Introduction
Third cranial nerve dysfunction, oculomotor nerve
palsy (ONP), can result from lesions anywhere along the nerve
between the midbrain and the orbit (1). This can include the oculomotor nucleus,
the fascicles in the midbrain tegmentum and the spaces it passes
through, including the subarachnoid space, the cavernous sinus and
the superior orbital fissure (1).
The major causes for ONP include congenital factors, trauma,
migraine, aneurysm, diabetes, microvascular ischemia and
inflammation (1,2), with neoplasms and tumors being less
common (2).
Cranial nerves and their surrounding leptomeninges
and cavernous sinus are often known to be involved in lymphomas
(3). ONP as a presenting feature of
an underlying benign or malignant tumor is rare. However, its
occurrence as the first presenting manifestation of a lymphoma is
particularly unusual, especially when no other lymphoma
manifestations have initially been identified (3). We describe a rare case of a 67-year old
man who presented with an isolated ONP as the primary manifestation
of a diffuse large B cell lymphoma (DLBCL), exhibiting without
palpated masses, enlarged lymph nodes or peripheral blood
abnormalities.
Case report
A 67-year old right-handed Caucasian male with no
significant past medical history developed a sudden, continuous
sharp right-sided retro-orbital pain, 8/10 in intensity. He denied
having photophobia, phonophobia, blurry vision, nausea or vomiting.
Upon an outside hospital admit (in-patient), he was found to have
an elevated serum C-reactive protein levels, and he underwent a
temporal artery (TA) biopsy and was prescribed steroids
(prednisone, 60 mg/day with taper over 1 month). The patient was
discharged with a working diagnosis of temporal arteritis. Once the
TA biopsy came back as negative, the steroids were stopped.
A month later, he returned to the same hospital with
a right eye ptosis. A computed tomography angiography of the head
and neck and magnetic resonance imaging (MRI) of the brain were
unremarkable. Upon transfer to our University of Louisville
hospital for further work-up, the patient revealed that his
previous retro-orbital pain had improved but that now he was now
experiencing double vision. Notably, his family history revealed a
very strong disposition for cancer (father died from lymphoma, two
sisters passed away from breast cancer and a brother died of
testicular cancer).
Upon physical examination he had right upper eyelid
ptosis, diplopia, right dilated pupil (not reactive to light), was
not able to move the right eye medially or upward and he denied
having any periorbital pain. A dilated ophthalmoscopic exam was
unremarkable, including the remainder of the neurological exams,
such as assessing for motor/sensory and other cranial nerves
function, gait and co-ordination examinations, which were normal. A
stroke assessment (including a brain MRI and CT angiogram of the
head and neck images, and blood tests) was unremarkable. Evaluation
for infections [including human immunodeficiency virus (HIV) and
rapid plasma regain test for syphilis] were also negative. The
patient was diagnosed having an ONP, prescribed aspirin (325 mg,
daily) and atorvastatin (80 mg, daily) and discharged from the
stroke service.
Later on, the same day, the patient developed B
symptoms, including chest pain, sweating and shortness of breath
accompanied by double vision and fever, and was readmitted to the
hospital. The electrocardiogram was unremarkable. A coincidental
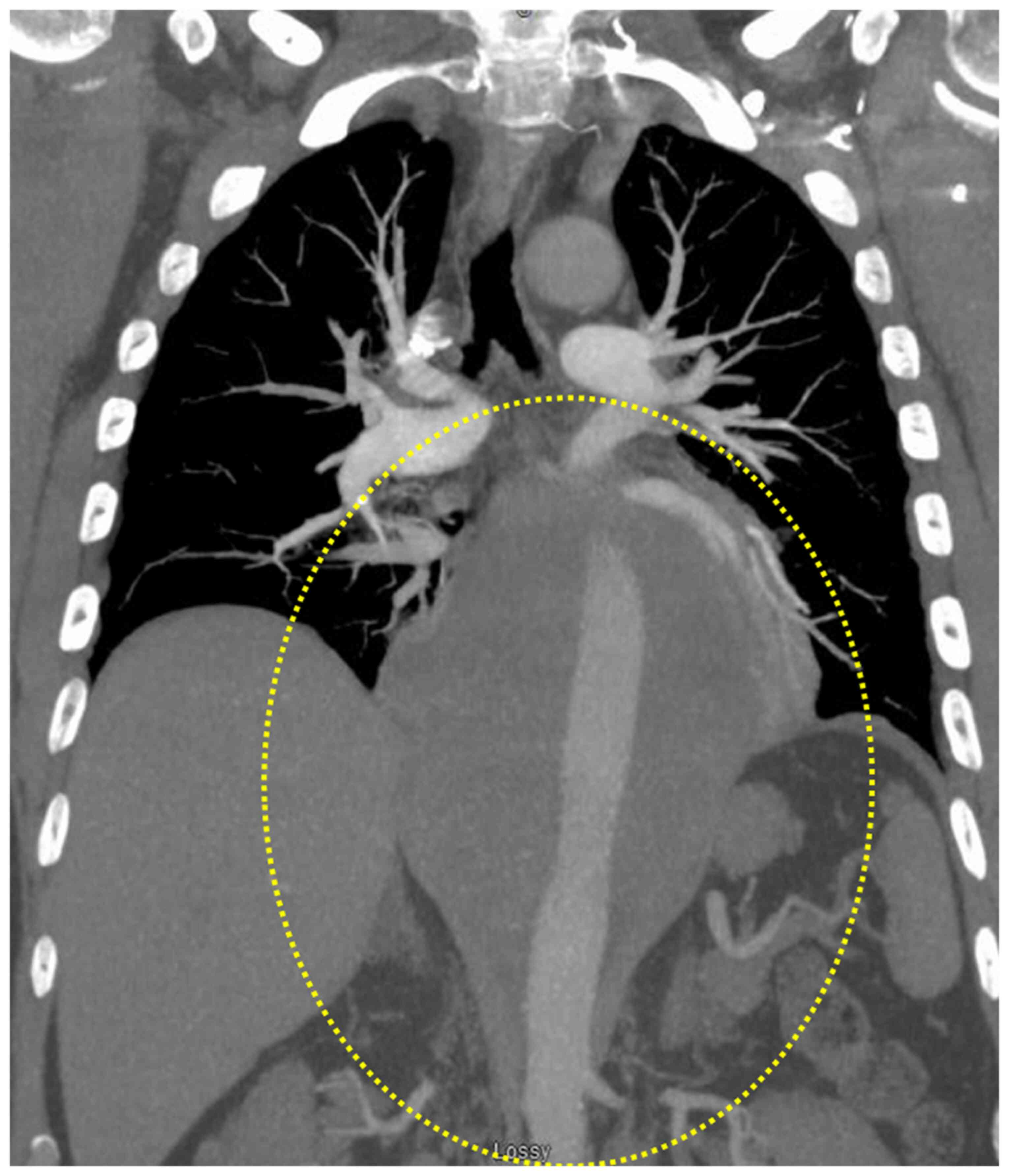
chest CT (pulmonary embolism protocol) showed a large posterior
mediastinal mass extending from the level of the carina into the
upper abdomen behind the crura of the diaphragm. The mass
completely encased the esophagus and aorta, measuring ~16.7 cm in
craniocaudal dimension. It had a maximum size on the axial images
of ~11.8×14.1 cm (Fig. 1). Staging
and prognostic evaluation of the mass revealed a stage IV DLBCL
with a revised international prognostic index (R-IPI) score of
4.
Formalin-fixed (10%, 24 h at room temperature),
paraffin-embedded mediastinal biopsy sections were cut into 4 µm
thickness sections, deparaffinized, and stained with hematoxylin
and eosin (H&E) and immunohistochemistry (IHC) markers as per
manufacturers' instructions. Briefly, after deparaffinization,
sections were washed with ethanol gradient (100, 95, 75 and 50% for
5 min each) then rehydrated and treated with 3%
H2O2 for 5 min at room temperature to block
endogenous peroxidase activity. Slides were steamed in retrieval
solution (citrate buffer, pH 6 or Tris/EDTA buffer, pH 9) for 15
min and then cooled for 15 min, and blocked in 5% FBS (Jackson
ImmunoResearch Laboratories, Inc.) that was diluted in wash buffer
with 1% BSA at room temperature for 15 min. For IHC, the following
antibodies were used: CD20 (1:150; cat. no. ab78237; Abcam), CD10
(1:300; cat. no. ab256494; Abcam), BCL6 (1:500; cat. no. ab172610;
Abcam), MUM1 (1:200; cat. no. ab133590; Abcam), Ki-67 (1:50; cat.
no. MIB-1; Labvision), BCL2 (1:300; cat. no. ab32124; Abcam) and
c-MYC (5 µg/ml; cat. no. ab32072; Abcam) at 4°C overnight. After
being washed three times with PBS, the sections were stained with
goat anti-rabbit secondary antibody (1:500; cat. no. ab150077;
Abcam) for 30 min at 37°C. After applying 3,3′diaminobenzidine
(DAB) for color development at room temperature for 5 min, the
sections were subsequently counterstained with hematoxylin. Each
slide was individually reviewed and scored by two experienced
pathologists using light microscopy.
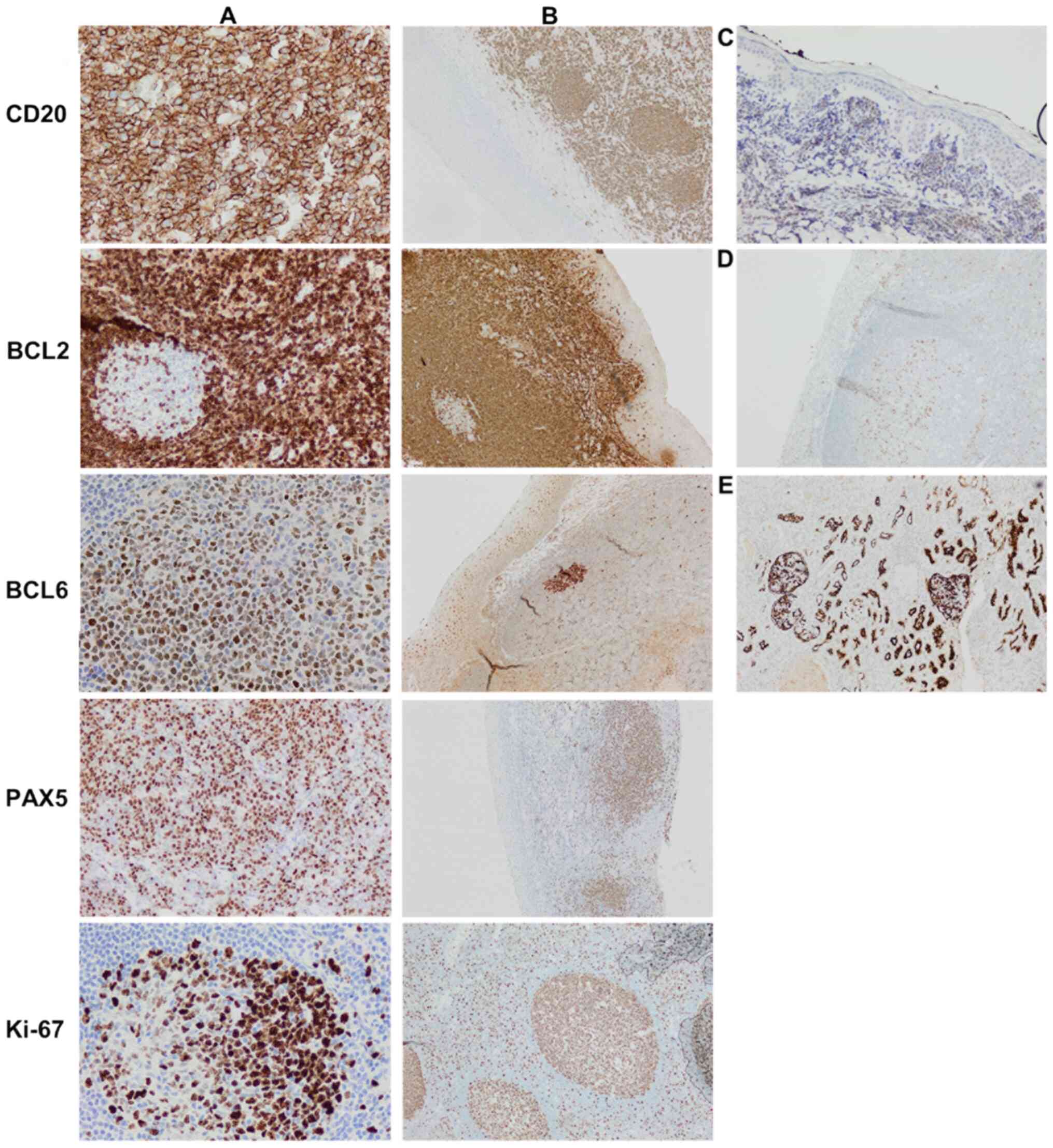
The tumor cells were positive for CD20, B
cell-specific activator protein, CD10 (cell surface marker), B cell
lymphoma (BCL)-6 [>50%, positive cells (from a total of 200)
were counted from three different areas of the slide and averaged
to get the percentage], but had a low expression for multiple
myeloma 1 (MUM1, <5%; Fig. 2).
These results categorized this large B cell lymphoma as a
germinal-center phenotype. The Ki-67 (proliferative marker)
staining showed high nuclear labeling (80%) (Fig. 2I) indicating a highly proliferative
aggressive lymphoma. The tumor cells were diffusely positive for
BCL2 expression (>50%) (Fig. 2F)
but showed very low expression for c-MYC (Fig. 2H), suggesting a possible BCL2
rearrangement that was later confirmed by fluorescent in
situ hybridization (FISH; performed by NeoGenomics
Laboratories). However, no c-MYC or BCL6 rearrangements were
detected. All IHC controls [using normal tonsil tissue having both,
positively stained (lymphoid tissue) and the negatively stained
(squamous mucosa) areas] showed specific staining patterns
(Fig. 3). The concurrent flow
cytometric analysis (performed at the Department of Pathology,
University of Louisville) detected an abnormal CD10 (+) monoclonal
large B cell population.
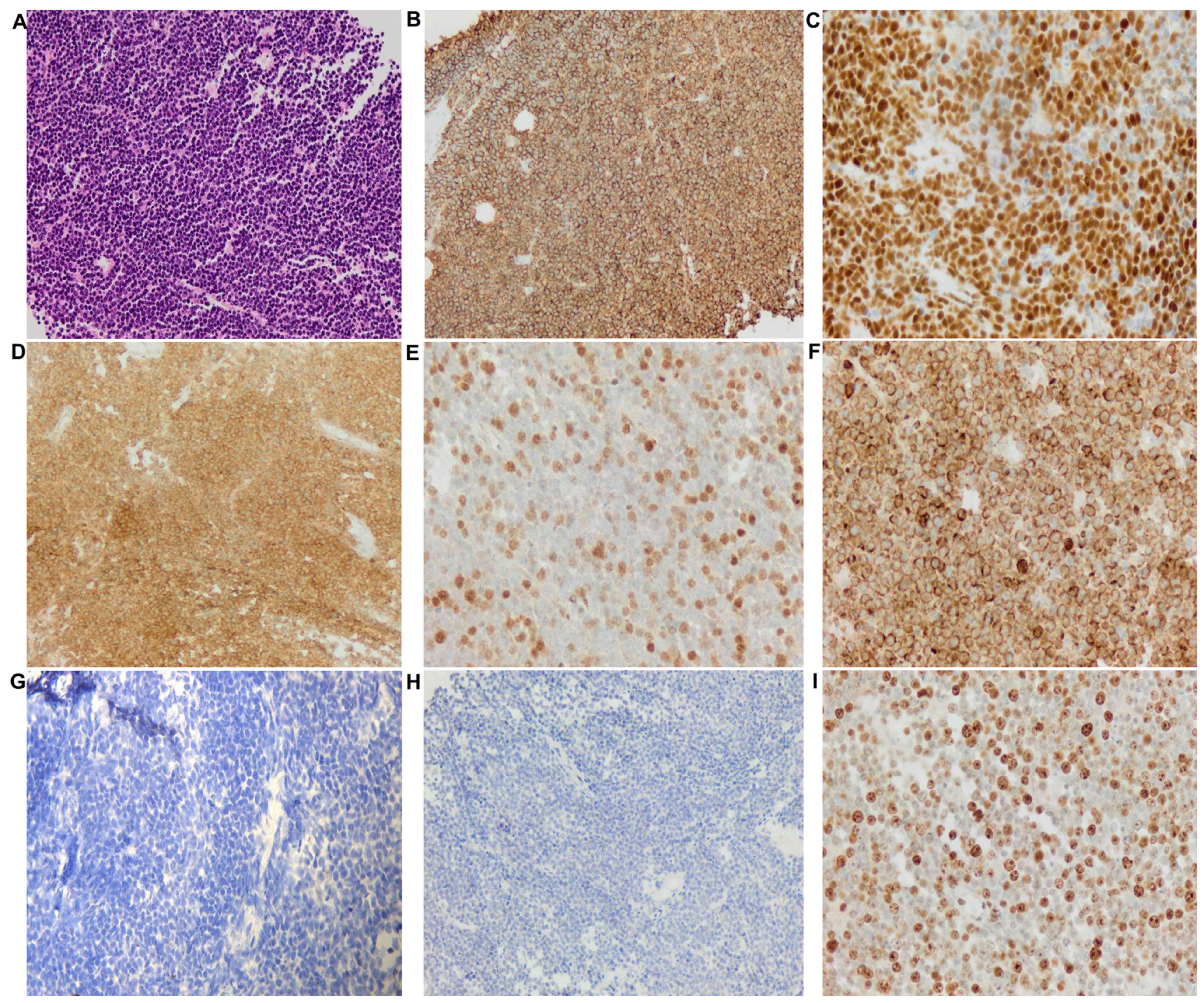 | Figure 2.Histopathological images of the
mediastinal biopsy specimen. (A) Large numbers of lymphocytes,
medium to large, with oval or round nuclei containing fine
chromatin and scanty cytoplasm (hematoxyclin and eosin staining,
×20 magnification). Immunohistochemical staining (compared with
appropriate controls) revealed strong, positive expression for (B)
CD20 (membranous, diffuse), (C) BSAP (nuclear, partial), (D) CD10
(membranous, diffuse), (E) BCL6 (nuclear), and (F) BCL2
(membranous). Tissue sections showed low expression for (G)
multiple myeloma 1 (no nuclear staining) and (H) MYC. (I) High
expression of proliferative marker Ki-67 (80%). |
Apart from slightly elevated total protein levels,
the lumbar puncture was otherwise normal. The cerebrospinal fluid
(CSF) culture was negative for any microorganisms (no growth for
five consecutive days), and the cytology identified no malignant
cells. A brain MRI (with and without contrast) did not show any
leptomeningeal enhancement. An aggressive chemotherapy treatment
was followed wherein the patient received six cycles of etoposide
phosphate, prednisone, oncovin (vincristine sulfate),
cyclophosphamide, hydroxydaunorubicin (doxorubicin hydrochloride)
with rituximab and pegfilgrastim, and two cycles of high-dose
intrathecal methotrexate. Despite this, the ONP did not resolve. A
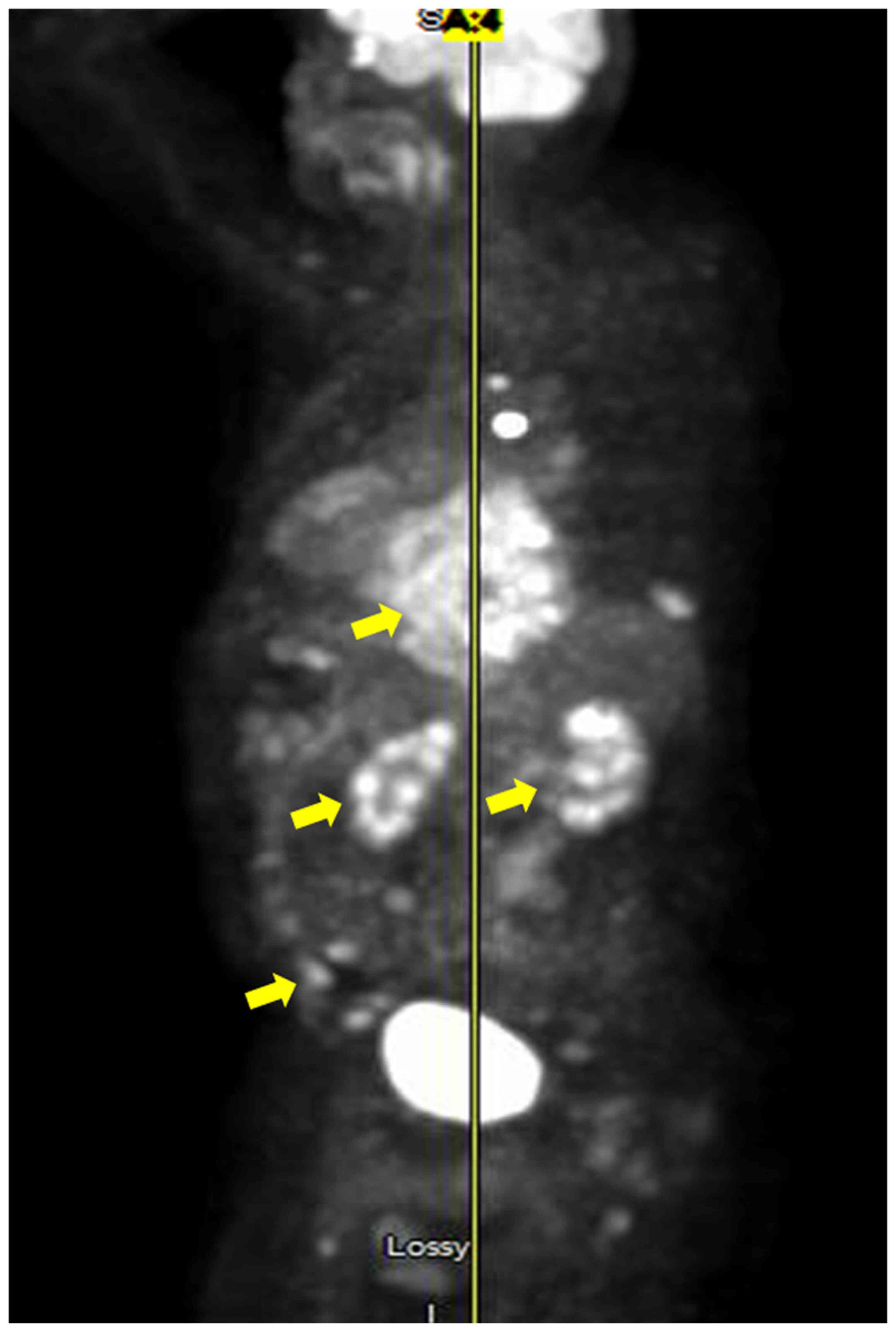
positron emission tomography (PET) scan after chemotherapy
completion showed new areas of lymphoma spread including the ribs
and mediastinum (Fig. 4). The
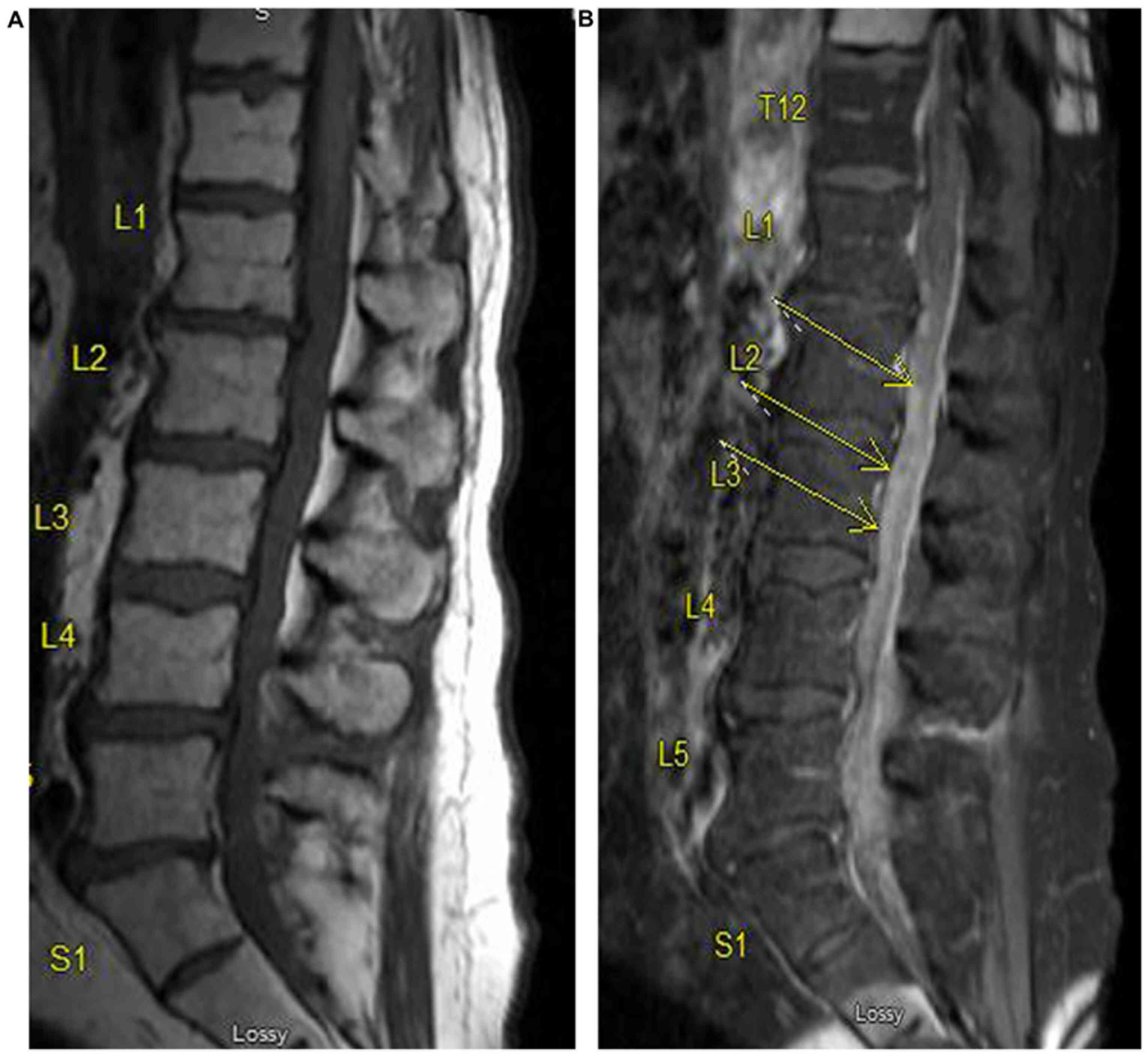
patient developed back pain and an MRI of the lumbar spine showed
diffuse leptomeningeal enhancement, suspicious for the spread of
his lymphoma (Fig. 5). Based on the
poor prognosis, the patient opted for palliative care and later
passed away.
Discussion
An isolated ONP occurring as a neurological
manifestation of an underlying lymphoma is rare with only a few
cases having been reported (1,2). The
present case was peculiar as, apart from an isolated ONP finding,
no other physical examination or laboratory results indicated
lymphoma. Usually, patients with lymphoma display other signs, such
as increased leucocyte count, B symptoms and the development of
mediastinal masses (3). The latter
was only identified from a coincidental CT chest in the present
patient, at a later stage. Retrospectively, it is also interesting
to note that the patient had gradual weight loss which could have
been another manifestation of the underlying lymphoma, but it went
unrecognized. Cases presenting with ONP due to lymphomas and
sarcomas have been previously summarized (4,5). A
summary of new cases reported between 2011 and 2019 are presented
in Table I. Out of the 12 lymphoma
cases identified, seven had DLBCL, three had Burkitt lymphoma, one
had Hodgkin lymphoma and one had non-Hodgkin lymphoma.
 | Table I.Summary of new cases of CN III palsy
(ONP) due to lymphomas between 2011 and 2019. |
Table I.
Summary of new cases of CN III palsy
(ONP) due to lymphomas between 2011 and 2019.
| Author, year | ONP side/other
palsies | Age, years/sex | Associated
lymphoma | Stage/IPI | CT scan | MRI | FDG-PET |
Outcome/Treatment | (Refs.) |
|---|
| Mori et al,
2019 | Left (including other
palsies) | 50/Male | DLBCL (detected
first) | NS | NS | + (lumbar spine)
detected enhanced lesions along the cauda equina his neurological
conditions were | +, abnormal | An MRI first revealed
the mass lesion (later confirmed as DLBCL); patient developed ONP
during three courses of chemotherapy (R-CHOP), improved based on a
PET scan after 3 R-CHOP courses | (9) |
| Liu et al,
2018 | Right pupil-sparing
(including other palsies) | 60s/Male | DLBCL/prior CIDP | NS | NS | +, abnormal | NS | Patient died; autopsy
disclosed primary NL, DLBCL of activated B cell subtype | (16) |
| Kumar et al,
2017 | Left (including other
palsies) | 75/Male | DLBCL | NS | + (chest, abdomen,
pelvis), abnormal | + (normal); MRA
revealed an aneurysm | NS | Patient died;
endoscopic evaluation revealed benign polyps in the stomach,
duodenum, descending colon, and sigmoid colon; the patient
developed resistant hypercalcemia and tumor lysis syndrome | (6) |
| Liang et al,
2017 | Right
pupil-sparing | 29/Male | BL-early CNS
invasion) | NS | +, revealed that
orbital and the optic nerve were normal | +, diffuse abnormal
bone signals | NS | Patient eventually
died after a relapse despite improvements in right eye palsy after
chemotherapy | (13) |
| Taga et al,
2017 | Right
pupil-sparing | 37/Male | Disseminated BL | NS | NS | +, (brain and
orbital) and MRA, both unremarkable | +, disclosed
mediastinal a mass | A high dose steroid
therapy resulted in almost complete recovery, but relapse of ONP
three weeks later. Histopathology confirming ‘starry sky’ BL | (12) |
| Kalantri et
al, 2017 | Left isolated
pupil-sparing | 3/Male | BL | IV; CNS
involvement | + (brain,
contrast-enhanced) showed infiltration | NS | Avoided, as child was
very sick | Chemotherapy started
immediately. Child treated with two cycles of R-CODOX-M/R-IVAC, ONP
resolved completely. Follow-up PET-CT revealed no metabolically
active disease. Patient was in complete remission (9 months
after). | (7) |
| Marttini et
al, 2017 | Right (including
other palsies) | 83/Female | DLBCL/cervical cancer
(30 years earlier) | High-grade | + (brain) showed
chronic inflammation | +, suggestive tumor
mass | +, confirmed of
DLBCL | Nasal drainage,
biopsy and anatomic-pathological analysis confirmed DLBCL. Patient
responded well to chemotherapy (R-CHOP) | (17) |
| Hirose et al,
2016 | Right (including
other palsies) | 62/Male | DLBCL | NS | NS | +, (Post-contrast
T1/T2 weighted) abnormal 6/8 weeks after
admission | NS | Histopathological
examination of the tonsil biopsy reveals DLBCL | (18) |
| Furudoi et al,
2016 | Right (6 months after
the last treatment of the primary malignant lymphoma of the
cheek) | 78/Female | Primary non-Hodgkin's
lymphoma (DLBCL) | Intermediate | + (chest, abdomen)
after first recurrence-unremarkable; after first chemo
cycles-showed no lesions | + (cranial repeats)
after first chemo cycles post recurrence- no lesions; but two
months after-abnormal (bilateral intraorbital tumors) | NS | Patient developed
secondary bilateral orbital involvement after initial treat ment
for primary non-Hodgkin lymphoma (DLBCL) of the cheek. The patient
died about 10 months after recurrent orbital tumor onset | (19) |
| Yan et al,
2014 | Left isolated
pupil-sparing | 72/Female | Gastric DLBCL | stage IVB | +
(contrast-enhanced), abnormal | +,
Gadolinium-enhanced (normal) | +, abnormal |
Panendoscopy/histopathology/bone narrow
biopsy confirmed DLBCL; ONP resolved two weeks after systemic
chemotherapy without any CNS-directed treatment, suggesting a
likely paraneoplastic process | (1) |
| Meireles et
al, 2014 | Left isolated
pupil-involving | 69/Female | Hodgkin's
lymphoma | stage
IVB/prognostic score:3 | + (angiogram): no
aneurysm; (thoracic): normal; (whole-body single photon emission):
abnormal | + (cranial): showed
CN III enhancement, otherwise normal | NS | Bone marrow
biopsy/histopathology confirmed Hodgkin's lymphoma. She was treated
with four chemotherapy cycles; no cranial radiotherapy was needed-a
complete hematological remission obtained; patient main tained mild
partial ONP | (3) |
| Tsai et al,
2013 | Right | 51/Female | DLBCL | NS | NS | + (abnormal); MRA
showed no aneurysm | NS | A surgery and
pathology revealed DLBCL; despite chemo- and radio therapy
treatment, the ONP did not recover | (20) |
A tumor can associate with the cranial nerves
locally, via direct infiltration or by a paraneoplastic process
(6). Cranial nerve involvement
depends on its anatomical course and the site of the tumor
(1). The most common reason
suggested for the occurrence of third nerve palsy is the invasion
of the cavernous sinus and the surrounding leptomeninges with or
without oculomotor nerve infiltration (4,5,7). In the current case, the MRI brain and
orbit (with and without contrast) did not show any pathology. A
possible reason for this could be that the patient received
steroids (for a suspected TA) prior to the brain MRI, which may
have resulted in a false negative image and thus masking signs of
infiltration or a leptomeningeal enhancement. As the patient had
pain in the same eye before starting steroids, it would have been
highly probable that signs of infiltration would have been
identified with a cranial MRI, as this would have justified the
pain and may contributed to an earlier diagnosis of the lymphoma.
Notably, cranial nerve palsies have been reported even in the
presence of normal MRIs (5,6). Intravascular lymphoma, one of the DLBCL
subtypes, has a high frequency of nerve infiltration including the
CNS (4), but unfortunately no biopsy
was performed in the present case to confirm this possibility.
Lymphatic micro-infiltration seemed an unlikely cause, due to a
negative CSF, making paraneoplastic process as another possible
mechanism of how the tumor came to affect the third cranial nerve.
This process may precede the actual lymphocytic infiltration by
weeks to months (1). Paraneoplastic
syndromes are a class of heterogeneous disorders with diverse
presentations caused by an immune response to an underlying
malignancy (6). While rarely
associated with Hodgkin's and non-Hodgkin's lymphoma, these often
develop into lymphoma during the end stages of the disease
(6). However, early detection of
asymptomatic gall bladder cancer in a patient with multiple cranial
palsies during possible paraneoplastic neurological syndrome has
also been reported (8).
In some of the previously patients (Table I), including the present patient, the
pupil was involved (right dilated pupil). Previous reports have
shown a direct infiltration of the oculomotor nerve upon
histological or MRI examination, suggesting that this is a major
pathological mechanism of pupil involvement (4,5). MRI and
PET scans are the imaging modalities of choice for evaluation of
patients with lymphoma and suspected neural involvement (9). In a report reviewing 14 lymphoma cases
with ONP, ten were assessed using brain MRIs and eight of these
patients had CNS involvement (5).
CSF analysis and brain MRIs may not always yield positive results,
and repeat testing may be essential for improving chances of
identification (5,7), which was also true for the present case
as only during a repeat MRI of the lumbar spine was the lymphoma
identified.
DLBCL is the most common of the aggressive
non-Hodgkin's lymphoma in the United States of America (10). Based on the cell of origin, it is
categorized into three different subgroups: Germinal center B
cell-like; activated C cell-like and unclassifiable (11). Accuracy of classification has become
increasingly sophisticated as it appears to have prognostic value
(in terms of overall survival) and bears strong significance in
making treatment decisions (11).
Different immunohistochemical (IHC) algorithms, such as the Hans
algorithm, have been proposed in the last decade to classify DLBCL
subgroups (11). This IHC
classification and molecular studies (Figs. 2 and 3) were used to confirm the diagnosis of
DLBCL in our patient.
New reports have identified an isolated ONP as a
neurological manifestation for Burkitt's lymphoma (7,12,13).
Distinguishing between Burkitt lymphoma and DLBCL was critical, as
the two diseases require different management (14). Almost all forms of Burkitt's lymphoma
are identified with a MYC locus rearrangement (translocation
at 8q24) that results in increased MYC protein levels, promoting
cell proliferation (11). The Ki-67
staining pattern in such cases is usually >95% (15). In the current case, the Ki-67
staining pattern (80%) together with the IHC and FISH data
confirmed the absence of MYC involvement, thus reducing the
likelihood of the tumor being ascribed as a Burkitt's lymphoma. In
addition, the patient tested negative for HIV thus further reducing
the possibility of the ONP presenting as a Burkitt lymphoma
manifestation instead of a DLBCL. A recent report identified only
three cases of ONP as the Burkitt manifestation, occurring in
patients with HIV infection (12).
Burkitt's is rapidly fatal if left untreated but curable with
intensive chemotherapy (doxorubicin, alkylators, vincristine and
etoposide); however, it cannot be cured by the protocol used for
DLBCL (15). Diagnostic accuracy is
therefore essential to prevent mistreatment.
Cranial neuropathies are known to be associated with
lymphomas, but as observed in the present report, occurrence of an
isolated ONP can be considered as a potential differential for an
underlying DLBCL. The current report suggests that ordering a PET
scan or a chest, abdomen and pelvis CT (with contrast) may permit
an early diagnosis of this cancer in patients with unexplained ONP,
particularly if brain vessel imaging does not show a posterior
cerebral artery aneurysm as a cause.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MMK collected the data. SN, HAAD and MMK analyzed
the literature and wrote the manuscript. CMJ, AEP, KSR, RPF and JJS
reviewed and edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The University of Louisville Institutional Review
Board approved the present study (IRB no. 20.0131; approval no.
702074).
Patient consent for publication
The informed consent was obtained from next of kin,
including consent to publish the case study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MRI
|
magnetic resonance imaging
|
|
CSF
|
cerebrospinal fluid
|
|
PET
|
positron emission tomography
|
|
CNS
|
central nervous system
|
|
ONP
|
oculomotor nerve palsy
|
|
DLBCL
|
diffuse large B cell lymphoma
|
|
HIV
|
human immunodeficiency virus
|
|
IHC
|
immunohistochemistry
|
|
FISH
|
fluorescence in situ
hybridization
|
References
|
1
|
Yan SY, Peng YJ, Lin CS, Peng GS and Chang
PY: Isolated oculomotor nerve palsy as a paraneoplastic
manifestation of gastric diffuse large B-cell lymphoma: A case
report. Oncol Lett. 8:1983–1985. 2014.PubMed/NCBI
|
|
2
|
Kim T, Nam K and Kwon BS: Isolated
oculomotor nerve palsy in mild traumatic brain injury. Am J Phys
Med Rehabil. 99:430–435. 2020.PubMed/NCBI
|
|
3
|
Meireles J, Garrett MC and Abreu P:
Isolated III cranial nerve palsy: A Hodgkin's lymphoma? BMJ Case
Rep. 2014:bcr20142039992014.PubMed/NCBI
|
|
4
|
Bhatti MT, Schmalfuss IM and Eskin TA:
Isolated cranial nerve III palsy as the presenting manifestation of
HIV-related large B-cell lymphoma: Clinical, radiological and
postmortem observations: Report of a case and review of the
literature. Surv Ophthalmol. 50:598–606. 2005.PubMed/NCBI
|
|
5
|
Sato H, Hashimoto T, Yoneda S, Hirabayashi
K, Oguchi K and Higuchi K: Lymphoma as a cause of isolated
oculomotor nerve palsy. J Clin Neurosci. 18:1256–1258.
2011.PubMed/NCBI
|
|
6
|
Kumar K, Ahmed R, Bajantri B, Singh A,
Abbas H, Dejesus E, Khan RR, Niazi M and Chilimuri S: Tumors
presenting as multiple cranial nerve palsies. Case Rep Neurol.
9:54–61. 2017.PubMed/NCBI
|
|
7
|
Kalantri SA, Nayak A, Datta S and
Bhattacharyya M: Isolated third nerve palsy: A rare neurological
presentation of Burkitt's lymphoma. BMJ Case Rep.
2017:bcr20172196702017.
|
|
8
|
Kaido M, Yuasa Y, Yamamoto T, Munakata S,
Tagawa N and Tanaka K: A case of possible paraneoplastic
neurological syndrome presenting as multiple cranial nerve palsies
associated with gallbladder cancer. Rinsho Shinkeigaku. 56:617–621.
2016.(In Japanese). PubMed/NCBI
|
|
9
|
Mori Y, Yamamoto K, Ohno A, Fukunaga M and
Nishikawa A: Primary central nervous system lymphoma with
peripheral nerve involvement: Case report. Cureus.
11:e56752019.PubMed/NCBI
|
|
10
|
Friedberg JW and Fisher RI: Diffuse large
B-cell lymphoma. Hematol Oncol Clin North Am. 22941–952.
(ix)2008.PubMed/NCBI
|
|
11
|
Swerdlow SH: Diagnosis of ‘double hit’
diffuse large B-cell lymphoma and B-cell lymphoma, unclassifiable,
with features intermediate between DLBCL and Burkitt lymphoma: When
and how, FISH versus IHC. Hematology Am Soc Hematol Educ Program.
2014:90–99. 2014.PubMed/NCBI
|
|
12
|
Taga A, Russo M, Florindo I and Pavesi G:
Isolated third cranial nerve palsy leading to the diagnosis of
disseminated Burkitt lymphoma: A case report and literature review.
Neurologist. 22:182–185. 2017.PubMed/NCBI
|
|
13
|
Liang Y, Ding L, Li X, Wang W and Zhang X:
Oculomotor nerve palsy as a preceding symptom of adult sporadic
Burkitt lymphoma: A case report and review of the literature. Oncol
Lett. 13:1315–1318. 2017.PubMed/NCBI
|
|
14
|
Bellan C, Stefano L, Giulia de F, Rogena
EA and Lorenzo L: Burkitt lymphoma versus diffuse large B-cell
lymphoma: A practical approach. Hematol Oncol. 28:53–56.
2010.PubMed/NCBI
|
|
15
|
Kalisz K, Alessandrino F, Beck R, Smith D,
Kikano E, Ramaiya NH and Tirumani SH: An update on Burkitt
lymphoma: A review of pathogenesis and multimodality imaging
assessment of disease presentation, treatment response, and
recurrence. Insights Imaging. 10:562019.PubMed/NCBI
|
|
16
|
Liu KC, Hennessey MA, McCall CM and Proia
AD: Ocular involvement in neurolymphomatosis. Am J Ophthalmol Case
Rep. 10:148–151. 2018.PubMed/NCBI
|
|
17
|
Marttini Abarca JDP, Portilla Franco ME
and Pastor Vicente E: A Third nerve palsy that reveals a diffuse
large B cell lymphoma in an octogenarian patient. Rev Esp Geriatr
Gerontol. 52:286–288. 2017.(In Spanish). PubMed/NCBI
|
|
18
|
Hirose T, Nakajima H, Shigekiyo T, Yokote
T, Ishida S and Kimura F: Malignant lymphoma presented as recurrent
multiple cranial nerve palsy after spontaneous regression of
oculomotor nerve palsy: A case report. Rinsho Shinkeigaku.
56:48–50. 2016.(In Japanese). PubMed/NCBI
|
|
19
|
Furudoi S, Yoshii T and Komori T:
Secondary bilateral orbital involvement from primary non-hodgkin
lymphoma of the cheek. Kobe J Med Sci. 62:E55–E57. 2016.PubMed/NCBI
|
|
20
|
Tsai CH, Lee EJ, Liu YS, Chen YC, Shih YH
and Chuang MT: Isolated oculomotor nerve palsy caused by diffuse
large B cell lymphoma. Acta Neurol Belg. 113:103–104.
2013.PubMed/NCBI
|