Introduction
Gastric cancer is the fifth most common tumor type
worldwide, with more than one million newly diagnosed cases
reported each year and considered to be the third-leading cause of
death by cancer (1). Although
several classifications have been suggested for gastric
adenocarcinoma, the most widely used classification is based on
Lauren's histological criteria, which divide gastric mucosa tumors
into two distinct main entities: Diffuse and intestinal subtypes
(2,3).
The standard treatment for primary gastric cancer is
radical surgery. However, as that the incidence of hematogenous and
peritoneal metastasis is high, systemic chemotherapy is also part
of the protocol, which does not discriminate between the two types
of gastric carcinoma, mainly resulting in poor survival outcomes
(4–7).
In addition, there is no standard treatment for
advanced or metastatic gastric cancer. Chemotherapeutic treatment
consisting of several drug combinations, including 5-fluorouracil
or capecitabine together with oxaliplatin or cisplatin, are
currently being used. These treatments are occasionally used in
combination with either an anthracycline or docetaxel and
irinotecan (3,8,9).
Although several meta-analyses have indicated that drug
combinations result in better outcomes compared to single-agent
chemotherapy (1 month above the 6.7 months of additional overall
survival observed with monotherapy), clinically effective doses
result in high toxicity for the patient (9,10).
Targeted therapy in combination with chemotherapy, mainly for
HER2-positive disease (10–30% of patients with gastric cancer), is
also the first-line treatment for this specific group, but patients
risk developing rapid tumor resistance to monoclonal antibody-based
therapy (10–14).
Several studies suggested that cancer cells have
higher requirements for cholesterol (15–17) and
have elevated levels of membrane cholesterol rich-lipid rafts
compared to normal cells. In addition, it was hypothesized that
altering these microdomains, such as cyclodextrin-induced
cholesterol depletion, may be a potential approach to treat cancer
metastasis (18).
Membrane cholesterol is produced in the mevalonate
pathway, which is one of the most important biosynthetic processes
in animal cells (19–21). This sterol and other lipids derived
from this pathway, such as isoprenoid groups that
post-translationally modify membrane proteins, have been implicated
in the survival and metastatic behavior of several types of cancer
(22). Inhibitors of this pathway,
such as plasma cholesterol-lowering statins, which inhibit the
first rate-limiting enzyme hydroxy methyl glutaryl CoA reductase
(HMGCR) and exert in vitro anti-proliferative and
pro-apoptotic effects (21), have
been clinically tested in patients with cancer (20,22,23).
Other drugs that interfere with the mevalonate pathway, such as
zoledronic acid and farnesyl and geranylgeranyl transferase
inhibitors that affect protein isoprenylation, have also been
tested. Terbinafine, an inhibitor of the mevalonate pathway
squalene epoxidase (24), was
suggested to be a possible treatment option for several
hepatocellular carcinoma tumors (25).
The present study assessed the toxic activity and
growth and migration inhibition of agents that affect membrane
lipid synthesis in cell lines widely used as models for
advanced-stage intestinal and diffuse gastric carcinomas, which
represent two genetic and phenotypically-different molecular
tumors. The present study indicated their differential sensitivity
to several potentially effective anticancer agents.
Materials and methods
Cell culture
NCI-N87 (ATCC CRL-5822) and Hs746T (ATCC HTB-135)
cell lines were obtained from the American Type Culture Collection.
The two cell lines were established from gastric carcinomas that
metastasized to the liver (NCI-N87) and the left leg muscle
(Hs746T). The NCI-N87 cell line is derived from an intestinal
gastric tumor, whereas Hs746T cells originate from a diffuse
gastric tumor. The cells were maintained in RPMI-1640 medium (cat.
no. R8758; Sigma-Aldrich; Merck KGaA) supplemented with 10% FBS
(cat. no. F2442; Sigma-Aldrich; Merck KGaA) and 100 U/ml
penicillin-streptomycin (cat. no. 15140122; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere containing 5%
CO2.
Growth curve analysis
Initially, 1.5×104 cells were seeded in
24-wells plates. Cells were counted using a hemocytometer in a 1:2
dilution with Trypan blue every 2 days. For each cell line, the
dividing time (DT) between days 2 and 4 was determined using the
following formula: DT=T ln2/ln (Xf/Xi), where T is the incubation
time, Xf the final cell number and Xi the initial number of cells
(26).
Cell viability assay
An MTT assay was used to determine the effect of
various drugs on the metastatic gastric cancer cell lines. Once the
cells were subjected to different treatments, the medium was
removed and a solution of MTT in RPMI-1640 medium (0.5 mg/ml) was
added. Cells were incubated for 2 h and the medium was subsequently
removed. Precipitated formazan crystals were dissolved in 95%
ethanol. Cells that were incubated with medium alone were used as a
control and defined as having 100% viability. Absorbance values
were determined at a wavelength of 570 nm using a microplate reader
(BioTek Cytation 3 Imaging Multi-Mode Reader; BioTek Instruments,
Inc.).
Cisplatin-induced cytotoxicity
NCI-N87 (1.6×104) and Hs746T
(8×103) cell suspensions were cultured in 96-well plates
and allowed to attach overnight. Cells were incubated with
different concentrations of cisplatin solution (6–500 µM; Pfizer,
Inc.) in 10% FBS supplemented with RPMI-1640 medium for 48 h. The
viability assay was then performed as mentioned above.
Inhibition of HMGCR
Simvastatin (cat. no. S6196; Sigma-Aldrich; Merck
KGaA) was used to inhibit HMGCR. To activate the drug, the protocol
described by Dong et al (27)
was used. NCI-N87 (1.6×104) and Hs746T
(8×103) cell suspensions were cultured in 96-well plates
and allowed to attach overnight. Cells were incubated with
different concentrations of the drug (3–100 µM) for 48 h in 10% FBS
supplemented RPMI-1640 medium. This range of simvastatin
concentrations was based on previous studies (28,29).
Mevalonolactone (1.25 µM; cat. no. M4667;
Sigma-Aldrich; Merck KGaA) and the isoprenoids geranylgeranyl
pyrophosphate (GGPP; cat. no. G6025; Sigma-Aldrich; Merck KGaA) and
farnesyl pyrophosphate (FPP; cat. no. F6892; Sigma-Aldrich; Merck
KGaA) were used to evaluate the effect of intermediary metabolites
of the mevalonate pathway. Cells were incubated simultaneously for
48 h with simvastatin and metabolites in medium supplemented with
10% FBS. In addition, the effect of the incorporation of the
metabolites in low-cholesterol media was evaluated using Advanced
RPMI media (cat. no. 12633012; Thermo Fisher Scientific, Inc.)
containing 1% FBS.
Inhibition of squalene epoxidase
Terbinafine (cat. no. T8826; Sigma-Aldrich; Merck
KGaA) was dissolved in DMSO to a final concentration of 150 mM. The
same number of cells used for simvastatin treatment was incubated
for 48 h with different concentrations of inhibitor (5–150 µM)
dissolved in medium supplemented with 10% FBS. The DMSO
concentration in the medium was <0.1%, which was not toxic to
the cells. For the control groups, cells were incubated with the
same DMSO concentrations utilized in the treatment groups. This
range of terbinafine concentrations was based on previous studies
(30,31). Cell viability at 48 h in the presence
of terbinafine in Advanced RPMI media containing 1% FBS was also
assessed.
Membrane cholesterol staining
NCI-N87 and Hs746T cells were plated in
96-black-well plates. Subsequently, cells were treated with
inhibitors, simvastatin or terbinafine for 48 h at the
IC50 value. Cells were fixed with 4% paraformaldehyde
for 10 min at room temperature. Cells were then incubated in 1.5
mg/ml glycine solution for 10 min at room temperature.
Subsequently, the cells were incubated in 0.05 mg/ml solution of
filipin (cat. no. F9765; Sigma-Aldrich; Merck KGaA) in PBS
supplemented with 10% FBS for 2 h at room temperature and protected
from light. As a positive control for the staining, cells were
incubated with 5 mM methyl-β-cyclodextrin (MβCD; cat. no. C4555;
Sigma-Aldrich; Merck KGaA) for 1 h to extract cholesterol from the
plasma membrane. Images were acquired by fluorescence microscopy
with a ×20 objective under the same conditions of LED exposure and
gain on a BioTek Cytation 3 Imaging Multi-Mode Reader.
Gen5 Image+ 3.09 software (BioTek Instruments, Inc.)
was used to quantify the fluorescence intensity. An experiment for
each cell line that included all the images (control and
treatments) was set and automatic image preprocessing for DAPI and
Brightfield channels were applied. The Cellular Analysis Tool was
used to select the areas that contained only cells in the
Brightfield images. For experiments with Hs746T cells, the
threshold value was set to 7,000 and object size selection was
between 20 and 200 µm. For NCI-N87 cells, the parameters were set
to a threshold value of 4,000 and object size selection was between
20 and 500 µm. For both cell lines, the primary edge objects were
included, and the entire image was analyzed.
The Object Sum Intensity [Tsf (DAPI 377,447)] and
the Object Sum Area [Tsf (Bright Field)] were estimated using the
software. For each experiment, 4–11 different images were analyzed.
The relative Object Sum Intensity/Object Sum Area was estimated for
all images. The mean value was determined, and statistical analyses
were performed.
Wound-healing assay and inhibition of
cell migration
NCI-N87 and Hs746T cells were grown on 24-well
plates at a density of 1×106 and 3×105
cells/well, respectively, and allowed to adhere overnight in the
presence of 10% FBS. Wounds were scraped in the middle of the well
with a sterile pipette tip. Prior to treatment, cell monolayers
were washed twice with culture media to remove floating cells.
Treatment with simvastatin (10 µM) and terbinafine (20 µM) was
performed in duplicate samples for each cell line in media
containing 10% FBS. A total of 2 light microscopy images were
captured on the BioTek Cytation 3 from two visual fields of the
wells. Images were acquired at 0 and 24 h for Hs746T cells and 0
and 48 h for NCI-N87 cells. The percentage of migration was
calculated by the average obtained from three random measurements
and expressed as a percentage of the control (time 0 for each
group) using ImageJ v1.49 software (National Institutes of
Health).
Sulforhodamine B assay
To determine the effect on cell proliferation of
simvastatin and terbinafine during the aforementioned wound-healing
assay, as well as the effect of the presence of FBS in the media,
the Sulforhodamine B assay was performed as previously described
(32). Treated cells were fixed in
10% trichloroacetic acid for 1 h at 4°C and stained with 0.04%
Sulforhodamine B solution (cat. no. S1402; Sigma-Aldrich; Merck
KGaA) for 30 min at room temperature. Subsequently, the wells were
rinsed with 1% acetic acid to remove the excess dye. Protein-bound
dye was dissolved in 10 mM Tris base solution and the absorbance
was measured at 530 nm, using a microplate reader (BioTek Cytation
3 Imaging Multi-Mode Reader). To determine the percentage of cell
proliferation relative to a non-treated control, cells without
treatment were also analyzed.
Statistical analysis
For all cell viability assays, dose-response curves
were plotted and the IC50 value was calculated using
Slide Write Plus 6.10 software (Advanced Graphics Software, Inc).
To evaluate the significance of differences in results between two
groups, an independent t-test was performed. One-way ANOVA was
performed to compare three or more groups, followed by Bonferroni's
post-hoc test. All results were analyzed with GraphPad Prism 5
(GraphPad Software, Inc.) and SPSS 22 software (IBM Corp.).
Results
Characteristics of metastatic gastric
cancer cell lines
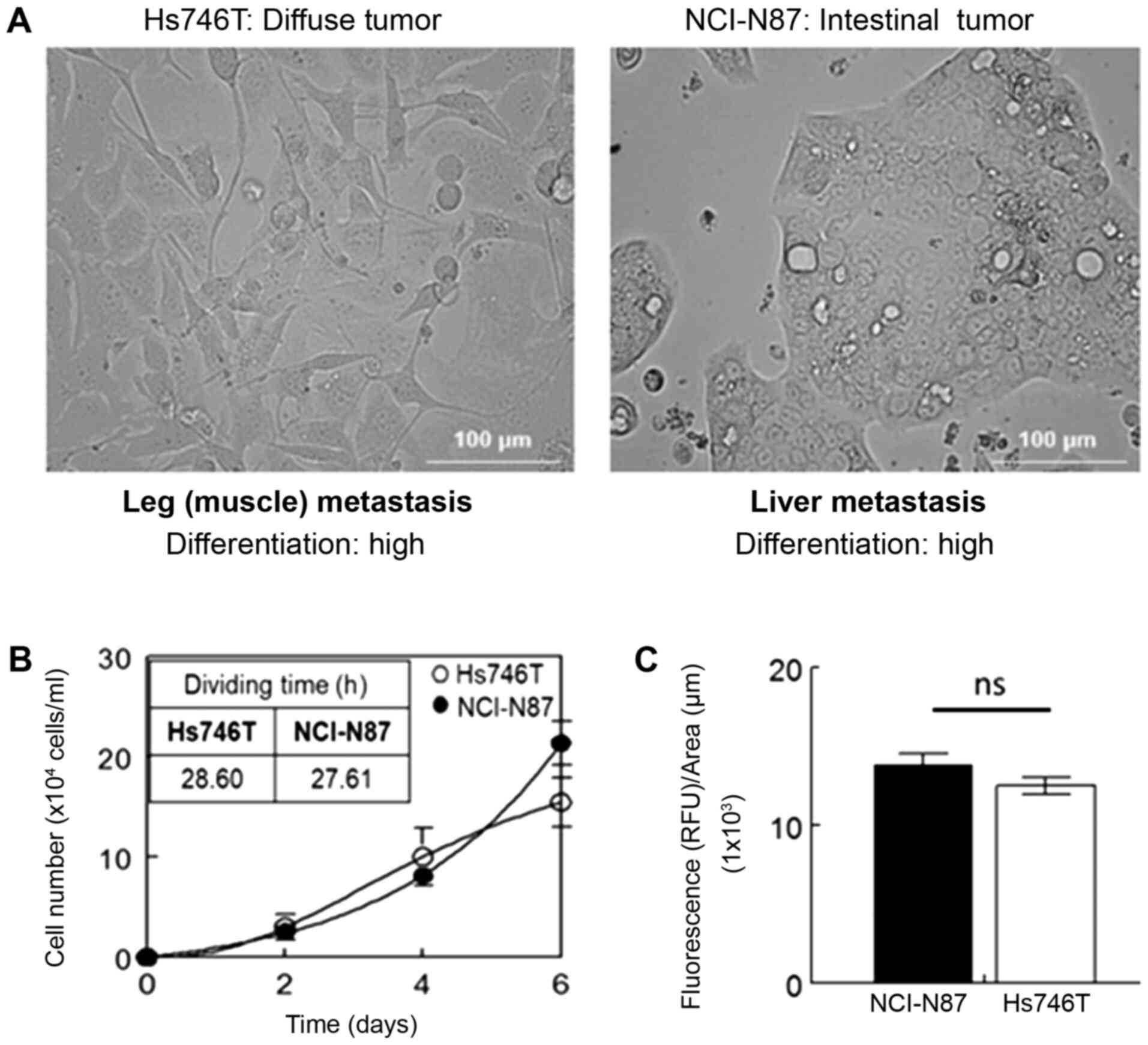
Two model cell lines representing the two major
subtypes of gastric metastatic carcinoma were characterized in
terms of proliferation and cholesterol content (Fig. 1). Both cell lines, derived from
different metastasis sites, exhibited significant differences in
morphology and levels of differentiation (Fig. 1A). The two cell lines had a similar
proliferation rate (Fig. 1B) and
membrane basal cholesterol levels (Fig.
1C).
Cisplatin and mevalonate pathway
inhibitors induce cytotoxicity
The effect of the chemotherapeutic drug cisplatin
(commonly used in the treatment of gastric cancer) on the viability
of Hs746T and NCI-N87 cells was included for comparison. In terms
of IC50 values at 48 h, there was a significant
difference between the two cell lines (Table I), with NCI-N87 having a greater
sensitivity to the drug.
 | Table I.IC50 values for cisplatin
and simvastatin on Hs746T and NCI-N87 cells for 48 h. |
Table I.
IC50 values for cisplatin
and simvastatin on Hs746T and NCI-N87 cells for 48 h.
| Drug | Hs746T | NCI-N87 |
|---|
| Cisplatin | 39.2±1.8 |
26.8±2.3a |
| Simvastatin | 2.3±0.2 |
141.7±2.8b |
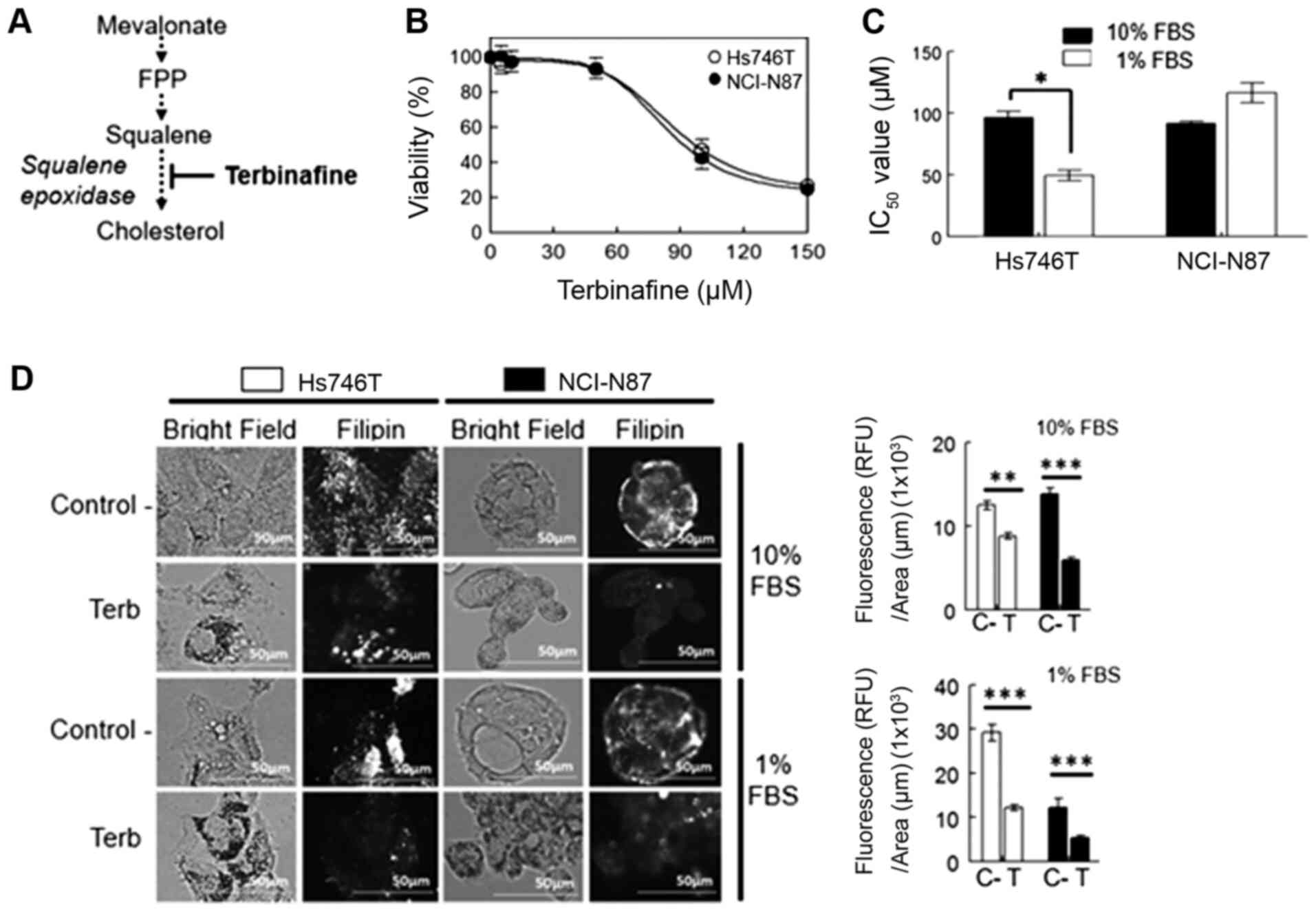
Upon inhibition of HMGCR with simvastatin (Fig. 2A), there was a strong effect on
Hs746T cells, whereas NCI-N87 cells were highly resistant. At 48 h
of treatment with the drug, the IC50 on Hs746T cells was
2.3±0.2 µM, whereas the IC50 on NCI-N87 cells was
141.7±2.8 µM (Table I; Fig. 2B). Despite the evident difference in
cell viability, when staining the cells with the macrolide filipin
(which binds to free cholesterol) to demonstrate the effect of
simvastatin on cholesterol metabolism, the staining intensity was
statistically similar to that of the negative controls in both cell
lines (Fig. 2C). The negative
controls corresponded to cells without simvastatin treatment, and
treatment with methyl-β-cyclodextrin, a cholesterol chelator, was
used as a positive control. Of note, all experiments were performed
in media supplemented with 10% FBS, which allowed the cells to
uptake cholesterol from the media.
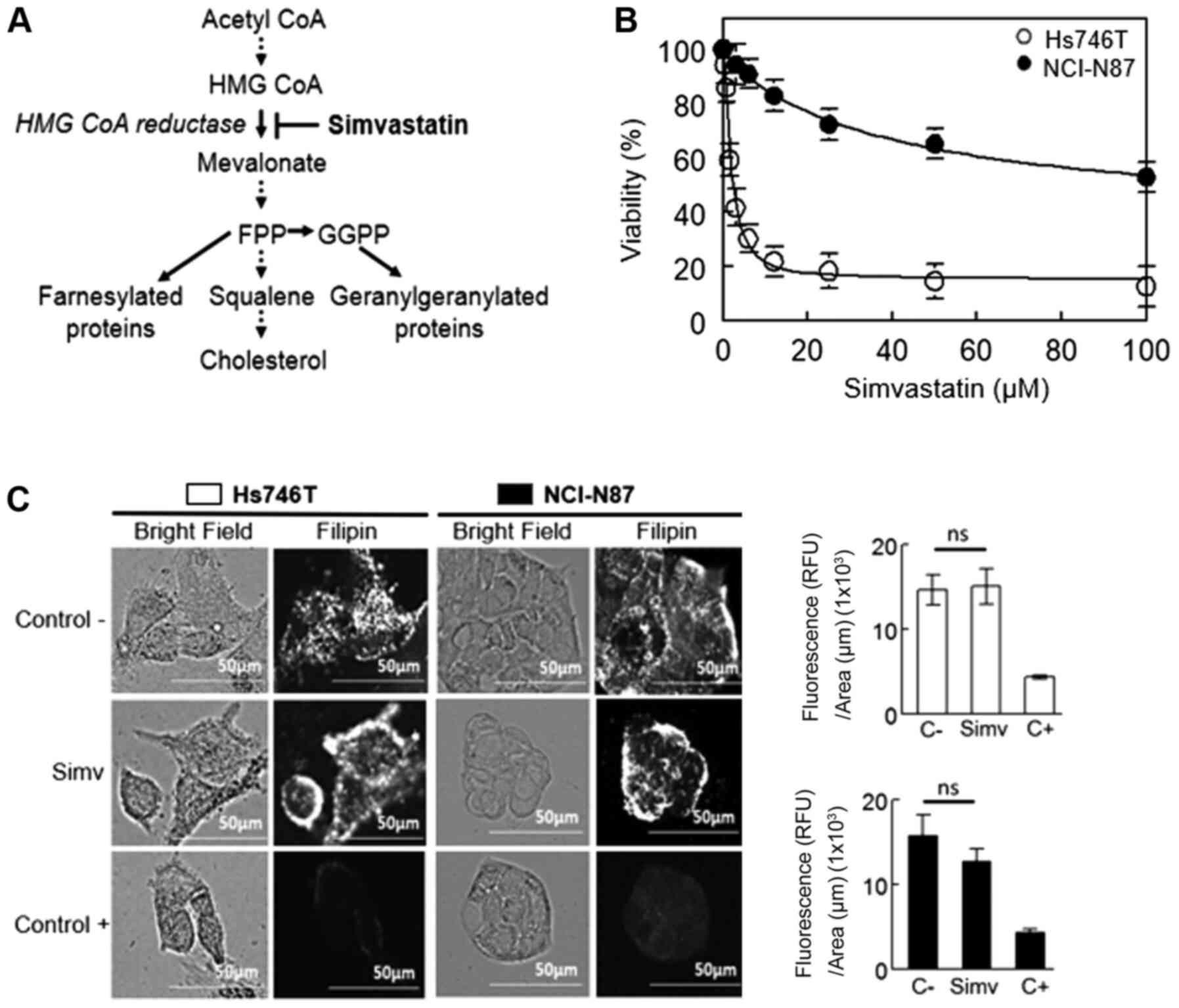 | Figure 2.Inhibitory effect of HMGCR on Hs746T
and NCI-N87 cell viability. (A) Schematic explaining the target
site of simvastatin on the mevalonate pathway. (B) Dose-response
curve of both cell lines treated with simvastatin for 48 h. Values
are expressed as the mean ± standard error of three independent
experiments performed in triplicate. (C) Left panel: Visualization
of membrane cholesterol by filipin staining after both cell lines
were incubated with simvastatin at the IC50 value for 48
h. As a positive control, cells were treated with 5 mM MβCD to
determine a decrease in cell membrane cholesterol (scale bars, 50
µm). Right panel: Fluorescence quantification adjusted by area.
Values are expressed as the mean ± standard error of ≥4 independent
wells. HMGCR, hydroxymethyl glutaryl CoA reductase; FPP, farnesyl
pyrophosphate; GGPP, geranylgeranyl pyrophosphate; MβCD,
methyl-β-cyclodextrin; simv, simvastatin; C-, negative control; C+,
positive control; Ns, no significance. |
Upon treatment with terbinafine, which is an
inhibitor of squalene epoxidase (Fig.
3A), no significant differences were observed in the
IC50 values between both cell lines in the media
supplemented with 10% FBS (Fig. 3B).
Reducing the concentration of FBS to 1% increased the toxicity of
the drug in the diffuse gastric tumor Hs746T cell line (Fig. 3C). Upon staining of cholesterol with
filipin following terbinafine treatment a significant reduction in
cholesterol levels in both cell lines was observed, which was
independent of the FBS concentration in the media (Fig. 3D).
Effects of treatment in the presence
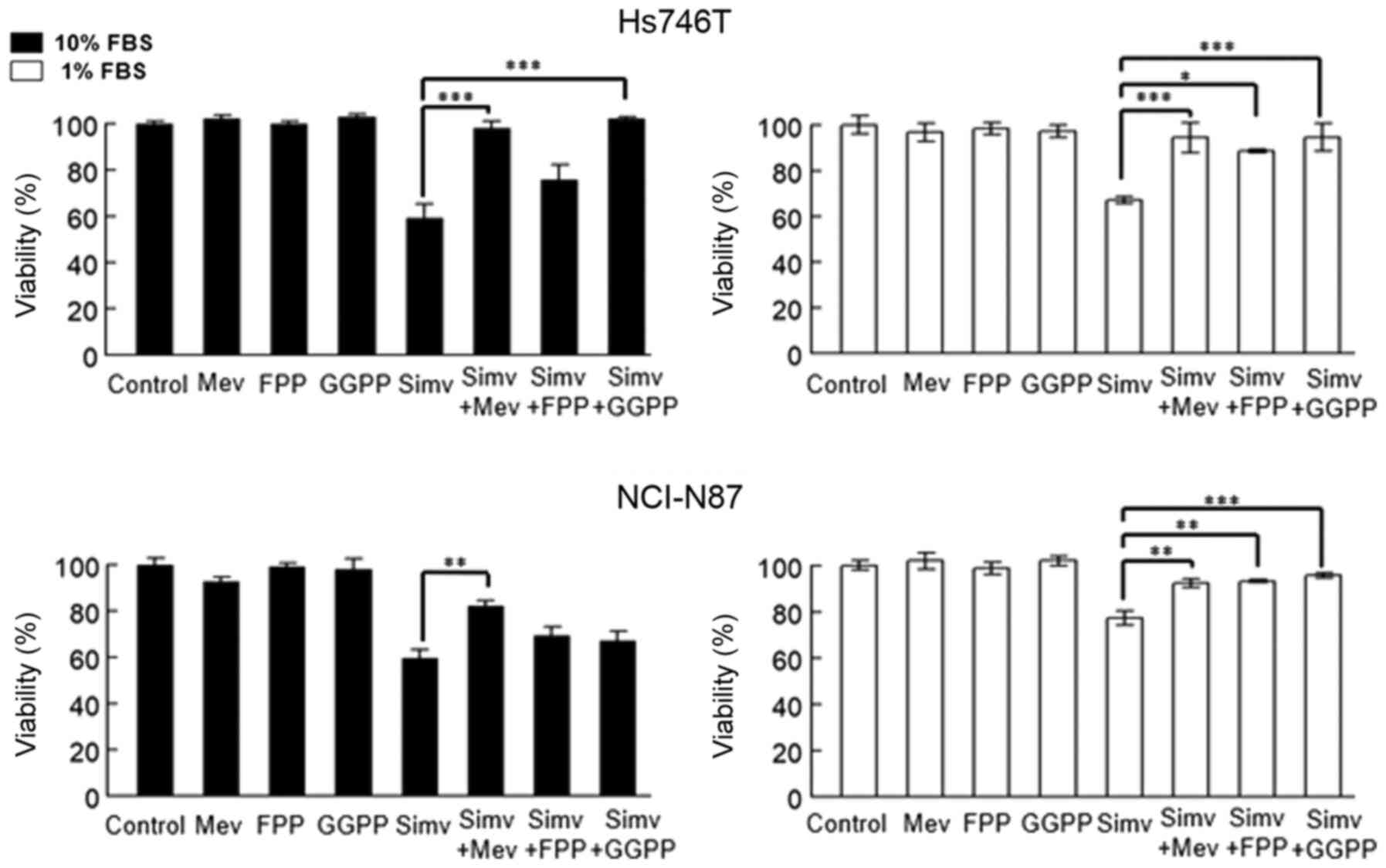
of intermediary metabolites mevalonolactone, FPP and GGPP
To determine the dependence of cholesterol and other
mevalonate-derived molecules (isoprenoids) on cell viability, cells
were co-incubated for 48 h with simvastatin in the presence of
mevalonolactone, FPP or GGPP in media supplemented with 10 or 1%
FBS.
The presence of mevalonolactone in the media
resulted in the complete recovery of viability in both cell lines,
independently of the FBS concentration. Furthermore, among the
cells replenished with isoprenoids in media supplemented with 10%
FBS, 1.25 µM GGPP was able to restore the viability only in Hs746T
cells. For NCI-N87 cells, the dose was increased to 7.5 µM to
obtain partial recovery (data not shown), since 1.25 µM did not
result in any significant effects in media supplemented with 10%
FBS. The addition of FPP to the media did not affect cell growth in
the presence of 10% FBS, whereas addition of FPP or GGPP in the
media containing 1% FBS, resulted in the complete recovery of
viability of Hs746T and NCI-N87 cells (Fig. 4).
Effects of simvastatin and terbinafine
on cell migration
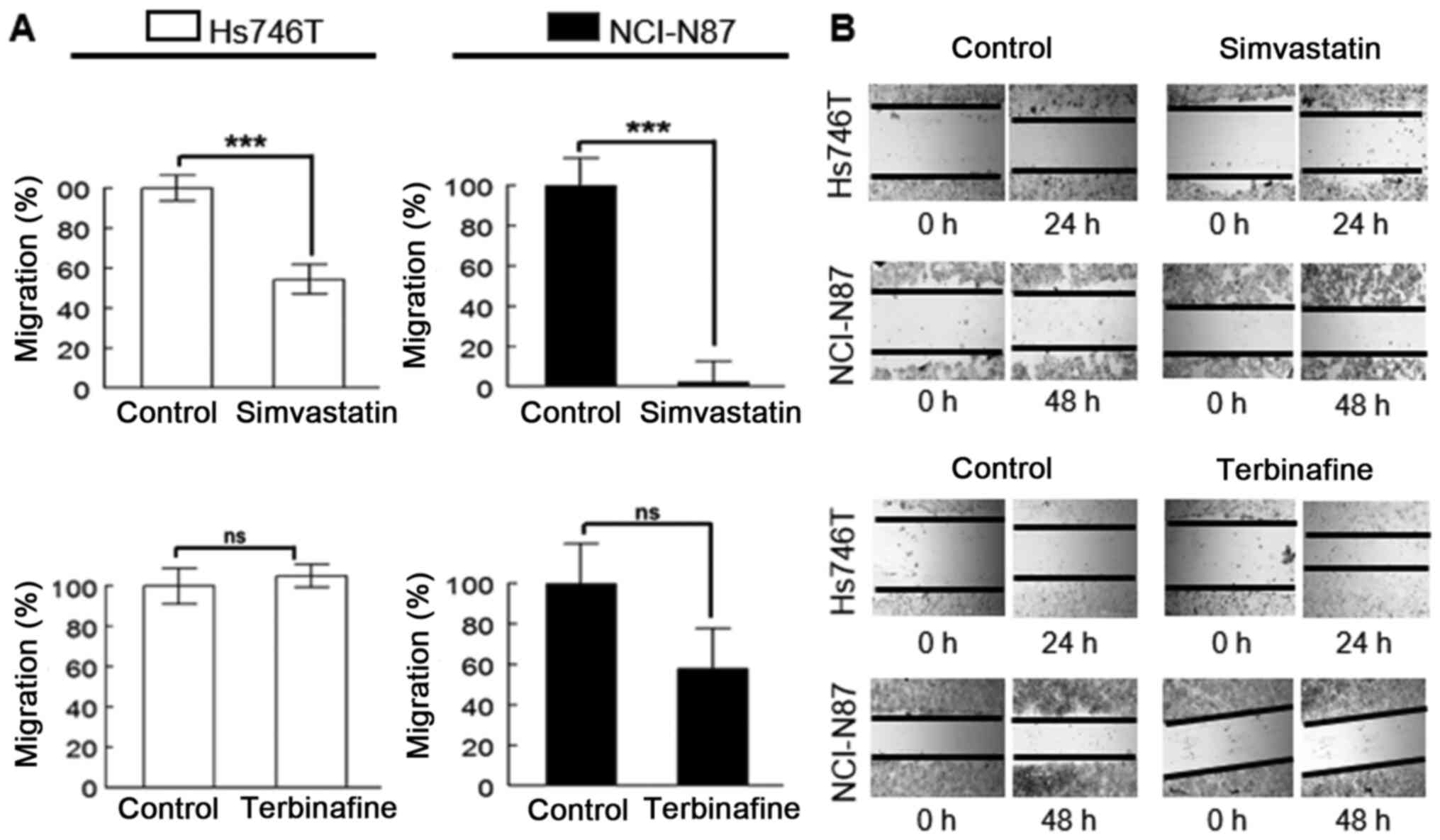
As presented in Fig.
5, simvastatin, but not terbinafine, was able to significantly
inhibit the migration of both cell lines in media containing 10%
FBS. For Hs746T cells, the incubation was performed for 24 h to
avoid the higher toxicity of simvastatin at 48 h. In addition, the
cytotoxicity and cell proliferation were monitored through the
entire wound-healing assay using the Sulforhodamine B assay (data
not shown), and the control groups were incubated with the same FBS
concentration as the treatment groups. For NCI-N87 cells, the
inhibition of migration was determined at 48 h, as the cells
migrated at a rate that was 4 times lower than that of Hs746T
cells.
Discussion
Advanced intestinal and diffuse subtypes of gastric
cancer are considered pathologically separate entities with
different origins and causes. However, they are clinically treated
similarly, even though patients should receive different therapies
(3). One of the major differences
between these two pathologies lies in their migration and invasion
characteristics: The diffuse subtype is mainly associated with
peritoneal dissemination, whereas the risk of liver metastasis is
higher in the intestinal subtype, due to hematogenous dispersion
(33).
Metastatic disease is practically incurable. In
terms of chemotherapy options, diffuse tumors result in worse
prognosis (34). Patients with the
intestinal subtype that overexpress HER2 have the option of
receiving monoclonal antibody therapy with trastuzumab (35). However, ~50% of patients exhibit
resistance to treatment (11).
In terms of cytotoxic agents used for chemotherapy
of gastric cancer, the present study evidenced that clinically-used
cisplatin alone exerted effects in both cell types, even when
differences in the IC50 were observed. These results
were expected, since platinum drugs are alkylating agents that bind
to DNA and inhibit its replication. Although they target dividing
cells at the beginning of DNA synthesis, they lack specificity,
resulting in differences when cells are not synchronized (36,37).
Hs746T cells were more resistant to cisplatin treatment probably
because, according to their proliferation rate, these cells did not
exhibit exponential growth, which may be a limitation for the
comparison. The lack of an exponential growth phase may be due to
cell contact-inhibition mechanisms (38).
Highly proliferating cancer cells have higher
requirements for cholesterol (16,17).
Statins have been used as chemopreventive agents and a treatment
option for several tumor types (22). To satisfy their cholesterol
requirements, cells may increase its endogenous synthesis or obtain
cholesterol from outside through low density-lipoprotein (LDL)
receptors (15). HMGCR, a mevalonate
pathway first rate-limiting enzyme, has been considered as a
metabolic oncogene. Although statins have been indicated to induce
its transcriptional upregulation, several tumor types have
deficient HMGCR feedback controls (39).
Caruso et al (40) reported that HMGCR activity is
significantly higher in neoplastic tissues compared with that in
normal gastric mucosa (in primary gastric tumors), whereas no
differences were observed between diffuse and intestinal subtypes.
They also suggested that LDL receptor levels were lower in primary
gastric neoplastic tissues, but only in the diffuse type. This may
explain the present results with simvastatin, since Hs746T diffuse
tumor cells were more sensitive to the inhibitor compared to the
intestinal type, NCI-N87 cells. However, the present study did not
indicate a decrease in free cholesterol when both cell lines were
treated with simvastatin, suggesting the triggering of a normal
feedback that maintains cell cholesterol at normal levels.
In terms of treatment, it has been indicated that
the statin concentration required to reach antitumoral therapeutic
doses is 10 times higher than the doses utilized for the treatment
of patients with hypercholesterolemia (41,42). The
doses of statins (such as simvastatin) used to treat
hypercholesterolemia are in the range of 10–40 mg per day (43), or 0.2–0.6 mg/kg/day. Doses of 1
mg/kg/day would give a serum level of ~0.1 µM, and therapeutic
doses of 2–4 µM are well tolerated in animal models (44). According to the present results, the
IC50 of simvastatin on Hs746T cells was 2.3 µM, which is
consistent with the aforementioned in vivo tolerated range,
whereas the IC50 on NCI-N87 cells was much higher (142
µM).
In the presence of 10% FBS, mevalonolactone and GGPP
(but not FPP) led to recovery of the cells from simvastatin-induced
toxicity. This indicates that isoprenoids are the most important
factor for maintaining cell proliferation when HMGCR is inhibited
under these conditions. However, when the FBS concentration was
lowered to 1%, FPP also led to the recovery from the cytotoxic
effects, indicating that these cells require isoprenoids and
cholesterol to proliferate normally. FPP is a downstream precursor
metabolite for cholesterol and GGPP synthesis in the mevalonate
pathway. Several proteins utilize FPP for farnesylation. However,
to be converted into GGPP, FPP requires other metabolites that may
not necessarily always be present in the cells (45). The differences in viability observed
with FPP reposition at high and low levels of FBS suggest that FPP
is being utilized to synthesize cholesterol.
Statins have been proposed as an option for the
treatment of several tumor types (20,22,23).
Based on the present results, simvastatin may be an alternative
treatment for the diffuse subtype of gastric carcinoma, even though
certain effects may be due to other pleiotropic activities of the
drug (46).
The present study revealed that the cells exhibited
resistance upon treatment with the squalene-epoxidase inhibitor
terbinafine, which affects the downstream second rate-limiting
enzyme of the mevalonate pathway (24), in the presence of 10% FBS. However,
Hs746T cells have a higher sensitivity in the presence of 1% FBS,
indicating that cholesterol also influences cell viability.
Conversely, this does not appear to be the case for NCI-N87 cells,
which remained resistant to treatment.
The antifungal drug terbinafine has been proposed to
be a potential agent for the treatment of several specific types of
liver cancer (47). Terbinafine may
also be an option for advanced gastric cancer treatment, although
the high doses required may give rise to complications. In the
present study, dark precipitates were observed in cells treated
with terbinafine, similar to the squalene deposits observed in a
previous study (47) and
squalene-induced toxicity also occurred in these cancer cells.
Upon testing the effects of simvastatin and
terbinafine on cell migration, it was indicated that the statin
significantly affected both cell types. A comparison between cell
lines was not possible due to the different incubation times used
for each cell line, which was due to differences in drug
sensitivity and basal migration ability of these cells. A
limitation of the present study was that FBS was not removed from
the media, and sera may induce cell proliferation, but due to the
poor migratory ability observed (especially for NCI-N87), long
time-points had to be used in these experiments, making it
difficult to remove the sera due to loss of viability. However,
controls were also in the presence of 10% FBS, which helped to
correct for the potential effect induced by proliferation.
Terbinafine did not exert any significant effect on
cell migration, suggesting that the effect of simvastatin may be
due to the inhibition of protein prenylation. However, since the
migration experiments were performed in the presence of 10% FBS,
cholesterol may also have a role in the process. Prenylated
proteins such as Rho are associated with migration and invasion of
cancer cells (48). In addition, the
role of HMGCR in gastric cancer cell migration has also been
established (49).
Even when no normal gastric cells were used in the
present study as a control, since they require specific nutrients
and growth factors that may affect the interpretation of the data,
the present study suggested that model cell lines representing the
two advanced gastric tumor subtypes (intestinal and diffuse)
respond differently to potential anti-neoplastic agents, including
cholesterol and the isoprenoid-lowering drugs simvastatin and
terbinafine. Preliminary results from our group on primary gastric
tumor cells indicated a similar level of sensitivity to simvastatin
as Hs746T cells (data not shown).
Another limitation of the present study is that only
one cell line from each histological subtype of the tumor was
tested; however, these are probably some of the best characterized
gastric cancer cells commercially available and they have been
widely used as a model of these subtypes of metastatic tumors
(50,51). They also have interesting features
regarding the overexpression of two genes that are associated with
tumor resistance, c-Met (Hs746T) and HER2 (NCI-N87). Even though
the cell signaling pathways associated with these oncogenes were
not characterized in the present study, this may be worth exploring
in the future.
The present study provided possible novel
therapeutic alternatives for advanced gastric cancer, alone or in
combination with other drugs. The results also established that
different drug treatment protocols should be provided for these two
subtypes of gastric tumor, mainly in the advanced stages and in the
presence of metastases. These two types of gastric tumors are
different molecular entities at the genotypical and phenotypical
level, and exhibit resistance to certain drugs and treatments. This
may be due to their specific genetic alterations and levels of
differentiation associated with epithelial-mesenchymal transition
events. Hence, this should be taken into consideration during the
selection of more appropriate and effective therapeutic
options.
Acknowledgements
Not applicable.
Funding
This study was partially supported by Vicerrectoría
de Investigación (grant no. 422-B7-098) and Sistema de Estudios de
Posgrado, both from the Universidad de Costa Rica.
Availability of data and materials
All data generated or analyzed during this study
were included in this published article.
Authors' contributions
NO and CD conceived and designed the study, analyzed
the results and wrote and reviewed the manuscript. Both authors
read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cisło M, Filip AA, Arnold Offerhaus GJ,
Ciseł B, Rawicz-Pruszyński K, Skierucha M and Polkowski WP:
Distinct molecular subtypes of gastric cancer: From Laurén to
molecular pathology. Oncotarget. 9:19427–19442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma J, Shen H, Kapesa L and Zeng S: Lauren
classification and individualized chemotherapy in gastric cancer.
Oncol Lett. 11:2959–2964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dittmar Y and Settmacher U: Individualized
treatment of gastric cancer: Impact of molecular biology and
pathohistological features. World J Gastrointest Oncol. 7:292–302.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quéro L, Guillerm S and Hennequin C:
Neoadjuvant or adjuvant therapy for gastric cancer. World J
Gastrointest Oncol. 7:102–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roukos DH and Kappas AM: Perspectives in
the treatment of gastric cancer. Nat Clin Pract Oncol. 2:98–107.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shirabe K, Shimada M, Matsumata T, Higashi
H, Yakeishi Y, Wakiyama S, Ikeda Y, Ezaki T, Fukuzawa S, Takenaka
K, et al: Analysis of the prognostic factors for liver metastasis
of gastric cancer after hepatic resection: A multi-institutional
study of the indications for resection. Hepatogastroenterology.
50:1560–1563. 2003.PubMed/NCBI
|
|
8
|
Kanat O and O'Neil BH: Metastatic gastric
cancer treatment: A little slow but worthy progress. Med Oncol.
30:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wagner AD, Unverzagt S, Grothe W, Kleber
G, Grothey A, Haerting J and Fleig WE: Chemotherapy for advanced
gastric cancer. Cochrane Database Syst Rev. CD0040642010.PubMed/NCBI
|
|
10
|
Wagner AD, Syn NL, Moehler M, Grothe W,
Yong WP, Tai BC, Ho J and Unverzagt S: Chemotherapy for advanced
gastric cancer. Cochrane Database Syst Rev.
8:CD0040642017.PubMed/NCBI
|
|
11
|
Apicella M, Corso S and Giordano S:
Targeted therapies for gastric cancer: Failures and hopes from
clinical trials. Oncotarget. 8:57654–57669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JS, Kim SH, Im SA, Kim MA and Han JK:
Human epidermal growth factor receptor 2 expression in unresectable
gastric cancers: Relationship with CT characteristics. Korean J
Radiol. 18:809–820. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Ling Y, Qi Q, Zhu M, Wan M, Zhang Y
and Zhang C: Trastuzumab increases the sensitivity of
HER2-amplified human gastric cancer cells to oxaliplatin and
cisplatin by affecting the expression of telomere-associated
proteins. Oncol Lett. 9:999–1005. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pazo Cid RA and Antón A: Advanced
HER2-positive gastric cancer: Current and future target therapies.
Curr Rev Oncol Hematol. 85:350–362. 2013. View Article : Google Scholar
|
|
15
|
Rodrigues dos Santos C, Domingues G,
Matias I, Matos J, Fonseca I, de Almeida JM and Dias S:
LDL-cholesterol signaling induces breast cancer proliferation and
invasion. Lipids Health Dis. 13:162014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang B, Song B and Xu C: Cholesterol
metabolism in cancer: Mechanisms and therapeutic opportunities. Nat
Metab. 2:132–141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Göbel A, Rauner M, Hofbauer LC and Rachner
TD: Cholesterol and beyond-the role of the mevalonate pathway in
cancer biology. Biochim Biophys Acta Rev Cancer. 1873:1883512020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang R, Bi J, Ampah KK, Ba X, Liu W and
Zeng X: Lipid rafts control human melanoma cell migration by
regulating focal adhesion disassembly. Biochim Biophys Acta.
1833:3195–3205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bathaie SZ, Ashrafi M, Azizian M and
Tamanoi F: Mevalonate pathway and human cancers. Curr Mol
Pharmacol. 10:77–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clendening JW and Penn LZ: Targeting tumor
cell metabolism with statins. Oncogene. 31:4967–4978. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharpe LJ and Brown AJ: Controlling
cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA
reductase (HMGCR). J Biol Chem. 288:18707–18715. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iannelli F, Lombardi R, Milone MR, Pucci
B, De Rienzo S, Budillon A and Bruzzese F: Targeting mevalonate
pathway in cancer treatment: Repurposing of statins. Recent Pat
Anticancer Drug Discov. 13:184–200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmadi Y, Ghorbanihaghjo A and Argani H:
The balance between induction and inhibition of mevalonate pathway
regulates cancer suppression by statins: A review of molecular
mechanisms. Chem Biol Interact. 273:273–285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshioka H, Coates JW, Chua NK, Hashimoto
Y, Brown AJ and Ohgane K: A key mammalian cholesterol synthesis
enzyme, squalene monooxygenase, is allosterically stabilized by its
substrate. Proc Natl Acad Sci USA. 117:7150–7158. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gill S, Stevenson J, Kristiana I and Brown
AJ: Cholesterol-dependent degradation of squalene monooxygenase, a
control point in cholesterol synthesis beyond HMG-CoA reductase.
Cell Metab. 13:260–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bernstein DL, Le Lay JE, Ruano EG and
Kaestner KH: TALE-mediated epigenetic suppression of CDKN2A
increases replication in human fibroblasts. J Clin Invest.
125:1998–2006. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong W, Vuletic S and Albers JJ:
Differential effects of simvastatin and pravastatin on expression
of Alzheimer's disease-related genes in human astrocytes and
neuronal cells. J Lipid Res. 50:2095–2102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen YY, Yuan Y, Du YY and Pan YY:
Molecular mechanism underlying the anticancer effect of simvastatin
on MDA-MB-231 human breast cancer cells. Mol Med Rep. 12:623–630.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Buranrat B, Suwannaloet W and Naowaboot J:
Simvastatin potentiates doxorubicin activity against MCF-7 breast
cancer cells. Oncol Lett. 14:6243–6250. 2017.PubMed/NCBI
|
|
30
|
Lee WS, Chen RJ, Wang YJ, Tseng H, Jeng
JH, Lin SY, Liang YC, Chen CH, Lin CH, Lin JK, et al: In vitro and
in vivo studies of the anticancer action of terbinafine in human
cancer cell lines: G0/G1p53-associated cell cycle arrest. Int J
Cancer. 106:125–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ho PY, Zhong WB, Ho YS and Lee WS:
Terbinafine inhibits endothelial cell migration through suppression
of the Rho-mediated pathway. Mol Cancer Ther. 5:3130–3138. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vichai V and Kirtikara K: Sulforhodamine B
colorimetric assay for cytotoxicity screening. Nat Protoc.
1:1112–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Riihimäki M, Thomsen H, Hemminki A,
Sundquist K and Hemminki K: Comparison of survival of patients with
metastases from known versus unknown primaries: Survival in
metastatic cancer. BMC Cancer. 13:362013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ge S, Xia X, Ding C, Zhen B, Zhou Q, Feng
J, Yuan J, Chen R, Li Y, Ge Z, et al: A proteomic landscape of
diffuse-type gastric cancer. Nat Commun. 9:10122018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gunturu KS, Woo Y, Beaubier N, Remotti HE
and Saif MW: Gastric cancer and trastuzumab: First biologic therapy
in gastric cancer. Ther Adv Med Oncol. 5:143–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim SM and Park SH: Chemotherapy beyond
second-line in advanced gastric cancer. World J Gastroenterol.
21:8811–8816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shah MA and Schwartz GK: Cell
cycle-mediated drug resistance: An emerging concept in cancer
therapy. Clin Cancer Res. 7:2168–2181. 2001.PubMed/NCBI
|
|
38
|
Basque JR, Chénard M, Chailler P and
Ménard D: Gastric cancer cell lines as models to study human
digestive functions. J Cell Biochem. 81:241–251. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Clendening JW, Pandyra A, Li Z, Boutros
PC, Martirosyan A, Lehner R, Jurisica I, Trudel S and Penn LZ:
Exploiting the mevalonate pathway to distinguish statin-sensitive
multiple myeloma. Blood. 115:4787–4797. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Caruso MG, Notarnicola M, Cavallini A and
Di Leo A: 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity
and low-density lipoprotein receptor expression in diffuse-type and
intestinal-type human gastric cancer. J Gastroenterol. 37:504–508.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim ST, Kang JH, Lee J, Park HP, Park O,
Park YS, Lim Y, Hwang G, Lee SC, Park KW, et al: Simvastatin plus
capecitabine-cisplatin versus placebo plus capecitabine-cisplatin
in patients with previously untreated advanced gastric cancer: A
double-blind randomized phase 3 study. Eur J Cancer. 50:2822–2830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lytras T, Nikolopoulos G and Bonovas S:
Statins and the risk of colorectal cancer: An updated systematic
review and meta-analysis of 40 studies. World J Gastroenterol.
20:1858–1870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jones P, Kafonek S, Laurora I and
Hunninghake D: Comparative dose efficacy study of atorvastatin
versus simvastatin, pravastatin, lovastatin, and fluvastatin in
patients with hypercholesterolemia (The CURVES Study). Am J
Cardiol. 81:582–587. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gerson RJ, MacDonald JS, Alberts AW,
Kornbrust DJ, Majka JA, Subbs R and Bokelman DL: Animal safety and
toxicology of simvastatin and related hydroxy-methyl
glutaryl-Coenzyme A reductase inhibitors. Am J Med. 87:28S–38S.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wong WW, Clendening JW, Martirosyan A,
Boutros PC, Bros C, Khosravi F, Jurisica I, Stewart AK, Bergsagel
PL and Penn LZ: Determinants of sensitivity to lovastatin-induced
apoptosis in multiple myeloma. Mol Cancer Ther. 6:1886–1897. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liao JK and Laufs U: Pleiotropic effects
of statins. Annu Rev Pharmacol Toxicol. 45:89–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mahoney CE, Pirman D, Chubukov V, Sleger
T, Hayes S, Fan ZP, Allen EL, Chen Y, Huang L, Liu M, et al: A
chemical biology screen identifies a vulnerability of
neuroendocrine cancer cells to SQLE inhibition. Nat Commun.
10:962019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Al-Haidari AA, Syk I and Thorlacius H:
HMG-CoA reductase regulates CCL17-induced colon cancer cell
migration via geranylgeranylation and RhoA activation. Biochem
Biophys Res Commun. 446:68–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chushi L, Wei W, Kangkang X, Yongzeng F,
Ning X and Xiaolei C: HMGCR is up-regulated in gastric cancer and
promotes the growth and migration of the cancer cells. Gene.
587:42–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ebert K, Mattes J, Kunzke T, Zwingenberger
G and Luber B: MET as a resistance factor for afatinib therapy and
motility driver in gastric cancer cells. PLoS One. 14:e02232252019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sethunath V, Hu H, De Angelis C,
Veeraraghavan J, Qin L, Wang N, Simon LM, Wang T, Fu X, Nardone A,
et al: Targeting the mevalonate pathway to overcome acquired
anti-HER2 treatment resistance in breast cancer. Mol Cancer Res.
17:2318–2330. 2019.PubMed/NCBI
|