Introduction
Biliary tract cancers (BTCs) are rare tumors arising
from the biliary tree epithelium, from the small peripheral hepatic
ducts to the distal common bile duct. The progress on the treatment
strategies for patients with BTCs has been slow in the past
decades. The disease prognosis remains poor, with a modest
improvement from 11 to 17% in terms of 5-year overall survival (OS)
rates (1).
Complete surgical resection or liver
transplantation, when feasible, are the only potentially curative
treatments in the early stages of BTCs (2). In advanced stages, standard
chemotherapy (CT) in combination with palliative supportive care,
such as biliary drainage or stenting, is the only available
therapeutic option, providing a survival advantage with a modest
impact and benefit in terms of quality of life (3,4).
Gemcitabine plus cisplatin regimen is the current standard
first-line treatment, with a median OS of less than 1 year
(4). However, no standard
second-line CT regimens have been established.
In recent years, whole-genome tumor profiling
studies have identified a wide variety of genetic alterations, many
of them considered targetable therapeutic options (HER2, BRAF,
FGFR1-3, IDH1/2, MET and MEK) (5,6). In
addition, preliminary trials in selected populations treated with
targeted therapies have demonstrated promising improvement in
survival outcome (7–9). Immunotherapy is considered a
revolutionary treatment method for selected patients and
preliminary preclinical data have revealed encouraging results,
even in BTCs (10).
C-ros oncogene 1 (ROS1), a proto-oncogene,
encodes a receptor tyrosine kinase (RTK) without a known ligand.
ROS1 shares a high structural homology with the insulin receptor
family and the anaplastic lymphoma kinase (ALK) (11). When ROS1 is constitutively
activated by gene rearrangement, the RTK is overexpressed and is
likely detected using immunohistochemistry (IHC). It has been
reported that chromosomal rearrangements lead to fusion of
ROS1 with several partner genes, resulting in the formation
of a constitutively active fusion kinase (12). This kinase induces mitogen-activated
protein kinase, signal transducer and activator of transcription 3
and phosphoinositide 3-kinase pathways, among others, subsequently
promoting cellular transformation (12). These rearrangements, also evidenced
by the aberrant expression of the RTK ROS1, have been detected in
several types of cancer, including 1–2% of lung adenocarcinoma
cases, glioblastoma, cholangiocarcinoma (CAC) and others (13,14). In
lung cancer, clinical and epidemiological published trials have
already described the incidence and prevalence of ROS1 as
well as its predictive and prognostic role. However, there is
currently a lack of consistent evidence regarding ROS1 gene
rearrangements and its protein expression in other neoplasms,
including BTCs (14).
It has been shown that ROS1 and ALK share
significant homology within their respective tyrosine kinase (TK)
domains. This finding led to the hypothesis that ALK tyrosine
kinase inhibitors (TKIs) may also inhibit ROS1 expression (15). Based on promising preclinical data
with different ALK TKIs, several clinical studies have been
performed in ROS1-positive NSCLC patients with interesting results.
For example, Shaw et al (15)
demonstrated a progression free survival (PFS) of 19.2 months and a
response rate of 72% in 50 ROS1-positive lung cancer patients
treated with crizotinib (16–19).
In a case series of various tumors, ROS1
rearrangements were detected in 2 out of 23 patients (8.7%) with
BTCs (20). However, in a cohort of
56 Chinese CAC patients no ROS1 rearrangements were observed
(21). Additionally, Graham et
al (22) reported one case with
ROS1 rearrangements among 100 CAC cases. Of note, the
ROS1 positive case also harbored an IDH1 mutation
(22,23). Recently, two additional studies on
Asiatic cohorts of BTCs patients reported ROS1
rearrangements in 0 and 1.1%, respectively (24,25). The
present study aimed to identify the incidence of ROS1
rearrangements in a retrospective, Italian and multicentric cohort
of patients with BTCs. All cases were tested using IHC and the
results from three different commercially available ROS1 primary
antibodies (Abs) (clones D4D6, PA1-30318 and EPMGHR2) were
compared. Positive cases were further analyzed by fluorescence
in situ hybridization (FISH) to confirm the presence of
ROS1 rearrangements.
Materials and methods
Study aim and design
The present multicenter, retrospective study was
conducted by the Italian Clinical Oncology Research Group (GOIRC)
and included eight Italian centers as follows: Azienda
Ospedaliero-Universitaria Careggi, Florence; Regional Hospital
Parini, Aosta; Santa Maria delle Croci Hospital, Ravenna; Santa
Chiara Hospital, Pisa; Santa Maria Nuova Hospital, Reggio Emilia;
IRSST, Meldola; Maggiore Hospital, Parma; and San Luca Hospital,
Lucca. In the present study, 150 cases of BTCs, diagnosed between
January 2012 and December 2015 using surgical specimens (n=98) or
liver biopsy (n=52), were enrolled. All cases were eligible for
inclusion in the study and sufficient material was available for
IHC and FISH analyses. At the time of diagnosis patients were ≥8
years old. All subjects provided written informed consent according
to the Local Ethical Committees.
Histopathological samples were centrally reviewed
and analyzed at the Pathology Units of the Regional Hospital Parini
and Santa Maria delle Croci Hospital. The clinicopathological
characteristics, including age, sex, disease stage, treatment and
survival rate, were analyzed from the patients' medical records and
referring physicians.
The association between ROS1 expression and survival
parameters and clinicopathological characteristics, collected from
the available medical records, was considered as the secondary
endpoint of the study. OS was defined as the time between diagnosis
and death, resulting from any cause. In particular, the secondary
end point was to evaluate the potential prognostic role of ROS1
expression by comparing the ROS1-positive population with
ROS1-negative tumors. The study was conducted in accordance with
the precepts of the Good Clinical Practice guidelines and the
Declaration of Helsinki. In addition, the present study was
approved by the Ethics Committee of each institute and written
informed consent was obtained from all participants.
IHC staining
IHC staining was performed on 4-µm sections obtained
from formalin-fixed and paraffin-embedded tissue blocks that were
subsequantelly mounted on charged slides. Following
deparaffinization and rehydration, antigen retrieval was carried
out using a Cell Conditioning 1 (CC1) solution for 64 min at 95°C.
ROS1 IHC assay was performed using three different primary Abs,
monoclonal EPMGHR2 (1:200 dilution; Abcam), monoclonal D4D6 (1:50
dilution; Cell Signaling Technology, Inc.) and polyclonal PA1-30318
(1:200 dilution; Thermo Fisher Scientific, Inc.). All assays were
carried out using an automated immunostainer (VENTANA. BenchMark
ULTRA system; Ventana Medical Systems, Inc.). The intensity of
immunostaining in samples was scored by an experienced pathologist
using the H-score method. The scoring system was based on intensity
(0, no staining; 1+, weak staining; 2+, moderate staining; and 3+,
strong staining) and the percentage of positive tumor cells.
Therefore, a H-score range of 0–300 was recorded in each case.
Based on a previous study in NSCLC (26), a case was considered positive when
the H-score was >100. In all batches, a negative (without a
primary antibody) and a positive (lung adenocarcinoma previously
evaluated as ROS1-positive using IHC and FISH analyses) control
were employed to evaluate the appropriateness of the IHC
analysis.
FISH analysis
In the present study FISH analysis was performed
using a commercially available assay (The ZytoLight®
SPEC ROS1 Dual Color Break Apart Probe; ZytoVision GmbH) according
to the manufacturer's recommendations. At least 50 tumor cells from
each sample were analyzed and scored according to the guidelines of
the European recommendations (27).
The probes labeled the 5′ (telomeric) and 3′
(centromeric) ends of the fusion breakpoint with green and orange
fluorochromes, respectively. The criteria for ROS1 FISH
interpretation in the tested tumors were the following: i) The
break-apart pattern (‘conventional’ pattern) with one fusion signal
and two separated 3′ and 5′ signals; and ii) an atypical pattern
showing an isolated 3′ signal (usually one fusion signal and one
isolated 3′ green signal without the corresponding 5′ signal). The
cut-off of rearranged signals to quote ROS1 positivity was based on
detection of ≥15% among 50 neoplastic nuclei.
Statistical analysis
No statistical analysis was reported since the
expression value of ROS1 with FISH analysis was negative in all
cases and the association between ROS1 expression and no
associations with clinicopathological parameters of patients was
determined.
Results
Study population
In this study, 150 CAC samples were collected,
including 98 surgical specimens and 52 biopsies. The samples were
collected from the medical archive corresponding to cases diagnosed
between January 2012 and December 2015. The available clinical data
were derived from 100 patients' medical records and referring
physicians, including 69 males and 31 females with a median age at
diagnosis of 70 years (range, 35–84 years). The Eastern Cooperative
Oncology Group (ECOG) performance status at diagnosis was 0 in 59,
1 in 27, 2 in 11 patients and unknown in the remaining 3 cases. The
primary tumor origin was: intrahepatic bile ducts (n=56); hilar
(n=4); extrahepatic (n=32); gallbladder (n=6); while in 2 patients
the tumor arised from an unknown primary site. In addition, the
clinical stage distribution at diagnosis was as follows: 48 cases
exhibited locally recurrent disease; 24 locally advanced disease;
26 metastatic disease; and 2 cases were undetermined. Furthermore,
67 patients underwent surgery and among them, 58 patients
experienced radical R0 resection. At the time of data collection
(April 2018), 22 patients were still alive, while 78 died,
including 50 who exhibited disease relapse or progression.
Patients' baseline characteristics are presented in Table I.
 | Table I.Clinical characteristics of patients
with biliary tract cancer. |
Table I.
Clinical characteristics of patients
with biliary tract cancer.
| Parameters | Value, n (%) |
|---|
| Sex |
|
Male | 69/100 (69) |
|
Female | 31/100 (31) |
| Age, years (median,
range) | 70 (35–84) |
| Eastern Cooperative
Oncology |
| Group Performance
Status |
| 0 | 59/100 (59) |
| 1 | 27/100 (27) |
| 2 | 11/100 (11) |
|
Unknown | 3/100
(3) |
| Disease stage |
|
Localized | 48/100 (48) |
|
Locally-advanced | 24/100 (24) |
|
Metastatic | 26/100 (26) |
|
Unknown | 2/100
(2) |
| Surgery | 67/100 (67) |
| R0 | 58/67 (87) |
| R1 | 5/67
(7) |
| R2 | 2/67
(3) |
|
Unknown | 2/67
(3) |
| Site |
|
Intrahepatic bile ducts | 56/100 (56) |
| Hilar
ducts | 4/100
(4) |
|
Extrahepatic ducts | 32/100 (32) |
|
Gallbladder | 6/100
(6) |
|
Unknown | 2/100
(2) |
According to the 2015 revised classification of
intrahepatic CAC, pathological diagnosis was consistent with
conventional small duct type (15 cases), bile ductular type (1
case), intraductal papillary type (1 case), intraductal tubular
type (1 case), squamous/adeno squamous cell type (1 case),
mucinous/signet ring cell type (5 cases) and undifferentiated type
(2 cases). Other rare types were reported in 9 cases, whereas in 13
cases the pathological subtype was undetermined. Regarding
perihilar and distal CAC, pathological subtype was conventional in
10 cases, intraductal papillary type in 1 case, squamous/adeno
squamous cell type in 1 case, mucinous/signet ring cell type in 1
case, while rare and undetermined types were reported in 3 and 12
cases, respectively. Finally, 6 cases of gallbladder adenocarcinoma
were included in the study.
Considering treatment strategy, 45/48 patients
(93.75%) with early stage BTC at diagnosis, received radical
surgery as first-line treatment. In 41 of these cases (91%), no
residual tumor (R0) following surgery was observed, while 4 cases
exhibited positive surgical margins (R1). Following surgery, 18
patients were treated with gemcitabine-based adjuvant CT. At
follow-up, 31 patients experienced disease relapse and among them,
21 patients were subsequently treated with alternative platinum- or
fluoropyrimidine-based CT treatment.
Furthermore, 24 patients were diagnosed with locally
advanced disease, therefore 19 of them underwent surgery and R0
status was observed in 16 cases. Of the remaining 3 patients, 1 was
treated with R1 surgery and 2 patients exhibited R2 positive
margins. Additionally, 7/24 locally advanced patients were treated
with gemcitabine-based adjuvant CT after surgery. Conversely, 2
patients received induction CT with platinum/gemcitabine regimen
prior to surgery. At follow-up, 12 patients experienced disease
relapse with 10 cases being subsequently treated with alternatively
platinum- or fluoropyrimidine-based CT. In addition, 26 patients
were diagnosed with metastatic disease. Nevertheless, 3 patients
received surgical treatment and among them, 1 patient exhibited R0
status.
Overall, 55 patients received a first-line CT for
advanced disease (alternatively platinum, gemcitabine- or
fluoropyrimidine-based; single agent or combination regimen).
Furthermore, following progression, 27 patients were treated with a
second-line CT.
ROS1 IHC and FISH findings
Although several studies with selected Abs have
shown significant correlation between molecular (extractive or
in situ) and IHC assays for ROS1 detection, the present
study is the first to directly compare various primary Abs against
ROS1. Therefore, a group of 150 BTCs was evaluated for ROS1
positivity, using three different primary Ab clones in order to
determine their relative sensitivity and specificity. ROS1 protein
expression was evaluated with clone D4D6 and two clones tested for
the first time, namely EPMGHR2 and PA1-30318. The main
characteristics of the three primary Abs used in the study are
summarized in Table II. The scoring
distribution of IHC results for each primary Ab is presented in
Table III. ROS1 was differentially
expressed, based on primary Ab clones used, whereas no
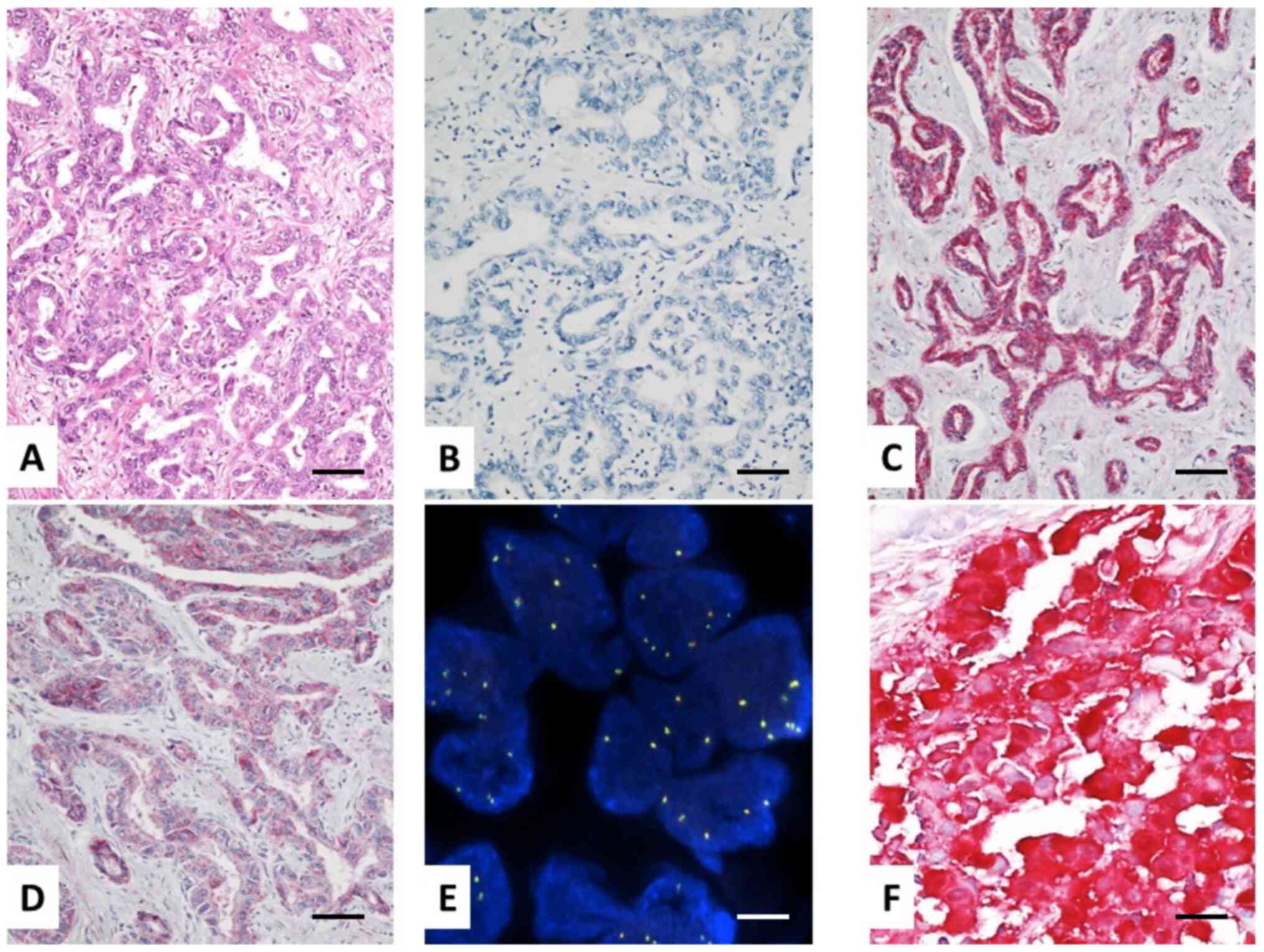
ROS1-positive tumors were observed when clone D4D6 was applied. By
contrast, 22 (14.6%) and 28 (18.6%) cases showed positive staining
when polyclonal PA1-30318 and monoclonal EPMGHR2 Abs were used,
respectively. Furthermore, both Abs exhibited higher
immunoreactivity in surgically-derived samples compared with that
noted to biopsies (Table III and
Fig. 1).
 | Table II.C-ros oncogene 1 antibodies tested in
the cohort of patients with biliary tract cancer. |
Table II.
C-ros oncogene 1 antibodies tested in
the cohort of patients with biliary tract cancer.
| Primary
antibody | Dilution | Commercial
source | Epitope | Isotype |
|---|
| Monoclonal
D4D6 | 1:50 | Cell Signaling
Technology, Inc. | Carboxy terminal
domain | Rabbit IgG |
| Monoclonal
EPMGHR2 | 1:200 | Abcam | aa 2050–2150 | Rabbit IgG |
| Polyclonal
PA1-30318 | 1:200 | Thermo Fisher
Scientific, Inc. | aa 39–57 | Rabbit IgG |
 | Table III.Distribution of C-ros oncogene 1
expression according to different primary antibody clones used in
immunohistochemistry experiments. |
Table III.
Distribution of C-ros oncogene 1
expression according to different primary antibody clones used in
immunohistochemistry experiments.
| Sample type | Clone D4D6 | Clone EPMGHR2 | Clone SP384 |
|---|
| Biopsy, n | 0/52 |
7/52 |
5/52 |
| Surgical sample,
n | 0/98 | 21/98 | 17/98 |
| Total, n (%) | 0/150 | 28/150 (18.6) | 22/150 (14.6) |
Subsequently, FISH analysis was performed in 75 BTC
cases, including 25 randomly selected negative cases that were
tested with all clones and, 22 and 28 ROS1 positive tumors
evaluated with PA1-30318 and EPMGHR2 Abs, respectively. Overall,
FISH was negative in all cases. The results obtained with clone
D4D6 were consistent with FISH analysis. Therefore, clone D4D6
exhibited the highest specificity and association with FISH assay
in detecting ROS1 rearrangements, while false positive
results were obtained using the other clones. The results of ROS1
immunostaining reported to date in the literature are summarized in
Table IV.
 | Table IV.Literature review: C-ros oncogene 1
expression (%) in cholangiocarcinoma using different antibodies and
techniques. |
Table IV.
Literature review: C-ros oncogene 1
expression (%) in cholangiocarcinoma using different antibodies and
techniques.
|
| IHC | FISH |
|
|
|
|---|
|
|
|
|
|
|
|
|---|
| Author, year | D4D6 | SP384 | EPMGHR2 | Break-apart | Exon 30 | RT-qPCR | Immunoaffinity | Refs. |
|---|
| Gu et al,
2011 | – | – | – | – | – | – | 8.7 | (20) |
| Liu et al,
2013 | – | – | – | – | – | 0 | – | (21) |
| Graham et
al, 2014 | – | – | – | 1 | – | – | – | (22) |
| Arai et al,
2014 | – | – | – | – | – | 0 | – | (28) |
| Lee et al,
2015 | 37.1 | – | – | – | 0 | – | – | (24) |
| Lim et al,
2017 | 19.1 | – | – | 1.1 | – | – | – | (25) |
Discussion
Due to controversial results having previously been
reported, ROS1 rearrangements in a large cohort of BTCs were
screened. To date, ROS1 rearrangements have been identified
only in 6 patients with BTCs (20,22,25). In
particular, Gu et al (20)
showed the presence of FIG-ROS1 rearrangement in 2/23
patients with CAC (8.7%), whereas Graham et al (22) reported a single case (1/100, 1%) with
ROS1 translocation and concurrent IDH1 mutation. In
addition, Lim et al (25)
demonstrated a frequency of ROS1 rearrangements of 1.1%
(3/261), in the largest cohort to date. However, the frequency of
ROS1 rearrangements has not been fully elucidated. Arai
et al (28) and Lim et
al (25) revealed that no
FIG-ROS1 fusion was detected in CAC when screened by RT-qPCR
and FISH, respectively. Screening of ROS1 rearrangements is
generally performed using IHC or FISH assays. In lung cancer, the
application of both assays is recommended in order to confirm
positive results (29).
In particular, we aimed to investigate the incidence
of ROS1 rearrangements in BTCs by exploiting IHC and FISH
techniques. The efficiency of IHC method was examined by comparing
three different commercially available primary Abs. To date, only a
study conducted by Conde et al (27) compared ROS1 expression using two
different ROS1 primary Abs, namely D4D6 and SP384, in a selected
cohort of NSCLC patients. This study suggested that SP384 clone was
less specific compared with D4D6, as a higher number of false
positive results were obtained (25 and 9% for each Ab,
respectively) (27). The results of
the present study confirmed that the D4D6 clone was more accurate
compared with polyclonal PA1-30318 and monoclonal EPMGHR2 Abs.
Furthermore, negative IHC results obtained with the D4D6 Ab, were
consistent with the results emerged by FISH analysis. By contrast,
the positive results obtained using PA1-30318 (14.6%) and EPMGHR2
(18.6%) Abs were not confirmed by FISH analysis. Unlike previous
studies, the present study did not detect ROS1 expression in 150
Italian patients with BTCs (20,22,25,30).
Previous works by Lee et al (24) and Lim et al (25) have detected ROS1 by IHC with clone
D4D6 in 37.1 and 19.1%, respectively. None out of 102 cases
(24) and 3 out of 261 cases (1.1%)
(25) were finally resulted as
positive at FISH analysis, respectively. Nevertheless, the authors
used a very low immunohistochemical cut-off of expression, namely
any staining in the study of Lim et al (25) and at least 5% of stained tumor cells
with at least 2+ of staining intensity in the report by Lee et
al (24). In the present study
we quoted a positive expression using a more robust cut-off, namely
H-score >100, then explaining the discrepancy of positive cases
between the present and previous observations (24,25).
In a recent study by Lowery et al (31), 195 patients with intrahepatic CAC
were prospectively examined by targeted next generation sequencing
(NGS) assay, including exons and selected introns from 410 tumor
genes. The results of this study demonstrated that the most
commonly altered genes in CAC were IDH1 (30%), ARID1A
(23%), BAP1 (20%), TP53 (20%) and FGFR2 gene
fusions (14%) (31). In addition,
the most common genetic alterations in extrahepatic CAC were
detected in KRAS, SMAD4 and STK11 genes. Of note, the
authors did not reveal ROS1 gene alterations in their large cohort
of patients, although an advanced sequencing technique was
performed (31).
In summary, the present study suggests that
ROS1 rearrangements in BTCs are not considered reliable
molecular targets for the development of novel and selective
therapeutic approaches. ROS1 gene alterations in BTCs, that
have been reported in previous studies, may be considered sporadic
cases or false-positive results. Therefore, no further studies are
required to detect ROS1 rearrangements in BTCs. However,
further research on alternative pathways for the detection of
consistent genetic alterations driving to BTC carcinogenesis is
needed. These pathways may serve as promising prognostic biomarkers
and therapeutic targets for BTCs.
Acknowledgements
The authors would like to thank Dr Luisa Di Cerbo
(Medical Oncology Unit, Azienda Ospedaliero-Universitaria Careggi,
Florence, Italy) for her work in data collection and
management.
Funding
The present study was supported by Gruppo Oncologico
Italiano di Ricerca Clinica (grant no. GOIRC07/2016) and a grant by
Pfizer (grant no. 2017GO-It-FI).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FM and LA conceived the study design. FM and SP
performed manuscript drafting. PP and SP were responsible for data
collection and analysis. EV, MP, ACG, FN, AL, CV, EG, GLF, LM, GJ
and AB collected data, enrolled patients and performed data
interpretation. GR performed data interpretation and wrote the
manuscript. LA revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with Good
Clinical Practice guidelines, the Declaration of Helsinki, and with
approval from each institutional Ethical Committee of the Centers
involved (Careggi Hospital-Florence, Regional Hospital
Parini-Aosta, Santa Maria delle Croci Hospital-Ravenna, Santa
Chiara Hospital-Pisa, Santa Maria Nuova Hospital-Reggio Emilia,
IRSST-Meldola, Maggiore Hospital-Parma and San Luca
Hospital-Lucca). Written informed consent was obtained from all
patients involved.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Italian Association Medical Oncology
association tumor register. The numbers of cancer in Italy. 2017,
simplehttp://www.registri-tumori.it/cms/pubblicazioni/i-numeri-del-cancro-italia-2017Published
September, 2017. July 6–2018
|
|
2
|
Huguet JM, Lobo M, Labrador JM, Boix C,
Albert C, Ferrer-Barceló L, Durá AB, Suárez P, Iranzo I, Gil-Raga
M, et al: Diagnostic-therapeutic management of bile duct cancer.
World J Clin Cases. 7:1732–1752. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Glimelius B, Hoffman K, Sjödén PO,
Jacobsson G, Sellström H, Enander LK, Linné T and Svensson C:
Chemotherapy improves survival and quality of life in advanced
pancreatic and biliary cancer. Ann Oncol. 7:593–600. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valle J, Wasan H, Palmer DH, Cunningham D,
Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira
SP, et al ABC-02 Trial Investigators, : Cisplatin plus gemcitabine
versus gemcitabine for biliary tract cancer. N Engl J Med.
362:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jain A and Javle M: Molecular profiling of
biliary tract cancer: A target rich disease. J Gastrointest Oncol.
7:797–803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jusakul A, Cutcutache I, Yong CH, Lim JQ,
Huang MN, Padmanabhan N, Nellore V, Kongpetch S, Ng AWT, Ng LM, et
al: Whole-Genome and Epigenomic Landscapes of Etiologically
Distinct Subtypes of Cholangiocarcinoma. Cancer Discov.
7:1116–1135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Javle M, Lowery M, Shroff RT, Weiss KH,
Springfeld C, Borad MJ, Ramanathan RK, Goyal L, Sadeghi S,
Macarulla T, et al: Phase II Study of BGJ398 in Patients With
FGFR-Altered Advanced Cholangiocarcinoma. J Clin Oncol. 36:276–282.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goyal L, Arkenau HT, Tran B, Soria J-C,
Bahleda R, Mak G, Zhu A, Javle M, Hiroshi H, Benedetti F, et al:
Early clinical efficacy of TAS-120, a covalently bound FGFR
inhibitor, in patients with cholangiocarcinoma. Ann Oncol. 28
(Suppl 3):202017. View Article : Google Scholar
|
|
9
|
Blair AB and Murphy A: Immunotherapy as a
treatment for biliary tract cancers: A review of approaches with an
eye to the future. Curr Probl Cancer. 42:49–58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Davies KD and Doebele RC: Molecular
pathways: ROS1 fusion proteins in cancer. Clin Cancer Res.
19:4040–4045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Uguen A and De Braekeleer M: ROS1 fusions
in cancer: A review. Future Oncol. 12:1911–1928. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bansal M, He J, Peyton M, Kustagi M, Iyer
A, Comb M, White M, Minna JD and Califano A: Elucidating
synergistic dependencies in lung adenocarcinoma by proteome-wide
signaling-network analysis. PLoS One. 14:e02086462019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bergethon K, Shaw AT, Ou SH, Katayama R,
Lovly CM, McDonald NT, Massion PP, Siwak-Tapp C, Gonzalez A, Fang
R, et al: ROS1 rearrangements define a unique molecular class of
lung cancers. J Clin Oncol. 30:863–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jänne PA and Meyerson M; J2012 PA, : ROS1
rearrangements in lung cancer: A new genomic subset of lung
adenocarcinoma. J Clin Oncol. 30:878–879. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shaw AT, Ou SH, Bang YJ, Camidge DR,
Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa
DB, et al: Crizotinib in ROS1-rearranged non-small-cell lung
cancer. N Engl J Med. 371:1963–1971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mazières J, Zalcman G, Crinò L, Biondani
P, Barlesi F, Filleron T, Dingemans AM, Léna H, Monnet I,
Rothschild SI, et al: Crizotinib therapy for advanced lung
adenocarcinoma and a ROS1 rearrangement: results from the EUROS1
cohort. J Clin Oncol. 33:992–999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiari R, Buttitta F, Iacono D, Bennati C,
Metro G, Di Lorito A, Iezzi M, Tiseo M, Mazzoni F, Cappuzzo F, et
al: Dramatic response to crizotinib in ROS1 fluorescent in situ
hybridization- and immunohistochemistry-positive lung
adenocarcinoma: a case series. Clin Lung Cancer. 15:470–474. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Landi L, Chiari R, Tiseo M, D'Incà F,
Dazzi C, Chella A, Delmonte A, Bonanno L, Giannarelli D, Cortinovis
DL, et al: Crizotinib in MET-Deregulated or ROS1-Rearranged
Pretreated Non-Small Cell Lung Cancer (METROS): A Phase II,
Prospective, Multicenter, Two-Arms Trial. Clin Cancer Res.
25:7312–7319. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petreni P, Mazzoni F, Meoni G, Lunghi A,
Cecere FL, Muto A and Di Costanzo F: Outcome of crizotinib
treatment in a young woman with heavily pretreated ROS1-positive
lung cancer. Tumori. 101:e103–e106. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu TL, Deng X, Huang F, Tucker M, Crosby
K, Rimkunas V, Wang Y, Deng G, Zhu L, Tan Z, et al: Survey of
tyrosine kinase signaling reveals ROS kinase fusions in human
cholangiocarcinoma. PLoS One. 6:e156402011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu P, Wu Y, Sun L, Zuo Q and Shi M: ROS
kinase fusions are not common in Chinese patients with
cholangiocarcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 33:474–478.
2013.PubMed/NCBI
|
|
22
|
Graham RP, Barr Fritcher EG, Pestova E,
Schulz J, Sitailo LA, Vasmatzis G, Murphy SJ, McWilliams RR, Hart
SN, Halling KC, et al: Fibroblast growth factor receptor 2
translocations in intrahepatic cholangiocarcinoma. Hum Pathol.
45:1630–1638. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kipp BR, Voss JS, Kerr SE, Barr Fritcher
EG, Graham RP, Zhang L, Highsmith WE, Zhang J, Roberts LR, Gores
GJ, et al: Isocitrate dehydrogenase 1 and 2 mutations in
cholangiocarcinoma. Hum Pathol. 43:1552–1558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee KH, Lee KB, Kim TY, Han SW, Oh DY, Im
SA, Kim TY, Yi NJ, Lee KW, Suh KS, et al: Clinical and pathological
significance of ROS1 expression in intrahepatic cholangiocarcinoma.
BMC Cancer. 15:7212015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lim SM, Yoo JE, Lim KH, Meng Tai DW, Cho
BC and Park YN: Rare Incidence of ROS1 Rearrangement in
Cholangiocarcinoma. Cancer Res Treat. 49:185–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rossi G, Jocollé G, Conti A, Tiseo M, Zito
Marino F, Donati G, Franco R, Bono F, Barbisan F and Facchinetti F:
Detection of ROS1 rearrangement in non-small cell lung cancer:
Current and future perspectives. Lung Cancer (Auckl). 8:45–55.
2017.PubMed/NCBI
|
|
27
|
Conde E, Hernandez S, Martinez R, De
Castro J, Collazo-Lorduy A, Jimenez B, Muriel A, Mate JL, Morán T,
Aranda I, et al: Evaluation of a Novel ROS1 Immunohistochemistry
Clone (SP384) for the Identification of ROS1 Rearrangements in
NSCLC Patients. J Thorac Oncol. 13 (Suppl 10):S553–S554. 2018.
View Article : Google Scholar
|
|
28
|
Arai Y, Totoki Y, Hosoda F, Shirota T,
Hama N, Nakamura H, Ojima H, Furuta K, Shimada K, Okusaka T, et al:
Fibroblast growth factor receptor 2 tyrosine kinase fusions define
a unique molecular subtype of cholangiocarcinoma. Hepatology.
59:1427–1434. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bubendorf L, Büttner R, Al-Dayel F, Dietel
M, Elmberger G, Kerr K, López-Ríos F, Marchetti A, Öz B, Pauwels P,
et al: Testing for ROS1 in non-small cell lung cancer: A review
with recommendations. Virchows Arch. 469:489–503. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abou-Alfa GK, Andersen JB, Chapman W,
Choti M, Forbes SJ, Gores GJ, Hong TS, Harding JJ, Vander Heiden
MG, Javle M, et al: Advances in cholangiocarcinoma research: Report
from the third Cholangiocarcinoma Foundation Annual Conference. J
Gastrointest Oncol. 7:819–827. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lowery MA, Ptashkin R, Jordan E, Berger
MF, Zehir A, Capanu M, Kemeny NE, O'Reilly EM, El-Dika I, Jarnagin
WR, et al: Comprehensive Molecular Profiling of Intrahepatic and
Extrahepatic Cholangiocarcinomas: Potential Targets for
Intervention. Clin Cancer Res. 24:4154–4161. 2018. View Article : Google Scholar : PubMed/NCBI
|