Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer in men and ninth in women, making HCC the second
leading cause of cancer-related deaths worldwide (1). According to global epidemiological
statistics, the number of deaths of hepatocellular cancer every
year has reached >700,000 (2). It
has been reported (3) that the
mortality rate of hepatocellular cancer has been increasing in many
parts of the world. However, the mortality rate has decreased in
some Asian countries (3) because of
the progress in hepatocellular cancer treatment.
HCC surgical treatment has become the most commonly
used and effective method for the treatment of HCC, due to its
application and development (4). The
severe pain after operation, if not intervened, leads to stress and
suppression of the immune function, which have serious effects on
the patient prognosis. Thus, effective postoperative analgesia is
needed (5). Patient-controlled
intravenous analgesia (PCIA) is an intravenous drug-using method
for analgesia, which is easy to implement and with a wide range of
drugs to use. The advantages are quick onset and wide application.
At the same time, PCIA's disadvantage lies in the wide range of
drugs, i.e., the analgesic effect of different drugs is quite
different. For example, the effect of dizoxacin is good, but the
dependence is strong; pethidine works quickly, but the analgesic
effect is general (6). Hydromorphone
is a semi-synthetic derivative of morphine, which has good
analgesic effect; however, at the same time, it has some side
effects, such as mental confusion and diarrhea (7). Sufentanil is a powerful opioid that
provides long-term central analgesia and has been successfully used
in postoperative analgesia after laparoscopic cholecystectomy.
However, a study has shown that the analgesic effect of this drug
alone on liver operation and other traumatic operations is limited
(8). It has been reported that 0.10%
of ropivacaine combined with 15 µg/ml of hydromorphone has good
analgesic effect, mild motor block and high safety (9). Considering the short analgesia time of
ropivacaine (10), it has been shown
that dizoxine combined with sufentanil can reduce the inhibitory
effect on NK cells and CD4+ activity, and inhibit the
activity of CD8+ cells (11). In the present study, whether
hydromorphone combined with sufentanil could have a similar
outcome, and the effect of this combination on pain, while
affecting the levels of immune factors, were investigated.
The clinical effect and safety evaluation of
hydromorphone combined with sufentanil in PCIA for patients with
HCC, as well as their effect on serum immune factors, were compared
with those of sufentanil treatment alone, in order to clarify the
analgesic effect of hydromorphone and provide reference for the
clinical treatment of HCC.
Patients and methods
General data
Clinical data from 385 patients with HCC (40–60
years of age), treated in the Hunan Provincial People's Hospital
(Changsha, China) from February 2015 to September 2018, were
retrospectively analyzed. The patients were divided into two groups
according to the method of analgesia used. A total of 205 patients
received the combination of hydromorphone and sufentanil PCIA
(study group), and 180 patients were treated with sufentanil PCIA
(control group). The study was approved by the Medical Ethics
Committee of Hunan Provincial People's Hospital. All patients and
their families were informed by letter or telephone, and signed
informed consents were obtained from the patients and/or
guardians.
Inclusion criteria: All patients were diagnosed with
HCC by pathology and met the diagnostic criteria (12); all patients underwent laparoscopic
hepatectomy; no radiotherapy, chemotherapy or related immunotherapy
was performed before serum specimen acquisition; patients had
complete clinical data.
Exclusion criteria: Patients with abnormal kidney
and cardiopulmonary function; pregnant or breast-feeding patients;
patients with mental illness or abnormal brain judgment.
Analgesia method
In the control group, the analgesic pump was filled
with sufentanil and tropisetron. The details are as follows: 2
µg/kg of sufentanil (SFDA approval no. H20120094; Yichang Renfu
Pharmaceuticals Co., Ltd.) and 5 mg of tropisetron (SFDA approval
no. H20050535; Qilu Pharmaceutical Co., Ltd.). In the study group,
the analgesic pump was filled with hydromorphone, sufentanil and
tropisetron. The details are as follows: 5 mg of hydromorphone
(SFDA approval no. H20120100; Yichang Renfu Pharmaceuticals Co.,
Ltd.) and the dosage of sufentanil and tropisetron was the same as
that of the control group. The drugs in both groups were diluted
into 100 ml with normal saline and the loading dose was 5 ml; the
continuous dose was 2 ml/h and the single dose of PCIA was 2
ml.
Observation indicators
The general data of the two groups were collected
and compared, including sex, age, body mass index (BMI), tumor
location, tumor size, alanine aminotransferase (ALT), operative
time and others.
The visual analogue scale (VAS) and numeric sedation
scale (NSS) scores at 12 and 24 h after operation were recorded in
both groups, as well as the and satisfaction score at 24 h after
operation. VAS system: 0 Point, no pain; 1–2 points, occasionally
mild pain; 3–4 points, often mild pain; 5–9 points, obvious pain;
10 points, intolerable pain. NSS system: 1 Point, not quiet,
restlessness; 2 points, quiet cooperation; 3 points, lethargy,
patient able to follow instructions; 4 points, sleep state, but the
patient can be awakened; 5 points, slow respiratory response; 6
points, deep sleep state, the patient can't be called to wake up.
Satisfaction score system: 1 Point, unsatisfied; 2 points,
basically satisfied; 3 points, satisfied; 4 points, quite
satisfied.
Peripheral venous blood (1.5 ml) was collected into
an Eppendorf (EP) tube and heparin was used for anticoagulation.
Another four EP tubes were used and numbered as 1, 2, 3 and 4. A
total of 150 µl of venous blood were added into each EP tube. Tube
1 was filled with antibodies IgG-FITC/IgG-PE (10 µl each); tube 2
was filled with antibodies CD3-FITC/CD4-PE (10 µl each); tube 3 was
filled with antibodies CD3-FITC/CD8-PE (10 µl each); tube 4 was
filled with antibodies CD3-FITC/CD16+56-PE (10 µl each). All tubes
were placed in the dark for 20 min. Hemolysin (2 ml) was added, and
the tubes were left for 10 min in the dark. Next, after
centrifugation at 1,000 × g for 5 min at 4°C, the precipitate was
rinsed with PBS. After a second centrifugation at 1,000 × g for 5
min at 4°C, the supernatant was discarded. A total of 3 ml of 1%
paraformaldehyde were added before the detection by FACScan flow
cytometer (BD Diagnostics). CD3, CD4, CD8 and CD16+56 detection
kits were purchased from Beckman Coulter, Inc. The analysis
software used was FACScan analysis software embedded in FACScan
flow cytometer.
In addition, the postoperative hospitalization time,
first flatulence time, first defecation time and first ambulation
time were recorded, as well as the occurrence of adverse reactions
after operation, such as nausea, emesis and diarrhea.
Statistical analysis
SPSS 19.0 software (AsiaAnalytics; formerly SPSS
China) was used to statistically analyze the data. Counting data
were expressed as n (%). The comparison of the rates between the
two groups was carried out by χ2 test. Enumeration data
were expressed as the mean ± standard deviation and their
comparison between the two groups was carried out by independent
samples t-test. Repeated measures ANOVA was used for the comparison
of the data at different time-points in the same group, and LSD
test was the post hoc test used. P<0.05 was considered to
indicate a statistically significant difference.
Results
General characteristics of the two
groups of patients
There were 180 patients in the control group,
including 118 males (65.56%) and 62 females (34.46%) with average
age of 51.6±10.8 years. In the study group, there were 205
patients, including 132 males (64.39%) and 73 females (35.61%) with
average age of 52.7±9.5 years. There was no significant difference
in sex, age, BMI, location of tumor, AST, ALT or other general
characteristics between the two groups (P>0.05), as presented in
Table I.
 | Table I.Patient general characteristics. |
Table I.
Patient general characteristics.
| Characteristics | Control group
(n=180) | Study group
(n=205) | χ2/t
value | P-value |
|---|
| Sex [n (%)] |
|
| 0.057 | 0.810 |
| Male | 118 (65.56) | 132 (64.39) |
|
|
|
Female | 62
(34.46) | 73
(35.61) |
|
|
| Age (years) | 51.6±10.8 | 52.7±9.5 | 1.063 | 0.288 |
| BMI
(kg/m2) | 25.1±3.9 | 24.9±4.2 | 0.552 | 0.518 |
| Tumor size (cm) | 6.4±2.5 | 6.6±3.2 | 0.677 | 0.499 |
| Tumor location [n
(%)] |
|
| 0.454 | 0.500 |
| Left | 86 (47.8) | 105 (51.2) |
|
|
|
Right | 94 (52.2) | 100 (48.8) |
|
|
| AST (U/l) | 73.5±40.4 | 70.7±46.4 | 0.989 | 0.323 |
| ALT (U/l) | 69.6±43.6 | 72.5±40.6 | 0.676 | 0.500 |
| TBiL (µmol/l) | 25.4±5.6 | 24.7±7.3 | 1.045 | 0.297 |
| Operative time
(min) | 176.1±46.8 | 181.5±37.5 | 1.273 | 0.204 |
| Hilar blocking time
(min) | 13.6±4.3 | 12.5±6.8 | 1.867 | 0.063 |
| Bleeding (ml) | 343.5±48.2 | 335.7±52.3 | 1.514 | 0.131 |
| Infusion (ml) | 2,320.7±463.5 | 2,230.5±500.8 | 1.826 | 0.069 |
| AFP (ng/ml) | 50.3±16.8 | 52.4±15.6 | 1.271 | 0.204 |
| CEA (ng/ml) | 19.8±7.6 | 20.6±8.9 | 0.942 | 0.347 |
Analysis of pain and sedation
index
Intragroup comparisons: VAS and NSS scores at 24 h
after operation were superior to those at 12 h after operation, and
the differences were statistically significant (P<0.05).
Intergroup comparisons: VAS and NSS scores in the study group were
better than those in the control group at 12 and 24 h after
operation, and the differences were statistically significant
(P<0.05; Table II).
 | Table II.Analysis of pain and sedation indexes
(scores). |
Table II.
Analysis of pain and sedation indexes
(scores).
| Scoring system | Control group
(n=180) | Study group
(n=205) | t value | P-value |
|---|
| VAS |
| 12 h
after operation | 4.5±0.6 | 4.1±1.6 | 3.164 | 0.002 |
| 24 h
after operation |
3.6±0.4a |
2.9±0.5a | 15.029 | <0.001 |
| NSS |
| 12 h
after operation | 2.3±0.5 | 3.2±0.6 | 15.861 | <0.001 |
| 24 h
after operation |
2.8±0.7a |
3.7±0.5a | 14.641 | <0.001 |
Analysis of patient satisfaction
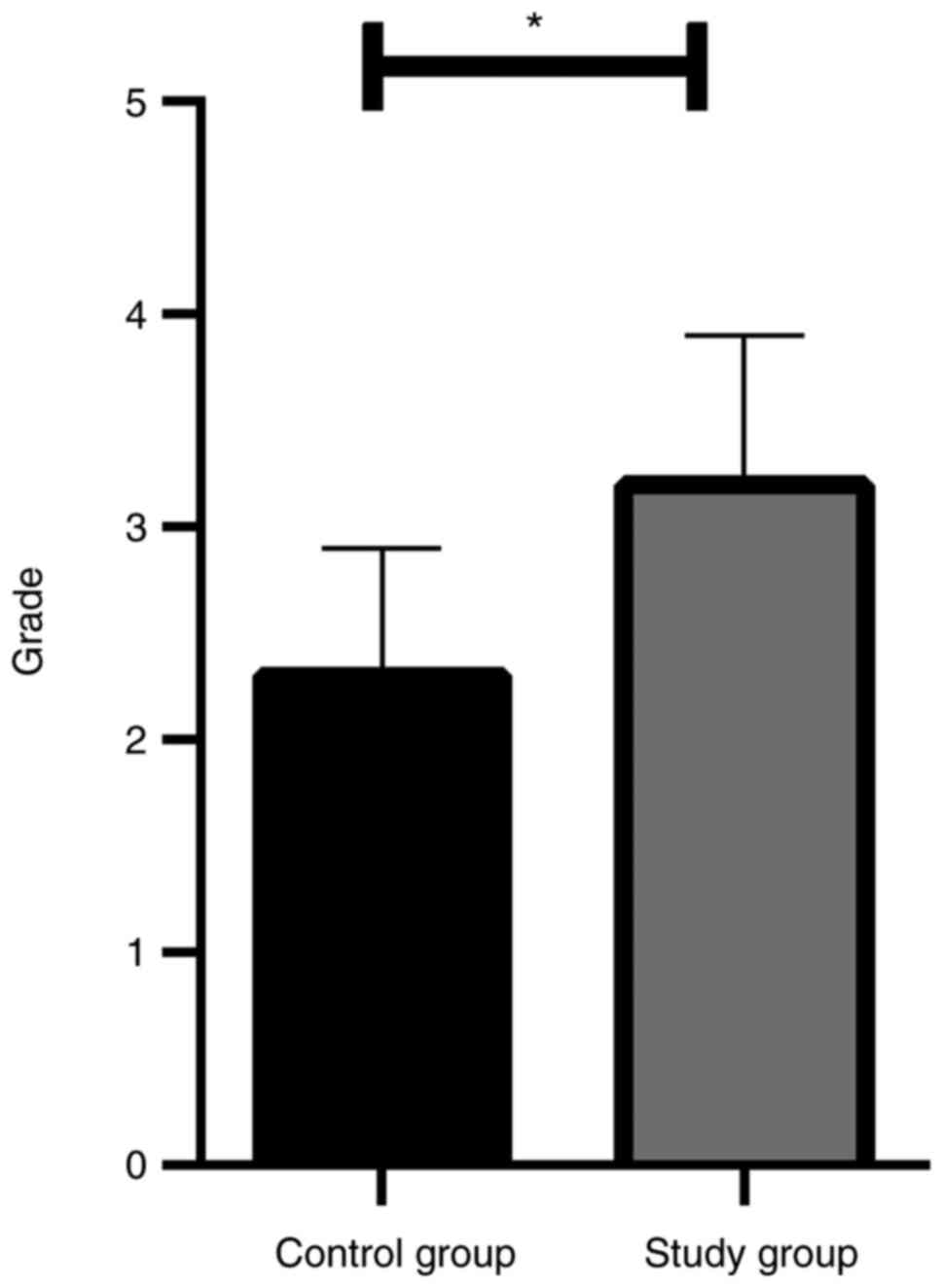
The satisfaction score at 24 h after operation in
the study group was significantly higher than that in the control
group (P<0.05; Fig. 1).
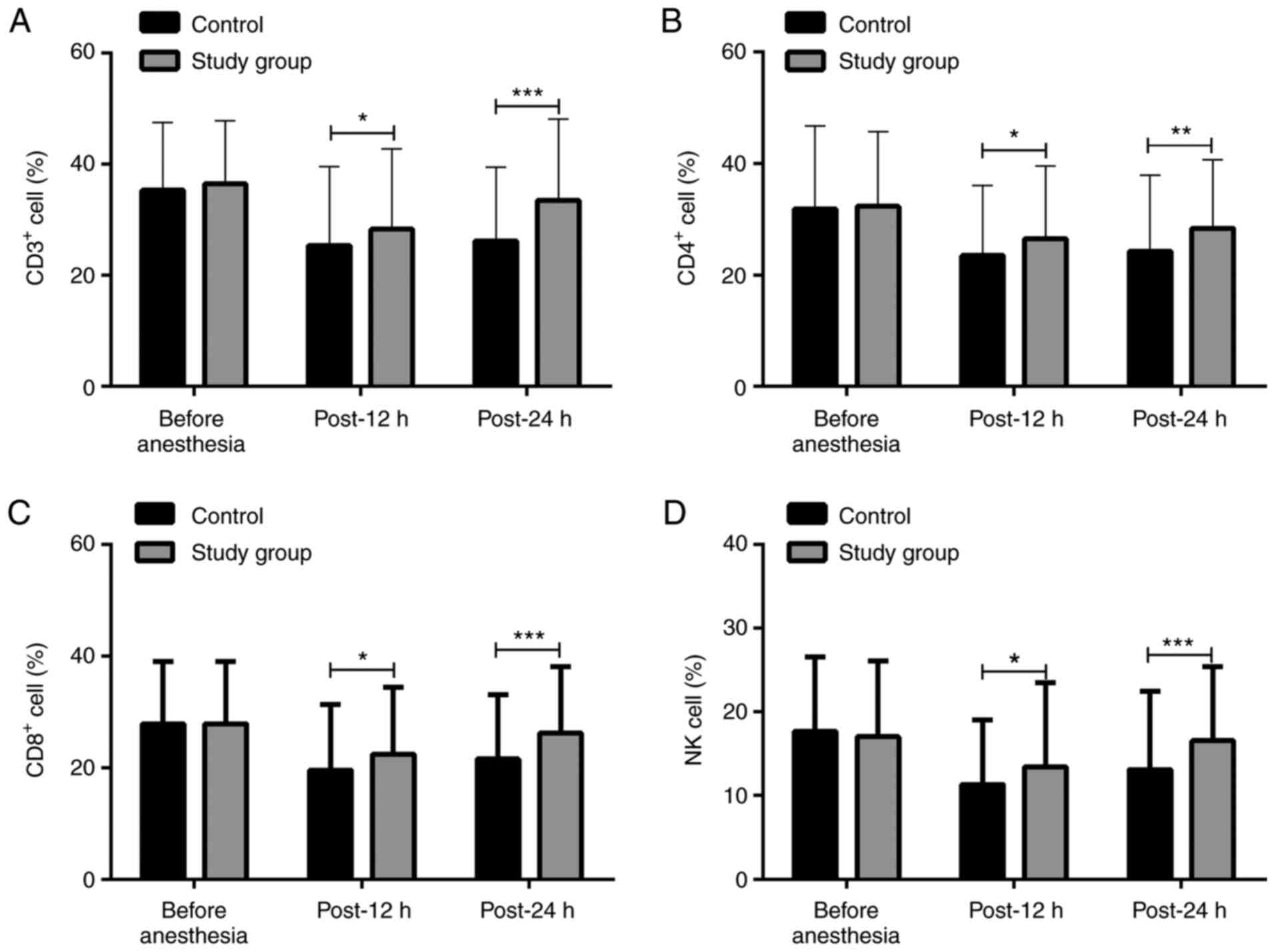
Analysis of immune cell levels
Intragroup comparisons: Compared with the levels
before anesthesia, the levels of CD3+, CD4+,
CD8+ and NK cells in both groups were significantly
decreased at 12 and 24 h after operation (P<0.05). Intergroup
comparisons: There was no significant difference in the activity of
CD3+, CD4+, CD8+ and NK cells
before anesthesia; however, the levels were significantly higher in
the study group compared with those in the control group at 12 and
24 h after operation (Figs. 2 and
3).
Analysis of postoperative
rehabilitation
The postoperative hospitalization time, first
flatulence time, first defecation time and first ambulation time
were shorter in the study group than those in the control group
(P<0.05; Table III).
 | Table III.Analysis of postoperative
rehabilitation. |
Table III.
Analysis of postoperative
rehabilitation.
| Variables | Control group
(n=180) | Study group
(n=205) | t value | P-value |
|---|
| Postoperative
hospitalization time (days) | 12.5±1.8 | 9.7±2.5 | 12.456 | <0.001 |
| First flatulence
time (h) | 55.8±10.6 | 47.6±8.3 | 8.500 | <0.001 |
| First defecation
time (h) | 88.6±11.6 | 82.4±9.3 | 5.815 | <0.001 |
| First ambulation
time (h) | 4.5±0.5 | 3.2±1.1 | 14.586 | <0.001 |
Analysis of adverse reactions
There was no significant difference in nausea,
emesis, diarrhea, dizziness or hear burn between the two groups
after operation (P>0.05). In addition, there was no significant
difference in the total adverse reactions (P>0.05) between the
two groups. There were no other serious adverse reaction in either
group (Table IV).
 | Table IV.Analysis of adverse reactions [n
(%)]. |
Table IV.
Analysis of adverse reactions [n
(%)].
| Adverse
reactions | Control group
(n=180) | Study group
(n=205) | χ2
value | P-value |
|---|
| Nausea | 7 (3.89) | 8 (3.90) |
4.70×10−5 | 0.995 |
| Vomiting | 7 (3.89) | 6 (2.93) | 0.272 | 0.521 |
| Diarrhea | 6 (3.33) | 8 (3.90) | 0.089 | 0.766 |
| Dizziness | 8 (4.44) | 10 (4.88) | 0.040 | 0.841 |
| Heart burn | 9 (5.00) | 12 (5.85) | 0.135 | 0.713 |
| Total adverse
reactions | 37 (20.56) | 44 (21.46) | 0.048 | 0.827 |
Discussion
HCC pathogenesis is not clear yet, which limits the
choices of treatment. Hepatectomy is still the first choice of
treatment. The 5-year survival rate of patients with HCC is between
30 and 40% (13). Postoperative pain
after hepatectomy is one of the serious challenges, thus it is
necessary for postoperative PCIA to control the pain of patients,
relieve their discomfort and enhance the recovery (14). A study has shown that dexmetomide can
effectively maintain the homeostasis of cellular immune function in
patients undergoing radical mastectomy, can effectively improve the
recovery of patients and reduce inflammation (15). Another study has reported that
dexmetomide can effectively reduce the release of inflammatory
factors in patients undergoing radical resection of gastric cancer
and can reduce the decrease of CD3+ and CD4+
cells to improve the impairment of immune function (16). These studies have shown that drug
PCIA has a positive effect on the immune function of the body. In
the present study, the clinical effect and safety assessment of
hydromorphone combined with sufentanil for PCIA in patients with
HCC were retrospectively analyzed, and the effect on the patients'
immune function was verified by examining the difference of the
immune cells in the serum to provide reference for the clinical
treatment of postoperative pain in patients with HCC.
A total of 385 patients with HCC were included in
the study, and the patients were divided into two groups according
to the different methods of drug analgesia that they received. The
analysis of the basic data of the two groups showed that there was
no significant difference between the two groups. VAS and NSS
scores at 12 and 24 h after operation, as well as the patient
satisfaction after 24 h, were significantly different between the
two groups, suggesting that the postoperative analgesia effect of
the combination of hydromorphone and sufentanil was better than
that of the sufentanil analgesia alone. At 12 h after operation,
the levels of immune factors in the study group were higher than
those in the control group, suggesting that the combination of
hydromorphone and sufentanil had a significant effect on improving
the immune level of the body. In addition, there was no significant
difference in postoperative adverse reactions between the two
groups, suggesting that the PCIA assisting role of hydromorphone is
desirable. A previous study has shown that there was no significant
difference in analgesic effect and adverse reactions between
hydromorphone alone and sufentanil alone (17). Another study (18) has shown that the administration of
dexmetiomide combined with sufentanil for postoperative analgesia
in patients with partial laryngectomy can reduce the dosage of
sufentanil and improve the analgesic effect, reduce the cough
frequency of patients and improve the sleep quality. However, the
rate of adverse reactions was still as high as 37.8% (18), and the incidence of adverse reactions
was 10.73% in this study.
The abnormal expressions of various pain mediators
in vivo will lead to acute pain, and the detection of the
level of patient mediators can objectively reflect the subjective
pain degree of patients. The effect threshold or stimulation range
of the combination of multiple drugs on pain mediators is wider
than that of drugs alone, and the effect on nervous system is
better than that of drugs alone (19,20).
Some studies have shown that sufentanil combined with butorphenol
has a stronger analgesic effect than butorphenol alone (21), the combination of dexmedetomidine and
sufentanil in the treatment of PCIA after thoracoscopic lobectomy
has better analgesic effect and more stable blood flow dynamics
than that of sufentanil alone, and can reduce the dose of
sufentanil and the adverse reaction (22). These studies have confirmed that the
effect of combined drugs is better than that of drugs alone. In
addition, it has been reported that the high density of
CD3+ and CD8+ T cells is closely related to
the recurrence rate of patients with breast cancer. The higher the
activity, the lower the recurrence rate, and the higher the
survival rate of the patients (23).
Another study showed that the survival time of patients with CD8CT
density >93 cells/mm2 was significantly longer than
that of patients with CD8CT density <93 cells/mm2
(24). One study demonstrated that
the density of CD3+, CD8+, and T-lymphocytes
can predict the survival rate of advanced colon cancer (25). Another study has shown that the
pro-inflammatory tumor micro-environment and infiltrating T
lymphocytes expressing CD8 are related to the improvement of
clinical outcomes of various tumor types. For example, bone
marrow-derived inhibitory cells and regulatory T cells seem to play
an important role in undermining the immune control of cancer
(26). On this basis, we believe
that PCIA with better results after operation may reduce the
recurrence rate and improve the survival rate, which could be
verified in future studies.
Although this study confirmed the effect of the
combination of hydromorphone and sufentanil for the PCIA after
hepatectomy, there are still some deficiencies. The study did not
investigate the PCIA of secondary hepatocellular cancer. This will
be the aim of our future research. In addition, there are some
limitations due to the retrospective character of the study. Serum
pain mediators would be useful in determining the clinical response
of patients; however, the serum pain medium data were not collected
in this study. Moreover, the lack of a larger sample size may have
produced inevitable deviation to the experimental results. These
shortcomings will be addressed in our future research.
In conclusion, PCIA with hydromorphone combined with
sufentanil can provide safe and effective analgesia, may improve
the patients' immune function and enhance the recovery ability of
the body, providing future reference for the clinical application
of hydromorphone combined with sufentanil PCIA.
Acknowledgements
Not applicable.
Funding
The study was financially supported by the Natural
Science Foundation of Hunan Province, ‘Study of Mechanism of
Targeted Temperature Management Induced Neuroprotection after
Cardiac Arrest Based on NPD1-Iduna pathway and a potential therapy
strategy’ project (grant no. 2020JJ4404).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JLi and YW analyzed and interpreted the patients'
data. YT and JLu were responsible for the flow cytometry. YL and ST
assisted with statistical analysis. JLi wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of Hunan Provincial People's Hospital (Changsha, China).
Patients who participated in this study had complete clinical data.
Signed written informed consents were obtained from the patients
and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ryerson AB, Eheman CR, Altekruse SF, Ward
JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM,
et al: Annual report to the nation on the status of cancer,
1975–2012, featuring the increasing incidence of liver cancer.
Cancer. 122:1312–1337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, He L, Du Y, Zhu P, Huang G, Luo J,
Yan X, Ye B, Li C, Xia P, et al: The long noncoding RNA lncTCF7
promotes self-renewal of human liver cancer stem cells through
activation of Wnt signaling. Cell Stem Cell. 16:413–425. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu XY, Hou PF, Li TT, Quan HY, Li ML, Lin
T, Liu JJ, Bai J and Zheng JN: The roles of Wnt/β-catenin signaling
pathway related lncRNAs in cancer. Int J Biol Sci. 14:2003–2011.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee MW and Lim HK: Management of
sub-centimeter recurrent hepatocellular carcinoma after curative
treatment: Current status and future. World J Gastroenterol.
24:5215–5222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Sun Y, Cai R, Wang G, Shu X and
Pang W: Long noncoding RNA: Multiple players in gene expression.
BMB Rep. 51:280–289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao X, Sun B, Liu T, Shao B, Sun R, Zhu
D, Zhang Y, Gu Q, Dong X, Liu F, et al: Long noncoding RNA n339260
promotes vasculogenic mimicry and cancer stem cell development in
hepatocellular carcinoma. Cancer Sci. 109:3197–3208. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brogan SE, Winter NB and Okifuji A:
Prospective observational study of patient-controlled intrathecal
analgesia: Impact on cancer-associated symptoms, breakthrough pain
control, and patient satisfaction. Reg Anesth Pain Med. 40:369–375.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Wu H, He R, Hu X and Liu S:
Oxycodone versus dezocine for postoperative analgesia in patients
with cervical cancer treated with radical surgery. J Cancer Res
Ther. 12 (Suppl 1):S27–S29. 2016. View Article : Google Scholar
|
|
9
|
Lu YY, Huang H, Mao WL, Liu RH, Hu MJ,
Shao LX, Hu MP and Li J: A concentration-response observation of
hydromorphone combined with ropivacaine in labor analgesia.
Zhonghua Yi Xue Za Zhi. 97:3297–3300. 2017.(In Chinese). PubMed/NCBI
|
|
10
|
Bhatia N, Mehta S, Saini V, Ghai B and
Kaman L: Comparison of intraperitoneal nebulization of ropivacaine
with ropivacaine-fentanyl combination for pain control following
laparoscopic cholecystectomy: A randomized, double-blind,
placebo-controlled trial. J Laparoendosc Adv Surg Tech A.
28:839–844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edwards BK, Noone AM, Mariotto AB, Simard
EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA,
et al: Annual report to the nation on the status of cancer,
1975–2010, featuring prevalence of comorbidity and impact on
survival among persons with lung, colorectal, breast, or prostate
cancer. Cancer. 120:1290–1314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bray F, Ferlay J, Laversanne M, Brewster
DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E,
Swaminathan R, Antoni S, et al: Cancer incidence in five
continents: Inclusion criteria, highlights from volume X and the
global status of cancer registration. Int J Cancer. 137:2060–2071.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du H, Le Y, Sun F, Li K and Xu Y: ILF2
directly binds and stabilizes CREB to stimulate malignant
phenotypes of liver cancer cells. Anal Cell Pathol (Amst).
2019:15750312019.PubMed/NCBI
|
|
14
|
Eheman C, Henley SJ, Ballard-Barbash R,
Jacobs EJ, Schymura MJ, Noone AM, Pan L, Anderson RN, Fulton JE,
Kohler BA, et al: Annual report to the nation on the status of
cancer, 1975–2008, featuring cancers associated with excess weight
and lack of sufficient physical activity. Cancer. 118:2338–2366.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang XH, Bai Q, Lv MM, Fu HG, Dong TL and
Zhou Z: Effect of dexmedetomidine on immune function of patients
undergoing radical mastectomy: A double blind and placebo control
study. Eur Rev Med Pharmacol Sci. 21:1112–1116. 2017.PubMed/NCBI
|
|
16
|
Sayed JA, Abd Elshafy SK, Kamel EZ, Fathy
Riad MA, Mahmoud AA and Khalaf GS: The impact of caudally
administered tramadol on immune response and analgesic efficacy for
pediatric patients: A comparative randomized clinical trial. Korean
J Pain. 31:206–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong W, Chen MH, Yang YH, Zhang X, Huang
MJ, Yang XJ and Wang HZ: The effect of dexmedetomidine on
expressions of inflammatory factors in patients with radical
resection of gastric cancer. Eur Rev Med Pharmacol Sci.
21:3510–3515. 2017.PubMed/NCBI
|
|
18
|
Peng Z, Zhang Y, Guo J, Guo X and Feng Z:
Patient-controlled intravenous analgesia for advanced cancer
patients with pain: A retrospective series study. Pain Res Manag.
2018:73235812018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jemal A, Simard EP, Dorell C, Noone AM,
Markowitz LE, Kohler B, Eheman C, Saraiya M, Bandi P, Saslow D, et
al: Annual report to the Nation on the status of cancer, 1975–2009,
featuring the burden and trends in human
papillomavirus(HPV)-associated cancers and HPV vaccination coverage
levels. J Natl Cancer Inst. 105:175–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim KH: Neurourological application of
neurogenesis and inflammation and pain mechanisms of rocuronium
bromide. Int Neurourol J. 20:274–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurozumi S, Matsumoto H, Kurosumi M, Inoue
K, Fujii T, Horiguchi J, Shirabe K, Oyama T and Kuwano H:
Prognostic significance of tumour-infiltrating lymphocytes for
oestrogen receptor-negative breast cancer without lymph node
metastasis. Oncol Lett. 17:2647–2656. 2019.PubMed/NCBI
|
|
22
|
Galon J, Mlecnik B, Bindea G, Angell HK,
Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et
al: Towards the introduction of the ‘Immunoscore’ in the
classification of malignant tumours. J Pathol. 232:199–209. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gabrielson A, Wu Y, Wang H, Jiang J,
Kallakury B, Gatalica Z, Reddy S, Kleiner D, Fishbein T, Johnson L,
et al: Intratumoral CD3 and CD8 T-cell densities associated with
relapse-free survival in HCC. Cancer Immunol Res. 4:419–430. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun C, Xu J, Song J, Liu C, Wang J, Weng
C, Sun H, Wei H, Xiao W, Sun R and Tian Z: The predictive value of
centre tumour CD8+ T cells in patients with
hepatocellular carcinoma: Comparison with Immunoscore. Oncotarget.
6:35602–35615. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwak Y, Koh J, Kim DW, Kang SB, Kim WH and
Lee HS: Immunoscore encompassing CD3+ and
CD8+ T cell densities in distant metastasis is a robust
prognostic marker for advanced colorectal cancer. Oncotarget.
7:81778–81790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barnes TA and Amir E: HYPE or HOPE: The
prognostic value of infiltrating immune cells in cancer. Br J
Cancer. 117:451–460. 2017. View Article : Google Scholar : PubMed/NCBI
|