Introduction
Lung cancer is one of the most common types (2.09
million cases; 11.6% among cancers; 2018) of cancer in the world,
and lung cancer-associated mortality (1.76 million deaths; 18.4% of
total cancer deaths; 2018) is higher than that of other types of
cancer (1–3). Only patients with early stage lung
cancer who undergo surgery have a reasonable chance of survival;
however, recurrence rates of lung cancer are high (2). In addition to conventional
chemotherapy, alternative therapy (4) or targeted therapy, such as therapies
targeting EGFR, VEGF or cyclooxygenase (COX), have been
investigated (5–8). COX is an essential enzyme to produce
prostaglandins and it is also involved in numerous cellular
responses, such as cell growth, survival and regulation of proteins
(9,10). COX can be inhibited by non-steroidal
anti-inflammatory drugs (NSAIDs), which are widely used to control
pain, to suppress inflammation and for cancer treatment (8). There are two types of COX enzymes,
namely COX-1, and COX-2. The use of NSAIDs for prolonged periods of
time can result in side effects, such as intestinal bleeding or
gastric ulcer due to inhibition of COX-1 (11). However, inhibition of COX-2 has few
side effects, and therefore COX-2 inhibitors are widely used to
relieve pain and to treat inflammatory diseases including
rheumatoid arthritis and osteoarthritis (12).
Celecoxib is a selective COX-2 inhibitor with little
effect on COX-1 (12). Celecoxib has
been reported to have anticancer effects in colon (13,14),
gastric (15,16), breast (17), lung (18,19) and
prostate (20,21) cancer. Celecoxib inhibits tumor
growth, induces apoptosis and cell cycle arrest of cancer cells,
and suppresses tumor angiogenesis (11,22,23).
Therefore, celecoxib has been investigated as an adjuvant agent in
cancer therapeutics. Clinicians have tried to use celecoxib as an
adjuvant agent to treat colorectal, lung, melanoma and breast
cancer, but it is not standard treatment yet (24).
The natural-killer group 2 member D (NKG2D) receptor
is a C-type lectin-like activating receptor (25,26).
NKG2D is a homodimeric receptor expressed by cytotoxic lymphocytes
and encoded by the natural killer (NK) cell gene complex (26). NKG2D is expressed by several types of
cells, including NK, CD8 αβ T, γδ T, NKT and a small subset of CD4
αβ T cells (25,26). Therefore, NKG2D recognition is
considered to be critical in tumor immune surveillance. NKG2D binds
to MHC class I-related chain A (MICA), MICB and UL16-binding
proteins 1–6 (ULBP-1-6) in humans (25–27).
NKG2D ligands are expressed on various cancer cells, but are rarely
expressed on healthy cells (28).
Expression of NKG2D ligands can induce heat shock stress, DNA
damage and post-transcriptional epigenetic modifications in cancer
cells (28). Cancer therapies that
increase NKG2D ligand expression may therefore have a therapeutic
effect. The present study investigated the induction of ULBP-1
expression in lung cancer cells after celecoxib treatment and
explored the susceptibility of these celecoxib-treated cells to NK
cell cytotoxicity.
Materials and methods
Cell culture
The human lung cancer A549 and H460 cell lines were
purchased from the American Type Culture Collection (ATCC) and were
maintained in RPMI-1640 medium supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin (all HyClone; Cytiva). The
human NK NK-92MI cell line (ATCC) was maintained in α Minimum
Essential Medium (HyClone; Cytiva) supplemented with 2 mM
L-glutamine, 0.2 mM inositol, 20 mM folic acid and 15% FBS. Cells
were grown in a humidified incubator at 37°C with 5%
CO2. NK-92MI cells were maintained with optimal cell
density between 2×105 and 8×105 cells/ml.
A549 and H450 cells were maintained between 5×103 and
5×104 cells/cm2.
Celecoxib and chemical treatment
A549 or H460 cells (1×104
cells/cm2) were initially placed in a culture dish.
After 8 h of incubation at 37°C, the attached cells were treated
with various concentrations (0, 25, 50, 75, 100 and 200 µM) of
celecoxib (Toronto Research Chemicals) at 37°C overnight. To
examine time-dependent increase of ULBP-1, A549 and H460 cells were
treated with 75 µM celecoxib for 2, 4, 6, 8, 10, 12 and 24 h. To
investigate the expression levels of NKG2D ligands, cells were
treated with sublethal concentrations (50 or 75 µM) of celecoxib.
For inhibition of PI3K or JNK pathway, cells were preincubated with
10 µM LY294002 (EMD Millipore), a PI3K inhibitor, or 10 µM SP600125
(EMD Millipore), a JNK inhibitor, for 1 h at 37°C before celecoxib
treatment. Cells were further treated with celecoxib as
aforementioned without removing the medium containing the
inhibitors.
Analysis of cytotoxicity
Cell viability was measured using the WST-1 assay
(Takara Bio, Inc.) according to the manufacturer's protocol. A549
and H460 cells (5×103/well) were seeded in 96-well
plates and incubated at 37°C for 24 h. Cells were treated with
various concentrations of celecoxib overnight, as aforementioned.
The next day, 10 µl of WST-1 was added to each well, and plates
were incubated at 37°C for 1 h. Absorbance was measured using a
microplate reader (Pierce; Thermo Fisher Scientific, Inc.) at a
wavelength of 450 nm.
RNA isolation and reverse
transcription (RT)-PCR
Total RNA was extracted from A549 and H460 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol and was
used to synthesize cDNA at 42°C for 1 h using oligo dT primers and
AccuPower® RT-premix (Bioneer Corporation). cDNA was
amplified using Tenuto PCR premix (Enzynomics Co., Ltd.) and a PCR
thermal cycler (Takara Bio, Inc.) with the following thermocycling
conditions: 5 min at 95°C (pre-denaturation), 30 cycles of 20 sec
at 94°C, 10 sec at 55°C (for MICA/B) or 65°C (for ULBPs), 30 sec at
72°C and 5 min at 72°C (final extension). The following primer
pairs were used: ULBP-1 forward, 5′-GCCAGGATGTCTTGTGAGCA-3′ and
reverse, 5′-CAGTGGTGAGTAGACAGGCG-3′; ULBP-2 forward,
5′-CCCTGGGGAAGAAACTAAATGTC-3′ and reverse,
5′-ACTGAACTGCCAAGATCCACTGCT-3′; ULBP-3 forward,
5′-GAGGCTCAGACTGGAACTGG-3′ and reverse, 5′-GCCTCTTCTTCCTGTGCATC-3′;
MICA forward, 5′-CAGACTGCCTGCAGGAACTA-3′ and reverse,
5′-TTTCTTCTTACAACAACGGACATA-3′; MICB forward, 5′-CGGACAGACTTTCCATAT
GTTT-3′ and reverse, 5′-TCCAACAACAATAAATAAGTG ATG-3′; and β-actin
forward, 5′-CATCGTGATGGACTCCGGTGAC-3′ and reverse,
5′-TCAGGTAGTCAGTCAGGTCC-3′. The PCR products were analyzed via 1%
agarose gel electrophoresis in TAE buffer with ethidium bromide and
a UV illuminator. The densities of the bands were measured using
ImageJ v1.53 software (National Institutes of Health).
Western blot analysis
Whole cell lysates from A549 and H460 cells
(4×105/60-mm culture dish) were prepared in RIPA lysis
buffer (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with a protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA).
Total protein in the cell lysates was determined using the BCA
assay, and sample proteins (10 µg/lane) were separated via 10%
SDS-PAGE. Separated proteins were transferred onto nitrocellulose
membranes after separation by electrophoresis. Membranes were
blocked with 5% skimmed milk at room temperature for 2 h and washed
three times with TBS-0.1% Tween (TBST). Primary antibodies against
ULBP-1 (1:1,000; cat. no. ab176566, Abcam) and β-actin (1:2,000;
cat. no. sc-69879, Santa Cruz Biotechnology, Inc.) were used.
Following incubation with primary antibodies in TBST at 4°C
overnight, unbound antibodies were washed away with TBST. Bound
primary antibodies were visualized via incubation of blots with
HRP-conjugated anti-rabbit secondary antibodies (1:5,000; cat. no.
7074; Cell Signaling Technology, Inc.) or HRP-conjugated anti-mouse
secondary antibodies (1:5,000; cat. no. 7076; Cell Signaling
Technology, Inc.) at room temperature for 1 h, and the signal was
detected using chemiluminescence detection reagents (Amersham;
Cytiva). The densities of bands were measured using ImageJ software
version 1.53 (National Institutes of Health).
Flow cytometry analysis and
fluorescence microscopy analysis of ULBP-1 expression
For flow cytometry, 1×106 cells of A549
and H460 were incubated with anti-ULBP-1 antibody (1 µg/ml; rabbit
IgG; cat. no. ab176566, Abcam) in PBS containing 0.1% FBS for 30
min on ice. Cells were washed and incubated with Alexa flour
488-conjugated secondary antibody for 30 min on ice (1:1,000; cat.
no. AP132JA4; Sigma-Aldrich; Merck KGaA). Labeled cells were washed
twice and resuspended in PBS. Flow cytometry data were obtained
using a MACSquant flow cytometer (Miltenyi Biotec GmbH) and
analyzed by MACSquant analysis software version 2.6.1517 (Miltenyi
Biotech, GmbH) embedded in the machine. For fluorescence
microscopy, labeled cells were collected onto glass slides using a
Cytospin centrifuge (Hanil Science Industrial Co., Ltd.). Cells
were analyzed via fluorescence microscopy (magnification, ×20)
using the iRiS digital cell imaging system (Logos Biosystems,
Inc.).
NK cytotoxicity assay
NK cell cytotoxic activity was determined using a
calcein-AM assay. Briefly, target cells (A549 and H460) were washed
twice with PBS and incubated with 5 mM calcein-AM (Sigma-Aldrich;
Merck KGaA) in serum-free RPMI medium for 10 min at 37°C. Cells
were then treated with/without celecoxib, as aforementioned.
Labeled target cells were distributed into the wells of U-bottom
microtiter plates at a concentration of 1×104
cells/well. NK-92MI cells were used as effector cells and were
added at various effector:target (E:T) ratios (0:1, 2.5:1, 5:1 and
10:1) in quadruplicate. Target cells in complete RPMI medium alone
were used to determine spontaneous calcein-AM retention. Maximal
lysis was determined by solubilizing three wells of target cells in
lysis buffer (0.1% Triton X-100; Sigma-Aldrich; Merck KGaA). After
incubation at 37°C for 8 h, assays were analyzed with 490/520 nm
(excitement/emission) using a fluorescence reader. Percent specific
cytotoxicity was calculated as calcein release relative to maximal
lysis.
Statistical analysis
Results are expressed as the mean ± standard
deviation. Statistical analysis was performed using GraphPad Prism
5.01 (GraphPad Software, Inc.). For comparisons among >2 groups,
data were analyzed using a one-way ANOVA with Bonferroni correction
to compare each two pairs between the indicated concentrations and
control. In the NK cytotoxicity assay, Student's unpaired two-sided
t-test was used to compare the lysis percentage between control and
each celecoxib-treated group, or a one-way ANOVA followed by the
post hoc Bonferonni test was used to compare multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
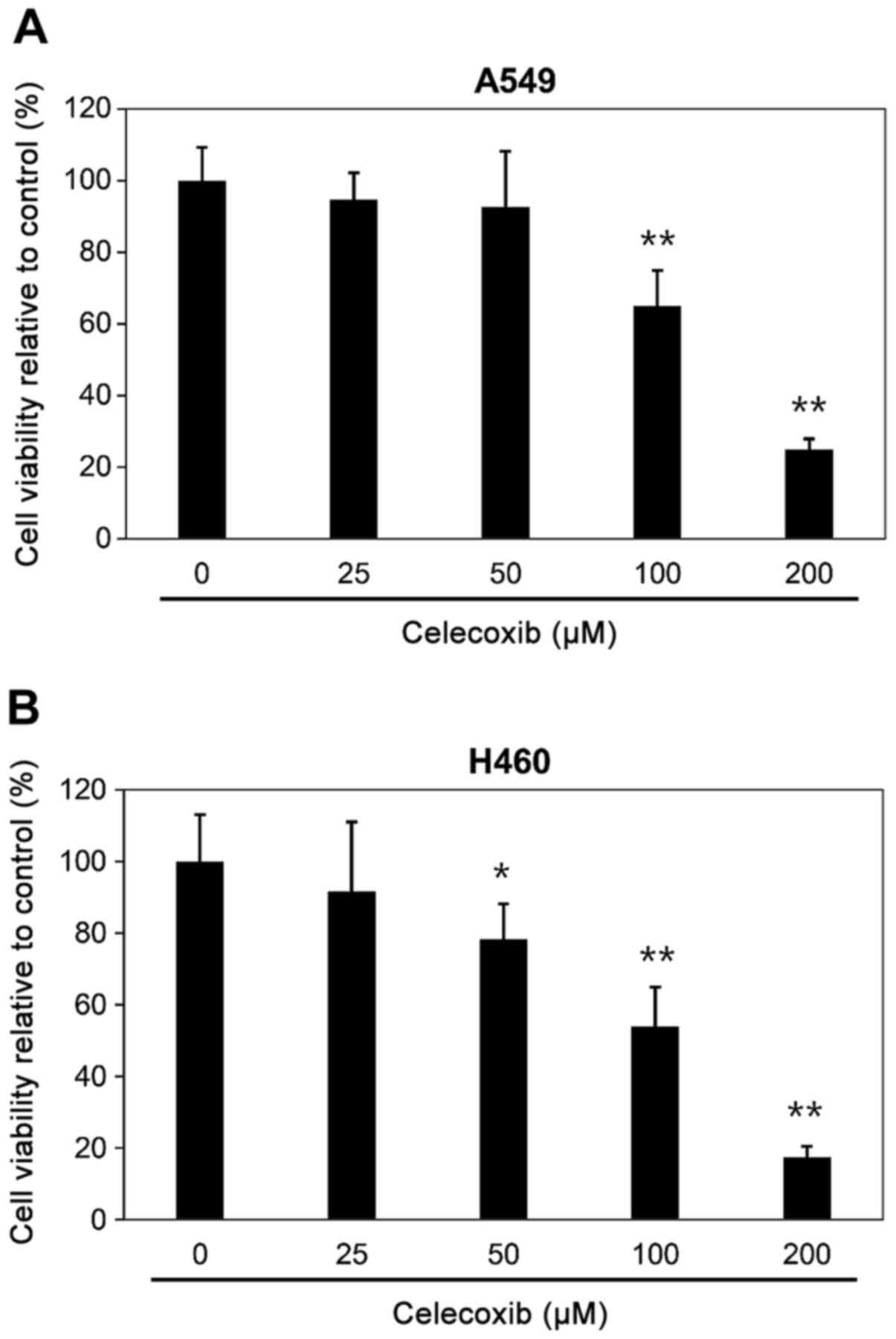
Cytotoxicity of celecoxib towards lung
cancer cells
Cell viability was determined using a WST-1 assay
after celecoxib treatment of the lung cancer A549 and H460 cell
lines. Lung cancer cells were incubated with various concentrations
of celecoxib (0, 25, 50, 100 and 200 µM) overnight. Cell
viabilities of A549 or H460 cells were significantly decreased when
treated with ≥100 µM celecoxib; therefore, 50 µM of celecoxib,
which exhibited minimal cell cytotoxicity, were the concentrations
used in subsequent experiments (Fig.
1).
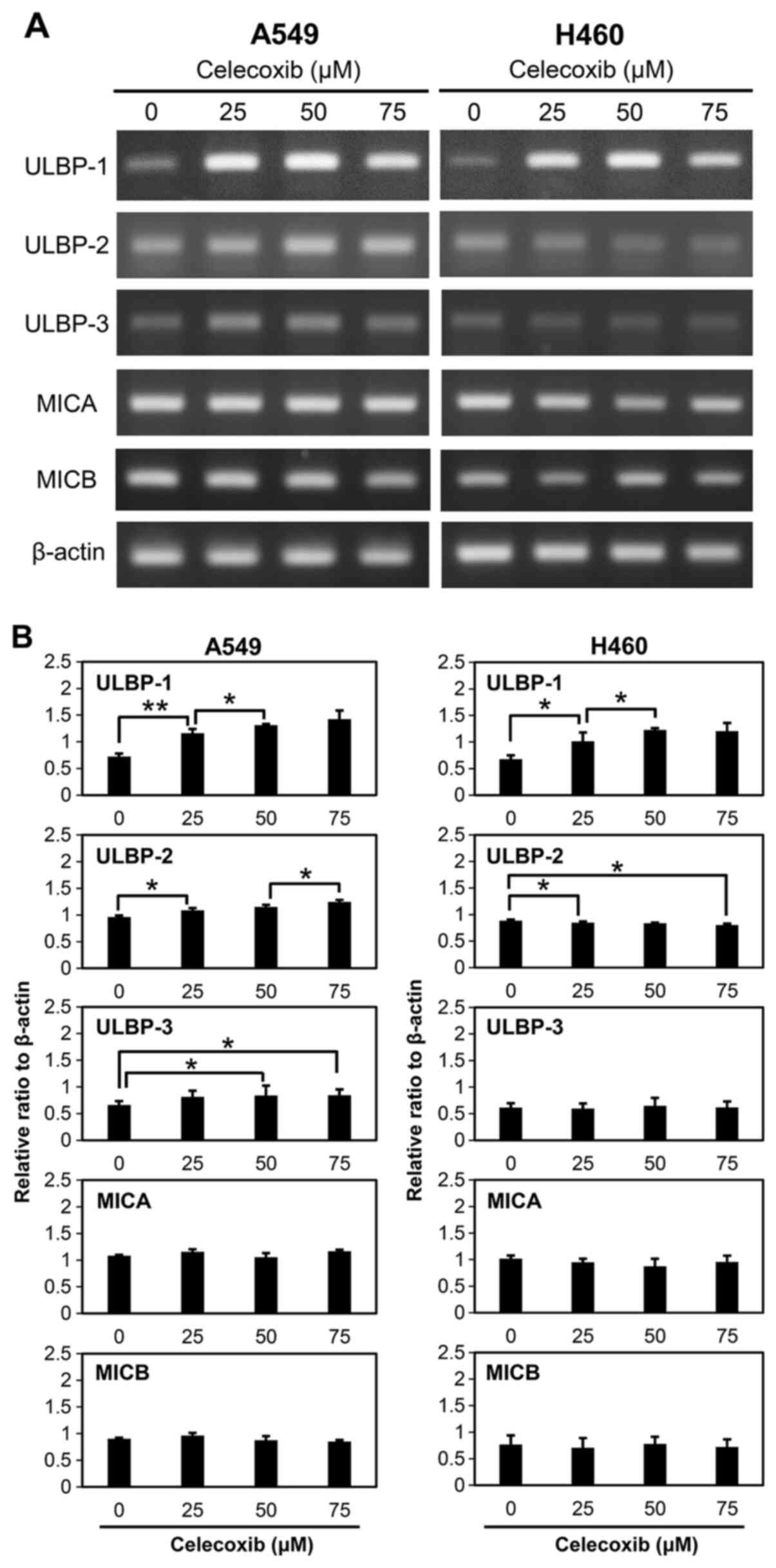
Expression levels of NKG2D ligands
after celecoxib treatment
Expression levels of NKG2D ligands, including
ULBP-1, ULBP-2, ULBP-3, MICA and MICB, were examined after
treatment of A549 and H460 cells with celecoxib (Fig. 2A). ULBP-1 mRNA expression increased
when A549 and H460 cells were treated with 25, 50 and 75 µM of
celecoxib. ULBP-2 expression significantly increased in A549 cells
treated with celecoxib compared with the control. In contrast,
ULBP-2 at 75 µM of celecoxib treatment slightly decreased in H460
cells compared with the control, but this was not statistically
significant (Fig. 2B). Celecoxib
treatment also increased ULBP-3 in A549 cells compared to the
control, however, this was not observed in H460 cells. Transcript
levels of other NKG2D ligands, namely MICA and MICB, did not
significantly change after celecoxib treatment of A549 and H460
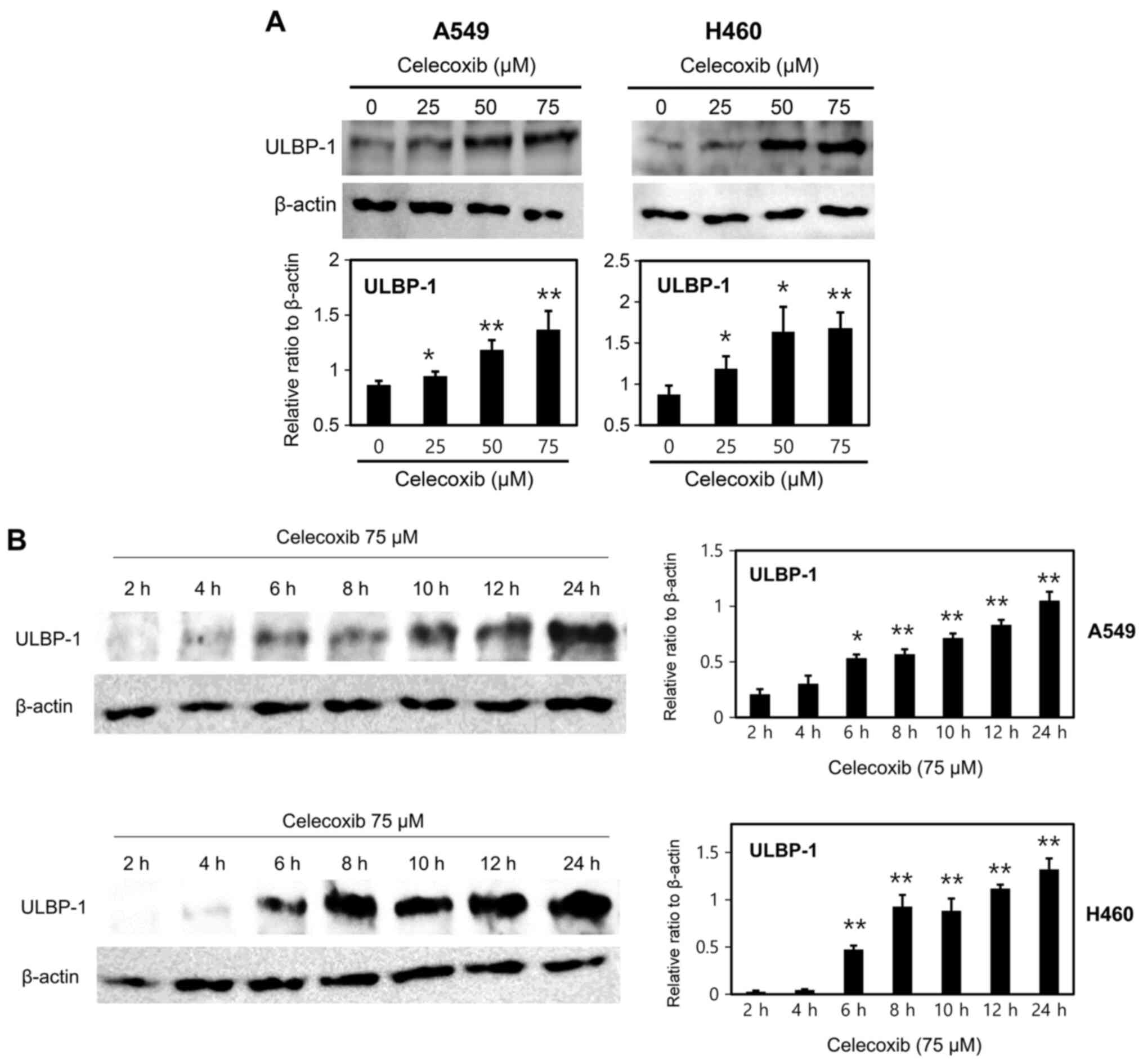
cells (Fig. 2B). Protein expression
of ULBP-1 was further investigated as its mRNA expression
distinctly. Other ULBPs proteins were also investigated and ULBP-2
protein was also increased on celecoxib-treated A549 cells (data
not shown), but the main focus was on ULBP-1 which demonstrated
distinct differences. It was further observed that the
celecoxib-induced increase in ULBP-1 expression was dose- (Fig. 3A) and time-dependent (Fig. 3B). ULBP-1 expression was
significantly increased in both A549 and H460 cells treated with
≥25 µM celecoxib (Fig. 3A).
Additionally, celecoxib significantly increased ULBP-1 protein
expression after 6 h of treatment (Fig.
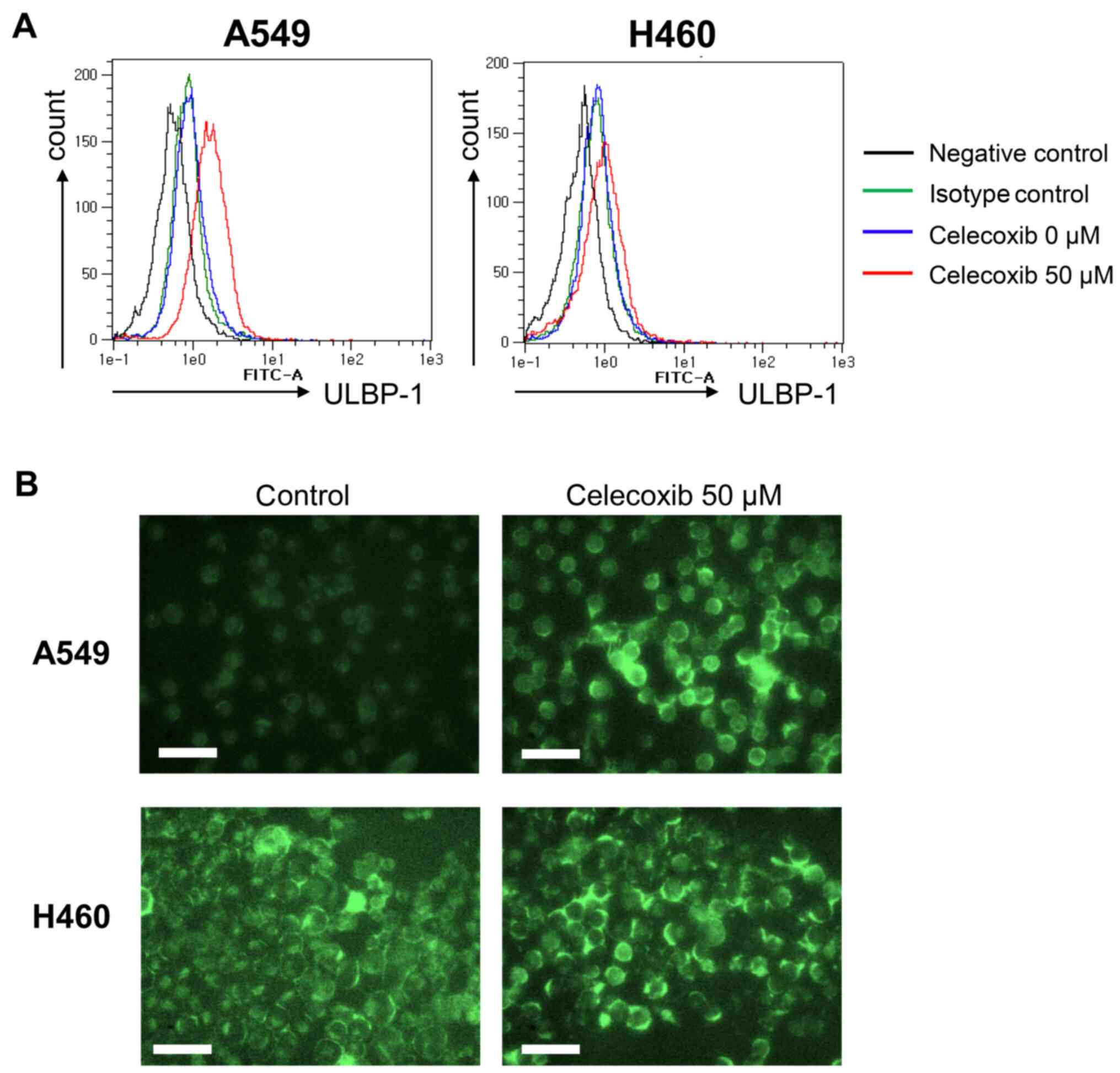
3B). The increase in ULBP-1 expression was also investigated
via flow cytometry analysis (Fig.
4A) and fluorescence microscopy (Fig. 4B). ULBP-1 was more strongly expressed
by A549 than H460 cells after celecoxib treatment (Fig. 4A). Fluorescence microscopic images
revealed abundant ULBP-1 in the cytoplasm after celecoxib treatment
(Fig. 4B).
Association between ULBP-1 expression
and JNK and PI3K signaling pathways
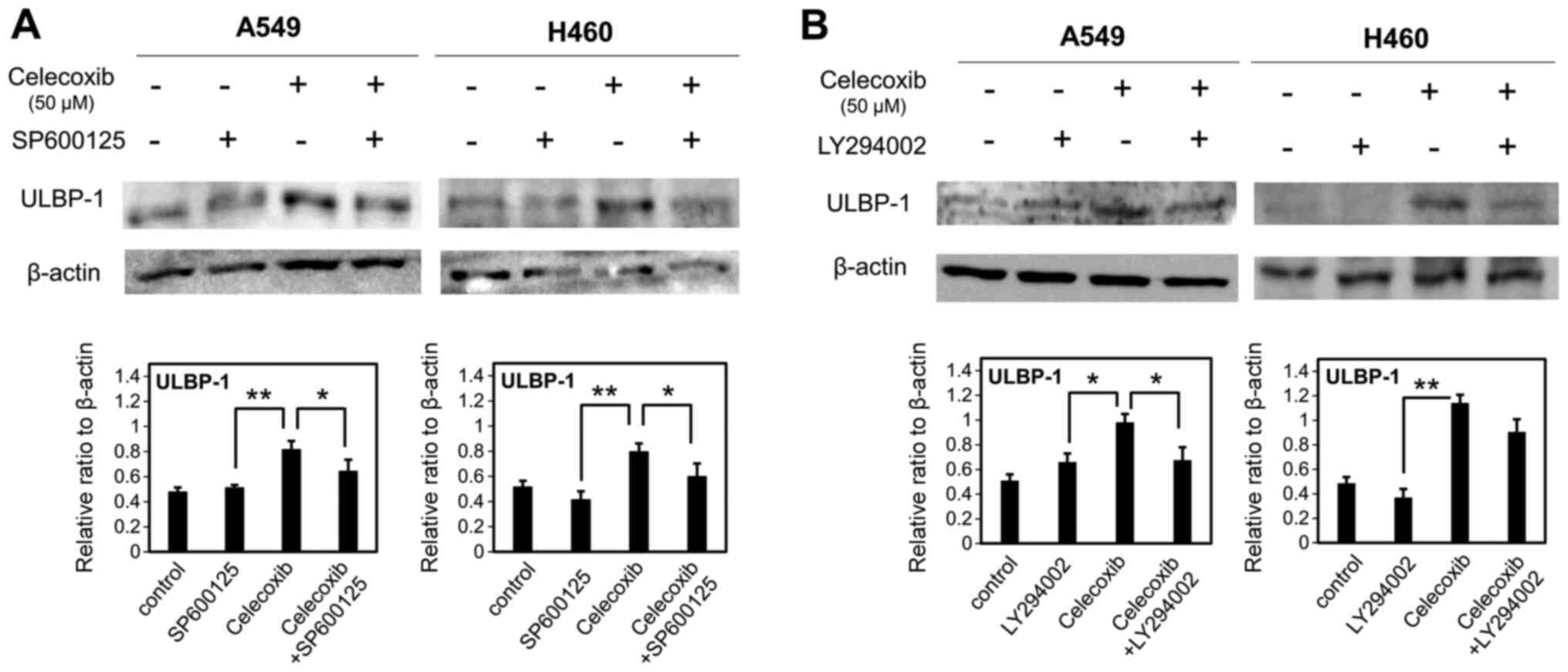
Cells were preincubated for 1 h with LY294002 (10
µM) as a PI3K inhibitor or SP600125 (10 µM) as a JNK inhibitor, and
were then treated with a sublethal concentration (50 µM) of
celecoxib. ULBP-1 expression was determined via western blotting.
SP600125 significantly attenuated the induction of ULBP-1
expression after celecoxib treatment of A549 and H460 cells
(P<0.05; Fig. 5A). Additionally,
LY294002 significantly attenuated ULBP-1 induction in A549
(P<0.05), but not H460 cells (P=0.071) (Fig. 5B).
Susceptibility of lung cancer cells to
NK cell-mediated lysis
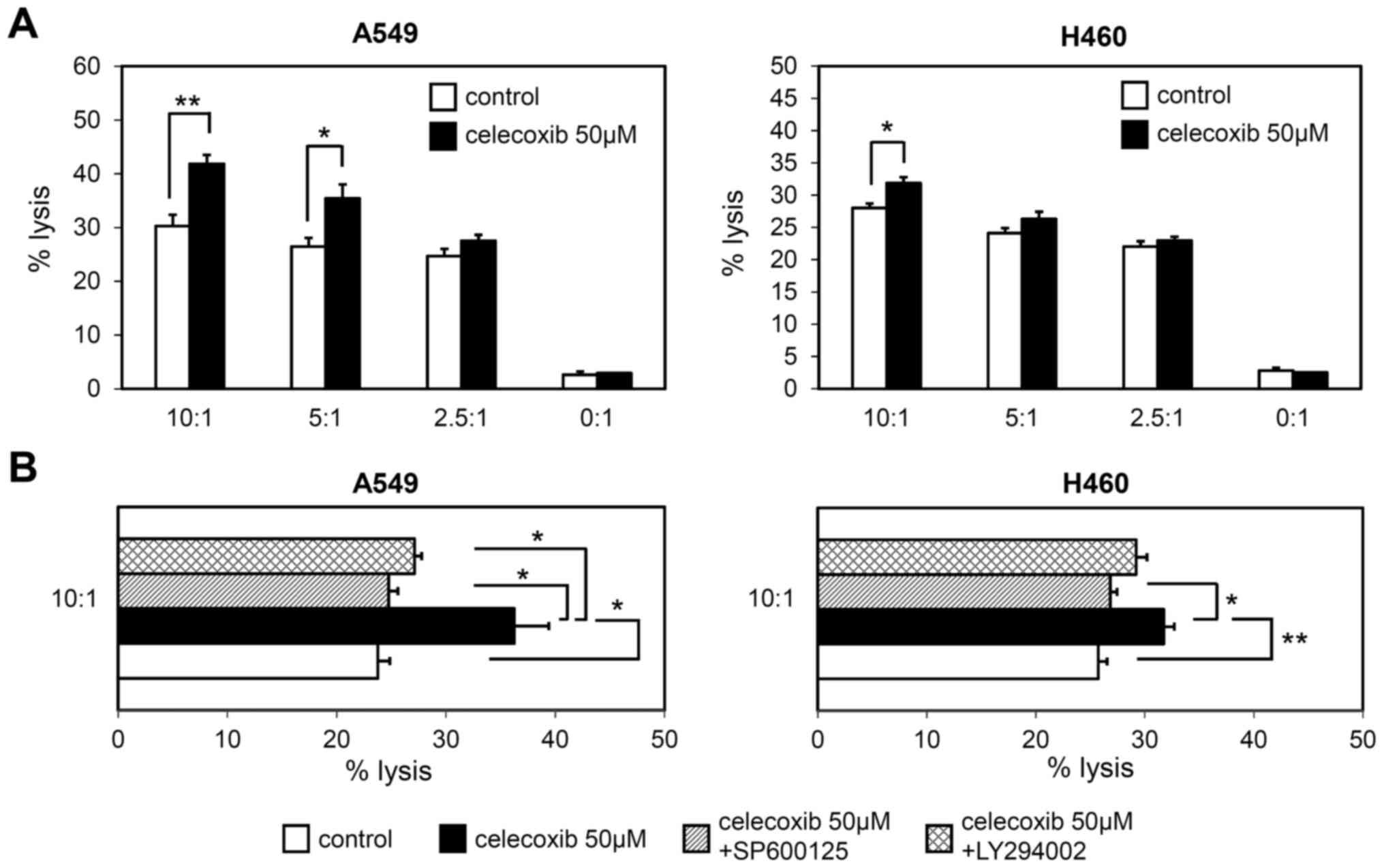
Finally, the present study investigated whether the
celecoxib-induced increase in ULBP-1 expression resulted in
increased NK cell-mediated lysis. Celecoxib-treated A549 and H460
cells were co-cultured with various ratios of NK-92MI cells, and
then NK cell toxicity was measured using a calcein assay. NK-91MI
cells were significantly cytotoxic to celecoxib-treated A549 cells
at 5:1 and 10:1 E:T ratios, while NK cell-mediated toxicity of
celecoxib-treated H460 cells was only observed at a 10:1 E:T ratio
(Fig. 6A). Both SP600125 and
LY294002 significantly attenuated NK cell susceptibility of A549
cells after celecoxib treatment (P<0.05; Fig. 6B). Additionally, SP600125, but not
LY249002, significantly attenuated NK cell-mediated toxicity of
celecoxib-treated H460 cells (P<0.05 and P=0.060, respectively;
Fig. 6B).
Discussion
Celecoxib was developed as a COX-2 selective
inhibitor, which is a type of NSAID that is used as an analgesic.
COX-2 is highly expressed in numerous types of cancer, such as
colorectal cancer, breast cancer and non-small cell lung cancer,
and is therefore a possible target of cancer therapy (7). By targeting COX-2, celecoxib has
attracted attention as an adjuvant therapeutic drug for cancer
treatment (8). However, it has been
reported that celecoxib can regulate intracellular functions
(autophagy or ER stress), and cell signaling (PI3K or MAPK), as
well as inhibit COX-2 function (16–23). In
the current study, an increase in transcript and protein expression
levels of ULBP-1, an activating receptor for NK cells, was observed
in lung cancer cells treated with celecoxib.
The increase in ULBP-1 expression was the most
prominent compared with other NKG2D, such as ULBP-2, ULBP-3, MICA
or MICB. Celecoxib was able to induce both mRNA and protein
expression levels of ULBP-1 in A549 and H460 cells in a dose- and
time-dependent manner. Kim et al (29) reported that celecoxib induced ULBP-1
expression in colon cancer cells in a COX-2 independent manner. The
present study revealed that not only ULBP-2 expression was
increased by treatment of A549 and H460 cells with celecoxib, but
also ULBP-3 expression was increased on A549 cells following
celecoxib treatment. ULBP-3 on H460 was not significantly changed.
MICA and MICB expression, on the other hand, was not affected by
celecoxib treatment. It was concluded that activating NKG2D ligands
(ULBPs) were more highly expressed by celecoxib-treated lung cancer
cells than inhibitory NKG2D ligands (MICA/B), as celecoxib-treated
lung cancer cells were susceptible to NK cell-mediated death.
However, interactions between NKG2D ligands and celecoxib treatment
should be studied further in other lung cancer cells that express
various types of EGFR and KRAS mutations (30), because both A549 and H460 have
wild-type EGFR.
Extrinsic stimuli, such as stress and drugs, can
activate the MAPK and PI3K signaling pathways (31). The MAPK signaling pathway was
reported as a regulator of NKG2D ligand expression, including ULBPs
(32). The PI3K signaling pathway is
also involved in NKG2D ligand regulation (33). Therefore, since celecoxib may produce
cell stress and modulate the MAPK or PI3K signaling pathways
(31–33), it may be involved in the regulation
of NKG2D ligands. In the present study, SP600125 (a JNK inhibitor)
and LY294002 (a PI3K inhibitor) decreased ULBP-1 expression in
celecoxib-treated lung cancer cells. However, the present study did
not investigate whether celecoxib may directly regulate the PI3K or
JNK signaling pathways. It is possible that other mediators
affected by JNK or PI3K may be associated with celecoxib-mediated
ULBP-1 expression. More precise experiments are required to
investigate this further.
Overall, the present results demonstrated that
treatment of lung cancer cells with a sublethal concentration of
celecoxib induced ULBP-1 expression without cell toxicity, and
increased the susceptibility of these cancer cells to NK cell
cytotoxicity. The current results indicated that celecoxib may
potentially increase the effects of conventional anticancer therapy
by making lung cancer cells more sensitive to NK cells, in addition
to targeting COX-2.
Acknowledgements
Not applicable.
Funding
The present study was supported by a 2016 research
grant from Inje University Busan Paik Hospital. This grant was an
internal research fund provided by the university itself.
Availability of data and materials
The data used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HKL and YSK contributed to conception and design and
interpretation of data. JK and MHN contributed to acquisition of
data and drafting the manuscript. DYH and BK contributed to
interpretation of data. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008.PubMed/NCBI
|
|
2
|
Bade BC and Dela Cruz CS: Lung Cancer
2020: Epidemiology, Etiology, and Prevention. Clin Chest Med.
41:1–24. 2020.PubMed/NCBI
|
|
3
|
Hirsch FR and Lippman SM: Advances in the
biology of lung cancer chemoprevention. J Clin Oncol. 23:3186–3197.
2005.PubMed/NCBI
|
|
4
|
Kasymjanova G, Tran AT, Cohen V, Pepe C,
Sakr L, Small D, Agulnik JS and Jagoe RT: The use of a standardized
Chinese herbal formula in patients with advanced lung cancer: a
feasibility study. J Integr Med. 16:390–395. 2018.PubMed/NCBI
|
|
5
|
Dy GK and Adjei AA: Novel targets for lung
cancer therapy: Part I. J Clin Oncol. 20:2881–2894. 2002.PubMed/NCBI
|
|
6
|
Singhal S, Vachani A, Antin-Ozerkis D,
Kaiser LR and Albelda SM: Prognostic implications of cell cycle,
apoptosis, and angiogenesis biomarkers in non-small cell lung
cancer: A review. Clin Cancer Res. 11:3974–3986. 2005.PubMed/NCBI
|
|
7
|
Ghosh N, Chaki R, Mandal V and Mandal SC:
COX-2 as a target for cancer chemotherapy. Pharmacol Rep.
62:233–244, 2010.7. PubMed/NCBI
|
|
8
|
Gasparini G, Longo R, Sarmiento R and
Morabito A: Inhibitors of cyclo-oxygenase 2: A new class of
anticancer agents? Lancet Oncol. 4:605–615. 2003.PubMed/NCBI
|
|
9
|
Kalinski P: Regulation of immune responses
by prostaglandin E2. J Immunol. 188:21–28. 2012.PubMed/NCBI
|
|
10
|
Williams CS, Mann M and DuBois RN: The
role of cyclooxygenases in inflammation, cancer, and development.
Oncogene. 18:7908–7916. 1999.PubMed/NCBI
|
|
11
|
Liggett JL, Zhang X, Eling TE and Baek SJ:
Anti-tumor activity of non-steroidal anti-inflammatory drugs:
Cyclooxygenase-independent targets. Cancer Lett. 346:217–224.
2014.PubMed/NCBI
|
|
12
|
Katori M and Majima M: Cyclooxygenase-2:
Its rich diversity of roles and possible application of its
selective inhibitors. Inflamm Res. 49:367–392. 2000.PubMed/NCBI
|
|
13
|
Gungor H, Ilhan N and Eroksuz H: The
effectiveness of cyclooxygenase-2 inhibitors and evaluation of
angiogenesis in the model of experimental colorectal cancer. Biomed
Pharmacother. 102:221–229. 2018.PubMed/NCBI
|
|
14
|
Huang S and Sinicrope FA:
Celecoxib-induced apoptosis is enhanced by ABT-737 and by
inhibition of autophagy in human colorectal cancer cells.
Autophagy. 6:256–269. 2010.PubMed/NCBI
|
|
15
|
Liu M, Li CM, Chen ZF, Ji R, Guo QH, Li Q,
Zhang HL and Zhou YN: Celecoxib regulates apoptosis and autophagy
via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer
cells. Int J Mol Med. 33:1451–1458. 2014.PubMed/NCBI
|
|
16
|
Kim N, Kim CH, Ahn DW, Lee KS, Cho SJ,
Park JH, Lee MK, Kim JS, Jung HC and Song IS: Anti-gastric cancer
effects of celecoxib, a selective COX-2 inhibitor, through
inhibition of Akt signaling. J Gastroenterol Hepatol. 24:480–487.
2009.PubMed/NCBI
|
|
17
|
Dai ZJ, Ma XB, Kang HF, Gao J, Min WL,
Guan HT, Diao Y, Lu WF and Wang XJ: Antitumor activity of the
selective cyclooxygenase-2 inhibitor, celecoxib, on breast cancer
in vitro and in vivo. Cancer Cell Int. 12:532012.PubMed/NCBI
|
|
18
|
Kim B, Kim J and Kim YS: Celecoxib induces
cell death on non-small cell lung cancer cells through endoplasmic
reticulum stress. Anat Cell Biol. 50:293–300. 2017.PubMed/NCBI
|
|
19
|
Schellhorn M, Haustein M, Frank M,
Linnebacher M and Hinz B: Celecoxib increases lung cancer cell
lysis by lymphokine-activated killer cells via upregulation of
ICAM-1. Oncotarget. 6:39342–39356. 2015.PubMed/NCBI
|
|
20
|
Bieniek J, Childress C, Swatski MD and
Yang W: COX-2 inhibitors arrest prostate cancer cell cycle
progression by down-regulation of kinetochore/centromere proteins.
Prostate. 74:999–1011. 2014.PubMed/NCBI
|
|
21
|
Katkoori VR, Manne K, Vital-Reyes VS,
Rodríguez-Burford C, Shanmugam C, Sthanam M, Manne U, Chatla C,
Abdulkadir SA and Grizzle WE: Selective COX-2 inhibitor (celecoxib)
decreases cellular growth in prostate cancer cell lines independent
of p53. Biotech Histochem. 88:38–46. 2013.PubMed/NCBI
|
|
22
|
Grösch S, Maier TJ, Schiffmann S and
Geisslinger G: Cyclooxygenase-2 (COX-2)-independent
anticarcinogenic effects of selective COX-2 inhibitors. J Natl
Cancer Inst. 98:736–747. 2006.PubMed/NCBI
|
|
23
|
Wu T, Leng J, Han C and Demetris AJ: The
cyclooxygenase-2 inhibitor celecoxib blocks phosphorylation of Akt
and induces apoptosis in human cholangiocarcinoma cells. Mol Cancer
Ther. 3:299–307. 2004.PubMed/NCBI
|
|
24
|
Tołoczko-Iwaniuk N, Dziemiańczyk-Pakieła
D, Nowaszewska BK, Celińska-Janowicz K and Miltyk W: Celecoxib in
cancer therapy and prevention - review. Curr Drug Targets.
20:302–315. 2019.PubMed/NCBI
|
|
25
|
Bryceson YT and Ljunggren HG: Tumor cell
recognition by the NK cell activating receptor NKG2D. Eur J
Immunol. 38:2957–2961. 2008.PubMed/NCBI
|
|
26
|
Waldhauer I and Steinle A: NK cells and
cancer immunosurveillance. Oncogene. 27:5932–5943. 2008.PubMed/NCBI
|
|
27
|
Raulet DH: Roles of the NKG2D
immunoreceptor and its ligands. Nat Rev Immunol. 3:781–790.
2003.PubMed/NCBI
|
|
28
|
Dhar P and Wu JD: NKG2D and its ligands in
cancer. Curr Opin Immunol. 51:55–61. 2018.PubMed/NCBI
|
|
29
|
Kim SJ, Ha GH, Bae JH, Kim GR, Son CH,
Park YS, Yang K, Oh SO, Kim SH and Kang CD: COX-2- and endoplasmic
reticulum stress-independent induction of ULBP-1 and enhancement of
sensitivity to NK cell-mediated cytotoxicity by celecoxib in colon
cancer cells. Exp Cell Res. 330:451–459. 2015.PubMed/NCBI
|
|
30
|
Reckamp KL: Molecular Targets Beyond the
Big 3. Thorac Surg Clin. 30:157–164. 2020.PubMed/NCBI
|
|
31
|
Kyriakis JM and Avruch J: Protein kinase
cascades activated by stress and inflammatory cytokines. BioEssays.
18:567–577. 1996.PubMed/NCBI
|
|
32
|
Soriani A, Borrelli C, Ricci B, Molfetta
R, Zingoni A, Fionda C, Carnevale S, Abruzzese MP, Petrucci MT,
Ricciardi MR, et al: p38 MAPK differentially controls NK activating
ligands at transcriptional and post-transcriptional level on
multiple myeloma cells. OncoImmunology. 6:e12645642016.PubMed/NCBI
|
|
33
|
Chen XH, Lu LL, Ke HP, Liu ZC, Wang HF,
Wei W, Qi YF, Wang HS, Cai SH and Du J: The TGF-β-induced
up-regulation of NKG2DLs requires AKT/GSK-3β-mediated stabilization
of SP1. J Cell Mol Med. 21:860–870. 2017.PubMed/NCBI
|