Introduction
Different macrophage subtypes have varying surface
molecules and secrete different cytokines. M1 macrophages secrete
pro-inflammatory factors, including TNF-α, IL-23 and IL-1β, which
exert pro-inflammatory and cytotoxic effects in the early stage of
the inflammatory response (1). On
the other hand, M2 macrophages secrete IL-10, which promotes
angiogenesis and is involved in the pathological processes of
tissue repair and wound healing (1).
Macrophages also exert important roles in iron metabolism due to
their versatile roles during innate immunity. For instance, M1
macrophages are often referred to as an ‘iron-sequestering
phenotype’. These M1 macrophages express high levels of ferritin,
divalent metal transporter 1 and CD91, but low levels of
transferrin receptor and ferroportin (FPN) (2). Moreover, M1 macrophages restrict the
growth and proliferation of pathogens by maximizing iron uptake and
storage, lowering iron excretion and decreasing the levels of iron
ions available to pathogens (3,4). M2 have
an iron-release phenotype, and thus can express more FPN and
release intracellular iron ions (3–5). M2
macrophages release iron ions from the labile iron pool, alter the
local microenvironment and increase the availability of iron ions
in adjacent cells, thereby promoting the proliferation of adjacent
cells, increasing collagen deposition and accelerating damage
repair and regeneration (5).
Fe3O4 nanoparticles are
commonly used as contrast agents and drug carriers (6,7).
Zanganeh et al (8) reported
that Fe3O4 nanoparticles could promote the
polarization of tumor-associated macrophages (TAMs) towards the M1
type, and significantly increase the production of reactive oxygen
species (ROS) in macrophages. Super paramagnetic iron-oxide
nanoparticles (SPIONs) are mainly phagocytized by macrophages, and
are degraded into iron ions in lysosomes (9), causing iron overload in macrophages and
ultimately promoting the repolarization of M2 macrophages to M1
macrophages (10).
Poly(lactic-co-glycolic) acid (PLGA) is a type of
polymer synthesized by the polymerization of lactic acid and
glycolic acid in a certain ratio (11). Copolymers with different degradation
periods can be obtained by adjusting the ratio and molecular weight
of the two polymers. The final metabolic products of PLGA in the
body are water and carbon dioxide, and therefore it is safe to use
and is non-toxic (12). PLGA, first
used as a long-acting controlled-release system in the 1970s, has
been certified by the US Food and Drug Administration, and is
officially included in the US Pharmacopoeia as a pharmaceutical
excipient (13,14). Currently, numerous studies have
focused on PLGA as a targeted nano-delivery system for delivering
chemotherapeutic cancer drugs to the target tissues (15). PLGA offers a number of advantages,
including decreased systemic toxicity, increased blood circulation
times and enhanced accumulation at the tumor site for the delivered
drug (16–19). Therapeutic nanoparticles can be
rapidly removed from the internal circulation by phagocytic immune
cells, mainly by the circulating monocytes and macrophages
(20). Previous studies have
revealed that the majority of the nanoparticles concentrate in the
liver and spleen, and only a small portion of the nanoparticles are
deposited in tumor tissues via the blood circulation (21,22).
Targeted ligands, such as antibodies and aptamers,
are often bound to the outer surface of nanoparticles during the
process of designing ‘active’ drug delivery systems, which helps to
deliver payloads specifically to the sites carrying homologous
receptors for targeted ligands (23). Ligands help to internalize
conjugates, and the payload carried by conjugates can be
transferred in cells (23–25).
The present study aimed to enhance antitumor
immunity by targeting the iron concentration in TAMs using
Fe3O4-based PLGA nanoparticles, which were
conjugated with anti-CD206 monoclonal antibody.
Materials and methods
Materials
Acid-terminated PLGA copolymer (50:50 ratio of
lactic acid to glycolic acid; molecular weight, 12 kDa) was
purchased from Sigma-Aldrich; Merck KGaA.
Fe3O4 nanocrystals (diameter, 10 nm) coated
with oleic acid and dispersed in chloroform (20 mg/ml) were
provided by Xi'an Ruixi Biological Technology Co., Ltd.
2-Morpholinoethanesulfonic acid (MES),
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC)
and N-hydroxysuccinimide (NHS) were purchased from
Sigma-Aldrich; Merck KGaA. Ferric citrate was also obtained from
Sigma-Aldrich; Merck KGaA. Invitrogen® FBS was purchased
from Thermo Fisher Scientific, Inc., while HyClone® DMEM
was obtained from Cytiva. Penicillin and Fungizone®
antimycotic and DMSO were provided by Thermo Fisher Scientific,
Inc.
GAPDH (cat. no. ab9485), anti-CD206 (cat. no.
ab64693), F4/80 (cat. no. ab100790) and CD86 (cat. no. ab119857)
monoclonal antibodies were obtained from Abcam. IL-1β (cat. no.
GTX74034), IL-10 (cat. no. GTX130513), TGF-β (cat. no. GTX21279),
TNF-α (cat. no. GTX110520), inducible nitric oxide synthase (iNOS;
cat. no. GTX130246) and Arginase 1 (Arg1; cat. no. GTX124113) were
purchased from GeneTex, Inc. Goat anti-rabbit secondary antibodies
labeled with Alexa Fluor® 647 (cat. no. ab150079), rat
anti-mouse secondary antibodies labeled with FITC (cat. no.
ab99572) and goat anti-rabbit secondary antibodies labeled with
tetramethylrhodamine (TRITC; cat. no. ab7087) were obtained from
Abcam. Horseradish peroxidase-labeled goat anti-mouse (cat. no.
A0216) and goat anti-rabbit (cat. no. A0208) secondary antibodies
were purchased from Beyotime Institute of Biotechnology.
The iron ion detection kit was purchased from
Sigma-Aldrich; Merck KGaA. BSA, DilC18(3) (DIL) and poly (vinyl
alcohol) (PVA) were obtained from Beyotime Institute of
Biotechnology. The primers used in the PCR experiments were from
BBI Life Sciences Corporation, and the sequences of these PCR
primers are presented in Table
I.
 | Table I.Sequences of the primers used for
reverse transcription-quantitative PCR. |
Table I.
Sequences of the primers used for
reverse transcription-quantitative PCR.
| Primer | Forward | Reverse |
|---|
| Arg1 |
5′-CCCCAGTACCAACAGGACTACC-3′ |
5′-TGAACGTGGCGGAATTTTGT-3′ |
| TNF-α |
5′-GGATCTCAAAGACAACCAAC-3′ |
5′-ACAGAGCAATGACTCCAAAG-3′ |
| iNOS |
5′-CTGCAGCACTTGGATCAGGAACCTG-3′ |
5′-GGAGTAGCCTGTGTGCACCTGGAA-3′ |
| TGF-β |
5′-ACCTGCAAGACCATCGACAT-3′ |
5′-GGTTTTCTCATAGATGGCGT-3′ |
| GAPDH |
5′-CACCCACTCCTCCACCTTTG-3′ |
5′-CCACCACCCTGTTGCTGTAG-3′ |
Preparation of nanoparticles
The oil in water method (26) was used to manufacture the
nanoparticles. PLGA (200 mg), Fe3O4 (400 µl
of a 20 mg/ml solution) and 9.4 µg DIL were dissolved in 2 ml
dichloromethane at room temperature for 30 sec as the oil phase,
and then 8 ml 4% PVA solution was added to serve as the aqueous
phase. The emulsion formed was homogenized at room temperature
using an ultrasonic oscillation instrument (Shanghai Yanyong
Ultrasonic Instrument Co., Ltd.) with the amplitude set at 70% for
1 min. The previous emulsion was subsequently added to 15 ml 0.2%
PVA aqueous solution, and the organic solvent was removed via
evaporation using a rotavapor for 90 min at room temperature. This
was followed by a subsequent centrifugation (15,000 × g for 10 min
at 4°C) to obtain the nanoparticles, which were then washed three
times with water.
Conjugation of anti-CD206 antibody to
the nanoparticles
Carbodiimide-mediated amide bond formation was used
to prepare the anti-CD206 monoclonal antibody-conjugated
nanoparticles, as described previously by Moura et al
(27). The prepared nanoparticles
were resuspended in 10 ml MES buffer (pH 6.0). A total of 1 ml EDC
(0.1 M) was added to the nanoparticle suspension with mild stirring
for 15 min at room temperature, then 1 ml NHS (0.7 M) was added and
the mixture was continually stirred for a further 45 min. The
remaining reagents in the coupling reaction were removed via
centrifugation (15,000 × g for 10 min at 4°C). Subsequently, the
nanoparticles were washed with MES (pH 8.0) for 5 min and repeat
three times, and finally re-dispersed in 2 ml double-distilled
water. Anti-CD206 antibody solution (100 µl) was added to the
activated nanoparticle suspension for antibody conjugation, and
incubated at room temperature for 2 h. The mixture was centrifuged
again (15,000 × g for 10 min at 4°C) in order to remove any excess
unconjugated anti-CD206 antibody, and washed three times with PBS
for 5 min.
Measurements of nanoparticles
The particle size, size distribution (polydispersity
index) and ζ potential of the nanoparticles produced were measured
using a laser particle size analyzer (Brookhaven Instruments
Corporation). Using ultrapure water as the medium, 50 mg
nanoparticles were distributed in ultrapure water and a 2 ml
suspension was then collected and placed in the colorimetric dish.
When the temperature of suspension had equilibrated at 25°C, the
distribution and particle size of the microspheres were analyzed
using a laser particle size analyzer. The nanoparticle freeze-dried
powder was adhered to the electron microscope plate with conductive
adhesive, and the surface characteristics of the sample were
observed at room temperature by scanning using an electron
microscope (S-3000N model; Hitachi, Ltd., magnification, ×50,000).
All samples were measured with three independent batch power
sources (six runs; 10 cycles each). Suspensions were prepared using
50 mg nanoparticle freeze-dried 173 powder dispersed in ultra9pure
water, and then 2 ml suspension was used to observe and collect
images with an optical microscope (LV100ND; Nikon instruments Co.,
Ltd, magnification, ×100).
Tissue immunofluorescence
The frozen sections of subcutaneous tumor tissue
(thickness, 5 µm) were prepared at −16°C and preserved at −4°C, and
the sections were washed three times with PBS and sealed at room
temperature for 1 h with 2% BSA. The sections were subsequently
washed three times with PBS and incubated with primary antibodies
of F4/80 (1:1,000) and CD86 (1:1,000) at room temperature for 12 h.
Next, the frozen sections were washed with PBS and incubated with
secondary antibodies (cat. nos. ab150079 and ab99572; 1:1,000) at
room temperature for 1 h. DAPI was used for nuclear staining at
room temperature for 5 min, and the tissue sections were sealed via
anti-fluorescent quenching. Under high-power magnification (×200),
tissue immunofluorescence micrographs were screened, and images
were captured using a fluorescence microscope (Leica Microsystems,
Inc.).
ROS level detection
Macrophages were cultured in 2 ml medium with 500 µl
suspension of PLGA (10 mg), Fe3O4-PLGA (10
mg) or CD206-Fe3O4-PLGA (10 mg) for 12 h at
37°C. ROS levels in macrophages were measured using a
2,7-dichlorofluorescin diacetate (DCFH-DA) probe (Beyotime
Institute of Biotechnology). Fluorescence microscopy was used to
observe the expression of green fluorescence in cells, and the
images were captured at a ×200 magnification.
Iron oxide nanoparticle association
efficiency
The Fe3O4 concentration was
analyzed using an iron assay kit (Sigma-Aldrich; Merck KGaA).
Aliquots of 10 µl of the 100 mM Iron Standard solution were diluted
with 990 µl water to generate a 1 mM standard solution. Different
amounts of the 1 mM standard solution (0, 2, 4, 6, 8 and 10 µl)
were added into a 96-well plate to generate 0, 2, 4, 6, 8 and 10
nmol/well standards at room temperature, respectively. Iron Assay
buffer was then added to each well to bring the volume to 100 µl,
prior to the addition of 5 µl Iron Reducer to each standard well.
Lyophilized powder (10 mg) of
CD206-Fe3O4-PLGA or
Fe3O4-PLGA nanoparticles was completely
dissolved in 500 µl DMSO. Subsequently, 50 µl this solution was
mixed with 50 µl Iron Assay buffer and 5 µl Iron Reducer. A
horizontal shaker was used to mix the samples, and the reaction
mixture was incubated for 30 min at room temperature, protecting
the plate from light during the incubation. Subsequently, 100 µl
Iron Probe was added to each well containing standard or test
samples. The samples were mixed using a horizontal shaker, and the
reaction was allowed to incubate in the dark for 60 min at room
temperature. Ultraviolet spectrophotometer (Thermo NanoDrop 3300,
Thermo Fisher 012 Scientific, Inc.) was used to determine the
absorbance value of each sample at 593 nm. The absorbance values
obtained from appropriate iron standards were then used to plot a
standard curve, and the concentration of iron was obtained via
value conversion. The carrier rate of Fe3O4
was determined using the following formula: Carrier rate of
Fe3O4 (%)=(the mass of
Fe3O4 in the sample)/(the total mass of added
Fe3O4) ×100%.
Effect of nanoparticles on mouse
model
A total of 15 female 6-week-old BALB/c-57 mice
weighing 18–22 g were purchased from the Experimental Animal Center
of Chongqing Medical University. Full value nutrient granulated
feed was used to raise mice, and the feed was added twice a week.
The water was supplied by drinking bottle, and the drinking bottle
was changed 2–3 times a week. The feeding environment was
controlled at 18–22°C, humidity was 50–60%, and the light/dark
cycle was 12:12 h.
Animals received humane care in accordance with the
guidelines provided by the Administrative measures of Chongqing
Municipality on laboratory animals (order no. 195 of Chongqing
Municipal People's Government). The animal protocols used in the
present study were evaluated and approved by the Animal Use and
Ethics Committee of The 2nd Affiliated Hospital of Chongqing
Medical University (protocol no. 2015-18).
A total of 15 subcutaneous tumorigenic BALB/c-57
mice were randomly divided into three groups (five mice/group). A
total of 5×106 murine mammary carcinoma cells (4T1 cell
line) were injected subcutaneously on the back of mice. About a
week later, the diameter of subcutaneous tumor reached 0.5 cm, and
each mouse was given 200 µl PLGA suspension, 200 µl
CD206-Fe3O4-PLGA suspension or 200 µl ferric
citrate solution via tail vein injection once a day for 14 days.
All mice were sacrificed by neck dislocation, and the tumor, liver,
spleen and lung were removed completely at the end of the
experiment.
Determination of iron content in
tissues
Subcutaneous tumors, livers, spleens and lungs were
harvested from 15 subcutaneous tumorigenic mice. Portions of tissue
(1.5 g) were carefully extracted and washed three times in PBS to
completely remove the red blood cells. RIPA lysis buffer (5 ml,
Beyotime Institute of Biotechnology) was then added, and the tissue
samples were ground sufficiently for 20 min with a homogenizer,
before the insoluble material was removed via centrifugation
(15,000 × g for 10 min at 4°C). The remaining procedures are the
same as those aforementioned for the measurement of iron oxide
nanoparticle association efficiency. The absorbance value of
control group was used as the control, and the ratio of absorbance
value between the experimental and control groups was regarded as
the relative iron concentration.
Anti-CD206 antibody conjugation
efficiency
Rabbit anti-mouse secondary antibodies (10 µl) to
anti-CD206 labeled with TRITC (Abcam) were added to the prepared
nanoparticle suspension, and the mixture was incubated for 1 h at
37°C. The mixture was subsequently centrifuged (15,000 × g for 10
min at 4°C) to remove the excess secondary antibodies, and then
washed three times with PBS. Fluorescence microscopy was used to
observe the conjugation of the fluorescent secondary antibodies,
and flow cytometry (FACSCalibur; BD Biosciences) was used to detect
the carrier rate of anti-CD206 antibodies at room temperature, and
without a blocking treatment.
Cell culture
RAW 264.7 cells (mouse monocyte macrophages) and 4T1
cells (murine mammary carcinoma cells) were purchased from the
American Type Culture Collection. Cells were cultured in
HyClone® DMEM (Thermo Fisher Scientific, Inc.) with 10%
FBS containing 1% (v/v) penicillin-streptomycin and 1% (v/v)
Fungizone® antimycotic. Cells were maintained in 37°C
humidified air containing 5% CO2.
1×106 macrophages were seeded in 6-well
plates and cultured with 2 ml medium for 3 h. A total of 2 µl IL-4
(SRP3211-20UG, Sigma-Aldrich; Merck KGaA)was then added into the
medium, and the cells were cultured for a further 12 h at 37°C to
obtain high expression levels of CD206 in the macrophages.
Macrophages were subsequently incubated at 37°C with 500 µl
CD206-Fe3O4-PLGA nanoparticles or with
Fe3O4-PLGA nanoparticle suspension, for 30
min. Then, the macrophages were washed three times with PBS,
incubated with rabbit anti-mouse secondary antibodies labeled with
TRITC (1:1,000) at room temperature for 1 h and washed with PBS for
a further three times. Fluorescence microscopy was used to observe
the targeting capacity of the nanoparticles (magnification,
×400).
Effect of nanoparticles on macrophage
polarization
1×107 macrophages were incubated with 2
µl IL-4 for at 37°C 12 h to obtain macrophages highly expressing
CD206. Subsequently, 500 µl CD206-Fe3O4-PLGA
(10 mg/ml), Fe3O4-PLGA (10 mg/ml), PLGA (10
mg/ml) or ferric citrate (1.2 mg/ml) was added into the medium, and
the cells were cultured at 37°C for a further 4 h. Protein and RNA
were then harvested from the macrophages.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the macrophages using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacture's protocol. A total of 30 ng
RNA/sample was reverse transcribed into cDNA using the PrimeScript™
RT Reagent kit (Takara Biotechnology Co., Ltd. cat. no. RR037A) at
37°C for 15 min, 85°C for 5 sec and 4°C hold. A two-step RT-PCR
program (Initial denaturation at 95°C for 5 sec, followed by the
annealing/elongation stage with 40 cycles for 30 sec at 60°C) was
used for amplification with specific primers (Sigma-Aldrich; Merck
KGaA). RT-qPCR was performed using SYBR® Green (Takara
Biotechnology Co., Ltd.) and the Applied Biosystems ABI Prism 7900
Sequence Detection system (Thermo Fisher Scientific, Inc.). Gene
expression was analyzed according to the 2−ΔΔCq method
(28), and GAPDH mRNA expression was
used as the control.
Western blotting
The concentration of protein was determined using
the BCA method. After determining the protein concentration, 200 µl
protein sample was mixed with 50 µl buffer and the sample protein
was denatured at 100°C for 10 min. Then, 10% SDS-PAGE was performed
with 30 mg aliquots of protein. The obtained PVDF membranes
(Beyotime Institute of Biotechnology) were blocked with 5% BSA at
room temperature for 60 min and incubated with primary antibodies
of TNF-α, IL-1β, Arg1, TGF-β, IL-10 and iNOS (all 1:1,000)
overnight at 4°C. Subsequently, the membranes were incubated with
the corresponding secondary antibodies (cat. nos. A0216 and A0208;
1:1,000) at 37°C for 1 h. The membranes were washed three times
with PBS-Tween-20 (0.3% Tween), and then analyzed and scanned using
Quantity One software (version 5.2.1; Bio-Rad Laboratories, Inc.)
after incubation with an enhanced chemiluminescence reagent (EMD
Millipore).
Statistical analysis
All data were analyzed using SPSS 17.0 software
(SPSS, Inc.). All experiments were performed in triplicate and data
are presented as the mean ± standard deviation. One-way ANOVA
followed by Tukey's test were used to compare the parameters among
>2 groups. The statistical differences between two groups were
determined using unpaired Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of nanoparticles
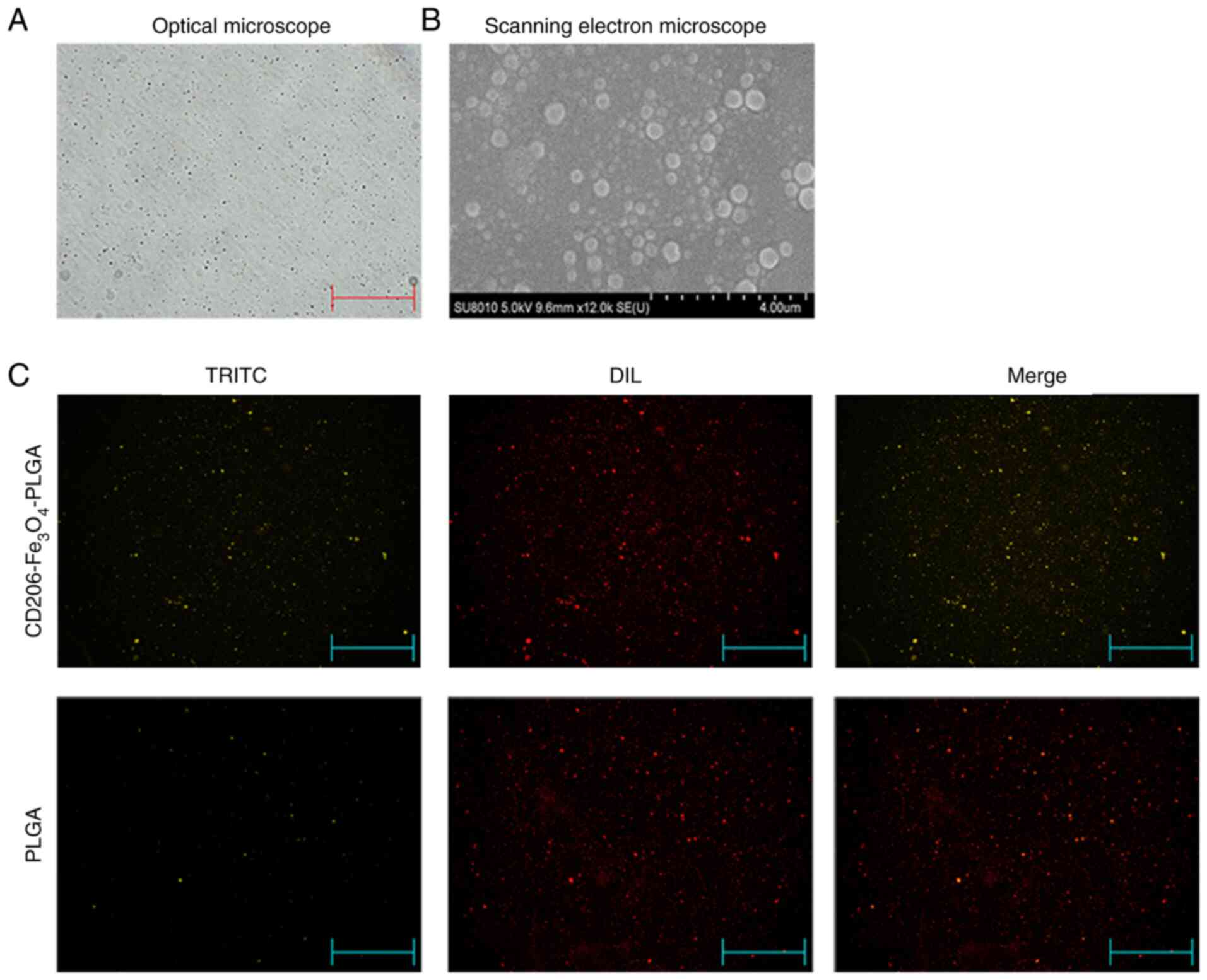
The optical microscopy and scanning electron
microscopy analyses identified that the nanoparticles had a
relatively uniform particle size, a spherical, smooth surface and a
good dispersion (Fig. 1A and B).
Fluorescence microscopy indicated that the anti-CD206 antibody
labeled with TRITC was successfully conjugated with PLGA
nanoparticles, and all the PLGA nanoparticles were labeled with DIL
(Fig. 1C). The particle size of
nanoparticles were 260–295 nm, and the ζ potential values were −19
to −33 mv. The Fe3O4 association efficiency
ranged from 65–75%, and the anti-CD206 conjunction efficiency
ranged from 65–70% (Table II). It
shows that the nanoparticles had a uniform particle size, good
dispersion in aqueous solution, good Fe3O4
association efficiency and anti-CD206 conjunction efficiency.
 | Table II.Characteristics of the NPs. |
Table II.
Characteristics of the NPs.
| Formula | Mean effective
diameter (nm) | ζ potential
(mV) | Anti-CD206
conjugation efficiency (%) |
Fe3O4 association
efficiency (%) |
|---|
| PLGA NPs | 261.4±22.5 | −25.6±4.4 | – | – |
|
Fe3O4-PLGA | 283.8±24.1 | −27.3±5.7 | – | 75.3±5.2 |
|
CD206-Fe3O4-PLGA | 294.3±17.8 | −32.2±3.5 | 67.8±2.5 | 66.7±3.7 |
Selective enhancement of the
intra-tumoral iron level
Our previous study reported that body iron
concentration at a high level in mice may inhibit tumor growth by
promoting M1 polarization in TAMs (29). Subsequent experimental results in the
present study demonstrated that tail vein injection of ferric
citrate in mice could significantly increase the iron content in
the liver, spleen and lung compared with the saline group (Fig. 2A), which may lead to potential organ
damage. RAW 264.7 cells were pretreated with IL-4 to obtain M2
macrophages, as TAM cells, which express high levels of CD206.
CD206-Fe3O4-PLGA nanoparticles were used to
transport Fe3O4 to CD206-positive
macrophages. Compared with the control group,
CD206-Fe3O4-PLGA significantly increased the
iron content in macrophages (Fig.
2B). Fluorescence assay results demonstrated that the
CD2060-positive macrophages (i.e., M2 macrophages) exhibited a
stronger PLGA-binding capability in the
CD206-Fe3O4-PLGA group after 30 min
co-culture, while the CD206-negative macrophages (M0/M1
macrophages) did not have a strong binding ability (Fig. 2C and D).
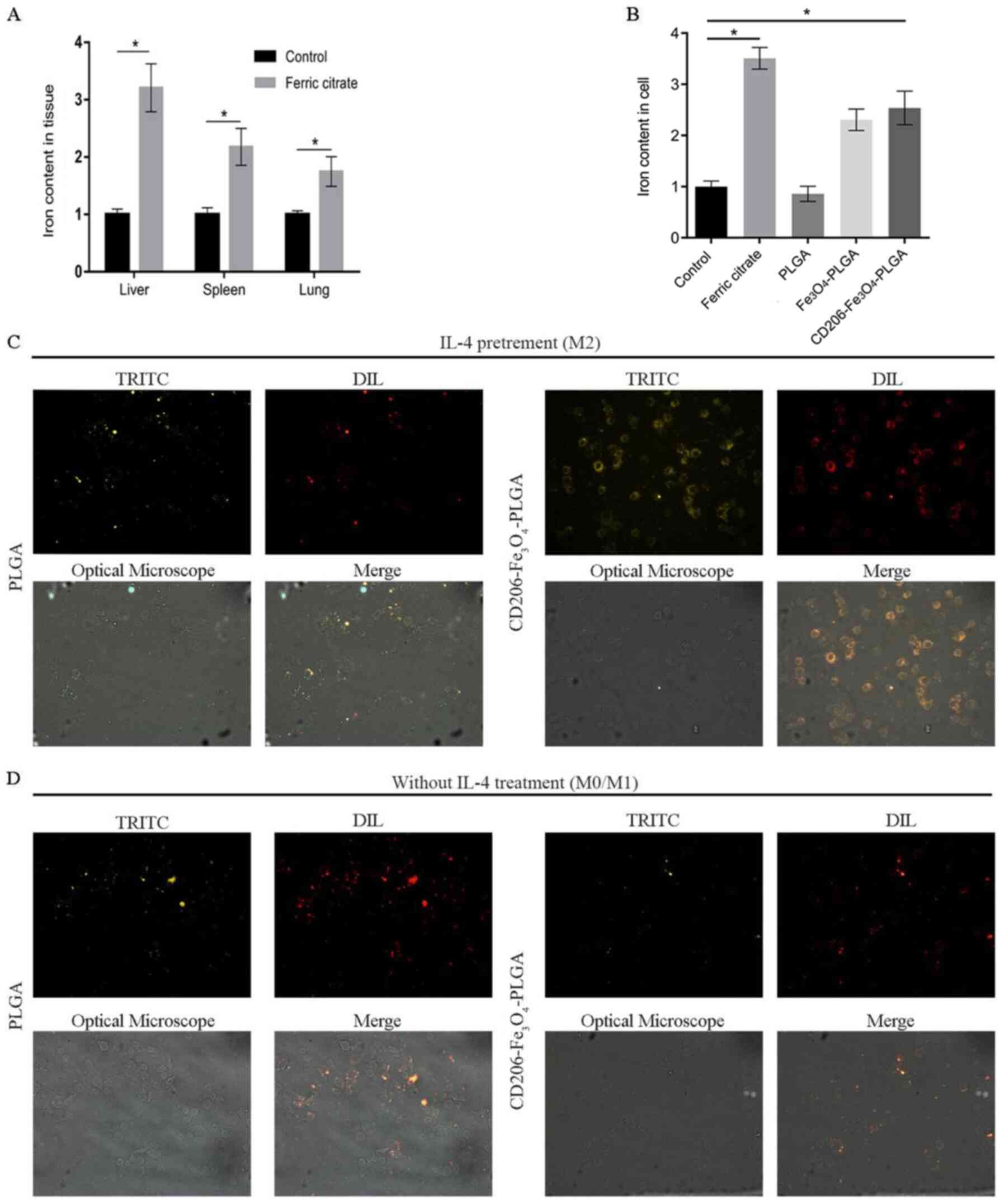 | Figure 2.Selective enhancement of the
intra-tumoral iron level. (A) Mice injected with ferric citrate had
a higher relative iron content in their liver, spleen, lung and
subcutaneous tumor compared with the control group. (B) Compared
with the control group, the relative iron content of macrophages,
pretreated with IL-4 and co-cultured with iron and
CD206-Fe3O4-PLGA for 24 h, was increased
significantly (*P<0.05), but not in the group co-culture with
PLGA alone. (C) Immunofluorescence analysis demonstrated that a
large number of CD206-Fe3O4-PLGA
nanoparticles surrounded the macrophages pretreated with IL-4
compared with the PLGA group (magnification, ×400). (D)
Immunofluorescence analysis demonstrated that only a few PLGA and
CD206-Fe3O4-PLGA nanoparticles surrounded the
macrophages without IL-4 pretreatment (magnification, ×400). PLGA,
poly(lactic-co-glycolic) acid; DIL, DiIC18(3); TRITC,
tetramethylrhodamine. |
Promoting macrophage polarization and
cellular ROS production
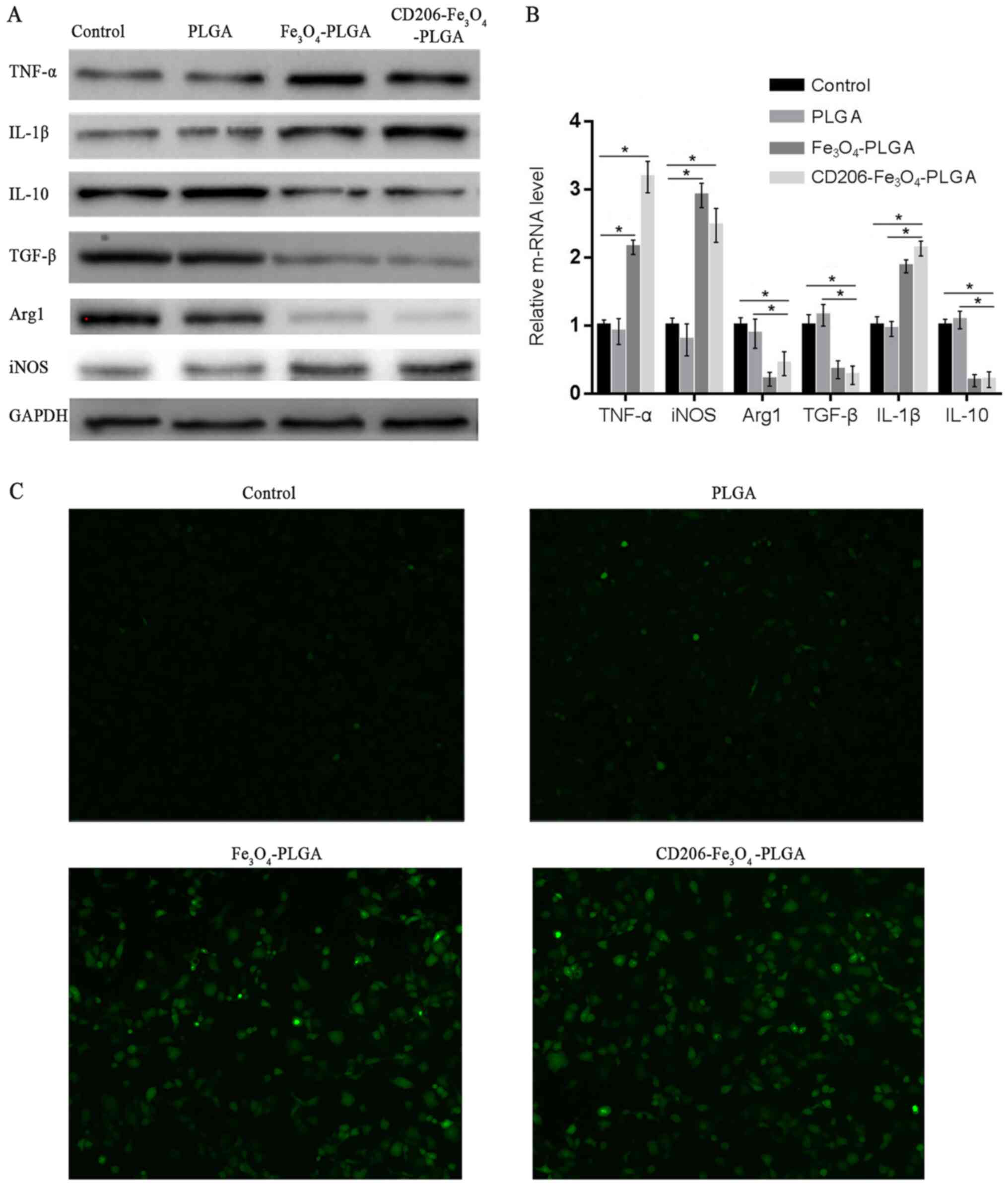
Western blotting and RT-qPCR were used to assess
whether nanoparticles could promote the polarization of
macrophages. Western blot and RT-qPCR analyses demonstrated that
CD206-Fe3O4-PLGA significantly promoted the
expression of TNF-α, IL-1β and iNOS (P<0.05), but inhibited the
expression of TGF-β, IL-10 and Arg1 (P<0.05; Fig. 3A and B). The results of these
experiments demonstrated that
CD206-Fe3O4-PLGA and
Fe3O4-PLGA nanoparticles promoted M1
polarization of the macrophages. Furthermore, it was found that the
PLGA nanoparticles alone were not able to promote macrophage
polarization.
Pretreatment of the
CD206-Fe3O4-PLGA and
Fe3O4-PLGA nanoparticles with IL-4 resulted
in increased ROS production in macrophages with elevated
intracellular iron content identified via DCFH-DA, a probe used for
detecting ROS in living cells (Fig.
3C).
Nanoparticles promote M1 polarization
of TAMs in an established mouse model
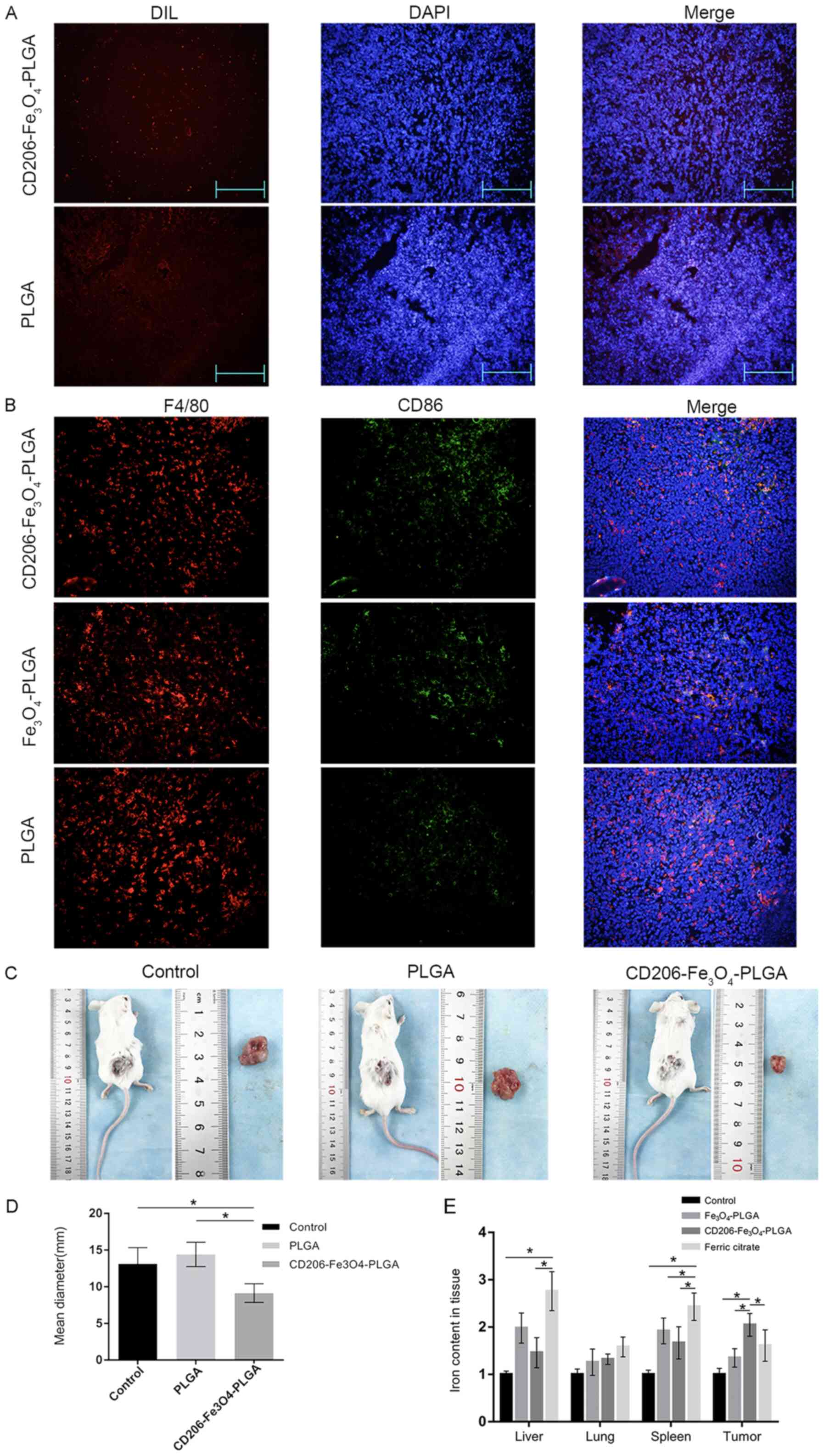
Tumor-bearing mice were injected with
CD206-Fe3O4-PLGA,
Fe3O4-PLGA, PLGA nanoparticles or ferric
citrate via the tail vein in order to study the targeting
properties of nanoparticles in vivo. An immunofluorescence
assay was subsequently used to observe the deposition of
nanoparticles in tumor tissues. The results indicated that there
were increased numbers of CD206-Fe3O4-PLGA
nanoparticles in the subcutaneous tissue compared with PLGA
nanoparticles (Fig. 4A).
Double-labeled tissue fluorescence was used to
detect the polarization of macrophages in tumor tissues. It was
identified that macrophages in the
CD206-Fe3O4-PLGA group expressed a higher
level of CD86, a specific marker of the M1 subtype (Fig. 4B). The diameter of subcutaneous tumor
of mice treated with PLGA microspheres was smaller compared with
control and PLGA groups (both P<0.05; Fig. 4C and D). The tumor tissue of the
CD206-Fe3O4-PLGA group contained a higher
iron concentration, whereas the liver and spleen contained less
iron, compared with the ferric citrate group (P<0.05; Fig. 4E).
Discussion
TAMs may be associated with up to 50% of the total
tumors, and often associated with a poor patient prognosis
(30). Therefore, TAM ablation (or
TAM reprogramming) has become a potential antitumor treatment
strategy.
M1 macrophages cannot only inhibit tumor cells via
secretion and phagocytosis, but can also block DNA replication of
tumor cells by absorbing and sequestering the iron found in the
microenvironment (31). M2
macrophages are major sites for taking up, metabolizing, storing
and exporting iron (31). M2
macrophages can release intracellular iron ions and promote iron
internalization and sequestration via known receptors, such as
megalin, contributing to cancer cell survival and metastasis
(32). Large numbers of iron ions
are required for the growth and proliferation of tumor cells
(33–35). Therefore, the introduction or
elimination of iron in cells to destroy the homeostasis of iron
metabolism has become a potential avenue for cancer treatment.
Iron chelators are able to both effectively decrease
the content of iron in tumor cells and inhibit the proliferation of
aggressive tumors, leading to G1/S cell cycle arrest and
apoptosis (36,37). Although iron chelators do exert
therapeutic effects on tumors, their side effects cannot be
ignored. Iron chelators have been reported to activate the
hypoxia-inducible factor-1α pathway, and induce the expression
levels of urokinase plasminogen activator and MMP-2, resulting in
enhanced metastasis via degrading the extracellular matrix and
increasing the level of VEGF, leading to toxic anemia and edema
(38–41).
Iron oxide nanoparticles (IONPs) have been widely
used for drug targeting and diagnostic applications (42). The concentration of intracellular
iron in dendritic cells can be increased by co-culturing with
IONPs; however, previous studies have revealed that an increase of
iron concentration in dendritic cells exerts little influence on
the phenotype and maturation of dendritic cells (43,44).
SPION treatment has been reported to produce ROS and activate the
extracellular signal-regulated kinase and AKT pathways (45). Moreover, SPION leads to impaired
chemotactic migration and an increased invasive capacity of M2
macrophages, but does not activate either the c-Jun terminal kinase
or the p38-mitogen-activated protein kinase pathways (45). It has been shown that IONPs induce
differential effects on antigen-specific cytokine expression
mediated by T cells, in which IFN-γ expression is sensitized, while
the effect on IL-4 expression is refractory (46). In addition, the suppressive effect of
IONPs on IRN-γ is closely associated with a decrease in the level
of glutathione (46).
It has been reported that exogenous administration
of iron nitrate leads to deposition of most iron in the liver and
spleen, and only a fraction of the hemosiderin-laden macrophages
were observed in the mammary tumors (47).
PLGA particles have been revealed to encapsulate
SPIONs and BSA, and the significantly improved uptake of
BSA/SPION-PLGA particles into RAW 264.7 cells is observed under the
influence of an external magnetic field compared with the uptake of
particles without an external magnetic field (48). These particles are shown to
significantly enhance bone marrow-derived dendritic cell maturation
by upregulating the expression levels of major histocompatibility
complex II, CD80 and CD86 (48).
In the present study, CD206 monoclonal
antibody-conjugated nanoparticles were able to specifically bind to
the surface of CD206-positive cells when circulating in the blood
vessels. Collectively, the current results demonstrated that the
nanoparticles could specifically bind to M2 macrophages in
vivo and in vitro. After being phagocytized by the TAMs,
the nanoparticles were gradually hydrolyzed, releasing
Fe3O4, which was degraded into iron ions in
the lysosomes, thus increasing the intracellular concentration of
iron in the macrophages and inducing reprogramming of the M2
macrophages.
In conclusion, the present study demonstrated that
CD206-Fe3O4-PLGA nanoparticles were
successfully constructed to increase the iron content in M2
macrophages, thus promoting the repolarization of M2 macrophages to
the M1 subtype. This effect lead to an inhibition of the growth of
tumors, and also decreased the deposition of physiological tissue
iron. Therefore, the present study provided a potential approach
for tumor immunotherapy; however, most targeted therapies are not
aimed at a specific type of cells. Thus, in a follow-up study,
further methods to improve the targeting ability, such as
identifying additional unique cell markers or coupling two or more
specific antibodies, will be developed.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81470899 and
81170442) and the Talent Project of The Second Affiliated Hospital
of Chongqing Medical University (grant no. 2016).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YZ and KTQ acquired, analyzed and interpreted the
data, and drafted the initial manuscript. HMT and PZ acquired,
analyzed and interpreted the data. QMF and ZJL conceived the
present study and designed the experiments, and analyzed and
interpreted the data. ZJL also helped draft the initial manuscript
and revise it for intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Use and Ethics Committee of The 2nd Affiliated Hospital of
Chongqing Medical University (approval no. 2015-18).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Juhas U, Ryba-Stanisławowska M, Szargiej P
and Myśliwska J: Different pathways of macrophage activation and
polarization. Postepy Hig Med Dosw (Online). 69:496–502. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jung M, Mertens C and Brüne B: Macrophage
iron homeostasis and polarization in the context of cancer.
Immunobiology. 220:295–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chlosta S, Fishman DS, Harrington L,
Johnson EE, Knutson MD, Wessling-Resnick M and Cherayil BJ: The
iron efflux protein ferroportin regulates the intracellular growth
of Salmonella enterica. Infect Immun. 74:3065–3067. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paradkar PN, De Domenico I, Durchfort N,
Zohn I, Kaplan J and Ward DM: Iron depletion limits intracellular
bacterial growth in macrophages. Blood. 112:866–874. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Recalcati S, Locati M, Gammella E,
Invernizzi P and Cairo G.: Iron levels in polarized macrophages:
Regulation of immunity and autoimmunity. Autoimmun Rev. 11:883–889.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lewin M, Carlesso N, Tung CH, Tang XW,
Cory D, Scadden DT and Weissleder R: Tat peptide-derivatized
magnetic nanoparticles allow in vivo tracking and recovery of
progenitor cells. Nat Biotechnol. 18:410–414. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Namdeo M, Saxena S, Tankhiwale R, Bajpai
M, Mohan YM and Bajpai SK: Magnetic nanoparticles for drug delivery
applications. J Nanosci Nanotechnol. 8:3247–3271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zanganeh S, Hutter G, Spitler R, Lenkov O,
Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M,
et al: Iron oxide nanoparticles inhibit tumour growth by inducing
pro-inflammatory macrophage polarization in tumour tissues. Nat
Nanotechnol. 11:986–994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thorek DL, Chen AK, Czupryna J and
Tsourkas A: Superparamagnetic iron oxide nanoparticle probes for
molecular imaging. Ann Biomed Eng. 34:23–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Laskar A, Eilertsen J, Li W and Yuan X:
SPION primes THP1 derived M2 macrophages towards M1-like
macrophages. Biochem Biophys Res Commun. 441:737–742. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Astete C and Sabliov CM: Synthesis and
characterization of PLGA nanoparticles. J Biomater Sci Polym Ed.
17:247–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kapoor DN, Bhatia A, Kaur R, Sharma R,
Kaur G and Dhawan S: PLGA: A unique polymer for drug delivery. Ther
Deliv. 6:41–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Postlethwait RW: Polyglycolic acid
surgical suture. Arch Surg. 101:489–494. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khatri K, Goyal A and Vyas S: Potential of
nanocarriers in genetic immunization. Recent Pat Drug Deliv Formul.
2:68–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Byrne JD, Betancourt T and Brannon-Peppas
L: Active targeting schemes for nanoparticle systems in cancer
therapeutics. Adv Drug Deliv Rev. 60:1615–1626. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu F, Zhang L, Teply BA, Mann N, Wang A,
Radovic-Moreno AF, Langer R and Farokhzad OC: Precise engineering
of targeted nanoparticles by using self-assembled biointegrated
block copolymers. Proc Natl Acad Sci USA. 105:2586–2591. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maeda H: Tumor-selective delivery of
macromolecular drugs via the EPR effect: Background and future
prospects. Bioconjugate Chem. 21:797–802. 2010. View Article : Google Scholar
|
|
18
|
Xu X, Ho W, Zhang X, Bertrand N and
Farokhzad O: Cancer nanomedicine: From targeted delivery to
combination therapy. Trends Mol Med. 21:223–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang G, Griffin JI, Inturi S, Brenneman B,
Banda NK, Holers VM, Moghimi SM and Simberg D: In vitro and in vivo
differences in murine third complement component (C3) opsonization
and macrophage/leukocyte responses to antibody-functionalized iron
oxide nanoworms. Front Immunol. 8:1512017.PubMed/NCBI
|
|
20
|
Jokerst JV, Lobovkina T, Zare RN and
Gambhir SS: Nanoparticle PEGylation for imaging and therapy.
Nanomedicine (Lond). 6:715–728. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwon IK, Lee SC, Han B and Park K:
Analysis on the current status of targeted drug delivery to tumors.
J Controlled Release. 164:108–114. 2012. View Article : Google Scholar
|
|
22
|
Ruenraroengsak P, Cook JM and Florence AT:
Nanosystem drug targeting: Facing up to complex realities. J
Control Release. 141:265–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Badkas A, Frank E, Zhou Z, Jafari M,
Chandra H, Sriram V, Lee JY and Yadav JS: Modulation of in vitro
phagocytic uptake and immunogenicity potential of modified
Herceptin®-conjugated PLGA-PEG nanoparticles for drug
delivery. Colloids Surf B Biointerfaces. 162:271–278. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Allen TM: Ligand-targeted therapeutics in
anticancer therapy. Nat Rev Cancer. 2:750–763. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peer D, Karp JM, Hong S, Farokhzad OC,
Margalit R and Langer R: Nanocarriers as an emerging platform for
cancer therapy. Nat Nanotechnol. 2:751–760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kamaly N, Yameen B, Wu J and Farokhzad OC:
Degradable controlled-release polymers and polymeric nanoparticles:
Mechanisms of controlling drug release. Chem Rev. 116:2602–2663.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moura CC, Segundo MA, Neves Jd, Reis S and
Sarmento B: Co-association of methotrexate and SPIONs into
anti-CD64 antibody-conjugated PLGA nanoparticles for theranostic
application. Int J Nanomedicine. 9:4911–4922. 2014.PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Y, Que KT, Zhang Z, Yi ZJ, Zhao PX,
You Y, Gong JP and Liu ZJ: Iron overloaded polarizes macrophage to
proinflammation phenotype through ROS/acetylp53 pathway. Cancer
Med. 7:4012–4022. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
DeNardo DG, Brennan DJ, Rexhepaj E,
Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD,
Junaid SA, et al: Leukocyte complexity predicts breast cancer
survival and functionally regulates response to chemotherapy.
Cancer Discov. 1:54–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Yu L, Ding J and Chen Y: Iron
metabolism in cancer. Int J Mol Sci. 20:952018. View Article : Google Scholar
|
|
32
|
Duan X, He K, Li J, Cheng M, Song H, Liu J
and Liu P.: Tumor associated macrophages deliver iron to tumor
cells via Lcn2. Int J Physiol Pathophysiol Pharmacol. 10:105–114.
2018.PubMed/NCBI
|
|
33
|
Pinnix ZK, Miller LD, Wang W, D'Agostino R
Jr, Kute T, Willingham MC, Hatcher H, Tesfay L, Sui G, Di X, et al:
Ferroportin and iron regulation in breast cancer progression and
prognosis. Sci Transl Med. 2:43ra562010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Majewska U, Banaś D, Braziewicz J, Góźdź
S, Kubala-Kukuś A and Kucharzewski M: Trace element concentration
distributions in breast, lung and colon tissues. Phys Med Biol.
52:3895–3911. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen L, Zhao HY, Du J and Wang F:
Anti-tumor activities of four chelating agents against human
neuroblastoma cells. In Vivo. 19:233–236. 2005.PubMed/NCBI
|
|
37
|
Li P, Zheng X, Shou K, Niu Y, Jian C, Zhao
Y, Yi W, Hu X and Yu A: The iron chelator Dp44mT suppresses
osteosarcoma's proliferation, invasion and migration: In vitro and
in vivo. Am J Transl Res. 8:5370–5385. 2016.PubMed/NCBI
|
|
38
|
Tsai SH, Huang PH, Hsu YJ, Peng YJ, Lee
CH, Wang JC, Chen JW and Lin SJ: Inhibition of hypoxia inducible
factor-1α attenuates abdominal aortic aneurysm progression through
the down-regulation of matrix metalloproteinases. Sci Rep.
6:286122016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kontoghiorghes GJ: Ethical issues and
risk/benefit assessment of iron chelation therapy: Advances with
deferiprone/deferoxamine combinations and concerns about the
safety, efficacy and costs of deferasirox. Hemoglobin. 32:1–15.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Di Nicola M, Barteselli G, Dell'Arti L,
Ratiglia R and Viola F: Functional and structural abnormalities in
deferoxamine retinopathy: A review of the literature. Biomed Res
Int. 2015:2496172015. View Article : Google Scholar
|
|
41
|
Hamilton JL, Hatef A, Imran ul-Haq M, Nair
N, Unniappan S and Kizhakkedathu JN: Clinicallyapproved iron
chelators influence zebrafish mortality, hatching morphology and
cardiac function. PLoS One. 9:e1098802014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xie J, Huang J, Li X, Sun S and Chen X:
Iron oxide nanoparticle platform for biomedical applications. Curr
Med Chem. 16:1278–1294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mou Y, Chen B, Zhang Y, Hou Y, Xie H, Xia
G, Tang M, Huang X, Ni Y and Hu Q: Influence of synthetic
superparamagnetic iron oxide on dendritic cells. Int J
Nanomedicine. 6:1779–1786. 2011.PubMed/NCBI
|
|
44
|
Verdijk P, Scheenen TW, Lesterhuis WJ,
Gambarota G, Veltien AA, Walczak P, Scharenborg NM, Bulte JWM, Punt
CJA, Heerschap A, et al: Sensitivity of magnetic resonance imaging
of dendritic cells for in vivo tracking of cellular cancer
vaccines. Int J Cancer. 120:978–984. 2006. View Article : Google Scholar
|
|
45
|
Rojas JM, Sanz-Ortega L, Mulens-Arias V,
Gutiérrez L, Pérez-Yagüe S and Barber DF: Superparamagnetic iron
oxide nanoparticle uptake alters M2 macrophage phenotype, iron
metabolism, migration and invasion. Nanomedicine. 12:1127–1138.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shen CC, Liang HJ, Wang CC, Liao MH and
Jan TR: A role of cellular glutathione in the differential effects
of iron oxide nanoparticles on antigen-specific T cell cytokine
expression. Int J Nanomedicine. 6:2791–2798. 2011.PubMed/NCBI
|
|
47
|
Leftin A, Ben-Chetrit N, Klemm F, Joyce JA
and Koutcher JA: Iron imaging reveals tumor and metastasis
macrophage hemosiderin deposits in breast cancer. PLoS One.
12:e01847652017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Saengruengrit C, Ritprajak P,
Wanichwecharungruang S, Sharma A, Salvan G, Zahn DRT and Insin N:
The combined magnetic field and iron oxide-PLGA composite
particles: Effective protein antigen delivery and immune
stimulation in dendritic cells. J Colloid Interface Sci.
520:101–111. 2018. View Article : Google Scholar : PubMed/NCBI
|