Introduction
Breast cancer (BC) is one of the most common types
of cancer in women worldwide and a number of patients exhibit an
increased risk of metastasis and recurrence (1–3). It is
estimated that ~50% of BC cases and 60% of BC-related deaths occur
in developing countries, with the majority of deaths caused by
cancer metastasis (4). With the
advancement and development of medical standards, the mortality
rate of patients with BC has steadily decreased in the past few
decades. However, although breast cancer death rates have dropped
34% since 1990, not all segments of the population have benefited
from this decrease (5).
MicroRNAs (miRNAs/miRs) are a group of endogenous,
non-coding, single-stranded RNAs, 18–24 nucleotides in length,
which mediate downstream gene expression at the
post-transcriptional level (6–8). miRNAs
are involved in several biological processes, and their expression
and functions are associated with numerous diseases, such as cancer
and diseases of the digestive, nervous and cardiovascular systems
(9). An increasing number of studies
have suggested that miRNAs are abnormally expressed in multiple
developmental processes of BC (10,11).
miR-1297 is a novel cancer-related miRNA that serves a crucial role
in the pathogenesis of human cancer (12–16).
Dysregulation of miR-1297 has been detected in various types of
human cancer (17–20). For example, Chen et al
(17) reported that miR-1297
expression was decreased in pancreatic adenocarcinoma tissues. Gao
et al (18) suggested that
miR-1297 expression was significantly lower in gastric cancer
tissue samples compared with adjacent healthy tissue samples.
Moreover, increases in miR-1297 expression levels were observed in
laryngeal squamous cell carcinoma (19) and BC (20) tissues. miR-1297 can exist as an
oncogene or a tumor suppressor gene in tumor cells (15,20–22).
Wang et al (15) demonstrated
that miR-1297 suppressed glioma cell proliferation via targeting
high mobility group A1. In addition, Liu et al (21) demonstrated that miR-1297 upregulation
attenuated cell proliferation via modulating enhancer of zeste
homolog 2, whereas Liang et al (22) revealed that miR-1297 was involved in
the development of oral squamous cell carcinoma via regulating
phosphatase and tensin homolog (PTEN). Furthermore, a previous
study suggested that miR-1297 was increased in BC tissues and cell
lines, and promoted BC cell proliferation (20). However, the effect of miR-1297 on BC
cells is not completely understood. The present study aimed to
investigate the effects of miR-1297 on BC cell
epithelial-mesenchymal transition (EMT) and proliferation, and the
underlying molecular mechanisms.
As a hydroxy fatty acid enzyme, fatty acid
2-hydroxylase (FA2H) promotes 2-hydroxylation of fatty acid N-acyl
chain (23). It has been reported
that 2-hydroxyceramide and FA2H, which are expressed in several
tissues (24,25), participate in multiple cell signaling
pathways, such as the AMPK and mTOR/p70 ribosomal protein S6 kinase
1 (S6K1)/glioma-associated oncogene homolog 1 pathways (26). FA2H also serves a crucial role in the
occurrence and development of tumors (26). FA2H downregulation has been observed
in triple negative breast cancer tissues and cell lines (27). FA2H expression levels were lower in
gastric tumor tissues compared with healthy control tissues
(26). Moreover, another study
revealed that FA2H levels were higher in adenocarcinoma compared
with squamous and neuroendocrine carcinoma (28). Previous studies have indicated that
FA2H may affect cell cycle and migration, enhance the sensitivity
of tumor cells to drugs and regulate drug resistance of tumor cells
(26–28). In terms of drug resistance, FA2H may
increase the therapeutic effect via enhancing the sensitivity of
tumor cells to drugs (26,29). Accumulating evidence has suggested
that FA2H exerts its role in promoting drug sensitivity via
regulating endocytosis and exocytosis of drugs via the cell
membrane (29). Additionally, it has
been reported that FA2H is associated with shorter tumor-free
survival in triple-negative BC (27). However, the specific roles of FA2H
and 2-hydroxy fatty acids, as components of the metabolism, in
regulating tumors and their underlying mechanisms are not
completely understood.
The current study aimed to investigate the effect of
miR-1297 and FA2H on breast cancer cell EMT and proliferation, and
the underlying molecular mechanisms.
Materials and methods
Cell culture and transfection
MDA-MB-231 cells were cultured in DMEM (HyClone;
Cytiva) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2. Subsequently, MDA-MB-231
cells (5×104 cells/well; 24-well plates) were
transfected with 100 nM inhibitor control (cat. no. MIH000000;
Applied Biological Materials, Inc.), 100 nM miR-1297 inhibitor
(cat. no. MIH01244; Applied Biological Materials, Inc.), 1 µg
control-plasmid (cat. no. sc-437275; Santa Cruz Biotechnology,
Inc.), 1 µg FA2H-plasmid (cat. no. sc-413143-ACT; Santa Cruz
Biotechnology, Inc.), 0.2 µM control-small interfering (si)RNA
(cat. no. sc-36869; Santa Cruz Biotechnology, Inc.), 0.2 µM
FA2H-siRNA (cat. no. sc-93418; Santa Cruz Biotechnology, Inc.),
miR-1297 inhibitor + control-siRNA or miR-1297 inhibitor +
FA2H-siRNA using Polyplus transfection reagent
(Polyplus-transfection SA) according to the manufacturer's
protocol. At 48 h post-transfection, cells were used for subsequent
experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from MDA-MB-231 cells using
TRIzol® reagent (Takara Bio, Inc.) according to the
manufacturer's protocol. The concentration of the RNA was detected
using a NanoDrop™ 2000 spectrophotometer (Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using the
HiScript II 1st Strand cDNA Synthesis kit (Vazyme Biotech Co.,
Ltd.). The RT temperature conditions were as follows: 70°C For 5
min, 37°C for 5 min and 42°C for 60 min. Subsequently, qPCR was
performed using a SYBR Green PCR kit (Vazyme Biotech Co., Ltd.).
The following thermocycling conditions were used for qPCR: Initial
denaturation at 95°C for 10 min, followed by 35 cycles of
denaturation at 95°C for 15 sec, annealing at 55°C for 40 sec and
extension at 72°C for 34 sec. The following primers were used for
qPCR: GAPDH, Forward, 5′-ATTCCATGGCACCGTCAAGGCTGA-3′ and reverse:
5′-TTCTCCATGGTGGTGAAGACGCCA-3′; U6, forward:
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse:
5′-CGCTTCACGAATTTGCGTGTCAT-3′; N-cadherin, forward:
5′-TTTGATGGAGGTCTCCTAACACC-3′ and reverse:
5′-ACGTTTAACACGTTGGAAATGTG-3′; E-cadherin, forward:
5′-CGAGAGCTACACGTTCACGG-3′ and reverse:
5′-GGGTGTCGAGGGAAAAATAGG-3′; matrix metalloproteinase (MMP)9,
forward: 5′-GAACCAATCTCACCGACAGG-3′ and reverse:
5′-CCACAACTCGTCATCGTCG-3′; FA2H, forward:
5′-AGTACTATGTGGGCGAACTGC-3′ and reverse:
5′-CAATAGCAGCATCTGTCTTCTGA-3′; miR-1297, forward:
5′-ACACTCCAGCTGGGTCCTTCATTCCA-3′ and reverse:
5′-GTGCAGGGTCCGAGGT-3′. miRNA and mRNA expression levels were
quantified using the 2−ΔΔCq method (30) and normalized to the internal
reference genes U6 and GAPDH, respectively.
Western blotting
Total protein was extracted from cells using RIPA
buffer (Beyotime Institute of Biotechnology). Total protein was
quantified using a bicinchoninic acid assay kit (Pierce; Thermo
Fisher Scientific, Inc.). Equal amounts of protein (40 µg per lane)
were separated via 12% SDS-PAGE for 40 min and transferred onto
PVDF membranes (EMD Millipore), which were blocked with 5% non-fat
milk for 1.5 h at room temperature. Subsequently, the membranes
were incubated at 4°C overnight with primary antibodies targeted
against: E-cadherin (cat. no. ab15148; 1:1,000; Abcam), N-cadherin
(cat. no. ab76057; 1:1,000; Abcam), MMP9 (cat. no. ab38898;
1:1,000; Abcam), FA2H (cat. no. ab128917; 1:1,000; Abcam) and GAPDH
(cat. no. ab9485; 1:1,000; Abcam). The following day, the membranes
were incubated with horseradish peroxidase-conjugated secondary
antibody (1:2,000; cat. no. 7074; Cell Signaling Technology, Inc.)
for 2 h at room temperature. Protein bands were visualized using
the enhanced chemiluminescence method (Cytiva). GAPDH was used as
the loading control.
Flow cytometry assay
Cell apoptosis was assessed using the Annexin-V/PI
Apoptosis Detection kit (Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. Briefly, cells were
seeded (2×105 cells/well) into 6-well plates and
cultured at 37°C overnight. Following transfection, cells were
harvested, centrifuged at 1,000 × g at 4°C for 5 min), and the cell
pellet was resuspended in 100 µl FITC-binding buffer. Subsequently,
the cell suspension was incubated with 5 µl ready-to-use Annexin
V-FITC (BD Bioscience) and 5 µl PI at room temperature in the dark
for 30 min. Cell apoptosis (late or early + late apoptosis) was
assessed via flow cytometry using a BD FACSCalibur flow cytometer
(BD Biosciences). Data were analyzed using CellQuest™ version 5.1
software (BD Biosciences).
Dual luciferase reporter assay
Bioinformatics analysis was performed using
TargetScan 7.2 (http://www.targetscan.org/vert_72/). And the results
showed the potential binding sites between miR-1297 and the 3′-UTR
of FA2H. The wild-type (WT) or mutant (MUT) 3′-untranslated regions
(3′-UTRs) of FA2H were cloned into the pmiRGLO vector (Promega
Corporation). The recombinant plasmids were acquired using an
EndoFree Plasmid Maxi kit (Vazyme Biotech Co., Ltd.). Subsequently,
293T cells (5×104 cells/well; American Type Culture
Collection) were seeded into 24-well plates and co-transfected with
50 nM miR-1297 mimic (cat. no. MCH01244; Applied Biological
Materials, Inc.) or 50 nM mimic control (cat. no. MCH00000; Applied
Biological Materials, Inc.) and 1 ng MUT FA2H 3′UTR or 1 ng WT FA2H
3′UTR using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C for 48 h. The pRL-TK plasmid
(Promega Corporation) containing the Renilla luciferase gene
was u firefly sed as an internal control. At 48 h
post-transfection, and Renilla luciferase activities were
detected using the Dual-Luciferase Reporter Assay System (Promega
Corporation). Firefly luciferase activity was normalized to Renilla
luciferase activity. Besides, 293T cells were transfected with
miR-1297 mimic or mimic control for 48 h, and the transfection
efficiency was confirmed using qRT-PCR.
MTT assay
Cell proliferation was assessed using an MTT assay.
Briefly, MDA-MB-231 cells were plated into a 96-well plate
(5×104 cells per well) and incubated at 37°C for 24, 48
or 72 h. Subsequently, 20 µl MTT reagent (5 mg/ml; Sigma-Aldrich;
Merck KGaA) was added to each well at 37°C for 4 h. In total, 150
µl DMSO (Beyotime Institute of Biotechnology) was used to dissolve
the purple formazan. The absorbance was measured at a wavelength of
570 nm using a multifunctional plate reader (BD Biosciences)
according to the assay manufacturer's instructions.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 6.0; GraphPad Software Inc.). Comparisons
among groups were analyzed using the unpaired Student's t-test or
one-way ANOVA followed by Tukey's post hoc test. Data are presented
as the mean ± SD from at least three independent experiments.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of miR-1297 knockdown on
MDA-MB-231 cell proliferation and apoptosis
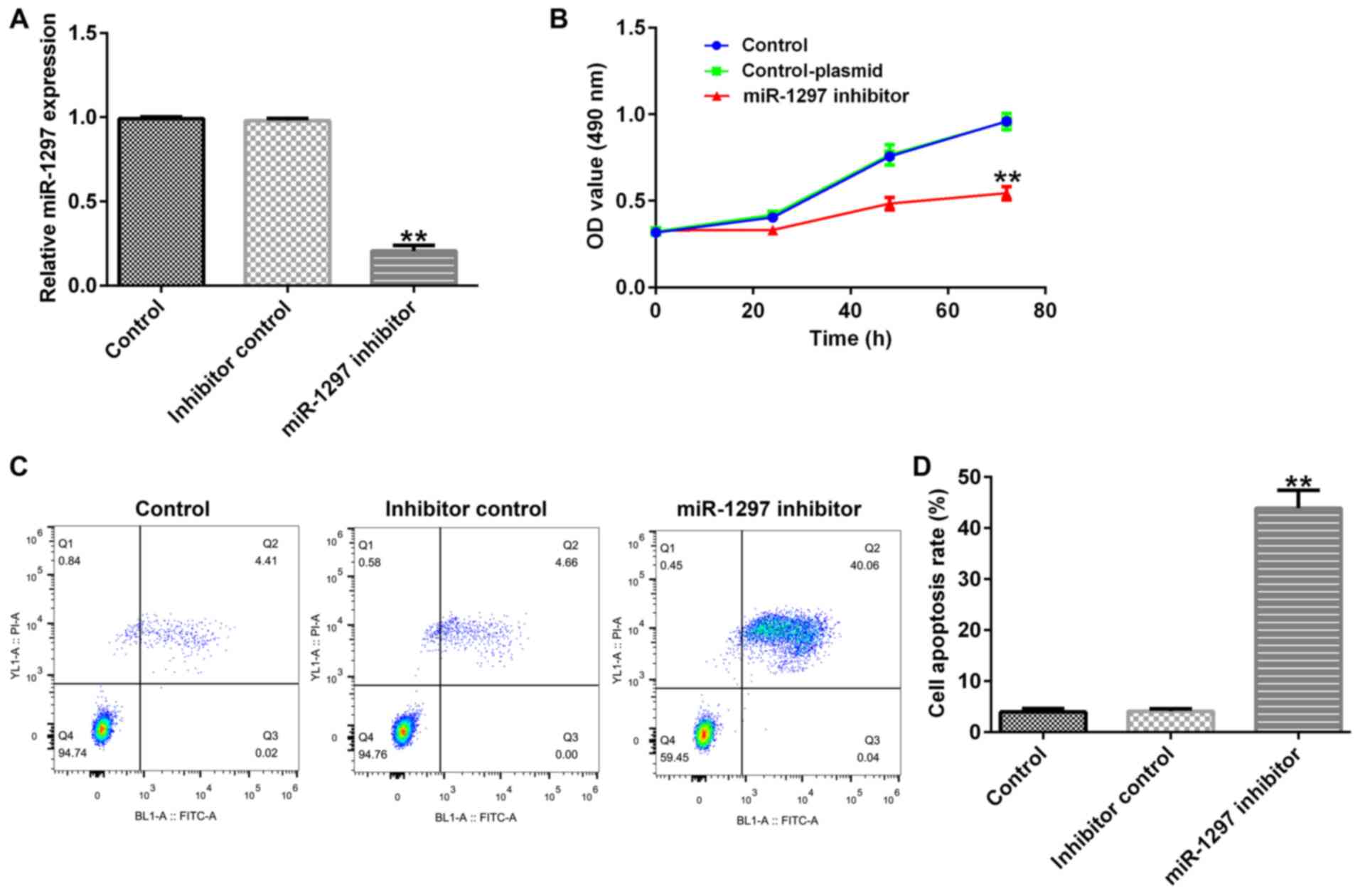
To investigate the biological effects of miR-1297 on
BC, MDA-MB-231 cells were transfected with inhibitor control or
miR-1297 inhibitor for 48 h. Compared with the inhibitor control
group, the RT-qPCR results indicated that miR-1297 inhibitor
significantly decreased miR-1297 expression in MDA-MB-231 cells
(Fig. 1A). In addition, the MTT
assay results suggested that miR-1297 inhibitor decreased
MDA-MB-231 cell proliferation at 24, 48 and 72 h compared with the
inhibitor control group (Fig. 1B).
Furthermore, the flow cytometry results indicated that miR-1297
inhibitor significantly increased MDA-MB-231 cell apoptosis
compared with the inhibitor control group (Fig. 1C and D).
Effects of miR-1297 knockdown on
MDA-MB-231 cell EMT
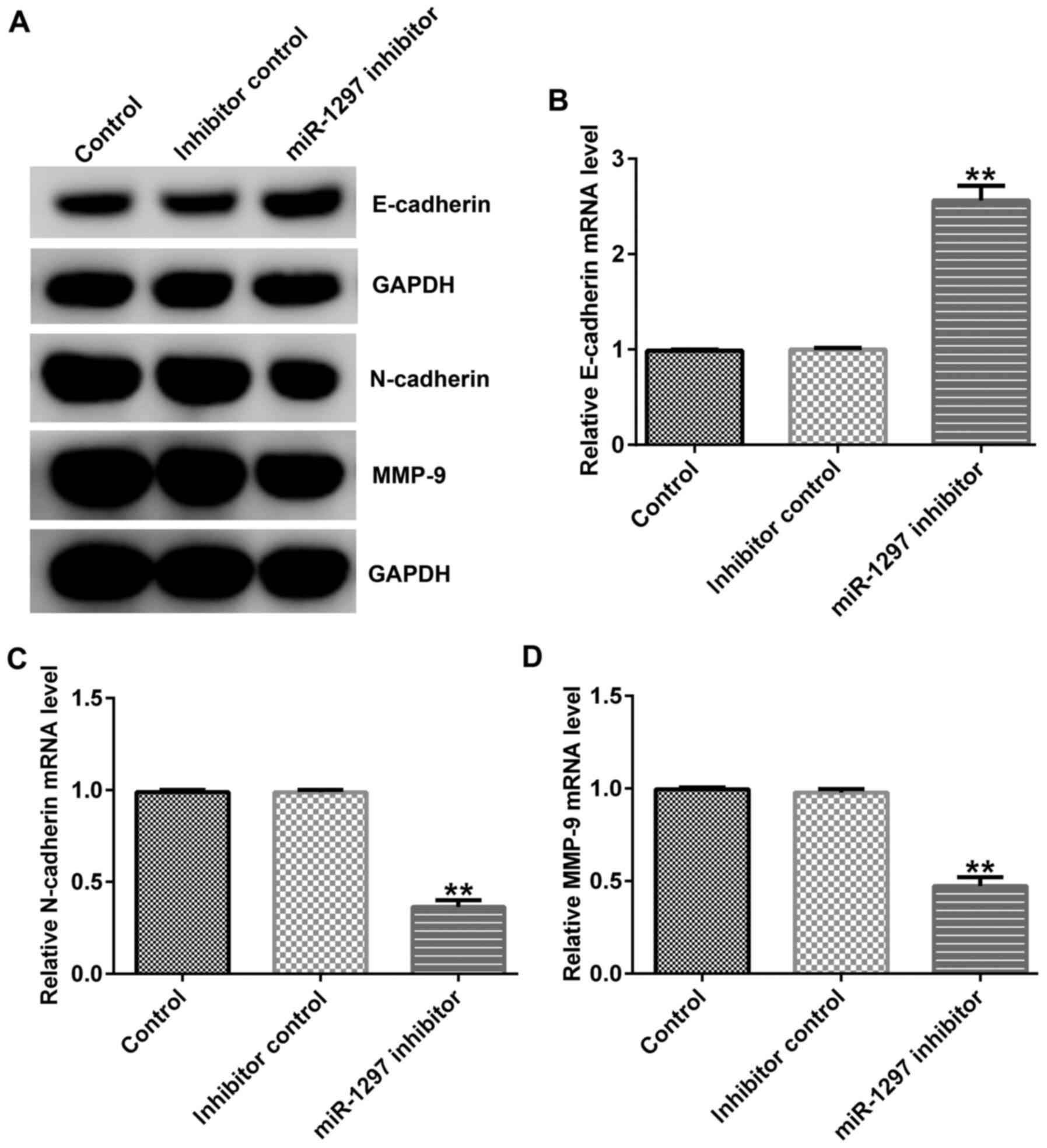
Subsequently, western blotting and RT-qPCR were
performed to detect the expression levels of EMT indicators, an
epithelial cell marker, E-cadherin, and two interstitial cell
markers, N-cadherin and MMP9. The results indicated that miR-1297
inhibitor upregulated E-cadherin expression levels, and decreased
N-cadherin and MMP9 expression levels at the protein and mRNA
levels compared with the inhibitor control group (Fig. 2A-D).
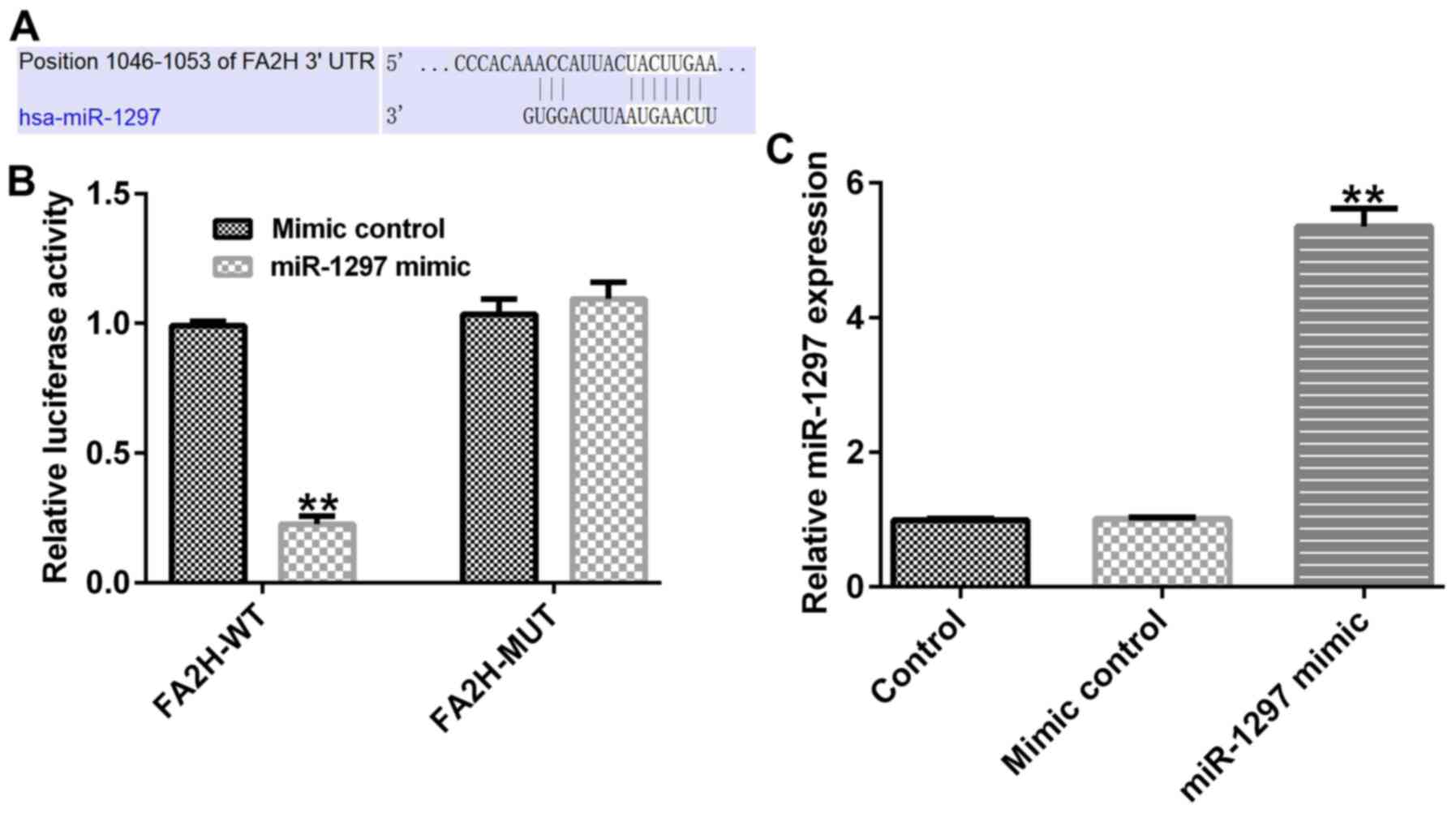
miR-1297 directly targets FA2H
To identify the mechanisms underlying FA2H,
bioinformatics analysis was performed using TargetScan. The results
indicated that FA2H might be a potential downstream target gene of
miR-1297 (Fig. 3A). Subsequently,
the association between FA2H and miR-1297 was assessed by
performing a dual luciferase activity assay. The results suggested
that miR-1297 mimic significantly inhibited the luciferase activity
of the WT FA2H 3′UTR reporter plasmid compared with mimic control.
However, miR-1297 mimic had no significant effect on the luciferase
activity of the MUT FA2H 3′UTR reporter plasmid compared with mimic
control (Fig. 3B). Moreover,
compared with the mimic control group, miR-1297 mimic significantly
increased miR-1297 expression in 293T cells (Fig. 3C). The results indicated that FA2H
was a direct target of miR-1297.
Effects of FA2H overexpression on
MDA-MB-231 cell proliferation and apoptosis
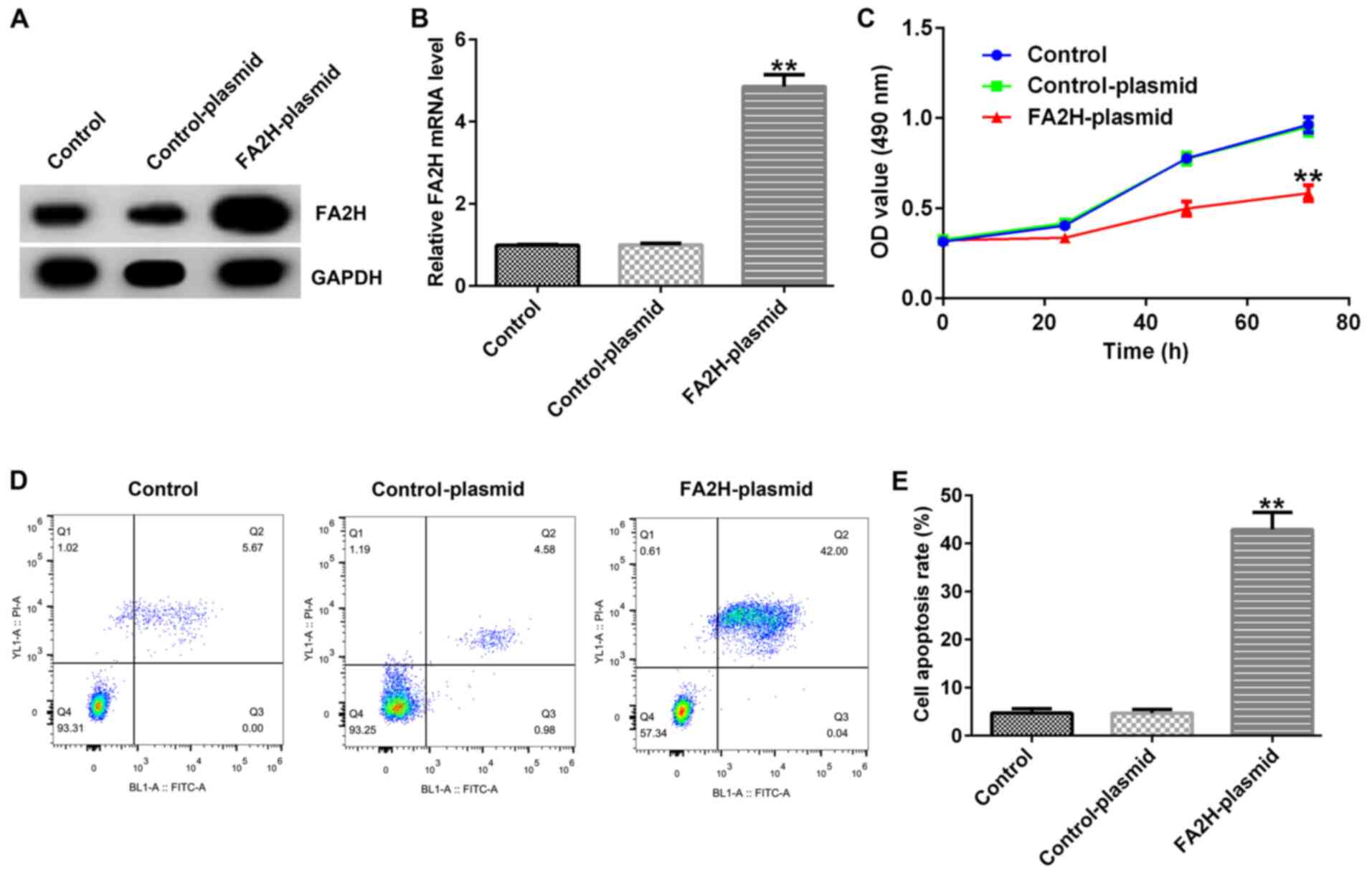
To further investigate the biological effects of
FA2H on BC, MDA-MB-231 cells were transfected with control-plasmid
or FA2H-plasmid for 48 h. Compared with the control-plasmid group,
FA2H overexpression increased FA2H mRNA and protein expression
levels in MDA-MB-231 cells (Fig. 4A and
B). Furthermore, compared with the control-plasmid group, FA2H
overexpression decreased MDA-MB-231 cell proliferation at 24, 48
and 72 h (Fig. 4C). By contrast,
FA2H overexpression significantly enhanced cell apoptosis compared
with the control-plasmid group (Fig. 4D
and E).
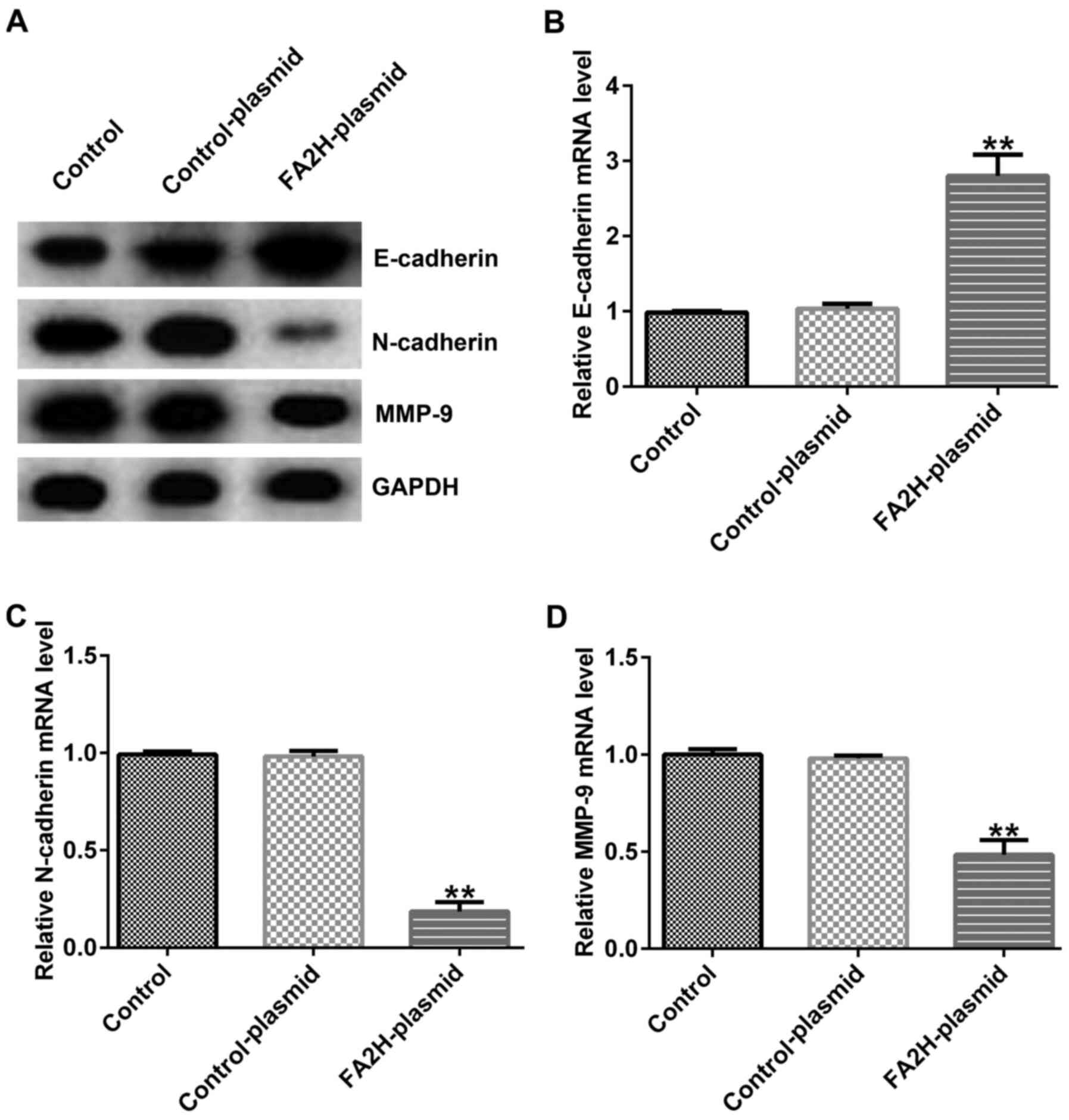
Effects of FA2H overexpression on
MDA-MB-231 cell EMT
Furthermore, the expression levels of EMT
indicators, E-cadherin, N-cadherin and MMP9, were assessed via
western blotting and RT-qPCR. FA2H overexpression upregulated
E-cadherin protein and mRNA expression levels, and decreased
N-cadherin and MMP9 protein and mRNA expression levels compared
with the control-plasmid group (Fig.
5A-D).
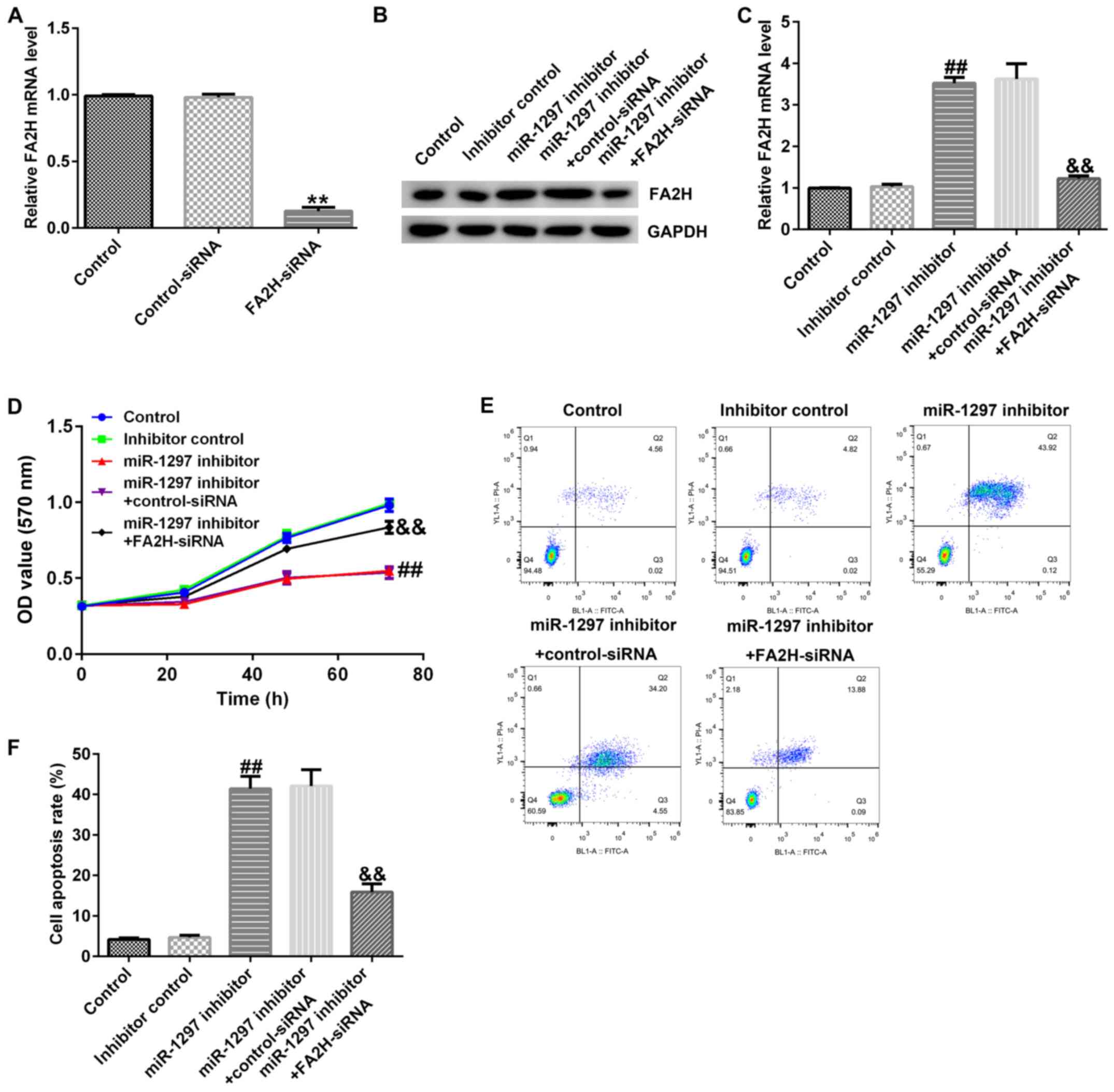
miR-1297 knockdown affects MDA-MB-231
cell proliferation by upregulating FA2H
Subsequently, the effects of miR-1297 and FA2H
expression on MDA-MB-231 cell proliferation, apoptosis and EMT were
investigated. MDA-MB-231 cells were transfected with control-siRNA,
FA2H-siRNA, inhibitor control, miR-1297 inhibitor, miR-1297
inhibitor + control-siRNA or miR-1297 inhibitor + FA2H-siRNA for 48
h. The RT-qPCR results indicated that FA2H-siRNA significantly
decreased FA2H mRNA expression levels in MDA-MB-231 cells compared
with the control-siRNA group (Fig.
6A). In addition, compared with the inhibitor control group,
miR-1297 inhibitor upregulated FA2H protein and mRNA expression
levels, which were reversed by co-transfection with FA2H-siRNA
(Fig. 6B and C). The MTT assay
indicated that miR-1297 inhibitor significantly reduced cell
proliferation at 72 h compared with the inhibitor control group
(Fig. 6D). In addition, miR-1297
inhibitor significantly increased apoptosis compared with the
inhibitor control group (Fig. 6E and
F). miR-1297 inhibitor-mediated alterations to MDA-MB-231 cell
proliferation and apoptosis were reversed by co-transfection with
FA2H-siRNA.
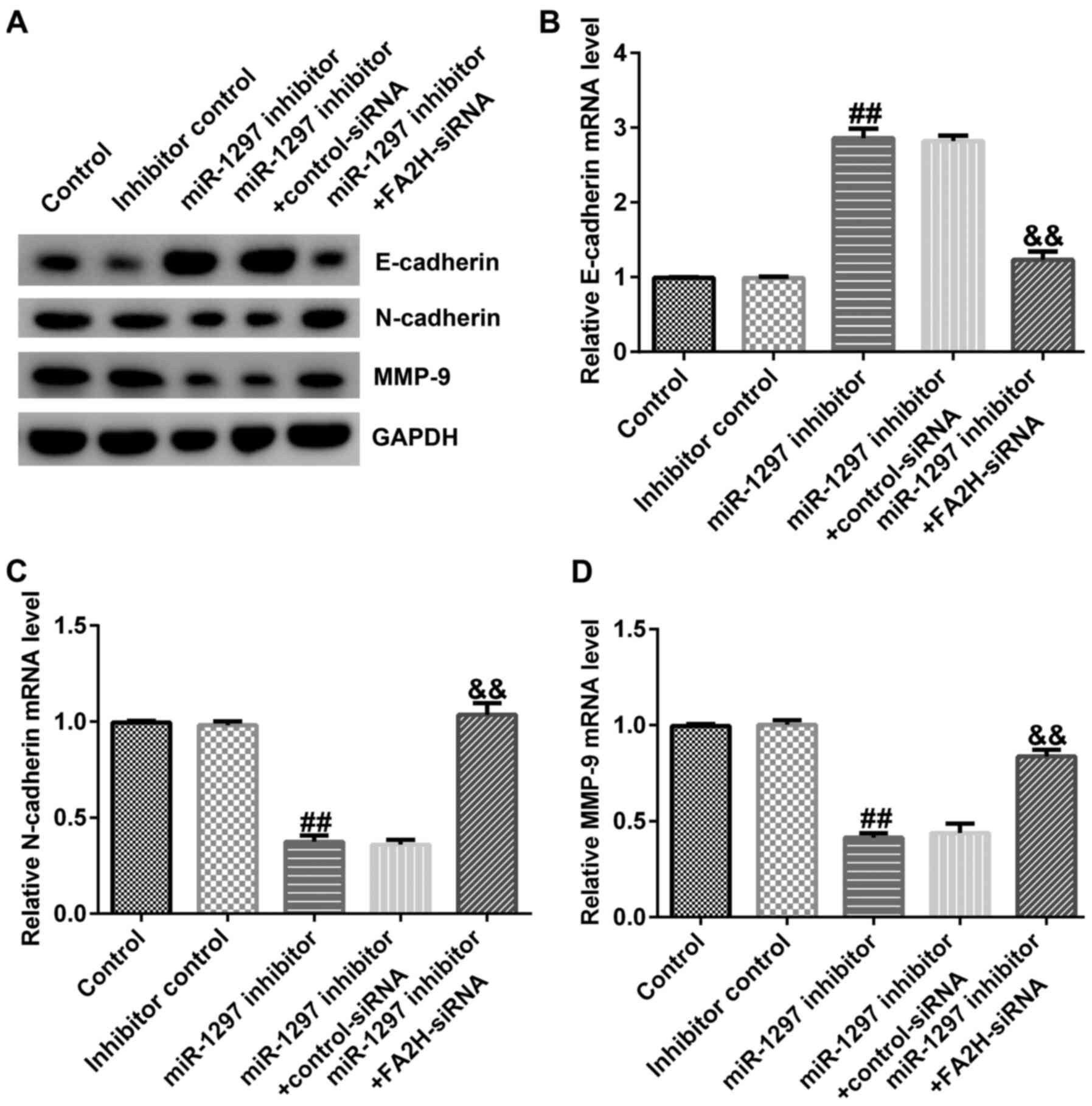
miR-1297 knockdown affects EMT by
upregulating FA2H
MDA-MB-231 cells were transfected with
control-siRNA, FA2H-siRNA, inhibitor control, miR-1297 inhibitor,
miR-1297 inhibitor + control-siRNA or miR-1297 inhibitor +
FA2H-siRNA for 48 h. The expression levels of EMT markers were
determined via RT-qPCR and western blotting. Compared with the
inhibitor control group, miR-1297 inhibitor obviously increased
E-cadherin protein and mRNA expression levels, and downregulated
N-cadherin and MMP9 protein and mRNA expression levels (Fig. 7A-D). Co-transfection with FA2H-siRNA
reversed miR-1297 inhibitor-mediated alterations to mRNA and
protein expression levels.
Discussion
Several advanced biotechnology studies have
demonstrated that abnormal miRNA expression in BC is a rule rather
than an exception (31,32). It has been reported that miR-21 is
expressed in human BC tissues and cells (33,34), and
may monitor the early occurrence of BC (35). Furthermore, miR-21 regulates cell
proliferation, G2/M checkpoints, metastatic spread
(36–38) and the expression of multiple target
genes, including tropomyosin-1, programmed cell death factor 4,
maspin and Bcl-2 (39). Wang et
al (40) demonstrated that
miR-21 promoted BC cell proliferation and metastasis. Emerging
evidence has indicated that several miRNAs are overexpressed in BC
cell lines, including the miR-221/222 cluster (41), miR-9, miR10b, miR-29a, miR-96,
miR-146a, miR-181, miR-373 and miR-589 (42). In addition, a previous study
suggested that miR-141 is involved in the development of BC
(43). Therefore, the present study
aimed to investigate the role of miR-1297 in BC.
Increasing evidence has indicated that miR-1297 is
associated with multiple types of cancer. Liang et al
(44) demonstrated that miR-1297
suppressed prostate cell proliferation and invasion via the
astrocyte elevated gene-1/Wnt signaling pathway (44). By contrast, other studies revealed
that miR-1297 promoted cell proliferation and affected several
biological behaviors in laryngeal squamous cell carcinoma and
testicular germ cell tumor cells via PTEN (19,45). The
present study demonstrated that miR-1297 knockdown decreased cell
proliferation and increased apoptosis in MDA-MB-231 cells compared
with the inhibitor control. miRNAs exert specific functions by
regulating the expression of their target genes (46–48).
Subsequently, bioinformatics and in vitro experiments were
performed to verify whether miR-1297 directly targeted FA2H. The
results indicated that FA2H was a direct target of miR-1297.
FA2H mediates the introduction of a chiral
(R)-hydroxyl group at the second carbon of long-chain FAs (24). FA2H is upregulated in multiple
organs, affects cell differentiation and regulates the membrane
transport capacity of nutrient transporters (49,50).
Alderson and Hama (51) indicated
that FA2H knockdown promoted D6P2T nerve sheath cell proliferation
and suppressed cAMP-induced cell cycle arrest, suggesting that FA2H
exhibited several functions in regulating signaling pathways
associated with cell proliferation. A previous study also suggested
that FA2H is associated with BC (27). In the present study, FA2H
overexpression decreased cell proliferation and increased apoptosis
compared with the control-plasmid group. Furthermore, the results
indicated that miR-1297 modulated EMT by regulating FA2H
expression.
EMT is the process whereby epithelial cells are
transformed into mesenchymal cells, thus gaining the ability to
migrate and invade (52). EMT is an
important component of cancer metastasis (53). During the early stages of cancer
metastasis, separation of tumoral cells from the primary tumor may
be mediated by attenuating EMT (54). The epithelial cell marker E-cadherin,
and the mesenchymal cell markers N-cadherin and MMP9 are primary
EMT markers (52,55). The results of the present study
revealed that FA2H overexpression increased the expression levels
of E-cadherin and decreased the expression levels of N-cadherin and
MMP9 compared with the control-plasmid group.
Collectively, the present study indicated that
miR-1297 regulated BC cell proliferation and EMT via regulating
FA2H expression. Compared with the inhibitor control, miR-1297
knockdown decreased BC cell EMT and proliferation in a
FA2H-dependent manner. Therefore, the present study suggested that
miR-1297 and FA2H may serve as potential therapeutic targets for
breast cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the First-Class
Discipline Construction Funded Project of Ningxia Medical
University and the School of Clinical Medicine (grant no.
NXYLXK2017A05) and the Ningxia Natural Science Foundation Project
(grant no. 2018AAC03165).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL contributed to the conception and design of the
study, data acquisition, analysis and interpretation, and drafted
and critically revised the manuscript. BL contributed to the
conception and design of the study, data acquisition, analysis and
interpretation and critically revised the manuscript. JL
contributed to conception and design of the study, data analysis
and interpretation, and drafted and critically revised the
manuscript. YL and DC contributed to data analysis and validation.
All authors gave final approval and agree to be accountable for all
aspects of the work. All authors read and reviewed the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics. 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Ma J, Goding Sauer A, Newman
LA and Jemal A: Breast cancer statistics, 2017, racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta I, Burney I, Al-Moundhri MS and
Tamimi Y: Molecular genetics complexity impeding research progress
in breast and ovarian cancers. Mol Clin Oncol. 7:3–14. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li P, Xu T, Zhou X, Liao L, Pang G, Luo W,
Han L, Zhang J, Luo X, Xie X and Zhu K: Downregulation of miRNA-141
in breast cancer cells is associated with cell migration and
invasion: Involvement of ANP32E targeting. Cancer Medicine.
6:662–672. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62.
2014.PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI
|
|
7
|
Mo YY: MicroRNA regulatory networks and
human disease. Cell Mol Life Sci. 69:3529–3531. 2012.PubMed/NCBI
|
|
8
|
Laffont B and Rayner KJ: MicroRNAs in the
pathobiology and therapy of atherosclerosis. Can J Cardiol.
33:313–324. 2017.PubMed/NCBI
|
|
9
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004.PubMed/NCBI
|
|
10
|
Ren Y, Chen Y, Liang X, Lu Y, Pan W and
Yang M: miRNA-638 promotes autophagy and malignant phenotypes of
cancer cells via directly suppressing DACT3. Cancer Lett.
390:126–136. 2017.PubMed/NCBI
|
|
11
|
Zhan MN, Yu XT, Tang J, Zhou CX, Wang CL,
Yin QQ, Gong XF, He M, He JR, Chen GQ and Zhao Q: MicroRNA-494
inhibits breast cancer progression by directly targeting PAK1. Cell
Death Dis. 8:e25292017.PubMed/NCBI
|
|
12
|
Wang C, Li QB, Liu F, Chen X, Nesa EU,
Guan SH, Liu BW, Han LH, Tan BX, Wang D, et al: Serum miR-1297: A
promising diagnostic biomarker in esophageal squamous cell
carcinoma. Biomarkers. 21:517–522. 2016.PubMed/NCBI
|
|
13
|
Ju HQ, Lu YX, Chen DL, Tian T, Mo HY, Wei
XL, Liao JW, Wang F, Zeng ZL, Pelicano H, et al: Redox regulation
of stem-like cells though the CD44v-xCT axis in colorectal cancer:
Mechanisms and therapeutic implications. Theranostics. 6:1160–1175.
2016.PubMed/NCBI
|
|
14
|
Zhang C, Chi YL, Wang PY, Wang YQ, Zhang
YX, Deng J, Lv CJ and Xie SY: miR-511 and miR-1297 inhibit human
lung adenocarcinoma cell proliferation by targeting oncogene TRIB2.
PLoS One. 7:e460902012.PubMed/NCBI
|
|
15
|
Wang J, Xu X, Mo S, Tian Y, Wu J, Zhang J
and Zhao J: Involvement of microRNA-1297, a new regulator of HMGA1,
in the regulation of glioma cell growth in vivo and in vitro. Am J
Transl Res. 8:2149–2158. 2016.PubMed/NCBI
|
|
16
|
Wu XJ, Pu XM, Zhao ZF, Zhao YN, Kang XJ,
Wu WD, Zou YM, Wu CY, Qu YY, Zhang DZ, et al: The expression
profiles of microRNAs in Kaposi's sarcoma. Tumour Biol. 36:437–446.
2015.PubMed/NCBI
|
|
17
|
Chen Z, Ma Y, Pan Y, Zhu H, Yu C and Sun
C: miR-1297 suppresses pancreatic cancer cell proliferation and
metastasis by targeting MTDH. Mol Cell Probes. 40:19–26.
2018.PubMed/NCBI
|
|
18
|
Gao W, Cao Y, Guo P, Bao X, Zhu H, Zheng
J, Yao C, Chen D, Yu S, Chen B, et al: Downregulation of miR-1297
predicts poor prognosis and enhances gastric cancer cell growth by
targeting CREB1. Biomed Pharmacother. 105:413–419. 2018.PubMed/NCBI
|
|
19
|
Li X, Wang HL, Peng X, Zhou HF and Wang X:
miR-1297 mediates PTEN expression and contributes to cell
progression in LSCC. Biochem Biophys Res Commun. 427:254–260.
2012.PubMed/NCBI
|
|
20
|
Liu C, Liu ZK, Li X, Tang XJ, He JJ and Lu
SY: MicroRNA-1297 contributes to tumor growth of human breast
cancer by targeting PTEN/PI3K/AKT signaling. Oncol Rep.
38:2435–2443. 2017.PubMed/NCBI
|
|
21
|
Liu F, He Y, Shu R and Wang S:
MicroRNA-1297 regulates hepatocellular carcinoma cell proliferation
and apoptosis by targeting EZH2. Int J Clin Exp Pathol.
8:4972–4980. 2015.PubMed/NCBI
|
|
22
|
Ling L, Feng L and Wei B: MicroRNA-1297
involves in the progression of oral squamous cell carcinoma through
PTEN. Saudi J Biol Sci. 25:923–927. 2018.PubMed/NCBI
|
|
23
|
Guo L, Zhang X, Zhou D, Okunade AL and Su
X: Stereospecifificity of fatty acid 2-hydroxylase and differential
functions of 2-hydroxy fatty acid enantiomers. J Lipid Res.
53:1327–1335. 2012.PubMed/NCBI
|
|
24
|
Alderson NL, Rembiesa BM, Walla MD,
Bielawska A, Bielawski J and Hama H: The human FA2H gene encodes a
fatty acid 2-hydroxylase. J Biol Chem. 279:48562–48568.
2004.PubMed/NCBI
|
|
25
|
Eckhardt M, Yaghootfam A, Fewou SN, Zöller
I and Gieselmann V: A mammalian fatty acid hydroxylase responsible
for the formation of alpha-hydroxylated galactosylceramide in
myelin. Biochem J. 388((Pt 1)): 245–254. 2005.PubMed/NCBI
|
|
26
|
Yao Y, Yang X, Sun L, Sun S, Huang X, Zhou
D, Li T, Zhang W, Abumrad NA, Zhu X, et al: Fatty acid
2-hydroxylation inhibits tumor growth and increases sensitivity to
cisplatin in gastric cancer. EBioMedicine. 41:256–267.
2019.PubMed/NCBI
|
|
27
|
Dai X, Zhang S, Cheng H, Cai D, Chen X and
Huang Z: FA2H exhibits tumor suppressive roles on breast cancers
via cancer stemness control. Front Oncol. 9:10892019.PubMed/NCBI
|
|
28
|
Lemay AM, Courtemanche O, Couttas TA,
Jamsari G, Gagné A, Bossé Y, Joubert P, Don AS and Marsolais D:
High FA2H and UGT8 transcript levels predict hydroxylated
hexosylceramide accumulation in lung adenocarcinoma. J Lipid Res.
60:1776–1786. 2019.PubMed/NCBI
|
|
29
|
Herrero AB, Astudillo AM, Balboa MA,
Cuevas C, Balsinde J and Moreno S: Levels of SCS7/FA2H-mediated
fatty acid 2-hydroxylation determine the sensitivity of cells to
antitumor PM02734. Cancer Res. 68:9779–9787. 2008.PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI
|
|
31
|
Andorfer CA, Necela BM, Thompson EA and
Perez EA: MicroRNA signatures: Clinical biomarkers for the
diagnosis and treatment of breast cancer. Trends Mol Med.
17:313–319. 2011.PubMed/NCBI
|
|
32
|
Shi M and Guo N: MicroRNA expression and
its implications for the diagnosis and therapeutic strategies of
breast cancer. Cancer Treat Rev. 35:328–334. 2009.PubMed/NCBI
|
|
33
|
Zhang ZJ and Ma SL: miRNAs in breast
cancer tumorigenesis (Review). Oncol Rep. 27:903–910.
2012.PubMed/NCBI
|
|
34
|
Corcoran C, Friel AM, Duffy MJ, Crown J
and O'Driscoll L: Intracellular and extracellular microRNAs in
breast cancer. Clin Chem. 57:18–32. 2011.PubMed/NCBI
|
|
35
|
Ozgun A, Karagoz B, Bilgi O, Tuncel T,
Baloglu H and Kandemir EG: MicroRNA-21 as an indicator of
aggressive phenotype in breast cancer. Onkologie. 36:115–118.
2013.PubMed/NCBI
|
|
36
|
Dong G, Liang X, Wang D, Gao H, Wang L,
Wang L, Liu J and Du Z: High expression of miR-21 in
triple-negative breast cancers was correlated with a poor prognosis
and promoted tumor cell in vitro proliferation. Med Oncol.
31:572014.PubMed/NCBI
|
|
37
|
Anastasov N, Höfig I, Vasconcellos IG,
Rappl K, Braselmann H, Ludyga N, Auer G, Aubele M and Atkinson MJ:
Radiation resistance due to high expression of miR-21 and G2/M
checkpoint arrest in breast cancer cells. Radiat Oncol.
7:2062012.PubMed/NCBI
|
|
38
|
Min W, Wang B, Li J, Han J, Zhao Y, Su W,
Dai Z, Wang X and Ma Q: The expression and significance of five
types of miRNAs in breast cancer. Med Sci Monit Basic Res.
20:97–104. 2014.PubMed/NCBI
|
|
39
|
Li J, Zhang Y, Zhang W, Jia S, Tian R,
Kang Y, Ma Y and Li D: Genetic heterogeneity of breast cancer
metastasis may be related to miR-21 regulation of TIMP-3 in
translation. Int J Surg Oncol. 2013:8750782013.PubMed/NCBI
|
|
40
|
Wang H, Tan Z, Hu H, Liu H, Wu T, Zheng C,
Wang X, Luo Z, Wang J, Liu S, et al: microRNA-21 promotes breast
cancer proliferation and metastasis by targeting LZTFL1. BMC
Cancer. 19:7382019.PubMed/NCBI
|
|
41
|
Piva R, Spandidos DA and Gambari R: From
microRNA functions to microRNA therapeutics: Novel targets and
novel drugs in breast cancer research and treatment (Review). Int J
Oncol. 43:985–994. 2013.PubMed/NCBI
|
|
42
|
Christodoulatos GS and Dalamaga M:
Micro-RNAs as clinical biomarkers and therapeutic targets in breast
cancer: Quo vadis? World J Clin Oncol. 5:71–81. 2014.PubMed/NCBI
|
|
43
|
Debeb BG, Lacerda L, Anfossi S,
Diagaradjane P, Chu K, Bambhroliya A, Huo L, Wei C, Larson RA,
Wolfe AR, et al: miR-141-mediated regulation of brain metastasis
from breast cancer. J Natl Cancer Inst. 108:djw0262016.
|
|
44
|
Liang X, Li H, Fu D, Chong T, Wang Z and
Li Z: MicroRNA-1297 inhibits prostate cancer cell proliferation and
invasion by targeting the AEG-1/Wnt signaling pathway. Biochem
Biophys Res Commun. 480:208–214. 2016.PubMed/NCBI
|
|
45
|
Yang NQ, Zhang J, Tang QY, Guo JM and Wang
GM: miRNA-1297 induces cell proliferation by targeting phosphatase
and tensin homolog in testicular germ cell tumor cells. Asian Pac J
Cancer Prev. 15:6243–6246. 2014.PubMed/NCBI
|
|
46
|
Lu TX and Rothenberg ME: MicroRNA. J
Allergy Clin Immunol. 141:1202–1207. 2018.PubMed/NCBI
|
|
47
|
Liu B, Li J and Cairns MJ: Identifying
miRNAs, targets and functions. Brief Bioinform. 15:1–19.
2014.PubMed/NCBI
|
|
48
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015.
|
|
49
|
Kota V and Hama H: 2′-Hydroxy ceramide in
membrane homeostasis and cell signaling. Adv Biol Regul.
54:223–230. 2014.PubMed/NCBI
|
|
50
|
Guo L, Zhou D, Pryse KM, Okunade AL and Su
X: Fatty acid 2-hydroxylase mediates diffusional mobility of
Raft-associated lipids, GLUT4 level, and lipogenesis in 3T3-L1
adipocytes. J Biol Chem. 285:25438–25447. 2010.PubMed/NCBI
|
|
51
|
Alderson NL and Hama H: Fatty acid
2-hydroxylase regulates cAMP-induced cell cycle exit in D6P2T
schwannoma cells. J Lipid Res. 50:1203–1208. 2009.PubMed/NCBI
|
|
52
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014.PubMed/NCBI
|
|
53
|
Ye X, Brabletz T, Kang Y, Longmore GD,
Nieto MA, Stanger BZ, Yang J and Weinberg RA: Upholding a role for
EMT in breast cancer metastasis. Nature. 547:E1–E3. 2017.PubMed/NCBI
|
|
54
|
Singh M, Yelle N, Venugopal C and Singh
SK: EMT: Mechanisms and Therapeutic Implications. Pharmacol Ther.
182:80–94. 2018.PubMed/NCBI
|
|
55
|
Zhao L, Pang A and Li Y: Function of GCN5
in the TGF-β1-induced epithelial-to-mesenchymal transition in
breast cancer. Oncol Lett. 16:3955–3963. 2018.PubMed/NCBI
|