Introduction
Gastric cancer (GC) is one of the most common,
lethal neoplasms of the human digestive system and is the second
most frequent cause of cancer-associated death worldwide (1). The highly aggressive advanced GC
promotes distant metastasis, with typical metastatic sites being
the lungs, liver and bones (2).
Angiogenesis mostly be essential for nourishing the primary tumor
(3). Therefore, it is imperative to
explore the possible mechanisms of angiogenesis in GC to expand the
current understanding of the disease etiology and prognosis and
offer novel treatment strategies.
Tumor angiogenesis is a complex process involving
activation of endothelial cells (ECs); degradation of the
extracellular matrix; migration of ECs; as well as proliferation,
tube formation and formation of adventitial membranes (4). Thus, ECs, usually generated from
mesenchymal stem cells, bone marrow-derived endothelial progenitor
cells and pre-existing ECs, have an essential role in tumor
angiogenesis (5–8). Numerous studies have demonstrated the
involvement of tumor cell-derived vascular ECs (VECs) in neoplasms
such as myeloma, osteosarcoma, glioblastoma, ovarian cancer and
neuroblastoma (9–13). Osteosarcoma cells transdifferentiate
into endothelial cells under hypoxic conditions (14). First osteosarcoma cells differentiate
into tumor stem cells under hypoxic conditions and then the tumor
stem cells further differentiate into endothelial cells (14). Therefore, tumor cell-derived VECs are
a typical source of ECs in tumor angiogenesis, although it remains
elusive whether GC cells are able to differentiate into ECs. The
present study aimed to determine whether human GC cells are able to
differentiate into ECs to take on their morphological and
functional properties.
Tumor cells are usually deficient in nutrients and
oxygen due to the failure of blood supply to meet the metabolic
requirements of these rapidly growing cells. Therefore, hypoxia is
a common phenomenon in the development of most solid tumor types
(15). Hypoxia-inducible factor
(HIF), a transcription factor produced by tumor cells, mediates the
growth, proliferation and metastasis of cancer cells under hypoxic
conditions (16). HIF is a
heterodimeric transcription factor containing a hypoxically
inducible HIF-α subunit and a constitutively expressed HIF-β
subunit (17). Therefore, the
activity of HIF is determined primarily by the expression level of
the α subunit (18). Hypoxia-induced
tumor angiogenesis and the important role of HIF-α in the process
have been widely reported (19–21). In
the present study, it was hypothesized that GC cells are able to
transdifferentiate into vascular EC-like cells under hypoxic
conditions.
To investigate morphological and molecular changes,
GC cells were cultured under hypoxic conditions, as well as on
Matrigel (tube formation assay, a property of ECs). The results
demonstrated that GC cells were able to transdifferentiate into
EC-like cells in vitro.
Materials and methods
Cell lines and culture
The human GC (HGC) cell line HGC-27 was obtained
from the Cell Bank of the Chinese Academy of Sciences. The cells
were cultured in RPMI-1640 medium (GIBCO; Thermo Fisher Scientific,
Inc.) supplemented with 20% fetal bovine serum (FBS; HyClone;
Cytiva) and 100 units/ml penicillin/streptomycin.
Induced transdifferentiation of HGC
cells
For the normoxia group, HGC cells were cultivated
under normoxia conditions (5% CO2, 95% air) in
endothelial differentiation medium, i.e. RPMI-1640 medium
supplemented with 20% FBS, 10 ng/ml vascular endothelial growth
factor (VEGF; Invitrogen; Thermo Fisher Scientific, Inc.), 1% N2
supplement, 10 ng/ml epidermal growth factor, 5 ng/ml bone-derived
fibroblast growth factor and 50 µg/ml heparin in vitro. For
the control group, HGC cells were cultivated under normoxia
conditions in RPMI-1640 medium only containing 10% FBS. For the
hypoxia group, HGC cells were cultivated under hypoxia conditions
in endothelial differentiation medium. For the HC group, HGC cells
were cultivated under hypoxia conditions in RPMI-1640 medium only
containing 10% FBS. The hypoxia group and GC group, HGC cells were
placed in an incubator (Precision Scientific) with 1% oxygen, 5%
CO2 and 94% nitrogen. The cells of each group were
cultured using their own specific aforementioned culture conditions
for 4 days, their appearance was observed.
Three-dimensional culture
Matrigel (BD Biosciences) was thawed at 4°C
overnight. 96-well plates were incubated at 4°C for 60 min.
Matrigel (BD Biosciences) was poured onto the 96-well dish at 30
µl/well and then the dish was placed in a CO2 incubator
(humidified atmosphere with 5% CO2/95% air) at 37°C for
30 min. Subsequently, the HGC-27 cell suspensions (1×105
cells/200 µl) in endothelial differentiation medium or basic medium
were added to the designated wells. In the hypoxia and GC groups,
the cells were cultivated under hypoxia conditions (1%
O2, 5% CO2, 94% nitrogen) and in the control
and normoxia groups, the cells were cultivated under normoxia
conditions (5% CO2, 95% air). The cells were
periodically observed by inverted phase-contrast microscopy and
images were acquired. The number of tubes and the length of the
branches were calculated using ImageJ software version 1.47
(National Institutes of Health).
RNA isolation and reverse
transcription-quantitative (RT-q)PCR
Total RNA was isolated from each group using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and
quantified spectrophotometrically (at 260 nm). RNAase-Free DNase I
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to remove
genomic DNA contamination. First-strand complementary (c)DNA was
synthesized from total RNA (500 ng), the ReverTra Ace kit (Toyobo)
and oligo (dT) 20 primers (performed according to the
manufacturer's protocol). PCR amplification of cDNA template was
performed using the LightCycler 480 real-time PCR system (Roche).
The reaction was performed in a 384-well plate using the
LightCycler 480 SYBR Green I Master kit (Roche). The PCR reaction
system consisted of 0.75 µl of template cDNA, 0.6 µl of 2.5 µM
primer mix, 2.5 µl of 2X SYBR Green I Master Mix and 1.15 µl
RNAse-free water in a final volume of 5 µl. The primer sequences
were as follows: CD31 forward, 5′-ACATGGCAACAAGGCTGTGTA-3′ and
reverse, 5′-CCTCAAACTGGGCATCATAAG-3′ (GenBank accession no.
NM_000442); CD34 forward, 5′-CCACTCGGTGCGTCTCTCTAGGAGC-3′ and
reverse, 5′-TTGTCTCTGGAGTTGAAACGTTGGC-3′ (GenBank accession no.
NM_001025109); von Willebrand factor (vWF) forward,
5′-CTGAAGAGTCATCGGGTCAACTGT-3′ and reverse,
5′-AGCATGAAGTCATTGGCTCCGTTCT-3′ (GenBank accession no. NM_000552);
β-actin forward, 5′-TTCTGTGGCATCCACGAAACT-3′ and reverse,
5′-GAAGCATTTGCGGTGGACGAT-3′ (GenBank accession no. NM_001101). The
optimal conditions for PCR amplification of the cDNA were as
follows: Initiation at 95°C for 30 sec, followed by 40 cycles of
95°C for 15 sec and 60°C for 60 sec. The fluorescence threshold
value was calculated using Lightcycler 480 series software. The
calculation of relative changes in mRNA levels was performed using
melting curve analysis with normalization to the housekeeping gene
β-actin. Data analysis of the relative real time PCR was based on
the 2−ΔΔCq method (22).
PCR amplification was repeated three times.
Immunofluorescence
HGC-27 cells were plated on sterile glass coverslips
with Lysin in a 6-well culture plate and cultivated under different
conditions according to the group. After 48 h of incubation, the
cells plated on glass coverslips were fixed with 4%
paraformaldehyde on ice for 30 min and treated with 0.3%
TritonX-100 solution (Sigma-Aldrich; Merck KGaA) for 10 min at room
temperature. Subsequently, the cells were blocked with 1% bovine
serum albumin (BSA) (Sigma-Aldrich; Merck KGaA) for 20 min at room
temperature. Subsequently, they were incubated overnight at 4°C
with antibodies against CD31 (1:2,000; mouse anti-human; cat. no.
ab218; Abcam), CD34 (1:2,000 rabbit anti-human; cat. no. ab81289;
Abcam) and vWF (1:2,000 mouse anti-human; cat. no. ab194405;
Abcam). The cells were incubated in the dark at room temperature
for 1 h with secondary antibodies: PE-conjugated anti-rabbit
antibodies (1:200; cat. no. P-2771MP; Invitrogen; Thermo Fisher
Scientific Inc.) or FITC-conjugated anti-mouse antibodies (1:200;
cat. no. A0568; Beyotime Institute of Biotechnology). Nuclei were
counterstained with DAPI (Sigma-Aldrich; Merck KGaA). Subsequently,
images were captured using an Olympus BX51 epifluorescent
microscope (Olympus BX51; Olympus Corp.).
Statistical analysis
Statistical analysis was performed using SPSS 20.0
(IBM Corp.) and GraphPad Prism software version 5.0 (GraphPad
Software, Inc.). Data were expressed as the mean ± standard
deviation. Groups were compared by one-way ANOVA followed by the
Newman-Keuls or Dunnett test. Comparison between groups H and GC
was performed by the Newman-Keuls test, while groups H, HC or N and
group C were compared by Dunnett's test. P<0.05 was considered
to indicate statistical significance.
Results
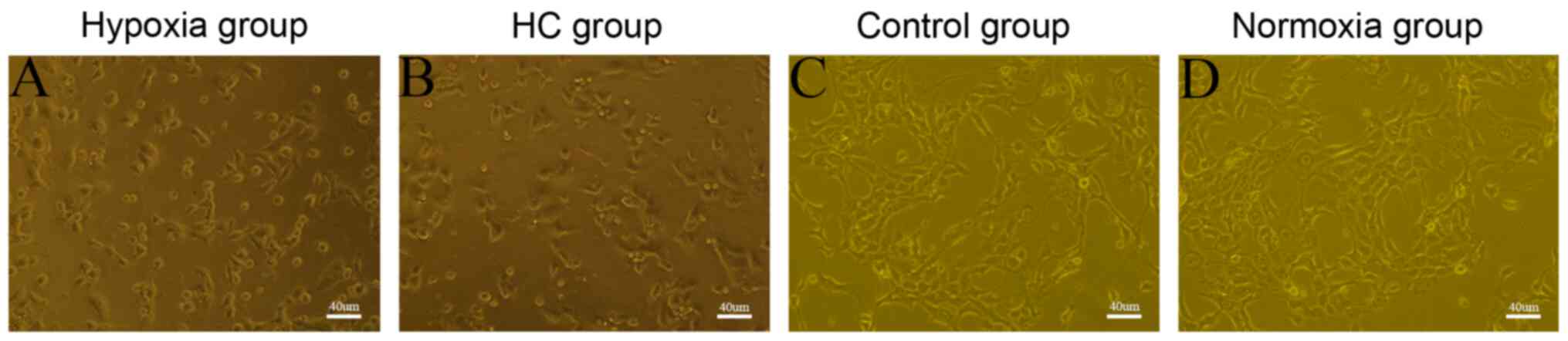
HGC-27 cells exhibit the morphological
features of ECs after transdifferentiation
To investigate the transdifferentiating capability
of the tumor cells, HGC-27 cells were cultured under different
conditions to determine the presence of the characteristic
‘flagstone’ appearance, as well as microtube formation on Matrigel.
The tube-formation capacity of the cells was evaluated by
determining the branch length and number of tubes. On day 4 of
HGC-27 cell cultivation under hypoxic conditions with or without
endothelial differentiation medium, a typical flagstone morphology
was noted (Fig. 1A and B). However,
HGC-27 cells cultured under normoxic conditions, with or without
endothelial differentiation medium, adhered to the culture dish
rather than developing the flagstone appearance (Fig. 1C and D). HGC-27 cells cultivated on
Matrigel under hypoxic conditions, with or without endothelial
differentiation medium, underwent a series of morphological
changes: From a single cell or a cluster of cells (0 h) to
discontinuous net-like structures (6 h), to continuous net-like
structures (12 h) and to a significant increase in the number of
the net-like structures (24 h), and the net-like structures
continued to exist at 48 h after seeding (Fig. 2A). Furthermore, the branch lengths
and the number of tubes at 24 h did not demonstrate any obvious
differences between H and HC groups (Fig. 2B and C). However, HGC-27 cells grown
on Matrigel under normoxic conditions, with or without endothelial
differentiation medium, did not form any obvious net-like
structures even at 48 h after seeding (Fig. 2A).
 | Figure 2.(A) HGC-27 cells were cultivated on
Matrigel under different conditions. Representative images
indicated that HGC-27 cells cultured for 0, 6, 12, 24 and 48 h
formed a network. The cells were cultured under hypoxia conditions,
H group and HC group (magnification, ×100; scale bar, 50 µm). (B)
Number of tubes in the photomicrographs of HGC-27 cells cultured
under different conditions. (C) Branch length measurements from
photomicrographs of tube formation. *P<0.05. Groups: H, HGC-27
cells in endothelial differentiation medium under hypoxia; HC,
HGC-27 cells in essential medium under hypoxia; N, HGC-27 cells in
endothelial differentiation medium under normoxia; C, HGC-27 cells
in essential medium under normoxia. NS, no significance. |
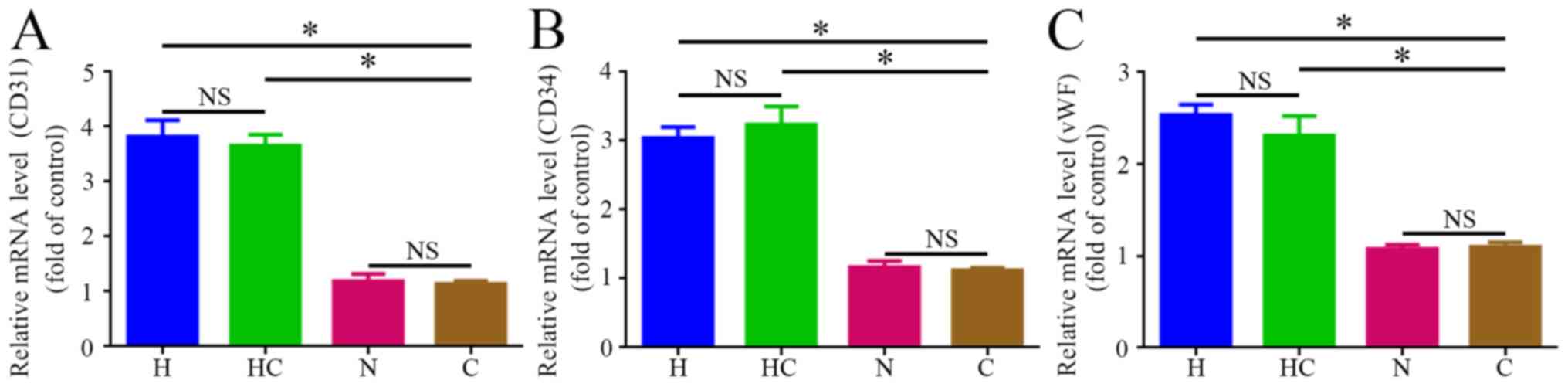
Increased expression of the EC markers
CD31, CD34 and vWF after transdifferentiation
To confirm whether HGC-27 cells transdifferentiated
to ECs under hypoxic conditions, the mRNA levels of the EC markers
CD31, CD34 and vWF were examined. Compared with the control group,
the transcription levels of these markers in HGC-27 cells were
significantly increased following exposure to hypoxic conditions, H
and HC groups. However, the transcription levels of these markers
were not significantly different between the N and C groups after
the cells were exposed to normoxic conditions (Fig. 3). In addition, immunofluorescent
staining was performed to assess the expression of these markers in
HGC-27 cells. Prior to treatment, HGC-27 cells were rarely positive
for CD31, CD34 and vWF. Of note, after exposure to hypoxic
conditions, most HGC-27 cells were positive for CD31, CD34 and vWF.
However, after culture under normoxic conditions, N (in endothelial
differentiation medium) and C groups (in RPMI-1640 medium only
containing 10% FBS), only a small percentage of HGC-27 cells were
positive for CD31, CD34 and vWF (Fig.
4). These results were consistent with those of the RT-qPCR
analysis.
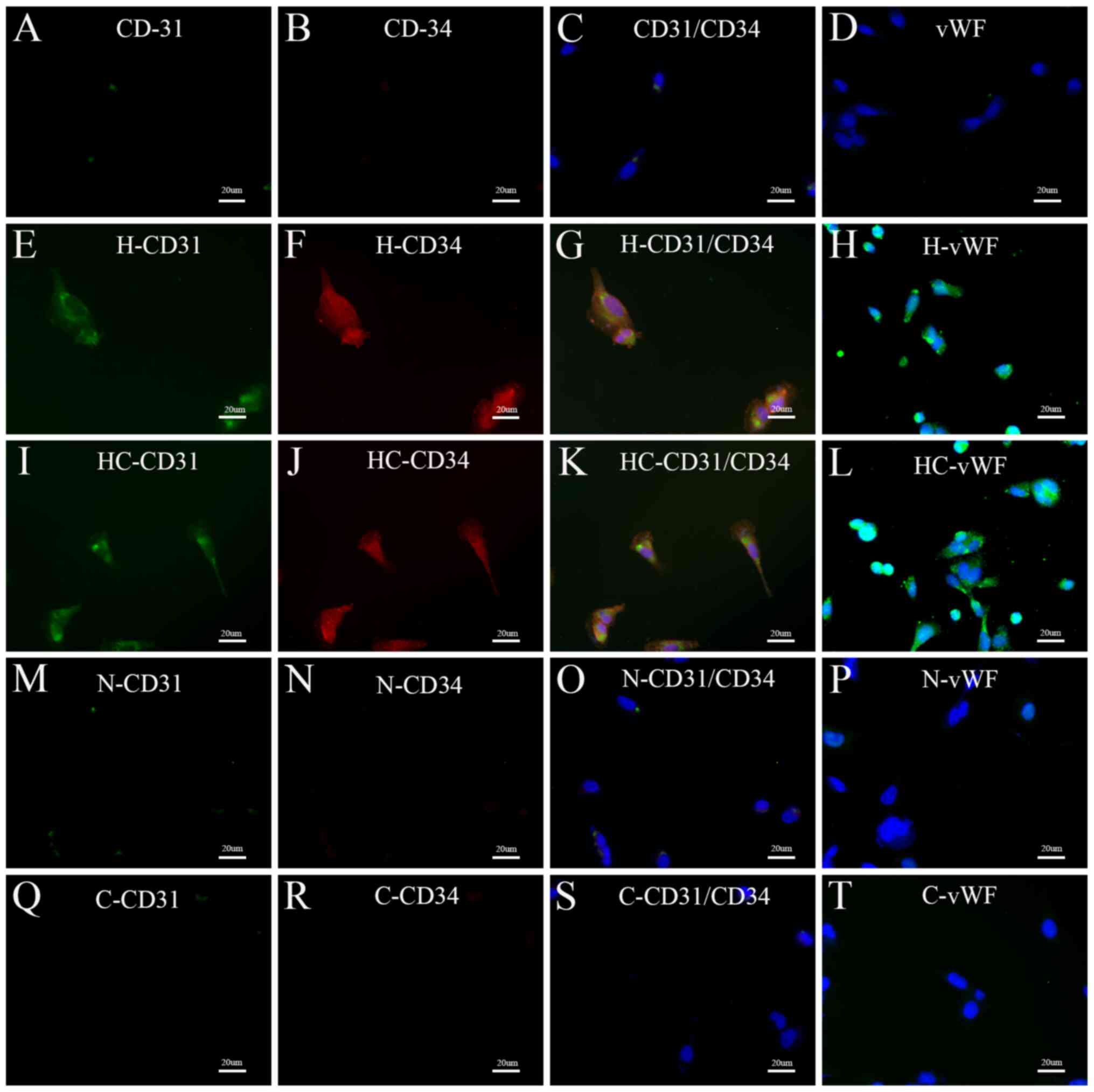 | Figure 4.Epifluorescent microscopy of HGC-27
cells prior to or after culture under different conditions. (A-D)
Immunofluorescent staining performed on HGC-27 cells prior to
culture under different conditions (A) CD-31, (B) CD-34, (C) CD31 +
CD34 merge and (D) vWF. (E-H) Immunofluorescent staining of cells
in the H group; (E) CD-31, (F) CD-34, (G) CD31 + CD34 merge and (H)
vWF. (I-L) immunofluorescent staining of cells in the GC group; (I)
CD-31, (J) CD-34, (K) CD31 + CD34 merge and (L) vWF. (M-P)
Immunofluorescent staining of cells in the N group; (M) CD-31, (N)
CD-34, (O) CD31 + CD34 merge and (P) vWF. (Q-T) Immunofluorescent
staining of cells in the C group (Q) CD-31, (R) CD-34, (S) CD31 +
CD34 merge and (T) vWF. CD31 is displayed in green, CD34 in red and
vWF in green (the nuclei stained with DAPI, blue) (magnification,
×400; scale bar, 20 µm). Groups: H, HGC-27 cells in endothelial
differentiation medium under hypoxia; HC, HGC-27 cells in essential
medium under hypoxia; N, HGC-27 cells in endothelial
differentiation medium under normoxia; C, HGC-27 cells in essential
medium under normoxia. vWF, von Willebrand factor. |
Discussion
Transdifferentiation occurs when a fully
differentiated cell loses its original phenotype under the
stimulation of certain factors and acquires a different cell
phenotype (23). In the present
study, HGC-27 cells were demonstrated to be able to
transdifferentiate into EC-like cells with characteristic
morphological and functional properties under hypoxic conditions.
GC cells originate from the endoderm (24), but the sole source of ECs appears to
be the mesoderm (25), implying that
the transformation of HGC-27 cells into EC-like cells was certainly
transdifferentiation.
Blood supply has a crucial role in tumor survival,
proliferation and metastasis (26).
Rapid growth of tumors causes a hypoxic microenvironment that
stimulates the formation of new blood vessels (27). An important part of the tumor
neovascularization process is VEC formation (28). In the present study, it was
demonstrated that HGC-27 cells transdifferentiated into EC-like
cells under hypoxic conditions. HGC-27 cells also expressed the EC
biomarkers CD31, CD34 and vWF. HGC-27 cells cultured under hypoxic
conditions had the characteristic flagstone appearance, the typical
morphological feature of ECs. Furthermore, HGC-27 cells cultured on
Matrigel gradually formed net-like structures.
Hypoxia is able to stimulate the formation of new
blood vessels (29). This mechanism
was thought to be hypoxia activating VEGF, which in turn stimulated
the proliferation and migration of endothelial cells and promoted
the production of new blood vessels (30). The present study provided evidence
for the transdifferentiation capability of HGC-27 cells into
EC-like cells under hypoxic conditions independent of exogenous
VEGF. These results suggested that hypoxia-induced
transdifferentiation of HGC-27 cells into EC-like cells is
exogenous VEGF-independent.
Several studies have demonstrated the pivotal role
of transcription factors in regulating a cell's fate (31). Cell fate is related to not only the
type of the transcription factor but also the proportion of
different transcription factors (32). These observations indicate that cell
type-specific programming may be revocable and the programming may
be re-regulated by modifying the activities of certain key
transcription factors (33). Several
types of malignant tumor cell may be transdifferentiated into
EC-like cells to acquire the phenotype of ECs, a process in which
transcription factors are involved (34,35).
However, the mechanisms of HGC-27 cell transdifferentiation into
EC-like cells and the transcription factors involved in the process
remain elusive, warranting further research.
There were certain shortcomings to the present
study. First, regarding the mechanism of HGC-27-cell
transdifferentiation into EC-like cells, the transcription factors
and potential other influencing factors involved in this process
remain elusive. Furthermore, the signaling pathways involved in the
mechanism of HGC-27-cell transdifferentiation under hypoxic
conditions requires further investigation. Finally, whether human
HGC-27 gastric cancer cells are able to transdifferentiate into
endothelial cells in vivo remains elusive and may be
assessed in a future study.
In conclusion, HGC-27 cells cultured in hypoxic
conditions demonstrated the morphological characteristics of ECs
and expressed EC markers CD31, CD34 and vWF. The present study
demonstrated that HGC-27 cells can transdifferentiate into
endothelial cells under hypoxic conditions in vitro.
Acknowledgements
The authors would like to thank Dr Jianhua Lin and
Dr Guangxian Zhong (Orthopaedic Research Institute, The First
Affiliated Hospital of Fujian Medical University, Fuzhou, China)
for providing technical assistance and useful discussions.
Funding
This study was supported by grants from the Science
and Technology Planning Project of Quanzhou City (grant no.
2019N040S).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CXC conducted the experiments and drafted the
manuscript. ZXH, MCW, ZCH, XBC and AYH contributed to statistical
analysis and manuscript writing. BBZ, LSW and YL participated in
performing the cell experiments. XWW and WFX conceived the present
study and helped revise the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Menbari MN, Nasseri S, Menbari N,
Mehdiabadi R, Alipur Y and Roshani D: The-160 (C>A) CDH1 gene
promoter polymorphism and its relationship with survival of
patients with gastric cancer in Kurdistan. Asian Pac J Cancer Prev.
18:1561–1565. 2017.PubMed/NCBI
|
|
2
|
Jmour O, Belaïd A, Mghirbi F, Béhi K,
Doghri R and Benna F: Gastric metastasis of bilateral breast
cancer. J Gastrointest Oncol. 8:E16–E20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Q, Li K, Tian S, Yu TH, Yu LH, Lin HD
and Bai DQ: Photodynamic therapy mediated by aloe-emodin inhibited
angiogenesis and cell metastasis through activating MAPK signaling
pathway on HUVECs. Technol Cancer Res Treat.
17:15330338187855122018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao C, Su Y, Zhang J, Feng Q, Qu L, Wang
L, Liu C, Jiang B, Meng L and Shou C: Fibrinogen-derived
fibrinostatin inhibits tumor growth through anti-angiogenesis.
Cancer Sci. 106:1596–1606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chamorro-Jorganes A, Lee M, Araldi E,
Landskroner-Eiger S, Fernández-Fuertes M, Sahraei M, Quiles Del Rey
M, van Solingen C, Yu J, Fernández-Hernando C, et al: VEGF-induced
expression of miR-17-92 cluster in endothelial cells is mediated by
ERK/ELK1 activation and regulates angiogenesis. Circ Res.
118:38–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garikipati V, Singh S, Mohanram Y, Gupta
A, Kapoor D and Nityanand S: Isolation and characterization of
mesenchymal stem cells from human fetus heart. PLoS One.
13:e01922442018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plummer P, Freeman R, Taft RJ, Vider J,
Sax M, Umer BA, Gao D, Johns C, Mattick JS, Wilton SD, et al:
MicroRNAs regulate tumor angiogenesis modulated by endothelial
progenitor cells. Cancer Res. 73:341–352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grigoras D, Pirtea L and Ceausu RA:
Endothelial progenitor cells contribute to the development of
ovarian carcinoma tumor blood vessels. Oncol Lett. 7:1511–1514.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen H, Campbell RA, Chang Y, Li M, Wang
CS, Li J, Sanchez E, Share M, Steinberg J, Berenson A, et al:
Pleiotrophin produced by multiple myeloma induces
transdifferentiation of monocytes into vascular endothelial cells:
A novel mechanism of tumor-induced vasculogenesis. Blood.
113:1992–2002. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Xu W, Wang S, Yu F, Feng J, Wang
X, Zhang L and Lin J: Transdifferentiation of human MNNG/HOS
osteosarcoma cells into vascular endothelial cells in vitro
and in vivo. Oncol Rep. 38:3153–3159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soda Y, Marumoto T, Friedmann-Morvinski D,
Soda M, Liu F, Michiue H, Pastorino S, Yang M, Hoffman RM, Kesari S
and Verma IM: Transdifferentiation of glioblastoma cells into
vascular endothelial cells. Proc Natl Acad Sci USA. 108:4274–4280.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang S, Xiang T, Huang S, Zhou J, Wang Z,
Xie R, Long H and Zhu B: Ovarian cancer stem-like cells
differentiate into endothelial cells and participate in tumor
angiogenesis through autocrine CCL5 signaling. Cancer Lett.
376:137–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pezzolo A, Marimpietri D, Raffaghello L,
Cocco C, Pistorio A, Gambini C, Cilli M, Horenstein A, Malavasi F
and Pistoia V: Failure of anti tumor-derived endothelial cell
immunotherapy depends on augmentation of tumor hypoxia. Oncotarget.
5:10368–10381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang H, Wu H, Zheng J, Yu P, Xu L, Jiang
P, Gao J, Wang H, Zhang Y, et al: Transforming growth factor β1
signal is crucial for dedifferentiation of cancer cells to cancer
stem cells in osteosarcoma. Stem Cells. 31:433–446. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin SC, Liao WL, Lee JC and Tsai SJ:
Hypoxia-regulated gene network in drug resistance and cancer
progression. Exp Biol Med (Maywood). 239:779–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang X, Yin H, Zhang Y, Li X, Tong H, Zeng
Y, Wang Q and He W: Hypoxia-induced autophagy promotes gemcitabine
resistance in human bladder cancer cells through hypoxia-inducible
factor 1α activation. Int J Oncol. 53:215–224. 2018.PubMed/NCBI
|
|
17
|
Joshi S, Singh A and Durden D: MDM2
regulates hypoxic hypoxia-inducible factor 1α stability in an E3
ligase, proteasome, and PTEN-phosphatidylinositol
3-kinase-AKT-dependent manner. J Biol Chem. 289:22785–22797. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park C, Ivanova I and Kenneth N: XIAP
upregulates expression of HIF target genes by targeting HIF1α for
Lys63-linked polyubiquitination. Nucleic Acids Res. 45:9336–9347.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim TH, Hur E, Kang SJ, Kim JA, Thapa D,
Lee YM, Ku SK, Jung Y and Kwak MK: NRF2 blockade suppresses colon
tumor angiogenesis by inhibiting hypoxia-induced activation of
HIF-1α. Cancer Res. 71:2260–2275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greenberger LM, Horak ID, Filpula D, Sapra
P, Westergaard M, Frydenlund HF, Albaek C, Schrøder H and Ørum H: A
RNA antagonist of hypoxia-inducible factor-1alpha, EZN-2968,
inhibits tumor cell growth. Mol Cancer Ther. 7:3598–3608. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toffoli S, Roegiers A, Feron O, Van
Steenbrugge M, Ninane N, Raes M and Michiels C: Intermittent
hypoxia is an angiogenic inducer for endothelial cells: Role of
HIF-1. Angiogenesis. 12:47–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen C, Burke Z and Tosh D:
Transdifferentiation, metaplasia and tissue regeneration.
Organogenesis. 1:36–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chera S, Ghila L, Dobretz K, Wenger Y,
Bauer C, Buzgariu W, Martinou JC and Galliot B: Apoptotic cells
provide an unexpected source of Wnt3 signaling to drive hydra head
regeneration. Dev Cell. 17:279–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lugus J, Park C, Ma Y and Choi K: Both
primitive and definitive blood cells are derived from Flk-1+
mesoderm. Blood. 113:563–566. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Crippa L, Gasparri A, Sacchi A, Ferrero E,
Curnis F and Corti A: Synergistic damage of tumor vessels with
ultra low-dose endothelial-monocyte activating polypeptide-II and
neovasculature-targeted tumor necrosis factor-alpha. Cancer Res.
68:1154–1161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tahmasebi Birgani Z, Fennema E, Gijbels M,
de Boer J, van Blitterswijk C and Habibovic P: Stimulatory effect
of cobalt ions incorporated into calcium phosphate coatings on
neovascularization in an in vivo intramuscular model in goats. Acta
Biomater. 36:267–276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li B, Nie Z, Zhang D, Wu J, Peng B, Guo X,
Shi Y, Cai X, Xu L and Cao F: Roles of circulating endothelial
progenitor cells and endothelial cells in gastric carcinoma. Oncol
Lett. 15:324–330. 2018.PubMed/NCBI
|
|
29
|
Acurio J, Troncoso F, Bertoglia P, Salomon
C, Aguayo C, Sobrevia L and Escudero C: Potential role of A2B
adenosine receptors on proliferation/migration of fetal endothelium
derived from preeclamptic pregnancies. Biomed Res Int.
2014:2745072014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Longchamp A, Mirabella T, Arduini A,
MacArthur MR, Das A, Treviño-Villarreal JH, Hine C, Ben-Sahra I,
Knudsen NH, Brace LE, et al: Amino acid restriction triggers
angiogenesis via GCN2/ATF4 regulation of VEGF and H2S
production. Cell. 173:117–129.e114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lam E, Francis R and Petkovic M: FOXO
transcription factors: Key regulators of cell fate. Biochem Soc
Trans. 34:722–726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sisci D, Maris P, Cesario MG, Anselmo W,
Coroniti R, Trombino GE, Romeo F, Ferraro A, Lanzino M, Aquila S,
et al: The estrogen receptor α is the key regulator of the
bifunctional role of FoxO3a transcription factor in breast cancer
motility and invasiveness. Cell Cycle. 12:3405–3420. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun X, Wang X, Tang Z, Grivainis M, Kahler
D, Yun C, Mita P, Fenyö D and Boeke JD: Transcription factor
profiling reveals molecular choreography and key regulators of
human retrotransposon expression. Proc Natl Acad Sci USA.
115:E5526–E5535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kohu K, Sato T, Ohno SI, Hayashi K, Uchino
R, Abe N, Nakazato M, Yoshida N, Kikuchi T, Iwakura Y, et al:
Overexpression of the Runx3 transcription factor increases the
proportion of mature thymocytes of the CD8 single-positive lineage.
J Immunol. 174:2627–2636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu T, Sims D and Baum B: Parallel RNAi
screens across different cell lines identify generic and cell
type-specific regulators of actin organization and cell morphology.
Genome Biol. 10:R262009. View Article : Google Scholar : PubMed/NCBI
|