Introduction
Carcinomatous meningitis (CM) is a condition in
which tumor cells spread to the subarachnoid space hematogenously,
via direct invasion, or through neuroinvasion (1), and occurs in at least 5% of patients
with disseminated cancer (2). CM is
diagnosed via neurological examination, computed Tomography,
Magnetic Resonance Imaging, cerebrospinal fluid (CSF) analysis
(cell count, opening pressure, levels of protein, glucose and
lactate) and CSF cytology (1).
Leukocyte counting and typing of CSF has previously
been performed manually or using various types of flow cytometers
(3,4). Normally, in healthy individuals the
manual leukocyte count is <5/µl (4). While, in 1987, Hayward et al
(5) described that 77% of malignant
meningitis (without specifying tumor types) showed >5 cells/ml
manual leukocyte count (5).
Subsequently, MacKenzie (6) reported
that in malignant meningitis including carcinomas, primary central
nervous system (CNS) tumors, and hematopoietic tumors, 22 cases out
of 26 confirmed malignancy cases showed raised manual leukocyte
counts. While Djukic et al (7) reported that 66 cases out of 132
confirmed malignancies in malignant meningitis showed increased
manual leukocyte counts. In terms of leukocyte subsets, diverse
results have been reported following analysis. For example, the
mononuclear cell count is elevated in ~50% of patients with
malignancy, and the polymorphonuclear cell count is increased in
20% of patients (8). while, the
frequencies of lymphocytes and granulocytes are also increased,
whereas those of monocytes are decreased (7).
Notably, leukocyte counting and typing are also
performed using flow cytometry (9).
For example, Illan et al (9)
analyzed absolute cell count, tumor cell count, and inflammatory
cell count in malignant lymphoma cases and leptomeningeal
carcinomaosis (9). On the other
hand, in terms of cytological specimens, the features of malignant
cells are the main focus of CSF cytology. For example, Singh et
al (10) summarized cytological
characteristics of tumor appearance patterns for breast carcinoma,
ovarian carcinoma, malignant melanoma among others (10). Ho et al (11) summarized cytomorphological
characteristics of primary CNS tumors in the CSF. Rao et al
(12) reported cytopathological
characteristics of CSF for 15 patients with breast cancer. Bell
(13) reported the pitfalls which
could interfere with the diagnostic accuracy in CSF cytology Bigner
(14) comprehensively summarized CSF
cytology from the preparation method to the cytological findings of
non-infectious condition, infectious conditions, hematopoietic
tumors, metastatic carcinomas and primary CNS tumors. Cutler and
Spertell (15) mentioned the
condition of sample preparation in order to maintain morphology of
cells in the CSF and achieve good cytological observation to
diagnose malignancy. In terms of characteristics of leukocytes, the
findings have been evaluated manually in cytological specimens. For
example, lymphocytes and monocytes mixed with erythrocytes and
fibrin are observed in the background of metastatic breast cancer
specimens (12), neutrophils are
identified in malignant specimens of the central nervous system and
eosinophils have been reported in patients with Hodgkin's lymphoma
(5). Moreover, reactive and
inflammatory infiltrates have been reported in the background when
only a few metastatic cancer cells are detected in CSF (14). In addition, none of these
aforementioned studies evaluated the leukocyte count and type using
cytological specimens via an objective method, such as
computer-assisted image analysis (CAIA). Furthermore, daily
practice of screening CSF cytological specimens usually focuses on
the detection of atypical cells, without much consideration given
to the leukocyte population (10–14).
Therefore, it is possible that the importance of background
leukocyte evaluation in cytology specimens may have be
overlooked.
Since 2016, the combination of whole slide imaging
(WSI) and CAIA has been used in the fields of pathology and
cytology (16). Yamada et al
(16) analyzed the size, shape and
texture of nuclei for specimens of proliferative breast lesions
using CAIA. The group described CAIA as a valuable tool for
distinguishing atypical hyperplasia and ductal carcinoma in
situ from typical ductal hyperplasia. Kosuge et al
(17) revealed that the nuclear
features of invasive urothelial carcinoma differed from those of
low-grade papillary urothelial carcinoma as assessed using the
combination of WSI and CAIA. Furthermore, Eyraud et al
(18) utilized CAIA to analyze the
immunohistochemical staining-positive area for indoleamine
2,3-dioxygenase or forkhead box protein 3 in a tissue microarray of
colorectal cancer tissue samples. Our previous study analyzed the
nuclear features of lung adenocarcinoma and found that some
adenocarcinomas showed low expression level of inner nuclear
membrane protein emerin (19), and
the low expression group showed enlarged size and oval shape of the
nucleus in comparison with the emerin expressing group (19). Overall, the aforementioned reports
indicated that WSI and CAIA are crucial for evaluating pathological
and cytological specimens. Thus, the present study evaluated CSF
cytology specimens utilizing WSI and CAIA to clarify the features
of leukocyte subsets.
Materials and methods
Sample selection
All CSF cytology samples, except ones of patients
who declined participation for the study, collected at Gunma
University Hospital (Maebashi, Japan) from January 2001 to December
2011 were reviewed using the hospital information system. Pediatric
cases, cases with no description of the clinical diagnosis,
unavailable cases and cases without a histological diagnosis were
excluded. Finally, 339 samples were examined by manually observing
Giemsa-stained specimens. For patients who underwent multiple
cytological examinations of CSF, the specimen with malignant
cytology that featured the highest number of cells was used for
subsequent analysis. The remaining 280 samples were utilized for
manual observation of the microscopically analyzed Giemsa
specimens. Of the 280 patients included in the present study, 145
were male and 135 female. The patients had a median age, 61.0
years; mean age, 56.1 years and age range, 20–86 years.
Ethics approval
The present study was approved by the Gunma
University Ethical Review Board for Medical Research Involving
Human Subjects of Gunma University School of Medicine (GUERB;
Gunma, Japan), and the written notification for the current study
was presented publicly on the webpage of Gunma University Hospital.
Furthermore, the possibility to decline participation in this study
was provided according to the Ethical Guidelines for Medical and
Health Research Involving Human Subjects of the Japanese government
(Ministry of Education, Culture, Sports, Science and Technology,
and Ministry of Health, Labour and Welfare). Informed consent was
waived by GUERB based on the above guidelines due to the
retrospective nature of the study.
Manual cell number evaluation and
specimen condition evaluation
Already prepared Giemsa-stained and Papanicolaou
(Pap)-stained specimens which were formerly prepared in the
Clinical Department of Pathology for daily practice were used. The
staining protocol for Pap smear used in Gunma University Hospital
is described below. All steps were done at room temperature. One
drop of 30% albumin was added to the CSF samples. Silane-coated
slide glass was assembled to Autosmear™ apparatus (Sakura Finetek
Japan Co., Ltd.) and the apparatus was set to roter of Autosmear™
centrifuge with CSF samples. Then the samples were centrifuged for
5 min at 48 × g. The supernatant was discarded and the slide glass
was removed from the apparatus. The specimen was fixed in 95%
ethanol for 15 min followed by 70% ethanol (30 sec), 50% ethanol
(30 sec) and running water (30 sec twice). Then the specimens were
stained by Gil's hematoxylin solution for 1.5 min followed by 0.5%
hydrochloric acid in 70% ethanol (30 sec), running water (5 min),
70% ethanol (30 sec), 80% ethanol (30 sec) and 95% ethanol (30
sec). Then the specimens were stained by OG-6 solution followed by
95% ethanol for 15 sec. Then the specimens were treated with 1%
phosphotungstic acid solution (30 sec) followed by 95% ethanol (30
sec). Then the specimens were stained using EA-50 solution 5 min)
followed by 95% ethanol (30 sec), 95% ethanol (1 min), 100% ethanol
(1 min, 4 times) and xylene (1 min, thrice). Finally the specimen's
coverslipping was performed. For Giemsa staining, all steps were
done at room temperature. One drop of 30% albumin was added to CSF
sample. Silane-coated slide glass was assembled to Autosmear™
apparatus (Sakura Finetek Japan Co., Ltd.,) and the apparatus was
set to roter of Autosmear™ centrifuge with CSF sample. Then the
samples were centrifuged for 5 min at 48 × g. The supernatant was
discarded and the slide glass was removed from the apparatus. The
specimen was cold dried by air. Then the specimens were stained by
May-Gruenwald solution for 2 min followed by running water for 30
sec. Then the specimens were stained by Giemsa solution for 15 min
followed by running water for 40 sec. Then the specimens were
completely cold dried by air. After rinsing in xylene for 1 min,
coverslipping of the specimens was performed. After evaluating the
specimens using a light microscope (BX40; Olympus Corporation), a
consensus was reached between a cytotechnologist (SK) and
cytopathologist (MS). Regarding manually assessed samples with
small cell numbers, 280 samples were observed using a light
microscope (magnification, ×40). In addition, the approximate
number of cells on the whole slide was evaluated, and the samples
were classified to contain <1,000 cells or ≥1,000 cells. Thus,
samples with < one cell in one high-power field (HPF) were
regarded as <1,000 cell cases because one HPF has an area of
0.785 mm2 and the whole area of the specimen (2×5 cm)
accounted for 1,273 HPFs. Moreover, the total number of cells in
the whole slide of the specimen was estimated to be <1,000 cells
if the number of cells per HPF was <one. The following samples
were excluded from the CAIA procedure in the consensus meeting
because the nuclei of leukocytes could not be appropriately
detected: Samples with i) markedly denatured cells, ii) possible
peripheral blood contamination, iii) >50% red blood cells
(RBCs), iv) a small number of cells (<1,000), v) almost
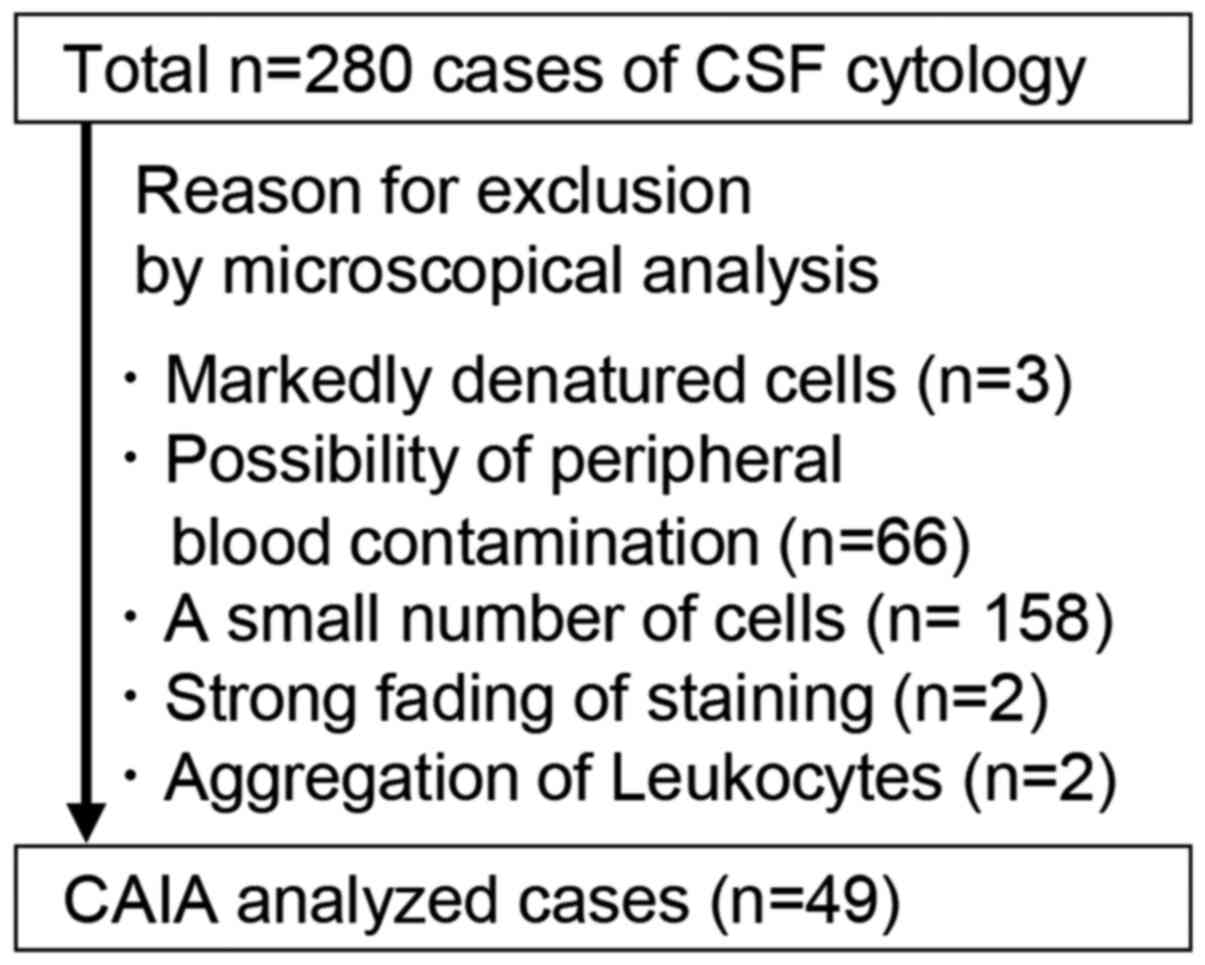
completely faded staining and vi) leukocyte aggregation (Fig. 1). Finally, CAIA was performed using
49 samples. Patients had a median age, 59.0 years; mean age, 55.8
years and age range, 20–80 years. Of the 49 patients 27 were male
and 22 female. The clinical diagnosis/cytological diagnosis of the
relevant patients are summarized in Table I as the comparison of original 280
cases and selected 49 cases.
 | Table I.Details of the clinical
diagnosis/cytological diagnosis of cases. |
Table I.
Details of the clinical
diagnosis/cytological diagnosis of cases.
|
Clinical/cytological diagnosis | Before
exclusion | CAIA |
|---|
| All cases | 280 | 49 |
|
Clinnon-tumor/Cytonegative,
n (%) | 120 (43) | 10 (21) |
| Non-HT, n (%) |
|
|
|
Clintumor/Cytonegative | 57 (20) | 7
(14) |
|
Clintumor/Cytomalignant | 17 (6) | 12 (24) |
| HT, n (%) |
|
|
|
Clintumor/Cytonegative | 65 (23) | 7
(14) |
|
Clintumor/Cytomalignant | 21 (8) | 13 (27) |
Definition of groups used in the
present study
The cases were grouped according to the combination
of clinical and cytological diagnoses as follows: Cases with no
tumor history or brain tumor according to CT, MRI or other
modalities and with a confirmed negative diagnosis of CSF cytology
(negative
cytology)(Clinnon-tumor/Cytonegative), cases
clinically suggestive of leptomeningeal carcinomatosis with
negative cytology (Clintumor/Cytonegative)
and cases clinically suggestive of leptomeningeal carcinomatosis
with positive cytology
(Clintumor/Cytomalignant). The present study
included cytological cases in which the primary tumor type was
confirmed by pathological diagnosis alone. The primary tumor
histological type was classified according to the World Health
Organization classification of tumors of the digestive system
(20), the central nervous system
(21), the lung, pleura, thymus and
heart (22) and hematopoietic and
lymphoid tissues (23) and is
presented in Table II. In terms of
tumor types, hematological malignancies were defined as described
in the World Health Organization classification of tumors of
hematopoietic and lymphoid tissues (23) as hematological tumor (HT) group, and
all another tumors as non-hematological tumor (non-HT) group in the
present study. The histological details of tumor types in CAIA
analyzed tumors were analyzed and it was found that 5 out of 9
cases of non-HT group tumor belonged to primary CNS tumor, the
other 4 cases belonged to metastatic carcinoma, 5 out of 7 HT group
tumor belonged to malignant lymphoma, and other 2 cases belonged to
leukemia (Table II).
 | Table II.Histological diagnosis of malignant
cases in the present study. |
Table II.
Histological diagnosis of malignant
cases in the present study.
| Histological
diagnosis | Cases, n (%) |
|---|
| Non-HT |
|
| Brain
medulloblastoma | 3 (33) |
| Stomach
poorly differentiated adenocarcinoma | 2 (22) |
| Brain
dysgerminoma | 1 (11) |
| Brain
malignant melanoma | 1 (11) |
|
Neuroendocrine carcinoma
(small-cell type) (rectum) | 1 (11) |
| Poorly
differentiated NSCLC | 1 (11) |
| HT |
|
| All
cases |
|
|
Malignant lymphoma | 5 (71) |
|
ALL | 1 (14) |
|
CML | 1 (14) |
WSI
The whole area of Pap-stained specimens and
Giemsa-stained specimens of the same sample was captured using a
virtual slide scanner (Nanozoomer SQ, Hamamatsu Photonics K.K.),
and WSI was performed in the ×40 mode (combination of 20X objective
lens and ×2 digital magnification). The specifications of the
Nanozoomer SQ are as follows: Camera pixels, 12 million pixels;
pixel size, 0.23 µm/pixel; and LED source, tiling capture via a
color CMOS sensor. Pap staining was focused manually, and Giemsa
staining was focused automatically.
CAIA
The WSI images of Pap- and Giemsa-stained specimens
were acquired using a Panoramic Viewer version 1.15.4, QuantCenter,
Histoquant module (3DHISTECK Ltd.). In addition, the protocols that
could detect the nuclei of lymphocytes, neutrophils, macrophages,
RBCs and all cells were determined independently, and each
population was separately analyzed to enumerate the cell counts
(Table III). The CAIA conditions
for each blood cell type and the total cells are presented in
Table III.
 | Table III.Image analysis conditions for each
blood cell type and all cells. |
Table III.
Image analysis conditions for each
blood cell type and all cells.
|
| Cell type |
|
|---|
|
|
|
|
|---|
| Factor | Lymphocytes | Lymphocytes | Macrophages | RBCs | All cells |
|---|
| Cell size,
pixels | 20-70 | 35-70 |
71-140 |
15-30 | 20-1,000 |
| Shape factor | 0.51–1 | 0-0.5 |
0-1 |
0.7–1 |
0-1 |
| Red range | 57-224 | 57-224 |
48-241 |
60-240 | 48-241 |
| Green range | 25-172 | 25-172 |
45-156 | 113-208 | 25-172 |
| Blue range | 85-205 | 85-214 | 111-222 | 120-215 | 85-222 |
Accordance rate between CAIA and
manual analysis
In total 29 representative cases were evaluated to
assess the accuracy of CAIA. At least 50 leukocytes/case were
evaluated. For this purpose, at least five images were captured
(magnification, ×40). If five images contained <50 leukocytes,
additional images were captured until the total cell count reached
at least 50 leukocytes. Then, one cytopathologist (M.S.) and one
cytotechnologist (S.K.) manually evaluated the images, and
consensus meetings were held to determine the cell types. Then, the
same images were utilized for CAIA. A two-way evaluation was
performed involving the accuracy of CAIA in evaluating manually
evaluated leukocytes (power of analysis) and the accuracy of
CAIA-detected cells under each condition shown in Table III in comparison with manually
evaluated cells (accuracy of analysis condition).
Statistical analysis
JMP Pro version 12.2.0 software (SAS Institute Inc.;
http://www.jmp.com/en_us/home.html)
was used for all statistical analyses. The Wilcoxon rank-sum test
was used for comparisons between the total cells of Pap- and
Giemsa-stained specimens. P-value was calculated by one-way
chi-square approximation for Wilcoxson rank-sum test. For multiple
comparisons, Wilcoxon's test should not be repeated, thus the
Steel-Dwass test was used for multiple comparisons involving each
blood cell component for each clinical diagnosis/cytological
diagnosis in the non-hematological tumor (non-HT) and hematological
tumor (HT) groups (24). Asymptotic
test was performed to calculate the P-values in Steel-Dwass test.
For the statistical examination of contingency tables in which one
of the groups was <5, the Fisher's exact test was used.
P<0.05 was considered to indicate a statistically significant
difference. As the present sample size was relatively small, in
addition to the aforementioned significant level, the power of the
statistical analysis was presented as 1-β using JMP Pro version
12.2.0 software. The cut-off for the population of macrophages in
the cytological diagnosis was calculated using the Youden index
(25) and the area under the curve
(AUC) was estimated.
Results
Increased total cell count in
malignant cytological CSF specimens using manual evaluation
All Giemsa-stained specimens were initially analyzed
by CAIA. However, due to several reasons summarized in Fig. 1, 231 cases could not be analyzed by
CAIA. The main reasons were a small number of cells (n=158) and
possibility of peripheral blood contamination (n=66), which account
for 97.0% of excluded cases (Fig.
1). Comparison of 280 cases and CAIA analyzed 49 cases for
clinical diagnosis and cytological diagnosis-based grouping were
shown in Table I. The association
between the cell count in Giemsa specimens and the clinical
background, cytological diagnosis and tumor type (HT or non-HT) was
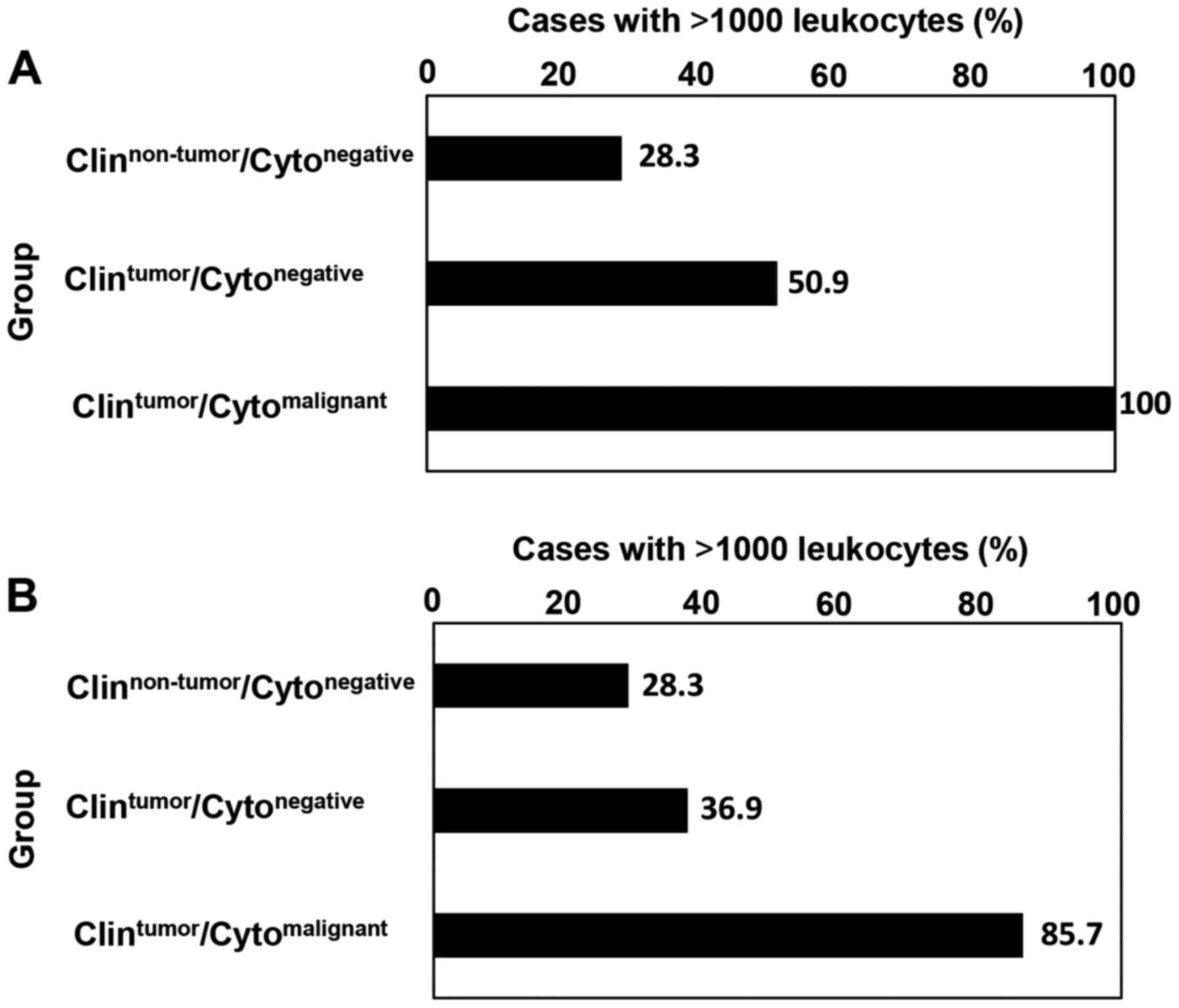
established via manual estimation (Fig.
2). In the non-HT group, the percentages of samples with cell
counts of >1,000 cells were 28.3% (34/120) in the
Clinnon-tumor/Cytonegative group, 50.9%
(29/57) in the Clintumor/Cytonegative group
and 100% (17/17) in the
Clintumor/Cytomalignant group (Fig. 2A). In the HT group, the percentages
of samples containing >1,000 cells were 28.3 (34/120), 36.9
(24/65), and 85.7% (18/21) in the
Clinnon-tumor/Cytonegative,
Clintumor/Cytonegative and
Clintumor/Cytomalignant groups, respectively
(Fig. 2B). These data suggested
frequency of cases that can be analyzed by CAIA were high in
cytologically malignant cases in both the HT and non-HT groups.
Giemsa staining is suitable for whole
slide leukocyte counting via CAIA
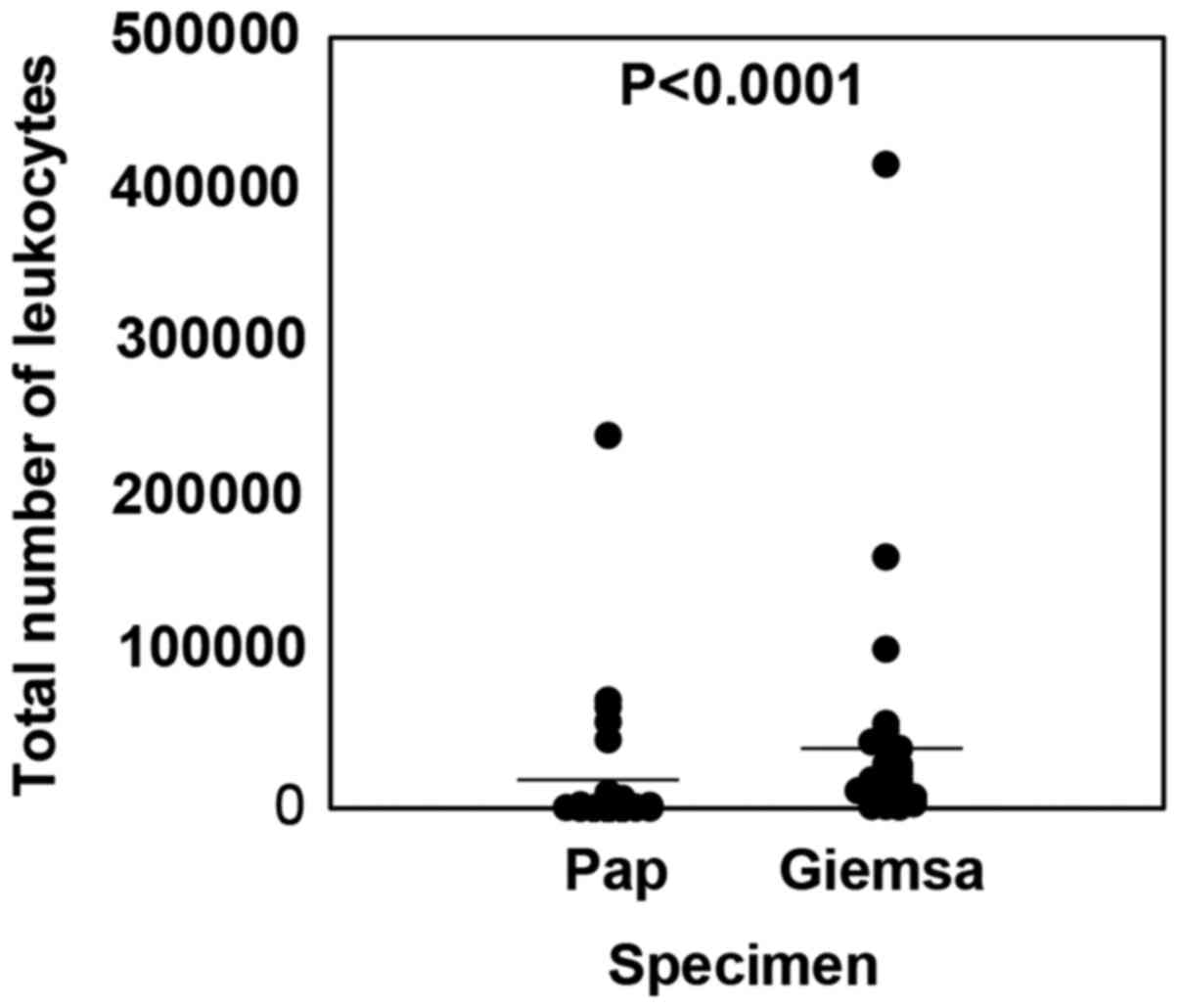
To compare the total cell number between WSI and
CAIA for Giemsa and Pap specimens prepared from the same samples,
the whole images of Pap and Giemsa specimens were analyzed with
respect to the number of nuclei to detect individual cells
(Fig. 3). The average total cell
counts in Pap and Giemsa specimens were 10,639±37,182 and
27,041±62,804, respectively; thus, the cell count was 2.54-fold
higher in Giemsa specimens (P<0.0001). These data suggested that
Giemsa specimens were superior to Pap smear in terms of cell
holding ability on the glass.
An increased percentage of macrophages
in cytological CSF specimens is an indicator of non-HT tumors
For the 49 samples subjected to CAIA, the number and
subtype ratio of the leukocyte population was evaluated. The
percentage of each leukocyte subtype was assessed according to the
combination of the clinical diagnosis at the time of sample
submission and the cytological diagnosis of the specimens using the
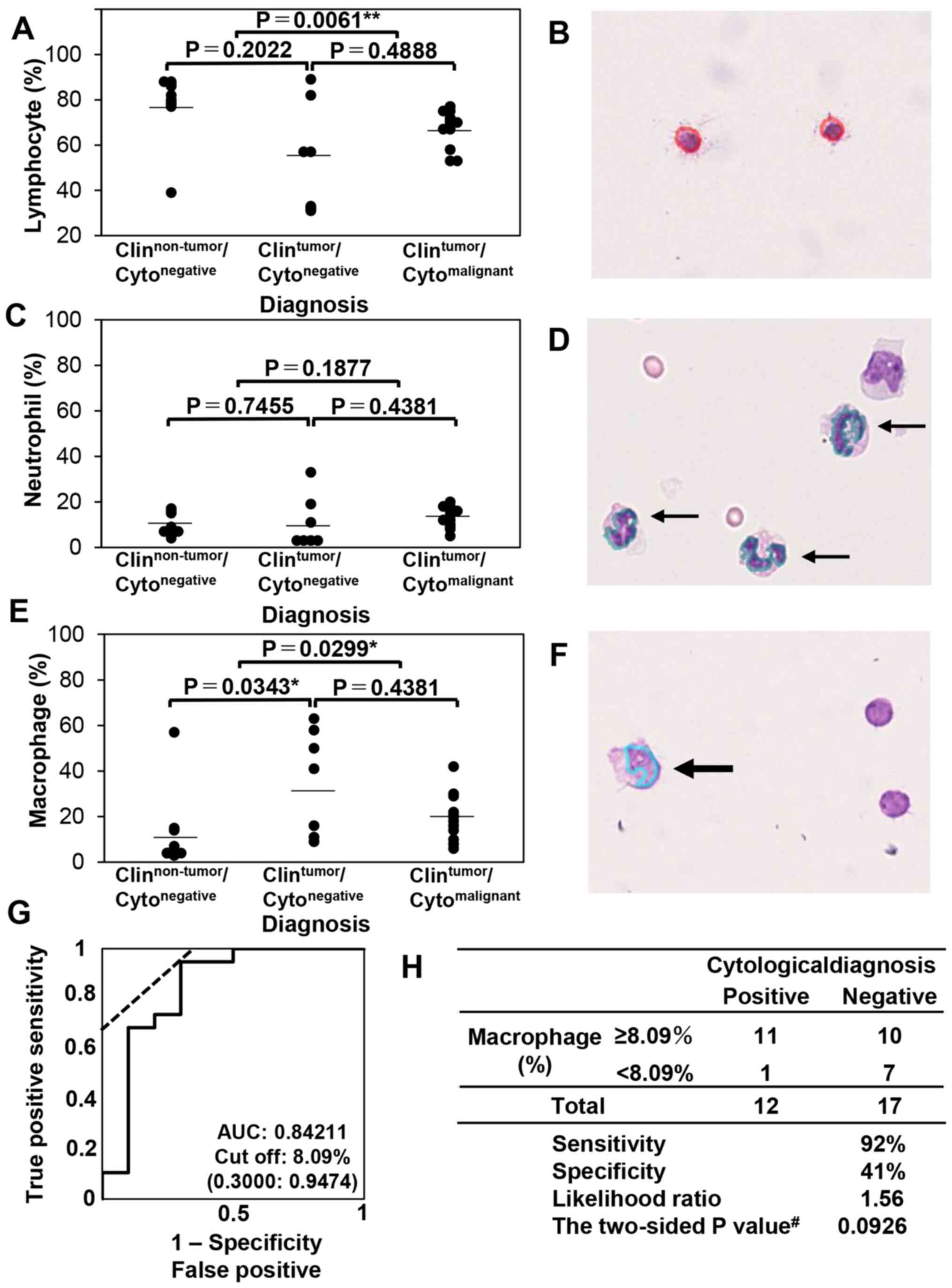
Steel-Dwass test. Next, samples from cases of malignancy in the
non-HT (Fig. 4A-F) were evaluated
and multiple comparisons among the clinical diagnosis/cytological
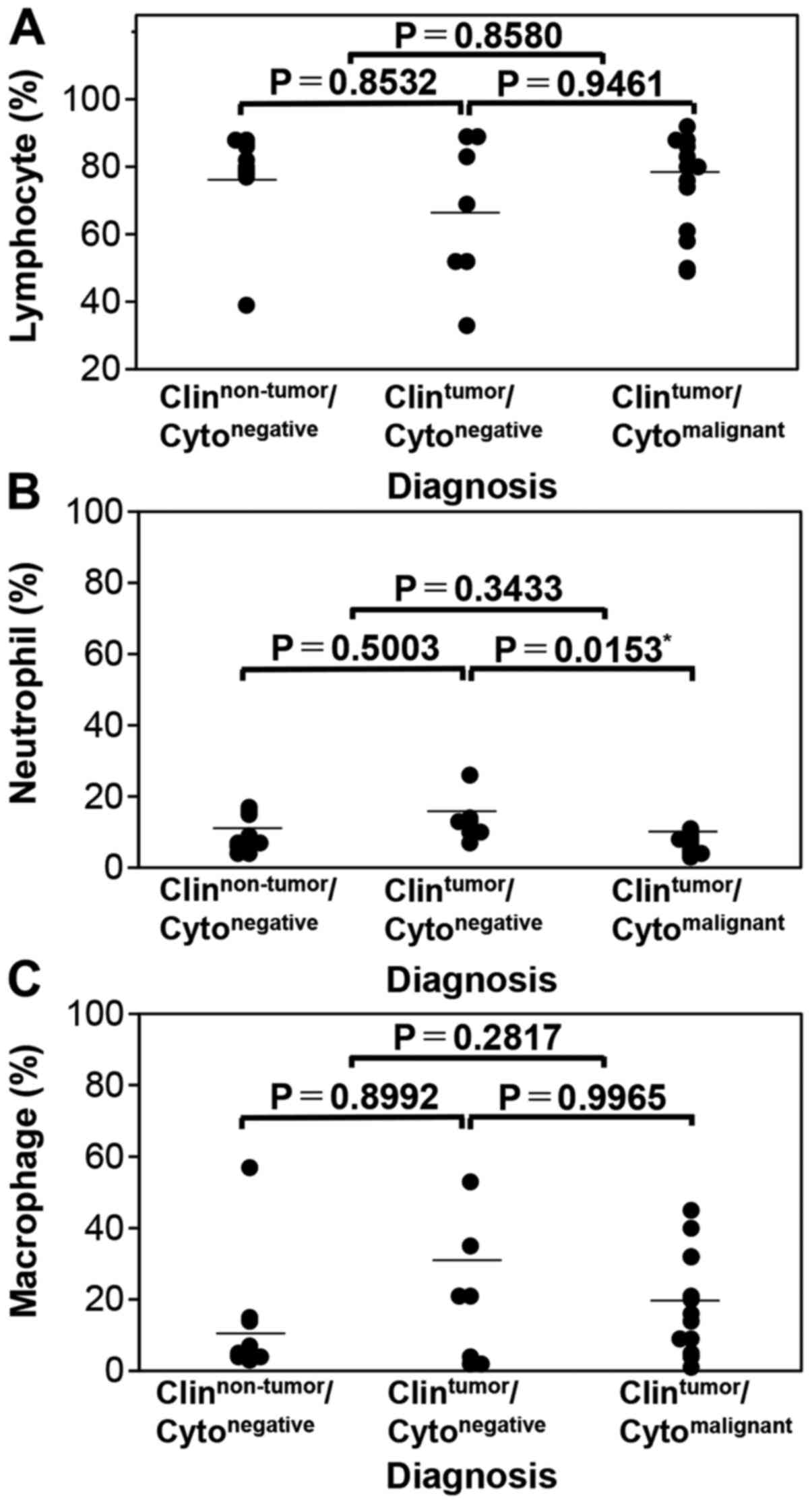
diagnosis groups were conducted. In the non-HT group, the
lymphocyte ratio was significantly different between the
Clinnon-tumor/Cytonegative and
Clintumor/Cytomalignant groups (P=0.0061,
α=5%, 1-β=57.30%) but not between the
Clinnon-tumor/Cytonegative and
Clintumor/Cytonegative groups (P=0.2022) or
between the Clintumor/Cytonegative and
Clintumor/Cytomalignant groups (P=0.4888;
Fig. 4A). The neutrophil ratio was
not significantly different among the three groups (Fig. 4C). Meanwhile, the macrophage ratio
significantly differed between the
Clinnon-tumor/Cytonegative and
Clintumor/Cytonegative groups (P=0.0343,
α=5%, 1-β=61.94%) and between the
Clinnon-tumor/Cytonegative and
Clintumor/Cytomalignant groups (P=0.0299,
α=5%, 1-β=24.45%) but not between the
Clintumor/Cytonegative and
Clintumor/Cytomalignant groups (P=0.4381;
Fig. 4E). Representative images
obtained using CAIA are presented in Fig. 4B, D and F. As the macrophage
population was higher in the Clinnon-tumor group
compared with Clintumor group including
Clintumor/Cytonegative and
Clintumor/Cytomalignant group, the
association between the ratio of macrophages among total leukocytes
and clinical negativity was analyzed. The receiver operating
characteristic curve of the macrophage ratio for distinguishing the
Clinnon-tumor/Cytonegative group from the
clinically non-HT≈group (including both cytologically negative and
positive cases) was examined, and the cut-off was calculated. As
presented in Fig. 4G, the AUC was
0.84211, and the cut-off was 8.09%. Moreover, the likelihood ratio
was 1.56, and the P-value using the Fisher's exact test was 0.0926
(Fig. 4H). These results suggested
that the macrophage population was increased in the non-HT tumor
group and that this increase could be detected by CAIA.
Malignant hematological tumors and the
reactive leukocyte population in cytological CSF specimens cannot
be distinguished via CAIA
Next, the leukocyte population in the HT group was
evaluated, and it was reported that the lymphocyte ratio did not
differ among any of the groups (Fig.
5A). Meanwhile, the neutrophil ratio significantly differed
only between the Clintumor/Cytonegative and
Clintumor/Cytomalignant groups (P=0.0153;
Fig. 5B). In addition, the
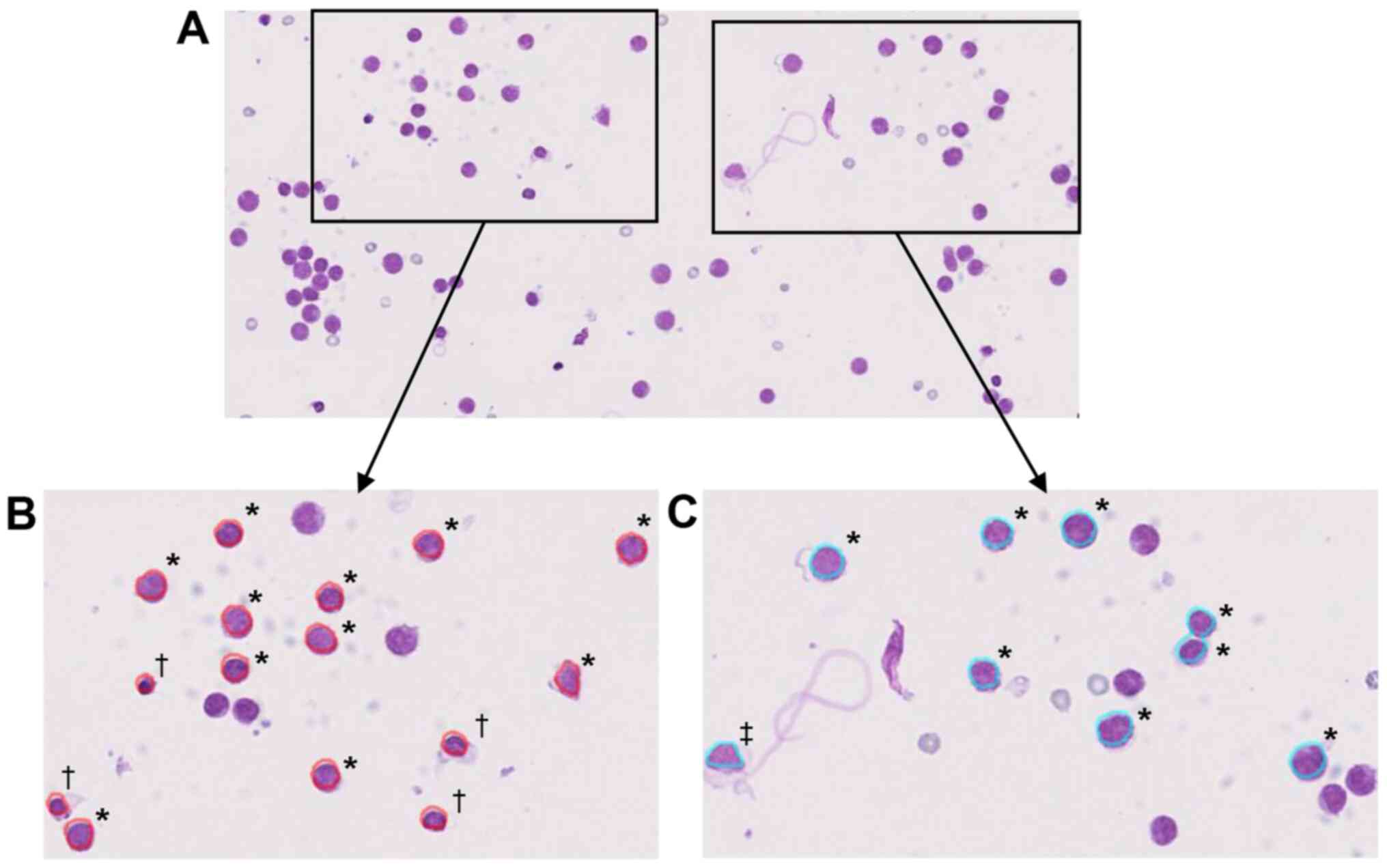
macrophage ratio did not differ among the groups (Fig. 5C). The representative malignant
lymphoma sample in which tumor nuclei were incorrectly identified
as normal lymphocytes or macrophages is presented in Fig. 6A-C. In this case, under both the
lymphocyte, and macrophage detection conditions, more than half of
the lymphoma cells were incorrectly recognized as normal. These
data indicated that in the present condition of CAIA, leukocytes
should not be evaluated in HT tumor cases.
Macrophage population is increased in
non-HT cases using manual and CAIA evaluation
To evaluate the power and accuracy of specific
subtype analyses, the rate of accordance between CAIA and manual
analysis in the non-HT group was compared (Table IV). The average accordance rate of
CAIA with manual evaluation was 68.33% for total leukocyte counts
in the non-HT cases analyzed in Fig.
4 (n=29). Conversely, the average accordance rate of manual
evaluation with CAIA was 67.30% for total leukocyte count in the
same cases. In addition, the average accordance rate of CAIA with
manual evaluation and that of manual evaluation with CAIA were
70.53 and 79.14%, respectively, for lymphocyte counts and 59.62 and
65.26%, respectively, for macrophage counts (Table IV). However, the average accordance
rate of CAIA with manual evaluation and that of manual evaluation
with CAIA were very low (6.37 and 7.52%, respectively) for
neutrophil counts, primarily due to the low number of cells and
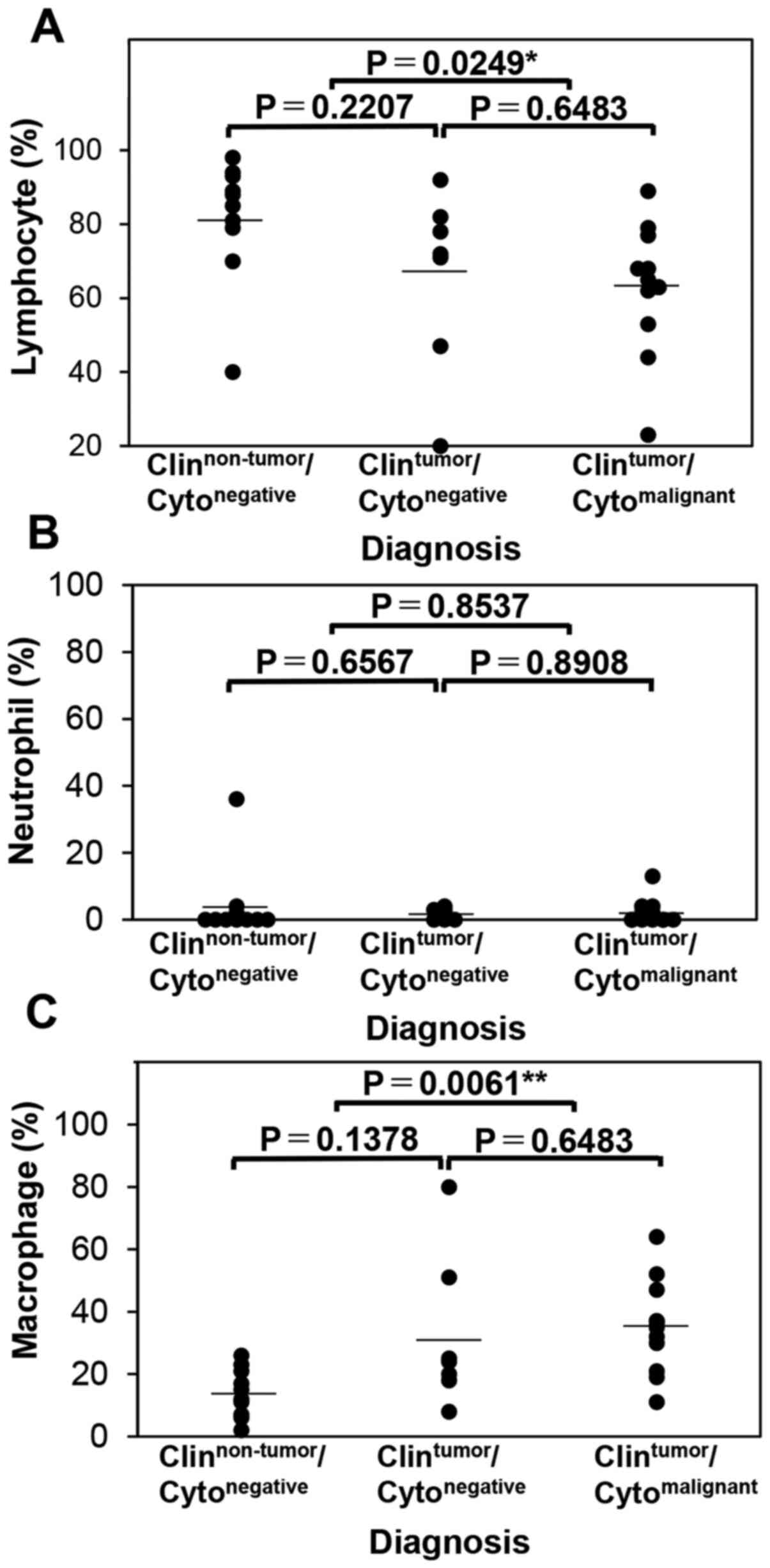
misrecognition of neutrophils as macrophages (Table IV). As the accordance rate between
CAIA and manual evaluation was not high, the specimens of non-HT
cases (Fig. 7A-C) was also evaluated
and multiple comparisons were performed among the
clinical/cytological diagnosis groups. In the non-HT group, the
lymphocyte ratio was significantly different between the
Clinnon-tumor/Cytonegative and
Clintumor/Cytomalignant groups (P=0.0249;
α=5%; 1-β=69.08%) but not between the
Clinnon-tumor/Cytonegative and
Clintumor/Cytonegative groups (P=0.2207) or
between the Clintumor/Cytonegative and
Clintumor/Cytomalignant groups (P=0.6483)
(Fig. 7A). The neutrophil ratio did
not significantly differ among the groups (Fig. 7B). Meanwhile, the macrophage ratio
significantly differed between the
Clinnon-tumor/Cytonegative and
Clintumor/Cytomalignant groups (P=0.0061;
α=5%; 1-β=96.82%), but not between the
Clinnon-tumor/Cytonegative and
Clintumor/Cytonegative groups (P=0.1378) or
between the Clintumor/Cytonegative and
Clintumor/Cytomalignant groups (P=0.6483;
Fig. 7C). These data suggested that,
although the accordance rate between CAIA analysis and manual
evaluation was not high, there was a tendency of a decreased
lymphocyte population and an increased macrophage population in
both evaluation methods. Overall, these data suggested that
increased percentage of monocyte/macrophage population within the
total leukocyte population in Giemsa-stained specimens should be
considered as a sign of non-HT leptomeningeal carcinomatosis, at
least in medulloblastoma, poorly differentiated adenocarcinoma of
the stomach, dysgerminoma of the brain, malignant melanoma of the
brain, neuroendocrine carcinoma (small-cell type) of the rectum and
poorly differentiated non-small cell lung carcinoma which was the
cases of non-HT tumors as summarized in Table II. Therefore, our data indicated
that an increase in the monocyte/macrophage population in
cytological specimens should prompt evaluation for the possible
presence of leptomeningeal carcinomatosis in patients with these
tumors.
 | Table IV.Average accordance rate between CAIA
and manual evaluation for each leukocyte type for 29 samples. |
Table IV.
Average accordance rate between CAIA
and manual evaluation for each leukocyte type for 29 samples.
| A, Accordance rate
of CAIA with manual evaluation |
|---|
|
|---|
| Lymphocytes, %
(Mean/SD) | Neutrophils, %
(Mean/SD) | Macrophages, %
(Mean/SD) | All leukocytes, %
(Mean/SD) |
|---|
| 70.53
(39.3/17.1) | 6.37
(3.7/15.4) | 59.62
(14.9/11.4) | 68.33
(57.9/34.5) |
|
| B, Accordance
rate of manual with CAIA evaluation |
|
| Lymphocytes, %
(Mean/SD) | Neutrophils, %
(Mean /SD) | Macrophages, %
(Mean /SD) | All leukocytes,
% (Mean/SD) |
|
| 79.14
(35.2/19.9) | 7.52 (3.9/6.8) | 65.26
(14.3/19.0) | 67.30
(53.3/33.2) |
Discussion
The present study emphasized the significance of the
examination of the background leukocyte population in cytological
specimens using CAIA. First, the association between the number of
cells in cytological CSF specimens and the cytological diagnosis
(benign or malignant) was examined. The frequency of specimens
containing >1,000 cells was increased in malignant cases
regardless of the tumor type. Regarding the cell count in CSF,
several reports have indicated that an increased cell count is
observed in the malignant CSF group via manual (4,6,7) and flow cytometric evaluation (9). In the present study, the number of
cells on the glass slide did not reflect the number of cells per
volume of CSF because the amount of CSF used varied between cases;
however, the number of cells might have been increased in
cytologically positive cases. The CSF volume used to prepare the
specimens could not be determined from medical records. Thus, when
recording the amount of CSF submitted to the laboratory, the amount
of CSF used to prepare specimens should be noted, and the leukocyte
composition of cytological specimens is important.
Next, it was determined whether Giemsa or Pap
staining was the optimal preparative method for CAIA. The number of
cells was significantly higher for Giemsa samples compared with
those of Pap samples. Anand et al (26) reported that hematoxylin and
eosin-stained and Pap-stained specimens had fewer cells loaded on
the glass, whereas this was not observed for Giemsa-stained
specimens because the cells adhered more firmly to the glass slide
during dry fixation. Beyer-Boon et al (27) reported that more cells remain on the
glass in Giemsa (dry fixation) specimens compared with those in Pap
(alcohol fixation) specimens among urine cytology cases. These
results are in agreement with the present findings. Thus, Giemsa
specimens are suitable for the leukocyte evaluation of cytological
CSF specimens using CAIA.
Then, the association between the percentage of each
leukocyte type and the cytological diagnosis was determined. Among
samples from the non-HT group, the lymphocyte ratio was
significantly decreased, macrophage ratio was significantly
increased and there was no difference in the neutrophil ratio
between the cytologically positive and cytologically negative
groups. In addition, the possibility of distinguishing between the
cytologically tumor-negative and tumor-positive cases based on the
cut-off macrophage ratio was demonstrated. Similar studies focusing
on inflammatory cells have been reported by Djukic et al
(7) and Illan et al (9). Djukic et al described the
relation of total cell density (include tumor cells and leukocytes)
and frequency of lymphocytes and granulocytes in the CFS, and
lymphocytes and granulocytes were frequently observed in total cell
number high cases (>4 cells/µl cases). However, they just
examined the cases of metastatic carcinomas, hematological
malignancies and primary CNS tumors altogether. Djukic et al
used a different grouping of cases compared with the current study,
and did not mention percentages of leukocyte subtypes, thereby
making it difficult to compare the results. Illan et al
compared the percentages of lymphocytes, neutrophils and monocytes
between cytologically negative and positive groups; however, the
percentage of neutrophils was significantly increased, whereas
those of lymphocytes and monocytes were not significantly
different. These results are not identical to those in the present
study. A possible explanation for this may be that Illan et
al used clinically malignant/cytologically negative samples,
unlike the negative group of the present study. In the present
study, the macrophage ratio was high in the non-HT cytologically
positive group compared with cytologically negative group. In the
tumor microenvironment, tumor-associated macrophages (TAMs)
infiltrate and promote tumor progression (28). Monocytes in the peripheral blood are
recruited to the tumor site and differentiate into macrophages in
response to chemokines and growth factors produced by tumor cells
(28). Among the non-HT samples
examined in the present study, the type of factors that contributed
to the TAM recruitment for gastric cancer, medulloblastoma and
dysgerminoma could not be identified. Reportedly, TAMs are
recruited by chemokines produced by non-small cell lung cancer
cells, homeoproteins produced by colon cancer cells, and secreted
and transmembrane protein 1 produced by melanoma cells (29–31).
These reports suggest that macrophage recruitment is increased by
tumor-produced factors. Thus, the increased percentage of
macrophages in cytological CSF specimens in clinically malignant
cases reflects TAM induction. To the best of our knowledge, no
studies have examined the association between cytokine/chemokine
production in the tumor microenvironment in the central nervous
system and the leukocyte population in the CSF. Thus, this
association should be examined in the future.
In terms of normal CSF, the median leukocyte ratios
are 86.5% for lymphocytes, 10.5% for monocytes and 2.0% for
macrophages (3). In leptomeningeal
carcinomatosis, the median leukocyte ratios are 59.7% for
lymphocytes, 24.0% for monocytes and 1.5% for neutrophils (9). Thus, these reports support the present
findings demonstrating that the macrophage population is increased
in non-HT tumor group. The present data suggested that an increased
number of macrophages is an indicator of leptomeningeal
carcinomatosis in cytological specimens of leptomeningeal spaces
even in atypical cells are not identified. Indeed, Chamberlain
et al (32) reported that the
cytological detection of malignant cells in leptomeningeal
metastasis is affected by the collection site, for example
ventricular or lumbar CSF. Thus, in cases where intracranial tumors
are detected, it is possible that spinal samples will be negative,
and the converse, that is, spinal tumor cases with intracranial
sample will be negative can also be true (32). Thus, cytopathologists and
cytotechnologists should monitor patients for leptomeningeal
carcinomatosis when they detect a higher macrophage count in CSF
samples. In order to investigate the possibility that macrophage
infiltration into CSF can occur in advance of malignant cells
exuding into the CSF, chronologically collected CSF samples of the
same patients are used. However, the present study could not
evaluate the cytological specimens of the same patients
chronologically due to lack of cases. Future studies including
chronological analysis are necessary to confirm monocyte/macrophage
characteristics and to assess changes in their numbers with the
emergence of tumor cells in the CSF.
Finally, the adequacy of the sample size for
statistical analysis must be discussed. In statistical analysis,
two types of errors exist (33). One
is α errors (type I errors), in which a significant difference is
incorrectly identified between two groups (33). The other is β errors (type II
errors), in which the absence of a significant difference is
incorrectly identified between two groups (33). Indeed, α for the present
statistically significant results was <5%. Thus, there was a low
possibility of a type I error. Usually, 1-β represents the power of
a statistical test. In statistical tests, the power increases as
the sample size increases (34).
Unfortunately, the present results exhibited low power, indicating
the possibility that the data were incorrectly considered
non-significant. Therefore, larger sample sizes should be used in
future research.
Overall, the analysis of the cytological specimens
in the present study revealed that leukocyte counts in the
background were higher in cytologically positive cases, and the
percentage of macrophages was elevated in non-HT cases. Therefore,
in the future, it is important to focus on the number of leukocytes
and the leukocyte ratio in the background while examining
cytological CSF specimens.
Acknowledgements
Not applicable.
Funding
This study was supported by the annual experimental
budget to MS and SK given by Gunma University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SK conducted clinical data collection, digital
imaging of the specimens via virtual slide scanning, image
analysis, statistical analysis, figure preparation and manuscript
preparation. MS developed the experimental design, conducted
experiments, and performed digital imaging of specimens via virtual
slide scanning, image analysis, figure preparation, statistical
analysis and manuscript preparation. MF assisted with clinical data
collection and reviewed the manuscript for manuscript preparation.
JH, TO and TF diagnosed independently by the request of MS for the
cases which original diagnosis and diagnosis in this study at
consensus meeting by SK and MS was not identical. JH, TO and TF
also reviewed the manuscript for manuscript preparation. All
authors read and approved the manuscript and agreed to be
accountable for all aspects of the research to ensure that the
accuracy or integrity of any part of the work was appropriately
investigated and resolved.
Ethics approval, consent to participate
The present study was approved by the Gunma
University Ethical Review Board for Medical Research Involving
Human Subjects of Gunma University School of Medicine (GUERB;
Gunma, Japan) (approval number, HS2017-120), and the written
notification for the current study was presented publicly on the
webpage of Gunma University Hospital. Furthermore, the possibility
to decline participation in this study was provided according to
the Ethical Guidelines for Medical and Health Research Involving
Human Subjects of the Japanese government (Ministry of Education,
Culture, Sports, Science and Technology, and Ministry of Health,
Labour and Welfare). Informed consent was waived by GUERB based on
the above guidelines due to the retrospective nature of the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roth P and Weller M: Management of
neoplastic meningitis. Chin Clin Oncol. 4:262015.PubMed/NCBI
|
|
2
|
Chamberlain MC: Neoplastic meningitis.
Oncologist. 13:967–977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sornas R: The cytology of the normal
cerebrospinal fluid. Acta Neurol Scand. 48:313–320. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rahimi J and Woehrer A: Overview of
cerebrospinal fluid cytology. Handb Clin Neurol. 145:563–571. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayward RA, Shapiro MF and Oye RK:
Laboratory testing on cerebrospinal fluid. A reappraisal. Lancet.
1:1–4. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
MacKenzie JM: Malignant meningitis: A
rational approach to cerebrospinal fluid cytology. J Clin Pathol.
49:497–499. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Djukic M, Trimmel R, Nagel I, Spreer A,
Lange P, Stadelmann C and Nau R: Cerebrospinal fluid abnormalities
in meningeosis neoplastica: A retrospective 12-year analysis.
Fluids Barriers CNS. 14:72017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Zanten AP, Twijnstra A and Ongerboer
de Visser BW: Routine investigations of the CSF with special
reference to meningeal malignancy and infectious meningitis. Acta
Neurol Scand. 77:210–214. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Illan J, Simo M, Serrano C, Castañón S,
Gonzalo R, Martínez-García M, Pardo J, Gómez L, Navarro M, Altozano
JP, et al: Differences in cerebrospinal fluid inflammatory cell
reaction of patients with leptomeningeal involvement by lymphoma
and carcinoma. Transl Res. 164:460–467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh G, Mathur SR, Iyer VK and Jain D:
Cytopathology of neoplastic meningitis: A series of 66 cases from a
tertiary care center. Cytojournal. 10:132013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ho CY, VandenBussche CJ, Huppman AR,
Chaudhry R and Ali SZ: Cytomorphologic and clinicoradiologic
analysis of primary nonhematologic central nervous system tumors
with positive cerebrospinal fluid. Cancer Cytopathol. 123:123–135.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rao R, Hoda SA, Marcus A and Hoda RS:
Metastatic breast carcinoma in cerebrospinal fluid: A
cytopathological review of 15 cases. Breast J. 23:456–460. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bell JE: Update on central nervous system
cytopathology. I. Cerebrospinal fluid. J Clin Pathol. 47:573–578.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bigner SH: Cerebrospinal fluid (CSF)
cytology: Current status and diagnostic applications. J Neuropathol
Exp Neurol. 51:235–245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cutler RW and Spertell RB: Cerebrospinal
fluid: A selective review. Ann Neurol. 11:1–10. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamada M, Saito A, Yamamoto Y, Cosatto E,
Kurata A, Nagao T, Tateishi A and Kuroda M: Quantitative nucleic
features are effective for discrimination of intraductal
proliferative lesions of the breast. J Pathol Inform. 7:12016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kosuge N, Saio M, Matsumoto H, Aoyama H,
Matsuzaki A and Yoshimi N: Nuclear features of infiltrating
urothelial carcinoma are distinguished from low-grade noninvasive
papillary urothelial carcinoma by image analysis. Oncol Lett.
14:2715–2722. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eyraud D, Granger B, Bardier A, Loncar Y,
Gottrand G, Le Naour G, Siksik JM, Vaillant JC, Klatzmann D,
Puybasset L, et al: Immunological environment in colorectal cancer:
A computer-aided morphometric study of whole slide digital images
derived from tissue microarray. Pathology. 50:607–612. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kobayashi S, Saio M, Fukuda T, Kimura K,
Hirato J and Oyama T: Image analysis of the nuclear characteristics
of emerin protein and the correlation with nuclear grooves and
intranuclear cytoplasmic inclusions in lung adenocarcinoma. Oncol
Rep. 41:133–142. 2019.PubMed/NCBI
|
|
20
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO Classification Of Tumours of the Digestive System.
WHO Classification of Tumours. 4th edition. 3. International Agency
for Research on Cancer; Lyon: 2010
|
|
21
|
Louis DN, Ohgaki H, Wiestler OD and
Cavenee WK: WHO Classification Of Tumours of the Central Nervous
System. WHO Classification of Tumours. 4th edition. 1.
International Agency for Research on Cancer; Lyon: 2007
|
|
22
|
Travis WD, Brambilla E, Burke AP, Marx A
and Nicholson AG: WHO Classification of Tumours of the Lung,
Pleura, Thymus, and Heart. WHO Classification of Tumours. 4th
edition. 7. International Agency for Research on Cancer; Lyon:
2015
|
|
23
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO Classification of
Tumours of the Haematopoietic and Lymphoid Tissues. WHO
Classification of Tumours. 4th edition. 2. International Agency for
Research on Cancer; Lyon: 2008
|
|
24
|
Douglas CE and Michael FA: On
distribution-free multiple comparisons in the one-way analysis of
variance. Commun Stat Theory Methods. 20:127–139. 1991. View Article : Google Scholar
|
|
25
|
Perkins NJ and Schisterman EF: The
inconsistency of ‘optimal’ cutpoints obtained using two criteria
based on the receiver operating characteristic curve. Am J
Epidemiol. 163:670–675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anand M, Kumar R, Jain P, Asthana S, Deo
SV, Shukla NK and Karak A: Comparison of three different staining
techniques for intraoperative assessment of nodal metastasis in
breast cancer. Diagn Cytopathol. 31:423–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beyer-Boon ME and Voorn-den Hollander MJ:
Cell yield obtained with various cytopreparatory techniques for
urinary cytology. Acta Cytol. 22:589–593. 1978.PubMed/NCBI
|
|
28
|
Kim J and Bae JS: Tumor-Associated
macrophages and neutrophils in tumor microenvironment. Mediators
Inflamm. 2016:60581472016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arenberg DA, Keane MP, DiGiovine B, Kunkel
SL, Strom SR, Burdick MD, Iannettoni MD and Strieter RM: Macrophage
infiltration in human non-small-cell lung cancer: The role of CC
chemokines. Cancer Immunol Immunother. 49:63–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu H, Zhang Y, Pena MM, Pirisi L and Creek
KE: Six1 promotes colorectal cancer growth and metastasis by
stimulating angiogenesis and recruiting tumor-associated
macrophages. Carcinogenesis. 38:281–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang T, Ge Y, Xiao M, Lopez-Coral A, Li L,
Roesch A, Huang C, Alexander P, Vogt T, Xu X, et al: SECTM1
produced by tumor cells attracts human monocytes via CD7-mediated
activation of the PI3K pathway. J Invest Dermatol. 134:1108–1118.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chamberlain MC, Kormanik PA and Glantz MJ:
A comparison between ventricular and lumbar cerebrospinal fluid
cytology in adult patients with leptomeningeal metastases. Neuro
Oncol. 3:42–45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gaddis GM and Gaddis ML: Introduction to
biostatistics: Part 3, sensitivity, specificity, predictive value,
and hypothesis testing. Ann Emerg Med. 19:591–597. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hazra A and Gogtay N: Biostatistics series
module 5: Determining sample size. Indian J Dermatol. 61:496–504.
2016. View Article : Google Scholar : PubMed/NCBI
|