu YC, Ko BJ, Hsu HT and
Wu ST: TNF-α augments CXCR2 and CXCR3 to promote progression of
renal cell carcinoma. J Cell Mol Med. 20:2020–2028. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Barata PC and Rini BI: Treatment of renal
cell carcinoma: Current status and future directions. CA Cancer J
Clin. 67:507–524. 2017. View Article : Google Scholar : PubMed/NCBI
|