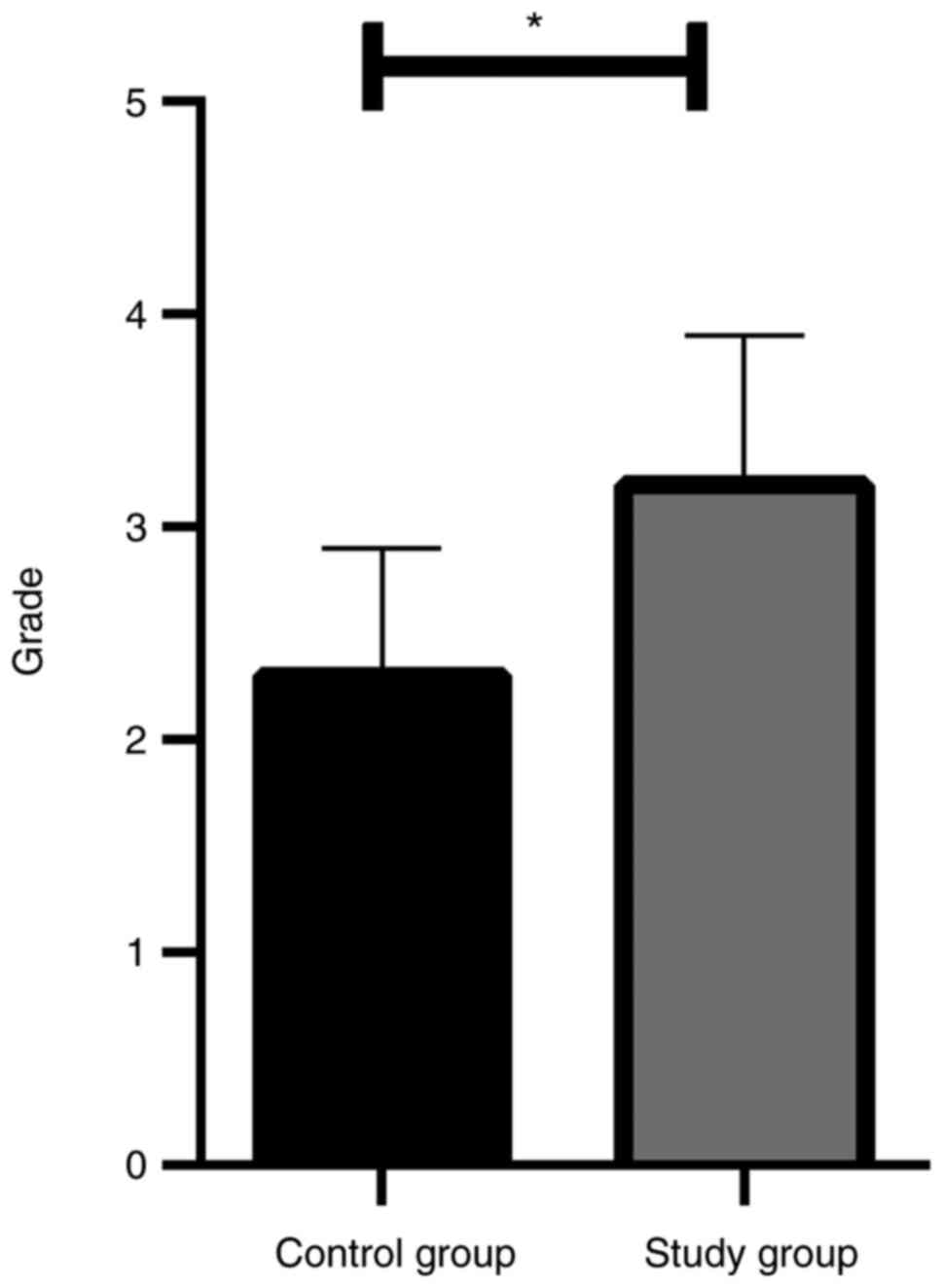
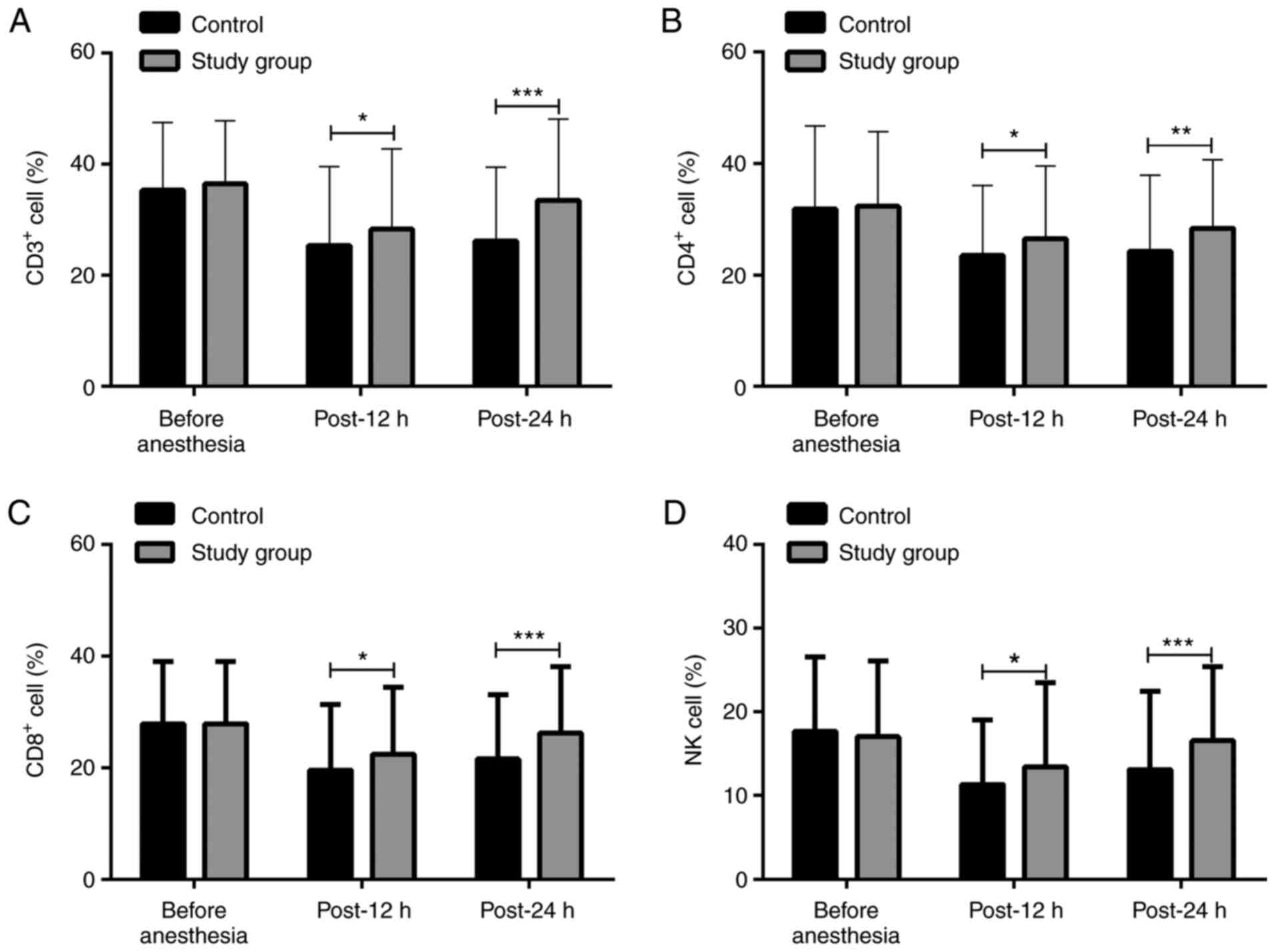
|
1
|
Ryerson AB, Eheman CR, Altekruse SF, Ward
JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM,
et al: Annual report to the nation on the status of cancer,
1975–2012, featuring the increasing incidence of liver cancer.
Cancer. 122:1312–1337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, He L, Du Y, Zhu P, Huang G, Luo J,
Yan X, Ye B, Li C, Xia P, et al: The long noncoding RNA lncTCF7
promotes self-renewal of human liver cancer stem cells through
activation of Wnt signaling. Cell Stem Cell. 16:413–425. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu XY, Hou PF, Li TT, Quan HY, Li ML, Lin
T, Liu JJ, Bai J and Zheng JN: The roles of Wnt/β-catenin signaling
pathway related lncRNAs in cancer. Int J Biol Sci. 14:2003–2011.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee MW and Lim HK: Management of
sub-centimeter recurrent hepatocellular carcinoma after curative
treatment: Current status and future. World J Gastroenterol.
24:5215–5222. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Sun Y, Cai R, Wang G, Shu X and
Pang W: Long noncoding RNA: Multiple players in gene expression.
BMB Rep. 51:280–289. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao X, Sun B, Liu T, Shao B, Sun R, Zhu
D, Zhang Y, Gu Q, Dong X, Liu F, et al: Long noncoding RNA n339260
promotes vasculogenic mimicry and cancer stem cell development in
hepatocellular carcinoma. Cancer Sci. 109:3197–3208. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brogan SE, Winter NB and Okifuji A:
Prospective observational study of patient-controlled intrathecal
analgesia: Impact on cancer-associated symptoms, breakthrough pain
control, and patient satisfaction. Reg Anesth Pain Med. 40:369–375.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Wu H, He R, Hu X and Liu S:
Oxycodone versus dezocine for postoperative analgesia in patients
with cervical cancer treated with radical surgery. J Cancer Res
Ther. 12 (Suppl 1):S27–S29. 2016. View Article : Google Scholar
|
|
9
|
Lu YY, Huang H, Mao WL, Liu RH, Hu MJ,
Shao LX, Hu MP and Li J: A concentration-response observation of
hydromorphone combined with ropivacaine in labor analgesia.
Zhonghua Yi Xue Za Zhi. 97:3297–3300. 2017.(In Chinese). PubMed/NCBI
|
|
10
|
Bhatia N, Mehta S, Saini V, Ghai B and
Kaman L: Comparison of intraperitoneal nebulization of ropivacaine
with ropivacaine-fentanyl combination for pain control following
laparoscopic cholecystectomy: A randomized, double-blind,
placebo-controlled trial. J Laparoendosc Adv Surg Tech A.
28:839–844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edwards BK, Noone AM, Mariotto AB, Simard
EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA,
et al: Annual report to the nation on the status of cancer,
1975–2010, featuring prevalence of comorbidity and impact on
survival among persons with lung, colorectal, breast, or prostate
cancer. Cancer. 120:1290–1314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bray F, Ferlay J, Laversanne M, Brewster
DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E,
Swaminathan R, Antoni S, et al: Cancer incidence in five
continents: Inclusion criteria, highlights from volume X and the
global status of cancer registration. Int J Cancer. 137:2060–2071.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du H, Le Y, Sun F, Li K and Xu Y: ILF2
directly binds and stabilizes CREB to stimulate malignant
phenotypes of liver cancer cells. Anal Cell Pathol (Amst).
2019:15750312019.PubMed/NCBI
|
|
14
|
Eheman C, Henley SJ, Ballard-Barbash R,
Jacobs EJ, Schymura MJ, Noone AM, Pan L, Anderson RN, Fulton JE,
Kohler BA, et al: Annual report to the nation on the status of
cancer, 1975–2008, featuring cancers associated with excess weight
and lack of sufficient physical activity. Cancer. 118:2338–2366.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang XH, Bai Q, Lv MM, Fu HG, Dong TL and
Zhou Z: Effect of dexmedetomidine on immune function of patients
undergoing radical mastectomy: A double blind and placebo control
study. Eur Rev Med Pharmacol Sci. 21:1112–1116. 2017.PubMed/NCBI
|
|
16
|
Sayed JA, Abd Elshafy SK, Kamel EZ, Fathy
Riad MA, Mahmoud AA and Khalaf GS: The impact of caudally
administered tramadol on immune response and analgesic efficacy for
pediatric patients: A comparative randomized clinical trial. Korean
J Pain. 31:206–214. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong W, Chen MH, Yang YH, Zhang X, Huang
MJ, Yang XJ and Wang HZ: The effect of dexmedetomidine on
expressions of inflammatory factors in patients with radical
resection of gastric cancer. Eur Rev Med Pharmacol Sci.
21:3510–3515. 2017.PubMed/NCBI
|
|
18
|
Peng Z, Zhang Y, Guo J, Guo X and Feng Z:
Patient-controlled intravenous analgesia for advanced cancer
patients with pain: A retrospective series study. Pain Res Manag.
2018:73235812018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jemal A, Simard EP, Dorell C, Noone AM,
Markowitz LE, Kohler B, Eheman C, Saraiya M, Bandi P, Saslow D, et
al: Annual report to the Nation on the status of cancer, 1975–2009,
featuring the burden and trends in human
papillomavirus(HPV)-associated cancers and HPV vaccination coverage
levels. J Natl Cancer Inst. 105:175–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim KH: Neurourological application of
neurogenesis and inflammation and pain mechanisms of rocuronium
bromide. Int Neurourol J. 20:274–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurozumi S, Matsumoto H, Kurosumi M, Inoue
K, Fujii T, Horiguchi J, Shirabe K, Oyama T and Kuwano H:
Prognostic significance of tumour-infiltrating lymphocytes for
oestrogen receptor-negative breast cancer without lymph node
metastasis. Oncol Lett. 17:2647–2656. 2019.PubMed/NCBI
|
|
22
|
Galon J, Mlecnik B, Bindea G, Angell HK,
Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et
al: Towards the introduction of the ‘Immunoscore’ in the
classification of malignant tumours. J Pathol. 232:199–209. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gabrielson A, Wu Y, Wang H, Jiang J,
Kallakury B, Gatalica Z, Reddy S, Kleiner D, Fishbein T, Johnson L,
et al: Intratumoral CD3 and CD8 T-cell densities associated with
relapse-free survival in HCC. Cancer Immunol Res. 4:419–430. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun C, Xu J, Song J, Liu C, Wang J, Weng
C, Sun H, Wei H, Xiao W, Sun R and Tian Z: The predictive value of
centre tumour CD8+ T cells in patients with
hepatocellular carcinoma: Comparison with Immunoscore. Oncotarget.
6:35602–35615. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwak Y, Koh J, Kim DW, Kang SB, Kim WH and
Lee HS: Immunoscore encompassing CD3+ and
CD8+ T cell densities in distant metastasis is a robust
prognostic marker for advanced colorectal cancer. Oncotarget.
7:81778–81790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barnes TA and Amir E: HYPE or HOPE: The
prognostic value of infiltrating immune cells in cancer. Br J
Cancer. 117:451–460. 2017. View Article : Google Scholar : PubMed/NCBI
|