Introduction
Prostate cancer is the most common cancer in men and
the second leading cause of cancer-associated mortality (1). Almost all cases prostate cancer are
adenocarcinomas and are characterized by slow growth, displaying no
signs or symptoms during the early stages. The 5-year survival rate
of localized prostate cancer is ~100%; however, the survival rate
decreases to 30% for metastatic prostate cancer (2). The major treatment modalities for
prostate cancer include prostatectomy, radiotherapy, hormonotherapy
and chemotherapy (3). The treatment
options depend on cancer stage, patient's age and potential
benefits (for example overall survival). The side effects of the
treatment and the development of drug resistance are the major
obstacles in prostate cancer management (4). Therefore, seeking novel therapeutic
approaches, such as medications derived from natural products, has
become a topic of great interest (5).
Herbs and plant extracts are considered as potential
candidates for drug development and serve as alternative therapies
for cancer treatment with minimal side effects (6). In recent years, plant-based drugs have
attracted great interest due to their anticancer properties, and
have gradually become a research focus (7). Previous studies indicate that the
majority of medicinal and aromatic herbs contain valuable compounds
with unique properties (8–10). Several popular drugs and compounds
have been isolated from medicinal plants and used to treat various
diseases; examples include artemisinin, schisandrin C, paclitaxel,
vincristine and vinblastine (11).
Linalool is a natural terpenoid alcohol substance
that may be found in several herbs, spices and fruits (12). Linalool has been reported to possess
anti-microbial, anti-inflammatory and antioxidant properties
(13). Moreover, linalool exhibits
anticancer potential against prostate cancer, colon cancer,
leukemia and cervical cancer (14,15). The
anticancer activity of linalool may be due to its apoptotic effect,
oxidative stress induction, cell cycle arrest and immunomodulatory
properties (14,15). In DU145 and PC-3 prostate cancer
cells, linalool was able to induce cell cycle arrest and the
extrinsic death receptor-dependent apoptosis pathway (16). Linalool was found to protect HDFa
cells from oxidative stress by inhibiting the phosphorylation of
the ERK1, JNK and p38 proteins of the mitogen-activated protein
kinase family and the activation of nuclear factor-κB/p65 (17). Linalool also induced Th1 cellular
immune response in T-47D cells by stimulating interferon-γ,
interleukin (IL)-13, IL-2, IL-21, IL-21R, IL-4, IL-6sR and tumor
necrosis factor (TNF)-α secretion (18). p53 and cyclin-dependent kinase
inhibitors were found to be upregulated in linalool-treated
leukemia cells (19). In addition,
caspase-3 and caspase-9 were activated in linalool-induced glioma
cell apoptotic death (20).
In the present study, the anti-proliferative effect
and mechanism of action of linalool in prostate cancer 22Rv1 cells
were investigated. The efficacy of the compound was evaluated and
compared in both an in vitro cell line-based model and an
in vivo xenograft tumor model.
Materials and methods
Materials
The 22Rv1 human prostate cancer cell line was
obtained from the Chinese Academy of Sciences Cell Bank (Shanghai,
China). Linalool (97% purity), Cell Counting Kit-8 (CCK-8) and the
Annexin V-FITC apoptosis detection kit were purchased from
Sigma-Aldrich; (cat. no. APOAF); Merck KGaA. The DNA content
quantification assay kit and the rhodamine 123 kit were purchased
from Nanjing KeyGen Biotech Co., Ltd. The FlowCellect Cytochrome
c kit (cat. no. FCCH100110) was purchased from Luminex
Corporation.
Cytotoxicity assay
Human prostate cancer 22Rv1 cells were maintained in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) medium
supplemented with 1 mM sodium pyruvate and 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.). Cells were seeded in clear
flat-bottomed 96-well plates at a density of 5×103
cells/well in 90 µl RPMI-1640 growth medium. After the cells
adhered to the bottom, linalool in PBS with 1% dimethyl sulfoxide
(50, 25, 10, 5, 1 and 0.5 mM) were added and incubated at 37°C with
5% CO2. After 48 h of treatment, 10 µl CCK-8 were added
to each well. Following incubation at 37°C for 4 h, the plates were
read at 450 and 630 nm on a plate reader.
Apoptosis
An Annexin V-FITC Apoptosis Detection kit was used
to study the apoptosis induction of linalool. Samples were prepared
according to the manufacturer's instruction. Briefly, 22Rv1 cells
were seeded in 6-well plates at a density of 5×105
cells/well in 1 ml RPMI-1640 growth medium. After the cells adhered
to the surface (12 h), they were treated with linalool (2.5 mM) for
24 h. The cells were collected, washed twice with PBS, resuspended
in 1X binding buffer (cat. no. B9796, Sigma-Aldrich; Merck KGaA),
and stained with Annexin V-FITC conjugate (cat. no. A9210,
Sigma-Aldrich, ~50 µg/ml in 50 mM Tris-HCl, pH 7.5, containing 100
mM NaCl) and propidium iodine (PI; cat. no. P2667; Sigma-Aldrich;
Merck KGaA; 100 µg/ml in 10 mM potassium phosphate buffer, pH 7.4,
containing 150 mM NaCl) for 10 min at room temperature in the dark.
Subsequently, the samples were quantified by a flow cytometer
(CyFlow Space; Sysmex Partec GmbH) and analyzed by FloMax cell
cycle analysis software, version 2.82 (Quantum Analysis GmbH).
Cell cycle analysis
Cell cycle analysis was performed using a DNA
content quantitation assay kit (Nanjing KeyGen Biotech Co., Ltd.).
The cells were seeded in a 6-well plate at a density of
5×105 cells/well in 1 ml RPMI-1640 growth medium and
treated with linalool (2.5 mM) for 24 h at 37°C. After the
treatment, the cells were collected and fixed with 70% ethanol at
4°C overnight. The cells were washed 3 times with PBS and stained
with PI in the presence of 1% DNase-free RNase A at 37°C for 30 min
prior to flow cytometry analysis. The distribution of cells among
the different cell cycle phases was determined using FloMax cell
cycle analysis software, version 2.82 (Quantum Analysis GmbH).
Mitochondrial transmembrane
potential
The rhodamine 123 kit was used to monitor
mitochondrial function in the cells. After linalool (2.5 mM)
treatment for 24 h at 37°C, the cells were collected and washed.
Subsequently, the cells were resuspended in 1 ml RPMI-1640 medium
with 10 µg/ml rhodamine 123 and incubated at 37°C for 10 min. The
cells were washed with PBS once and analyzed with a flow cytometer
(FloMax 2.82, CyFlow Space; Sysmex Partec GmbH).
Cytochrome c release
The FlowCellect Cytochrome c kit was used to
investigate the effect of linalool on cytochrome c release.
The assay was done according to the manufacturer's instructions.
Briefly, following treatment with linalool (2.5 mM) for 24 h at
37°C, the cells were collected and washed. Subsequently, the cells
were incubated with 100 µl permeabilization working solution on ice
for 10 min, followed by 100 µl fixation working solution at room
temperature for 20 min. Thereafter, the cells were washed with 1X
blocking buffer once. After centrifugation at 13,000 × g (4°C) for
10 min, the cells were incubated with 100 µl 1X blocking buffer for
30 min at room temperature, followed by addition of 10 µl
anti-cytochrome c-FITC and incubation at room temperature for 30
min. After another centrifugation step, blocking buffer (from the
kit) was added for flow cytometry analysis (FloMax 2.82, CyFlow
Space; Sysmex Partec GmbH).
Western blot analysis
The 22Rv1 cells (1×106) were seeded in a
25-cm2 flask and treated with linalool (2.5 mM) for 24 h
at 37°C. After the treatment, the cells were collected and lysed
with RIPA buffer on ice for 30 min. The protein concentration was
determined by a BCA kit (Beyotime Institute of Biotechnology). The
proteins (30 µg per lane) were separated on a 10% SDS-PAGE gel and
electrotransferred onto a PVDF membrane. The membrane was blocked
with 5% bovine serum albumin at room temperature for 1 h, and then
immunoblotted with antibodies against caspase-3, cleaved caspase-3
(cat. nos. 9665 and 9664, respectively; both 1:1,000; Cell
Signaling Technology, Inc.), caspase-8 (cat. no. SC56070; 1:500
dilution; Santa Cruz Biotechnology, Inc.), cleaved caspase-8 (cat.
no. 9748; 1:1,000; Cell Signaling Technology, Inc.), caspase-9,
cleaved caspase-9, DR4, DR5 (cat. nos. ab32539, ab2324, ab8414 and
ab8416, respectively; all 1:1,000; Abcam), p53 (cat. no. AF0879;
1:1,000; Affinity Biosciences, Inc.), Bcl-2 (cat. no. ab59348;
1:1,000; Abcam), Bax, and β-actin (cat. nos. GB11007 and GB12001,
respectively; 1:300 and 1:3,000, respectively; Wuhan Servicebio
Technology, Co., Ltd.). The BeyoECL Plus kit (Beyotime Institute of
Biotechnology) was used for visualization. The bands were analyzed
by AlphaEaseFC 4.0 software (Genetic Technologies, Inc.).
Efficacy study in nude mice
Male BALB/c nude mice (specific pathogen-free; 6
weeks old, 18–22 g) were purchased from HFK Bioscience Co, Ltd. The
animals were fed a standard commercial diet purchased from Beijing
Keao Xieli Feed Co., Ltd. The nude mice were housed in the SPF
Animal Experiment Center (no. of permit: SYXK K2015-0002) that is
specific and pathogen-free with a 12 h light-dark cycle. The room
was maintained at a temperature of 18 to 22°C, relative humidity of
40 to 60%. Food and water are accessible at all times.
A 22Rv1 cell suspension (1×107 cells in
0.1 ml PBS) was inoculated subcutaneously into the rear flanks of
the mice. Once xenograft tumors were palpable (~50 mm3),
the animals were randomly divided into two groups (five mice/group)
and treated with either a solvent control (15% polyethylene glycol
400) or linalool (100 mg/kg body weight) twice a week for 4 weeks
via subcutaneous injection (12).
Tumor growth was measured twice a week with calipers, and the tumor
volume was calculated by the following formula: Length × width ×
height × 0.5236. Animals were euthanized by cervical dislocation
once the xenograft tumor in control groups reached the limit of
tumor size (1,600 mm3).
All animal experimental procedures were in
compliance with the Guide for the Care and Use of Laboratory
Animals at the Kunming Medical University and were approved by the
Kunming Medical University Experimental Animal Ethics Committee
(approval no. KMMU 2015008).
Immunohistochemistry
All mice were euthanized after the last treatment on
the 28th day. Tumor tissues were collected, washed with PBS, fixed
in 10% neutral formalin at room temperature overnight, and embedded
in paraffin. Paraffin blocks were cut into 5-µm sections. Sections
were mounted onto slides and air dried for 30 min. Then the slides
were baked at 45°C in an oven overnight. After antigen retrieval at
100°C with 0.01 M pH 6.0 citric acid buffer for 10 min, the slides
‘Then the slides were incubated with primary antibodies, Ki-67
(1:250; clone H-300; cat. no. sc-15402; Santa Cruz Biotechnology,
Inc.) and proliferating cell nuclear antigen (PCNA; 1:250; clone
PC10; cat. no. sc-56; Santa Cruz Biotechnology, Inc.) for 1 h at
room temperature. Slides were washed and incubated with a secondary
antibody (1:50 dilution; m-IgGκ BP-HRP; cat. no. sc-516102; Santa
Cruz Biotechnology, Inc.) for 30 min at room temperature. Then, the
sections were visualized under a light microscope (400×
magnification) using a DAKO LSAB detection system (K0679; Dako;
Agilent Technologies) and immunosignal quantification was performed
with the following formula: (Positive cell number/total cell
number) ×100%.
Apoptosis in xenograft tumors
To detect apoptotic cell death in xenograft tumors,
the terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) assay was performed using the in situ TUNEL cell
apoptosis detection kit (KGA702, KeyGEN BioTECH). Slides were
prepared according to the manufacturer's instructions. Three fields
of each section were visualized (400× magnification). The
immunosignal indices were calculated as follows: (Apoptotic cell
number/total cell number) ×100.
Statistical analysis
GraphPad Prism 5 software (GraphPad Software, Inc.)
was used for statistical analysis. t-test was used for comparisons
between two means. P≤0.05 was considered to indicate a
statistically significant difference.
Results
In vitro cytotoxicity
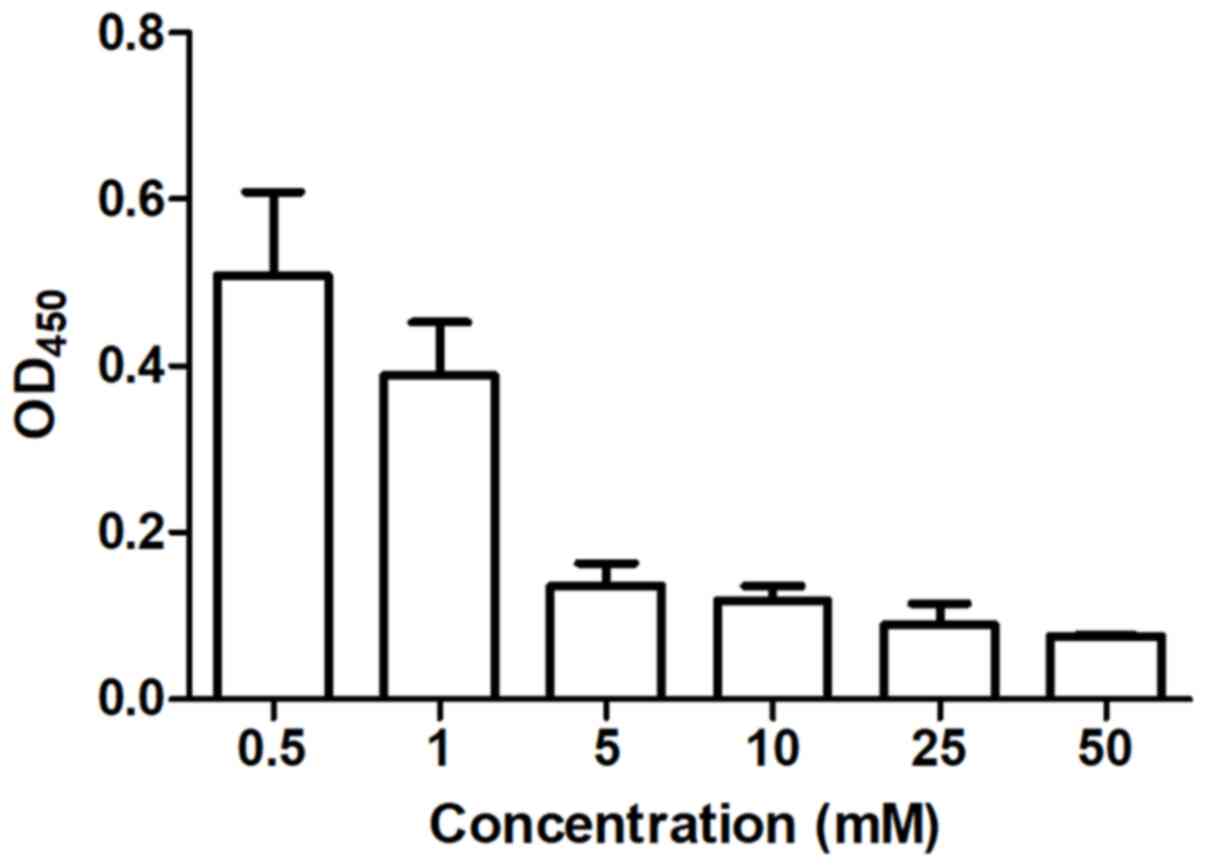
The inhibitory effect of linalool on human prostate
cancer cells was evaluated using a cytotoxicity assay. Cell
viability was inhibited after 48 h of treatment in a dose-dependent
manner (Fig. 1). The half maximal
inhibitory concentration in 22Rv1 cells was 3.384±0.118 mM.
Apoptosis
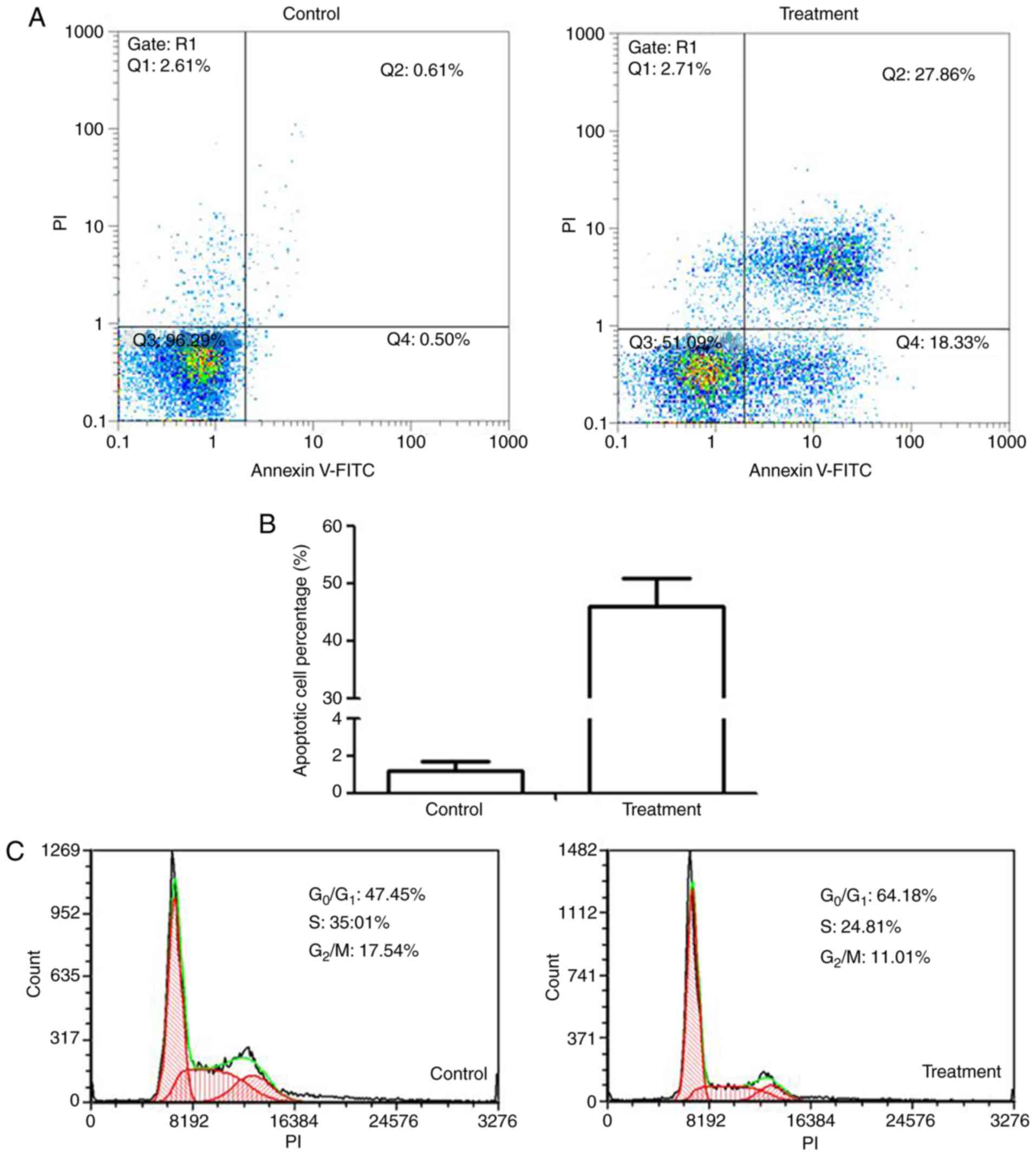
Annexin V/PI staining was used to study the
induction of apoptosis by linalool in 22Rv1 cells. The proportion
of total apoptotic cells (late and early apoptotic cells, Q2 + Q4,
respectively) was significantly higher in the 2.5-mM treatment
group compared with that in the untreated cells (P<0.001;
Fig. 2A).
Cell cycle arrest
The effect of linalool on cell cycle regulation was
investigated by flow cytometry after PI staining. PI binds to the
major groove of double-stranded DNA producing a fluorescent signal
(ex/em, 488/600 nm). Cells in the S phase have more DNA compared
with cells in the G1 phase. Therefore, the cells take up
proportionally more dye and fluoresce more brightly. G2
cells are approximately twice as bright as G1 cells. The
distribution of 22Rv1 cells after a 24-h treatment is shown in
Fig. 2C. It was observed that, after
the treatment, the percentage of the cells in the
G0/G1 phase was increased, whereas the number
of cells in the S and G2/M phases was reduced.
Mitochondrial transmembrane
potential
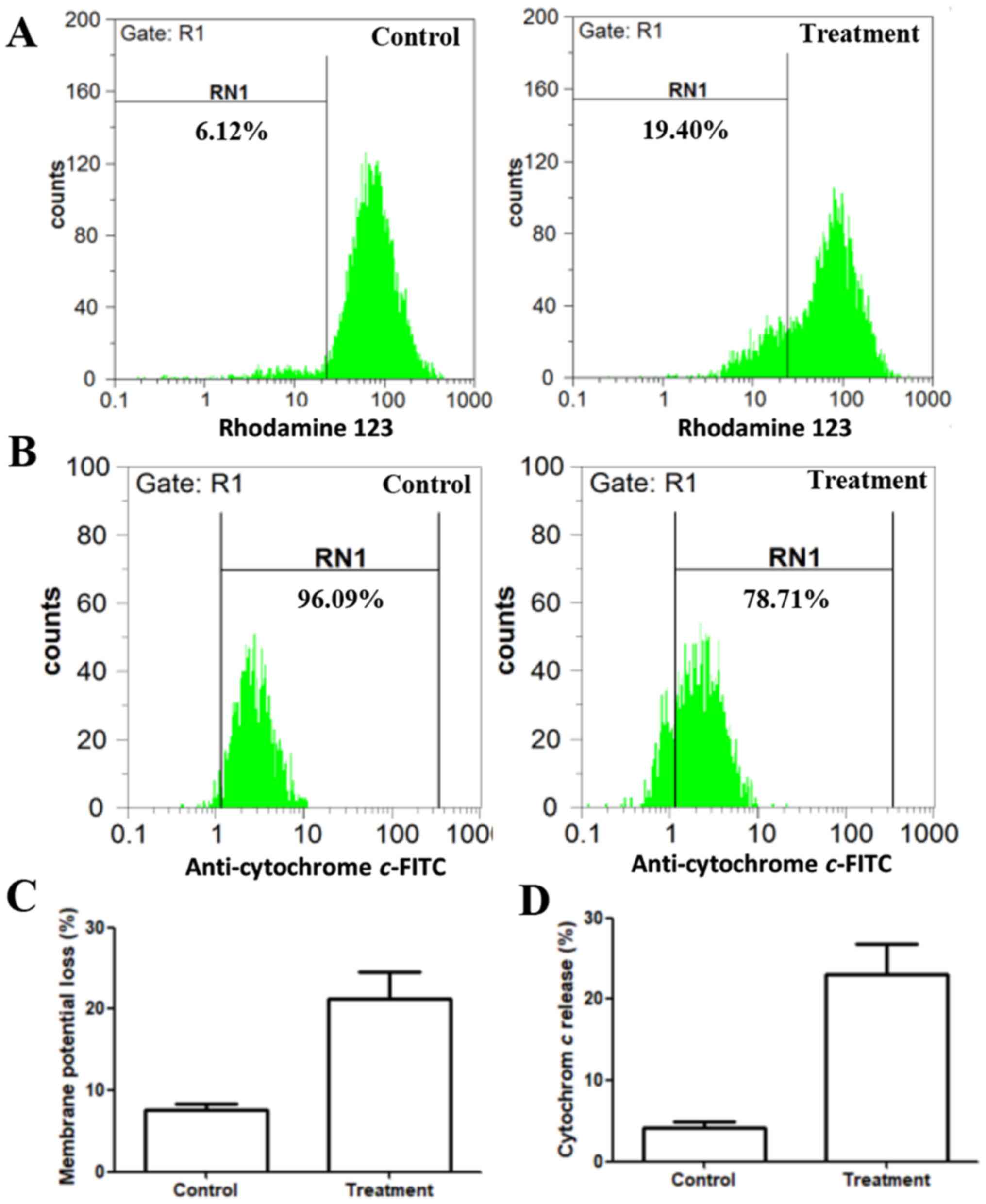
The effect of linalool on the mitochondrial membrane
potential in 22Rv1 cells was analyzed using rhodamine 123.
Depolarized mitochondria display decreased membrane potential,
which is an early characteristic of apoptosis, attributed to the
loss of the electrochemical gradient across the mitochondrial
membrane. Mitochondrial energization induces rhodamine 123
fluorescence quenching. The rate of fluorescence decay is
proportional to the decrease in the mitochondrial membrane
potential (21). In the present
study, the control group retained 93.9% fluorescence. After
treatment with 2.5 mM linalool for 24 h, the fluorescence declined
to 80.6% (Fig. 3A). This result
indicated that linalool induces apoptosis through the disruption of
mitochondria membrane potential.
Cytochrome c release
The release of cytochrome c from the
mitochondria to the cytosol has been considered as a critical early
event leading to caspase-induced apoptosis (22). Further involvement of the intrinsic
pathway of apoptosis was investigated. Live cells demonstrate
higher levels of cytochrome c staining, whereas apoptotic
cells with cytochrome c release to the cytoplasm demonstrate
reduced staining intensity. In the present study, flow cytometry
results revealed that the control group retained 96.09% cytochrome
c in the mitochondria. Linalool-treated 22Rv1 cells had
78.71% cytochrome c in the mitochondria, whereas 21.29% was
released to the cytoplasm (Fig. 3B).
This result indicates that apoptosis induction occurred by means of
the intrinsic pathway.
Western blot analysis for
apoptosis-related proteins
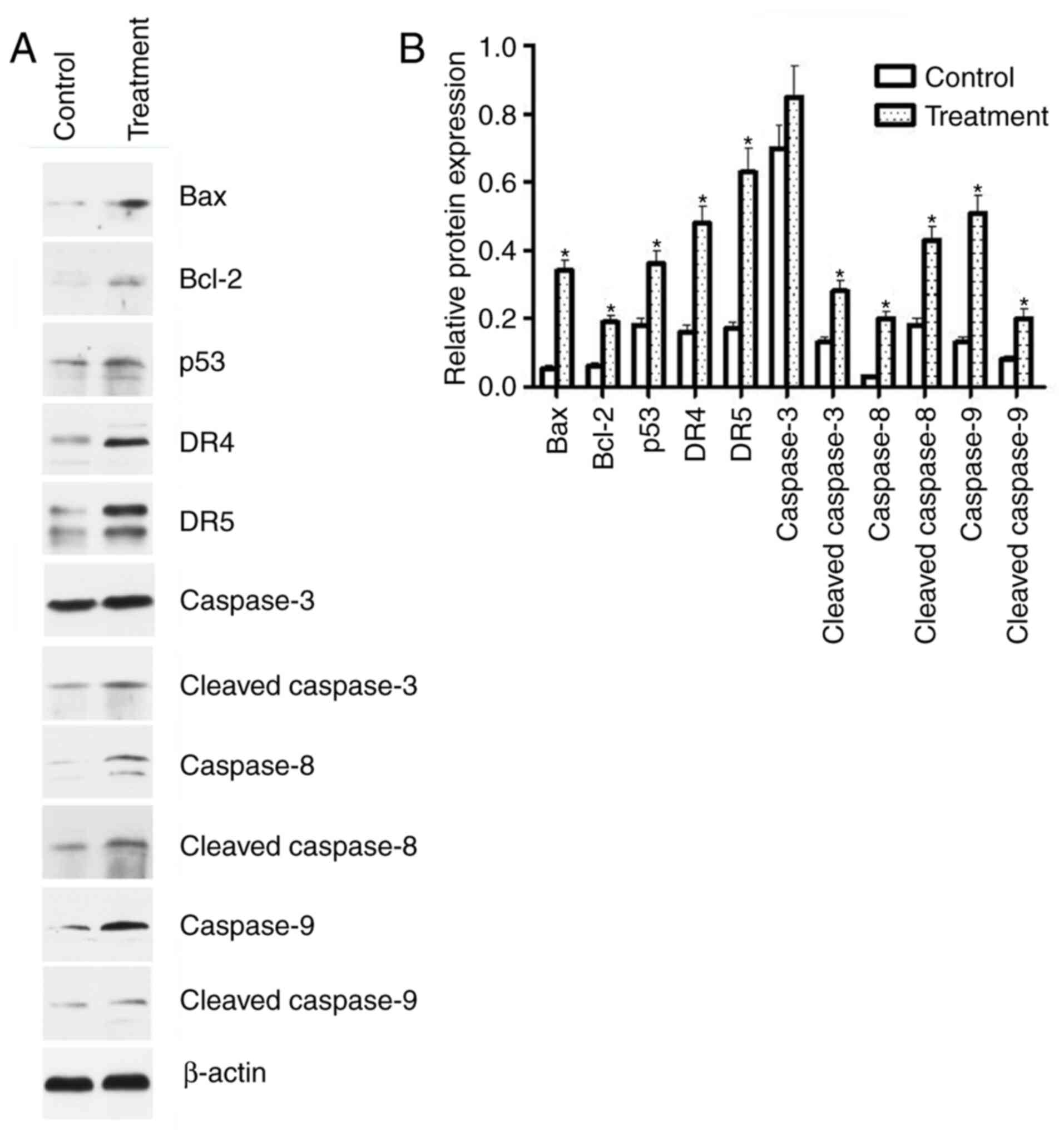
The mechanism underlying apoptosis induction by
linalool was demonstrated by western blot analysis. The expression
of Bax, Bcl-2, p53, DR4, DR5, cleaved caspase-3, cleaved caspase-8,
caspase-8, cleaved caspase-9 and caspase-9, but not caspase-3, were
significantly activated by treatment with 2.5 mM linalool for 24 h
(Fig. 4). These results indicated
that both the intrinsic mitochondria-dependent and extrinsic death
receptor-dependent pathways are involved in apoptosis induction by
linalool.
Animal efficacy study
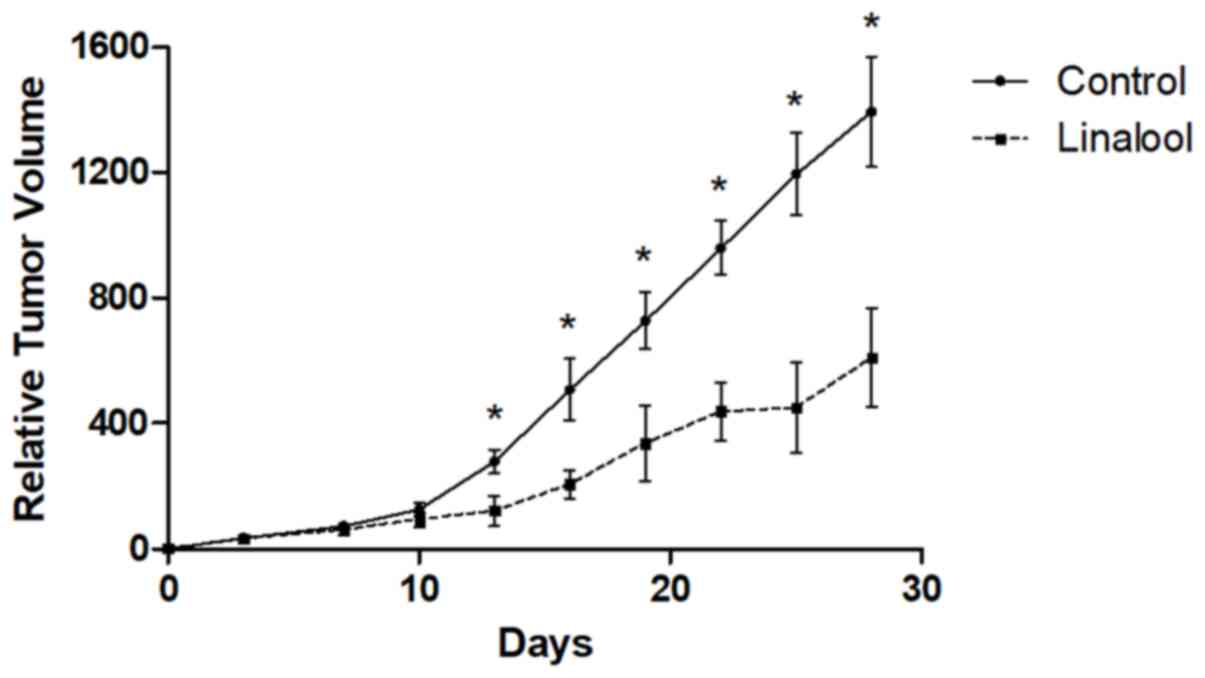
The antitumor effects of linalool were examined in a
mouse xenograft model. The maximum xenograft tumor diameter was
17.83 mm in the control group and 13.48 mm in the treatment group.
As shown in Fig. 5, xenograft tumor
growth was significantly suppressed at the end of treatment in the
linalool group compared with the control group (P<0.001). Tumor
volumes in the control group were 1208.97, 1309.25, 1394.95,
1477.08 and 1562.78 mm3. Tumor volumes in the treatment
group were 431.94, 536.02, 653.48, 696.19 and 749.28
mm3.
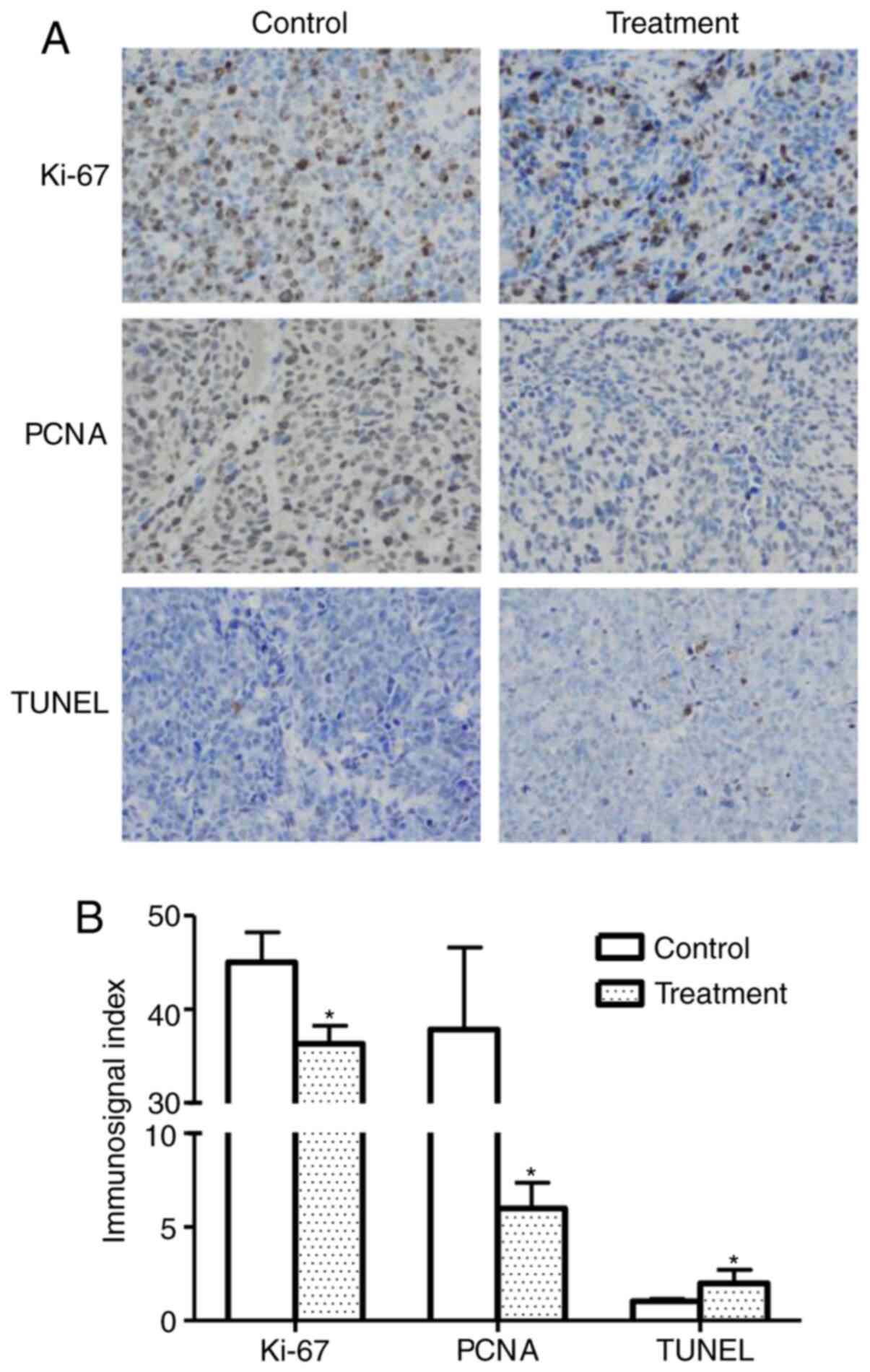
Immunohistochemistry
Tumor cell proliferation indices were assessed by
detecting PCNA and Ki-67 expression in xenograft tumors. As shown
in Fig. 6A, the expression of PCNA
and Ki-67 was markedly lower in the treatment group compared with
that in the control group. These results are consistent with the
tumor growth curves as measured by tumor size, indicating that
linalool suppresses tumor growth in vivo by inhibiting tumor
cell proliferation.
TUNEL assay
A TUNEL assay was performed to determine whether
linalool induces apoptotic cell death in xenograft tumors. Positive
immunosignals were observed in xenograft tumor sections from mice
treated with linalool. Quantitative analysis of the images
(Fig. 6B) indicated that the rate of
apoptosis in the linalool treatment group was significantly higher
compared with that in the control group.
Discussion
Approximately 80% of men who reach the age of 80
years are diagnosed with prostate cancer. To reduce this health
burden, it is important to discover medicinal herb- and
phytocompound-based therapies for prostate cancer treatment.
Therefore, complementary and alternative therapies are increasingly
being used in prostate cancer patients, particularly those that may
not be associated with the same side effects as conventional
therapy (23). However, several of
those therapies have not been thoroughly studied.
Linalool exerts its cytotoxic effects by apoptosis
induction and cell cycle arrest. The present study demonstrated
that linalool was able to induce cell death via the apoptotic
pathway and, thus, may hold promise as a chemotherapeutic agent.
Furthermore, linalool was reported to induce G1 phase
arrest in several types of cancer, such as oral cancer (24), leukemia (15) and hepatocellular carcinoma (25). These findings are consistent with
results of the present study, indicating that linalool blocks the
transition through the G1 checkpoint to inhibit cancer
growth.
p53 is a tumor suppressor protein that is involved
in growth arrest, DNA repair and apoptosis induction. p53 is
mutated in cell lines from various cancer types (26). In the present study, it was
demonstrated that the expression of the p53 protein in the linalool
treatment group was higher compared with that in the control group,
indicating that linalool may induce apoptosis by regulating p53
activity. Since there is a balance between p53 and androgen
receptor expression in prostate cancer progression (27), linalool may also be able to block
androgen signaling and play an important role in androgen-resistant
prostate cancer treatment.
There are two major apoptotic pathways, namely the
mitochondria-mediated intrinsic pathway and the death
receptor-mediated extrinsic pathway. Mitochondria play a pivotal
role in energy generation and cascade events associated with cell
death. The release of cytochrome c, a key mitochondrial
protein, is a hallmark of apoptosis. Mitochondrial membrane
perturbation is a consequence of intrinsic pro-apoptotic signaling.
Upon apoptosis induction and accompanying events, such as
mitochondrial depolarization, cytochrome c along with
pro-apoptotic proteins are released into the cytosol, activating
the intrinsic pathway. The quantification of cytochrome c
release may be used to characterize the mitochondrial-dependent
pathway. The present study, using rhodamine 123 as a fluorescent
probe, demonstrated a slight mitochondrial membrane depolarization
in the linalool treatment group. In addition, following linalool
treatment, 21.29% of cytochrome c was released from the
mitochondria into cytosol, whereas only 3.91% cytochrome c
was released in the control group. Moreover, the intrinsic pathway
is partly regulated by Bcl family members, including the negative
apoptosis regulatory protein Bcl-2, as well as the pro-apoptotic
regulatory protein Bax (28). It was
previously reported that prostate epithelial cells with an
increased Bax/Bcl-2 ratio are more sensitive to apoptotic stimuli
(29). In the present study, the
Bax/Bcl-2 ratio was ~2-fold higher in the linalool treatment group
compared with that in the control group. These results suggest that
the cellular apoptosis induced by linalool treatment is dependent
on alterations in the expression of the Bcl-2 family of proteins
and is associated with the mitochondrial pathway. Furthermore,
stress signals cause the binding of cytoplasmic proteins, such as
Bax and Bid, to the outer membrane of the mitochondria to trigger
the release of the internal content (30). The mitochondrial protein Bak
interacts with Bax and Bid to promote cytochrome c release
to the cytosol. Cytochrome c forms a complex with Apaf-1,
which triggers the activation of caspase-9, thereby initiating
caspase-3 activation, ultimately leading to apoptosis (31). It was also demonstrated that the
expression of cleaved caspase-3 and cleaved caspase-9 are higher in
the linalool treatment group compared with the control group. These
results are consistent with previous experiments, indicating that
linalool may induce 22Rv1 cell apoptosis via the intrinsic
pathway.
The extrinsic apoptosis pathway is mediated by death
receptors, including Fas, TNF, and TNF-related apoptosis-inducing
ligand (TRAIL) receptors (32). The
binding of a ligand to its receptors on the target cell triggers
multiple receptors to aggregate on its surface. This aggregation
recruits the adaptor proteins on the cytoplasmic side of the
receptors to form the death-inducing signaling complex (DISC). DISC
induces the activation of caspase-8 and initiates caspase-3
activation to initiate the degradation of the cell (33). Active caspase-8 also mediates the
cleavage of the Bid protein, which links the intrinsic and
extrinsic apoptotic pathways (34).
In addition, there is accumulating evidence that the upregulation
of DR4 (TRAIL receptors 1) and DR5 (TRAIL receptors-2) are
associated with TRAIL-induced apoptosis (35). The present study demonstrated that
DR4, DR5 and cleaved caspase-8 were upregulated in the linalool
treatment group. These results indicate that linalool may augment
TRAIL-induced apoptosis by increasing the expression of DR4 and DR5
in 22Rv1 cells.
The antiproliferative effect of linalool was studied
using a xenograft model. It was demonstrated that linalool
significantly suppressed tumor growth in 22Rv1-bearing mice.
Furthermore, Ki-67 and PCNA expression in prostatic carcinoma was
found to be correlated with Gleason score (36). The overexpression of these markers
was also associated with increased serum levels of
prostate-specific antigen, lymph node metastases, capsular
penetration, seminal vesicle invasion and surgical resection margin
positivity (37). Ki-67 and PCNA may
be used in early diagnosis of prostate carcinoma and as prognostic
markers of prostate cancer recurrence (38). In the present study, both Ki-67 and
PCNA were significantly lower in the treatment group compared with
the control group, indicating that linalool suppressed tumor cell
proliferation and may improve prostate cancer prognosis. In
addition, the TUNEL assay detected more apoptotic cells in the
linalool treatment group compared with the control group. These
data are consistent with the in vitro Annexin V-FITC and
western blotting experiments of the present study, suggesting that
linalool inhibited 22Rv1 cell growth by inducing apoptosis in both
in vitro and in vivo models. Although further studies
are required to elucidate the detailed anticancer mechanisms
underlying the effects of linalool, these data provide preliminary
evidence supporting the use of linalool in the treatment of human
prostate cancer and other similar conditions.
In conclusion, the present study demonstrated that
linalool can inhibit prostate cancer 22Rv1 cell growth and induce
apoptosis both in vitro and in vivo, and the effects
of linalool are mediated by both the intrinsic and extrinsic
apoptotic pathways. Therefore, linalool may hold promise as an
alternative therapeutic approach to prostate cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the 2017 Yunnan
Applied Basic Research Projects-Basic Research Kunming Medical
University Joint Project Special Funds [grant no. 2017FE468(−131)]
(YZ) and the Hundred-Talent Program of Kunming Medical University
(grant no. 60117190445) (YZ).
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YZ and CQ designed the experiments and interpreted
the data. XC, GW and YL performed the experiments and analyzed the
results. All authors drafted, reviewed, edited and approved the
final manuscript, and agree to be accountable for all aspects of
the study.
Ethics approval and consent to
participate
All animal experimental procedures were in
compliance with the Guide for the Care and Use of Laboratory
Animals of the Kunming Medical University (Kunming, China) and were
approved by the Kunming Medical University Experimental Animal
Ethics Committee (approval no. KMMU 2015008).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society, . Cancer Facts
and Figures 2020. Atlanta: American Cancer Society; 2020
|
|
3
|
Leonel Almeida P and Jorge Pereira B:
Local treatment of metastatic prostate cancer: What is the evidence
so far? Prostate Cancer. 2018:26545722018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Semenas J, Allegrucci C, Boorjian SA,
Mongan NP and Persson JL: Overcoming drug resistance and treating
advanced prostate cancer. Curr Drug Targets. 13:1308–1323. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cragg GM and Newman DJ: Natural products:
A continuing source of novel drug leads. Biochim Biophys Acta.
1830:3670–3695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olaku O and White JD: Herbal therapy use
by cancer patients: A literature review on case reports. Eur J
Cancer. 47:508–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Che CT and Zhang H: Plant natural products
for human health. Int J Mol Sci. 20:8302019. View Article : Google Scholar
|
|
8
|
Cowan MM: Plant products as antimicrobial
agents. Clin Microbiol Rev. 12:564–582. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Panche AN, Diwan AD and Chandra SR:
Flavonoids: An overview. J Nutr Sci. 5:e472016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sofowora A, Ogunbodede E and Onayade A:
The role and place of medicinal plants in the strategies for
disease prevention. Afr J Tradit Complement Altern Med. 10:210–229.
2013.PubMed/NCBI
|
|
11
|
Pan SY, Zhou SF, Gao SH, Yu ZL, Zhang SF,
Tang MK, Sun JN, Ma DL, Han YF, Fong WF and Ko KM: New perspectives
on how to discover drugs from herbal medicines: CAM's outstanding
contribution to modern therapeutics. Evid Based Complement Alternat
Med. 2013:6273752013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peana AT, D'Aquila PS, Panin F, Serra G,
Pippia P and Moretti MD: Anti-inflammatory activity of linalool and
linalyl acetate constituents of essential oils. Phytomedicine.
9:721–726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zengin H and Baysal AH: Antibacterial and
antioxidant activity of essential oil terpenes against pathogenic
and spoilage-forming bacteria and cell structure-activity
relationships evaluated by SEM microscopy. Molecules.
19:17773–17798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iwasaki K, Zheng YW, Murata S, Ito H,
Nakayama K, Kurokawa T, Sano N, Nowatari T, Villareal MO, Nagano
YN, et al: Anticancer effect of linalool via cancer-specific
hydroxyl radical generation in human colon cancer. World J
Gastroenterol. 22:9765–9774. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang MY, Shieh DE, Chen CC, Yeh CS and
Dong HP: Linalool induces cell cycle arrest and apoptosis in
leukemia cells and cervical cancer cells through CDKIs. Int J Mol
Sci. 16:28169–28179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao Y, Chen R, Wang Y, Qing C, Wang W and
Yang Y: In vitro and in vivo efficacy studies of lavender
angustifolia essential oil and its active constituents on the
proliferation of human prostate cancer. Integr Cancer Ther.
16:215–226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gunaseelan S, Balupillai A, Govindasamy K,
Ramasamy K, Muthusamy G, Shanmugam M, Thangaiyan R, Robert BM,
Prasad Nagarajan R, Ponniresan VK and Rathinaraj P: Linalool
prevents oxidative stress activated protein kinases in single
UVB-exposed human skin cells. PLoS One. 12:e01766992017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang MY and Shen YL: Linalool exhibits
cytotoxic effects by activating antitumor immunity. Molecules.
19:6694–6706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu Y, Ting Z, Qiu X, Zhang X, Gan X, Fang
Y, Xu X and Xu R: Linalool preferentially induces robust apoptosis
of a variety of leukemia cells via upregulating p53 and
cyclin-dependent kinase inhibitors. Toxicology. 268:19–24. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng Y, Dai C and Zhang J: SIRT3-SOD2-ROS
pathway is involved in linalool-induced glioma cell apoptotic
death. Acta Biochim Pol. 64:343–350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baracca A, Sgarbi G, Solaini G and Lenaz
G: Rhodamine 123 as a probe of mitochondrial membrane potential:
Evaluation of proton flux through F(0) during ATP synthesis.
Biochim Biophys Acta. 1606:137–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Q, Gong B and Almasan A: Distinct
stages of cytochrome c release from mitochondria: Evidence for a
feedback amplification loop linking caspase activation to
mitochondrial dysfunction in genotoxic stress induced apoptosis.
Cell Death Differ. 7:227–233. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Von Low EC, Perabo FG, Siener R and Muller
SC: Review. Facts and fiction of phytotherapy for prostate cancer:
A critical assessment of preclinical and clinical data. In Vivo.
21:189–204. 2007.PubMed/NCBI
|
|
24
|
Pan W and Zhang G: Linalool monoterpene
exerts potent antitumor effects in OECM 1 human oral cancer cells
by inducing sub-G1 cell cycle arrest, loss of mitochondrial
membrane potential and inhibition of PI3K/AKT biochemical pathway.
J BUON. 24:323–328. 2019.PubMed/NCBI
|
|
25
|
Rodenak-Kladniew B, Castro A, Starkel P,
De Saeger C, Garcia de Bravo M and Crespo R: Linalool induces cell
cycle arrest and apoptosis in HepG2 cells through oxidative stress
generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life
Sci. 199:48–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leroy B, Girard L, Hollestelle A, Minna
JD, Gazdar AF and Soussi T: Analysis of TP53 mutation status in
human cancer cell lines: A reassessment. Hum Mutat. 35:756–765.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cronauer MV, Schulz WA, Burchardt T,
Ackermann R and Burchardt M: Inhibition of p53 function diminishes
androgen receptor-mediated signaling in prostate cancer cell lines.
Oncogene. 23:3541–3549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hardwick JM and Soane L: Multiple
functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol.
5:a0087222013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perlman H, Zhang X, Chen MW, Walsh K and
Buttyan R: An elevated bax/bcl-2 ratio corresponds with the onset
of prostate epithelial cell apoptosis. Cell Death Differ. 6:48–54.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dewson G and Kluck RM: Mechanisms by which
Bak and Bax permeabilise mitochondria during apoptosis. J Cell Sci.
122:2801–2808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bratton SB and Salvesen GS: Regulation of
the Apaf-1-caspase-9 apoptosome. J Cell Sci. 123:3209–3214. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Valley CC, Lewis AK, Mudaliar DJ,
Perlmutter JD, Braun AR, Karim CB, Thomas DD, Brody JR and Sachs
JN: Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)
induces death receptor 5 networks that are highly organized. J Biol
Chem. 287:21265–21278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Young MM, Takahashi Y, Khan O, Park S,
Hori T, Yun J, Sharma AK, Amin S, Hu CD, Zhang J, et al:
Autophagosomal membrane serves as platform for intracellular
death-inducing signaling complex (iDISC)-mediated caspase-8
activation and apoptosis. J Biol Chem. 287:12455–12468. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fossati S, Ghiso J and Rostagno A: TRAIL
death receptors DR4 and DR5 mediate cerebral microvascular
endothelial cell apoptosis induced by oligomeric Alzheimer's Aβ.
Cell Death Dis. 3:e3212012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bantis A, Giannopoulos A, Gonidi M, Liossi
A, Aggelonidou E, Petrakakou E, Athanassiades P and Athanassiadou
P: Expression of p120, Ki-67 and PCNA as proliferation biomarkers
in imprint smears of prostate carcinoma and their prognostic value.
Cytopathology. 15:25–31. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sulik M and Guzinska-Ustymowicz K:
Expression of Ki-67 and PCNA as proliferating markers in prostate
cancer. Rocz Akad Med Bialymst. 47:262–269. 2002.PubMed/NCBI
|
|
38
|
Zhong W, Peng J, He H, Wu D, Han Z, Bi X
and Dai Q: Ki-67 and PCNA expression in prostate cancer and benign
prostatic hyperplasia. Clin Invest Med. 31:E8–E15. 2008. View Article : Google Scholar : PubMed/NCBI
|