Introduction
Pseudolaric acid B (PAB) is a diterpene-type acid
isolated from the root and trunk bark of Pseudolarix
kaempferi Gordon of the Pinaceae family, also termed
‘Tu-Jin-Pi’ in Traditional Chinese Medicine, which has been used to
treat dermatological fungal infections (1,2). PAB
exerts potent inhibition of cell proliferation in vitro in
various tumor cell lines by inducing cell cycle arrest and
apoptosis in human melanoma cell line A375-S2 and human breast
cancer cell line MCF-7 (1–5) or autophagy in murine fibrosarcoma cell
line L929 and human thyroid squamous cell line SW579 (6–8).
Rhabdomyosarcoma is the most common type of soft tissue sarcoma in
children worldwide and represents a high-grade neoplasm of skeletal
myoblasts, this tumor accounts for 5–10% of all childhood tumors
since 2002 (9). Surgery and
chemotherapy are currently the main treatment methods for
rhabdomyosarcoma. In the present study the antitumor effect of PAB
on rhabdomyosarcoma was investigated.
Previous studies have focused on apoptosis as a
process that can be used for antitumor drug development (10,11). In
addition to apoptosis, autophagy is extensively studied in the
field of oncology (12,13). Apoptosis, or programmed cell death,
refers to a series of biochemical changes in the cell that lead to
distinct morphological changes (14). The major mechanisms of apoptosis
include the extrinsic pathway, which activates caspase-8 and
caspase-10 in response to external stimuli, and the intrinsic
pathway, also termed the mitochondrial pathway, which induces the
cleavage of pro-caspase-9 in response to internal stimuli (14). Autophagy is a process during which
the components of the cell are delivered to lysosomes for
degradation (15). Under certain
circumstances, this process may promote cell death and morbidity;
however, under the majority of circumstances, autophagy promotes
survival by adjusting the cellular response to stress conditions
(16,17). Following the induction of apoptosis
or autophagy, cell cycle arrest is observed (6–8,18,19).
Cell cycle arrest is a tumor-suppressive mechanism, as the cells do
not enter the next phase of the retarded phase (4,7,12) and thus, cell proliferation is
inhibited.
The present study aimed to prove that PAB inhibited
human rhabdomyosarcoma proliferation and to confirm the inhibitory
mechanism of PAB, which provided potential opportunities to further
develop new drugs for improved clinical outcomes.
Materials and methods
Materials
PAB was purchased from the National Institute for
the Control of Pharmaceutical and Biological Products and was
dissolved in dimethyl sulfoxide (DMSO) to produce a stock solution.
The DMSO concentration was maintained <0.01% in the cell culture
and did not exert any detectable effect on cell proliferation or
death. Propidium iodide (PI), Hoechst 33258, RNase A,
3-methyladenine (3-MA),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
monodansylcadaverine (MDC), NBT and BCIP were purchased from
Sigma-Aldrich; Merck KGaA. Antibodies against Caspase-8 (cat. no.
66093-1-Ig), caspase-9 (cat. no. 66169-1-Ig), cyclin B1 (cat. no.
55004-1-AP), Beclin 1 (cat. no. 11306-1-AP), LC3 (cat. no.
14600-1-AP), beta actin (cat. no. 66009-1-Ig) and tubulin (cat. no.
10068-1-AP) were purchased from ProteinTech Group, Inc., whereas
the antibody against phosphorylated H2A histone family member X
(γ-H2AX; cat. no. 9718S) was purchased from Cell Signaling
Technology, Inc. Alkaline Phosphatase AffiniPure Goat Anti-Mouse
IgG (H+L) (cat. no. 115-055-003) and Alkaline Phosphatase
AffiniPure Goat Anti-Rabbit IgG (H+L) (cat. no. 111-055-003) were
obtained from Jackson ImmunoResearch Laboratories, Inc.
HRP-conjugated AffiniPure Goat Anti-Mouse IgG (H+L) (cat. no.
SA00001-1), HRP-conjugated AffiniPure Goat Anti-Rabbit IgG (H+L)
(cat. no. SA00001-2), Fluorescein (FITC)-conjugated Affinipure Goat
Anti-Rabbit IgG (H+L) (cat. no. SA00003-2) and ECL kit (cat. no.
B500014) were purchased from ProteinTech Group, Inc.
Cell culture
Human rhabdomyosarcoma RD cells (cat. no. CCL-136)
were obtained from the American Type Culture Collection and
cultured in DMEM (HyClone; Cytiva) supplemented with 10% fetal calf
serum (Gibco; Thermo Fisher Scientific, Inc.). The cells were
maintained at 37°C without CO2 in a humidified
atmosphere.
Cell proliferation inhibition
test
The inhibition of cell proliferation was determined
by MTT assay. RD cells (1×104 cells/well) were seeded
into 96-well culture plates (Nalge Nunc International; Thermo
Fisher Scientific, Inc.). Following 24-h culture, 0, 2, 4,6, 8, 10,
12, 14, 16, 18 and 20 µM of PAB were added to the plates. Following
incubation at 37°C for 12, 24, 36 or 48 h, respectively, cell
proliferation was measured at different time points by adding 20 µl
MTT (5 mg/ml) for 3 h at 37°C. DMSO (150 µl) was added to dissolve
the formazan crystals. The absorbance was measured at 492 nm with
an ELISA plate reader (Bio-Rad Laboratories, Inc.). The percentage
of inhibition was calculated as follows: Inhibitory ratio (%) =
[A492 (control)-A492
(sample)]/[A492 (control)-A492 (blank)]
×100%.
Immunofluorescence assay
RD cells (5×105 cells/well) were cultured
on the cover slips in a 6-well plate. Following 24-h culture, the
cells were treated with 4 µM PAB for 24 h at 37°C, and control
cells were treated with equal amount of solvent (DMSO) in 10% DMEM,
then washed with PBS and fixed in 3.7% formaldehyde at room
temperature for 15 min. The cells were subsequently rinsed three
times in PBS. The specimens were incubated in blocking buffer (1X
PBS; 5% normal cell culture fetal calf serum; 0.3% Triton X-100)
for 60 min at room temperature and subsequently with the 1:300
diluted primary antibody against β-tubulin overnight at 4°C. The
cells were rinsed three times with PBS and incubated with a 1:100
diluted FITC-conjugated secondary antibody for 2 h at room
temperature in the dark. The secondary antibody was aspirated,
rinsed once with PBS and stained with Hoechst 33258 (5 mg/l) for 30
min at room temperature. The FITC staining in three fields was
visualized by fluorescence microscopy (Leica Microsystems, Inc.;
magnification, ×400) with an excitation wavelength of 505 nm and an
emission wavelength of 534 nm. Nuclear changes were observed by
fluorescence microscopy with an excitation wavelength of 350 nm and
an emission wavelength of 460 nm.
Cell migration
RD cells (5×105 cells/well) in 6-well
plates were cultured for 24 h, and a scratch was made randomly with
10-µl pipette tips near the center of the well, the lines with same
width were chosen, and the cells were treated 4 µM PAB or equal
DMSO control in DMEM culture medium with 10% fetal calf serum
(7). Along the chosen line, the
photograph of the wound was recorded at 0, 12, 24 and 36 h with PAB
treatment by phase contrast microscopy (Leica Microsystems, Inc.;
magnification, ×200), respectively, and the width of wound was
labeled with two parallel lines. The test was repeated three
times.
Observation of morphological changes
by light microscopy
RD cells (5×105 cells/well) were cultured
in 6 wells plates for 24 h. Subsequently, 4 µM PAB was added to the
cells for 24 and 48 h, and the morphological changes in three
fields were observed by phase contrast microscopy (Leica
Microsystems, Inc.; magnification, ×200).
Determination of DNA fragmentation by
agarose gel electrophoresis
RD cells (5×105 cells/well) were cultured
in 6 wells plates for 24 h. Subsequently, 4 µM PAB was added to the
cells for 0, 12, 24 or 48 h, respectively. Adherent and floating
cells were collected by centrifugation at 1,000 × g for 5 min at
4°C. The cell pellet was suspended in cell lysis buffer (10 mM
Tris-HCl, pH 7.4; 10 mM EDTA, pH 8.0; 0.5% Triton-100) and
maintained at 4°C for 30 min. The lysate was centrifuged at 25,000
× g for 20 min at 4°C. The supernatant was incubated with 20 g/l
RNase A (2 µl) at 37°C for 1 h and with 20 g/l proteinase K (2 µl)
at 37°C for 1 h, and subsequently mixed with 5 M NaCl (20 µl) and
isopropanol (120 µl) and incubated at −20°C overnight. The samples
were centrifuged at 25,000 × g for 15 min at 4°C. The supernatant
was discarded, and the DNA sediment was dissolved in TE buffer (10
mM Tris-HCl, pH 7.4; 1 mM EDTA, pH 8.0) and separated by 2% agarose
gel electrophoresis at 100 V for 50 min.
Flow cytometric analysis of the cell
cycle
RD cells (5×105 cells/well) were cultured
in 6 wells plates for 24 h. Subsequently, 4 µM PAB or control
medium was added to the cells for 0, 12, 24 or 48 h, respectively.
Then RD cells were harvested and rinsed with PBS. The cell pellets
were fixed in 70% ethanol at 4°C overnight. Following washing twice
with PBS, the cells were stained with 1.0-ml solution containing 50
mg/l PI, 1 g/l RNase A and 0.1% Triton X-100 in 3.8 mM sodium
citrate on ice in the dark for 30 min. The cell cycle distribution
was analyzed by BD CellQuest Pro software version 5.1 (BD
Biosciences) in BD FACSCalibur flow cytometer (Becton Dickinson and
Company). The histograms were analyzed using ModFit LT version 3.0
software (Verity Software House Inc.) to determine the percentage
of cells in each phase of the cell cycle.
MDC staining
The fluorescent compound MDC has been proposed as a
tracer for autophagic vacuoles (6–8). RD
cells (2×105 cells/well) were cultured in 6 wells plates
for 24 h. 24 h later, RD cells were treated with 4 µM PAB for
another 24 h and incubated with 0.05 mM MDC at 37°C for 1 h.
Following incubation, the cells were washed once with PBS.
Intracellular MDC in three fields was measured by fluorescence
microscopy at an excitation wavelength of 380 nm and an emission
wavelength of 525 nm (Leica Microsystems, Inc.; magnification,
×200).
3-MA treatment
The RD cells were treated with PAB (4 µM)
together/or 3-MA (2 mM) for 24 h.
Western blot analysis of total
cytoplasmic and nuclear protein expression
RD cells (1×106 cells/well) were cultured
in a 25-ml culture bottle for 24 h and subsequently treated with 4
µM PAB for 24 h. Adherent and floating cells were collected and
frozen at −80°C. Western blot analysis was performed for the
determination of the total protein expression as previously
described (4). Briefly, protein (40
µg/lane) was loaded in 10% SDS-PAGE and transferred onto
nitrocellulose membrane. The membranes were blocked for 1 h at room
temperature in blocking buffer [5% nonfat dry milk in 1X TBST
(Tris-HCL 1.576 g/l, NaCl 8.00 g/l, Tween-20 0.1%)]. Then the
membranes were incubated with primary polyclonal antibody (1:1,000)
including caspase-8 (cat. no. 66093-1-Ig; ProteinTech Group, Inc.),
caspase-9 (cat. no. 66169-1-Ig; ProteinTech Group, Inc.), cyclin B1
(cat. no. 55004-1-AP; ProteinTech Group, Inc.), Beclin 1 (cat. no.
11306-1-AP; ProteinTech Group Inc.), LC3 (cat. no. 14600-1-AP;
ProteinTech Group, Inc.), β-actin (cat. no. 66009-1-Ig; ProteinTech
Group, Inc.) and tubulin (cat. no. 10068-1-AP; ProteinTech Group,
Inc.), or phosphorylated H2A histone family member X (γ-H2AX; cat.
no. 9718S; Cell Signaling Technology, Inc.) at 4°C overnight,
washed three times for 5 min each with TBST and incubated with the
appropriate secondary polyclonal antibody (1:2,000) including
Alkaline Phosphatase AffiniPure Goat Anti-Mouse IgG (H+L) (cat. no.
115-055-003; Jackson ImmunoResearch Laboratories, Inc.), Alkaline
Phosphatase AffiniPure Goat Anti-Rabbit IgG (H+L) (cat. no.
111-055-003; Jackson ImmunoResearch Laboratories, Inc.),
HRP-conjugated AffiniPure Goat Anti-Mouse IgG (H+L) (cat. no.
SA00001-1; ProteinTechGroup, Inc.) or HRP-conjugated AffiniPure
Goat Anti-Rabbit IgG (H+L) (cat. no. SA00001-2; ProteinTech Group,
Inc.) at room temperature for 1 h. The loading control used was
tubulin or actin. The proteins were visualized using NBT and BCIP
for the AP-conjugated secondary antibody for Fig. 5 and the data was collected by Canon
Scan (9000F MarkII; Canon); and using the ECL kit for the
HRP-conjugated secondary antibody for Fig. 6 and the data was collected by Azure
C500 (Azure Biosystems).
Statistical analysis
All experiments were performed independently ≥3
times, and the data are presented as the mean ± SD. Statistical
analysis was performed using SPSS 10.0 software (SPSS, Inc.).
Differences between two groups at various timepoints were assessed
using one-way repeated measures ANOVA. Differences among multiple
groups were assessed using one-way ANOVA with Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
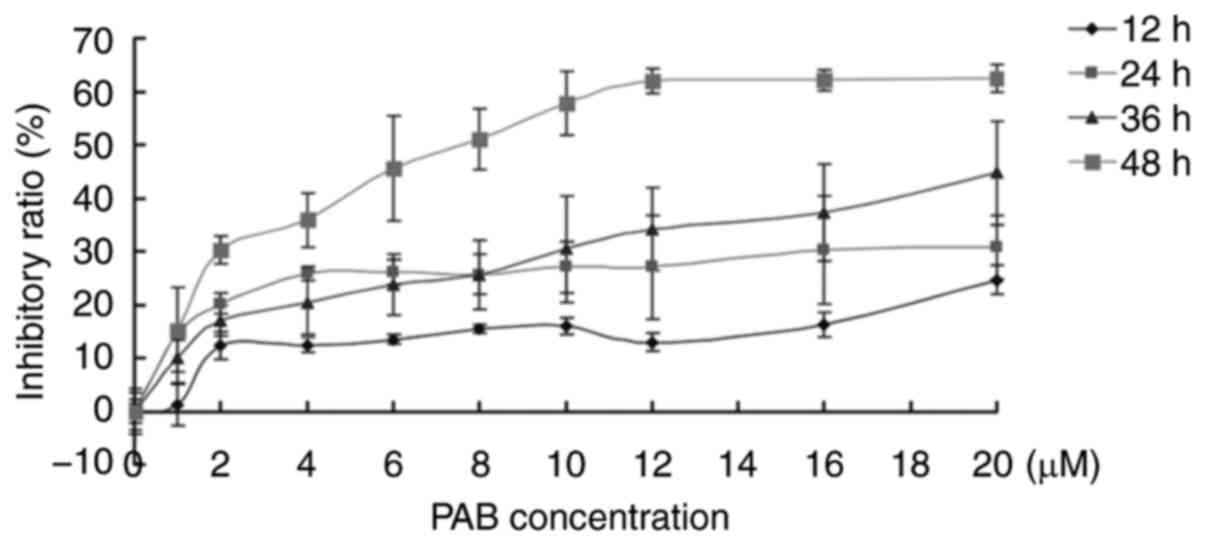
PAB inhibits RD cell
proliferation
In the present study, the inhibitory role of PAB was
investigated in human rhabdomyosarcoma RD cells. The results of the
MTT assay demonstrated that 1–20 µM PAB inhibited cell
proliferation, and between 12 and 48 h, the inhibitory ability of
PAB increased (Fig. 1). At 36 h, the
IC50 was estimated to be 41 µM, and at 48 h, the
IC50 was 7.5 µM. At 48 h, 4 µM of PAB inhibited RD cell
proliferation with an inhibitory ratio of 35% (Fig. 1). These results were consistent with
previous studies (4,5); therefore, 4 µM PAB was selected for
subsequent experiments.
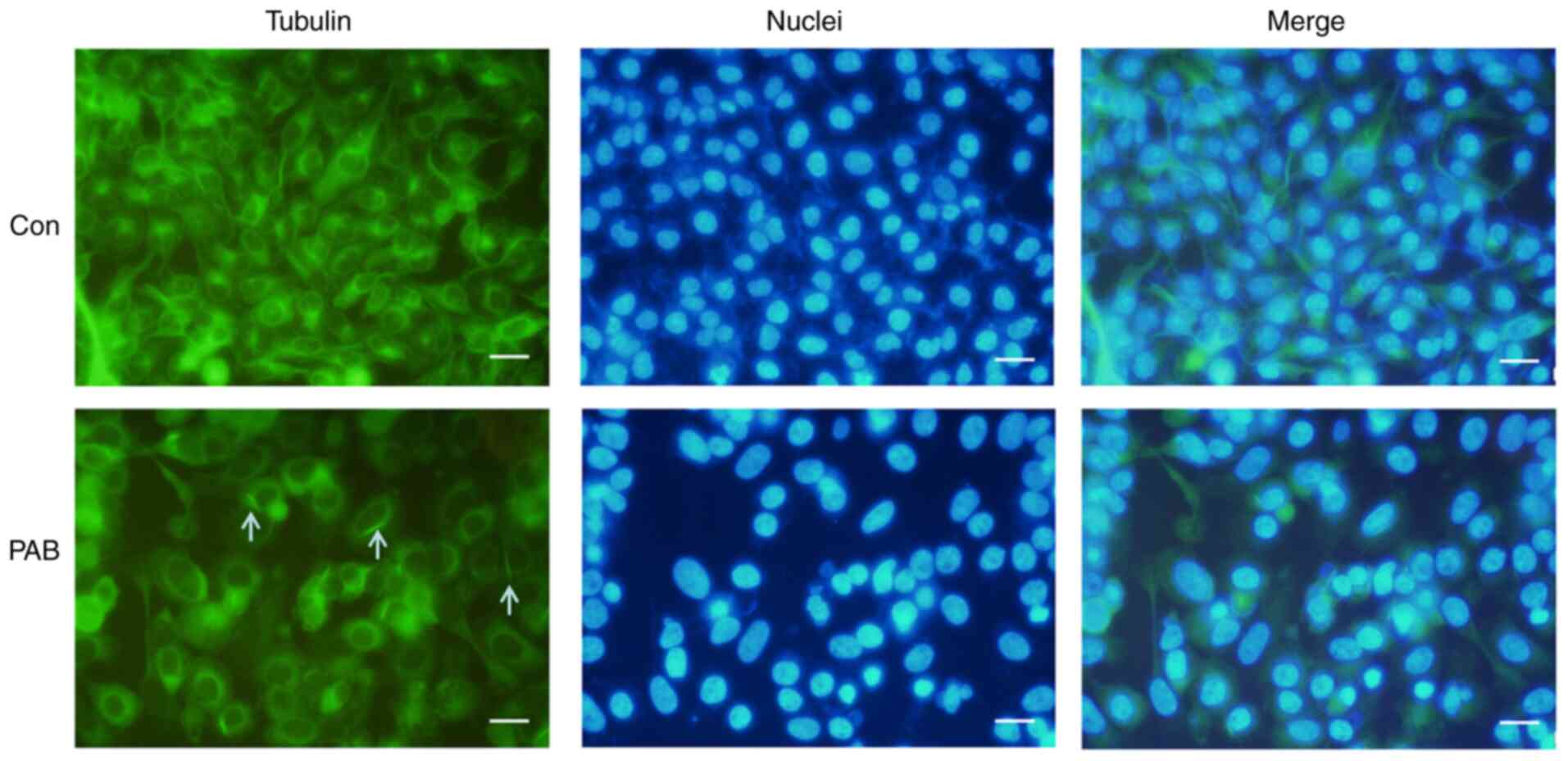
PAB alters tubulin distribution
PAB has been reported to target tubulin (20,21).
Therefore, the current study investigated the effects of PAB on the
microtubule networks of RD cells by tubulin immunofluorescence
staining. Treatment of RD cells with 4 µM PAB for 24 h resulted in
the aggregation of the microtubule fibers compared with that
observed in cells that had undergone a control treatment (Fig. 2). To verify the location of the
aggregation of the microtubule fibers, the cell nuclei were
stained, and the results demonstrated that the fibers were located
in the cytoplasm (Fig. 2). At the
same time, it was noted that cells became larger and nuclei became
larger in PAB-treated group, which was consistent with a previous
study (22), so it was speculated
that PAB resulted in the aggregation of the microtubule fibers, and
cells could not divide although DNA replication had been
completed.
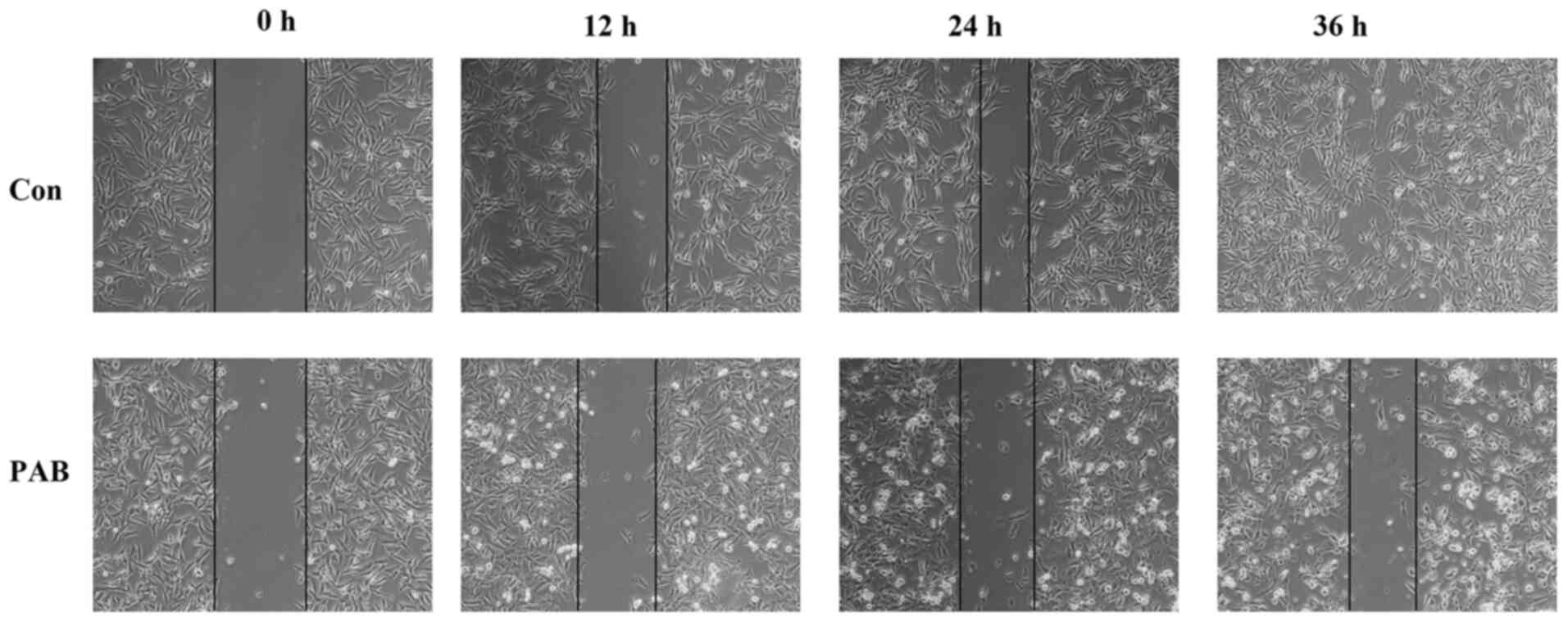
PAB inhibits cell migration
The effects of PAB on RD cell migration were
assessed by wound healing assay. The results demonstrated that RD
cells migrated over time (from 0 to 36 h), since the edge of the
wound gradually disappeared, which was consistent with the effects
noted in the aggregation of microtubule fibers. However, following
PAB treatment, the edge of the wound was visible by morphological
observation at 12, 24 and 36 h post-treatment (Fig. 3). Therefore, these results indicated
that PAB inhibited RD cell migration.
PAB induces apoptosis
To further determine whether PAB induced apoptosis
in RD cells, cell morphology was assessed. At 24 and 48 h, the
induction of apoptosis was evident in PAB-treated cells compared
with that in the cells that had undergone control treatment;
apoptotic bodies and condensed cells were observed in the
PAB-treated cells (Fig. 4A). In
addition, in the agarose gel electrophoresis assay, no DNA ladder
was present at 0 h, whereas the appearance of the DNA ladder was
noted at 24, 36 and 48 h following PAB treatment (Fig. 4B). Following 24 h post-PAB treatment,
the expression levels of pro-caspase-9 appeared to be decreased,
whereas those of active caspase 8 appeared to be increased compared
with the control group (Fig. 4C).
Although PAB changed the tubulin aggregation, PAB did not affect
tubulin expression, and the expression of tubulin was same as actin
and histone (data not shown). These results suggested that PAB
induced apoptosis in RD cells.
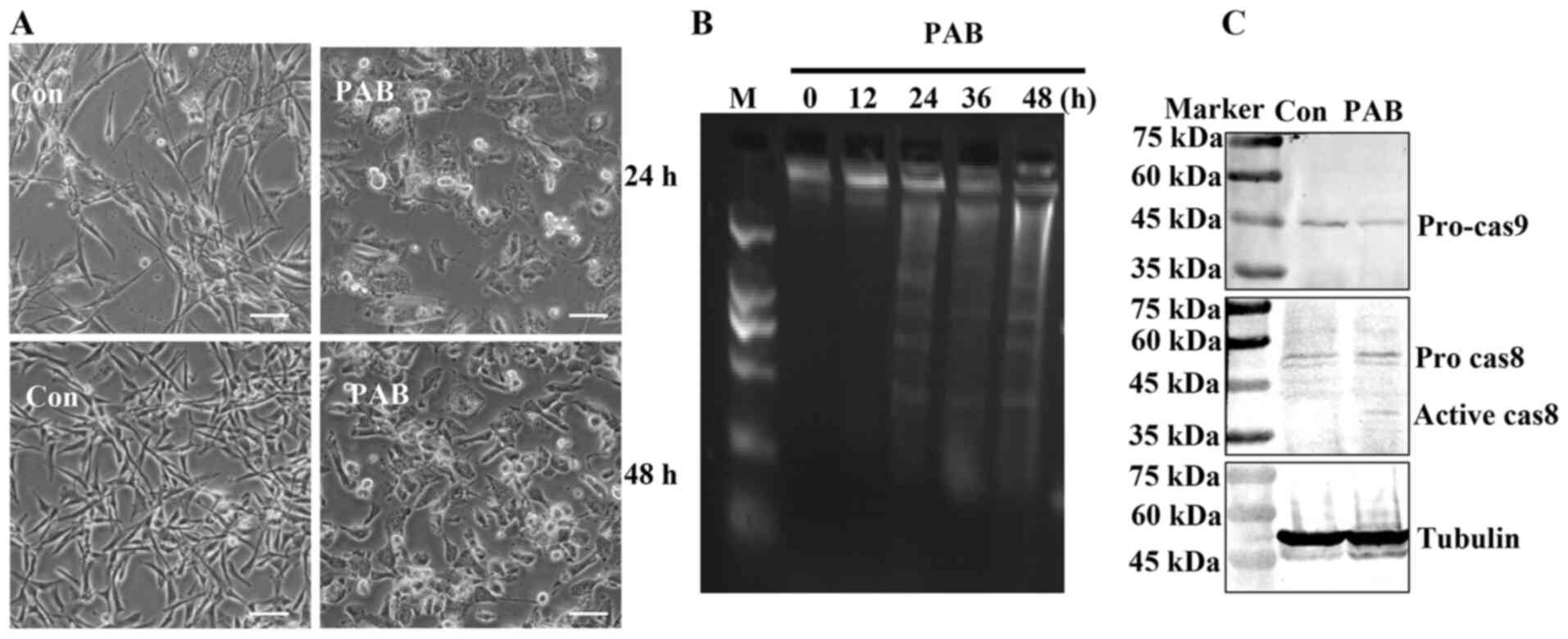 | Figure 4.PAB induces apoptosis. (A) Cell
morphology was visualized following 24- or 48-h PAB treatment. n=3.
Scale bar, 30 µm. (B) The fragmentation of chromosomal DNA was
noted in PAB-treated cells. At 0, 12, 24, 36 and 48 h, DNA was
extracted from RB cells treated with PAB, and the induction of
apoptosis was determined by agarose gel electrophoresis. (C) The
expression levels of pro-caspase-9 and active caspase-8 were
determined by western blotting at 24 h after 4 µM PAB treatment.
Representative images of pro-caspase-9, active caspase-8 and
tubulin were from the same batch of samples, but different gels due
to the molecular weights of the proteins. Tubulin was used as the
loading control. n=3. PAB, pseudolaric acid B; Con, control; M,
marker. |
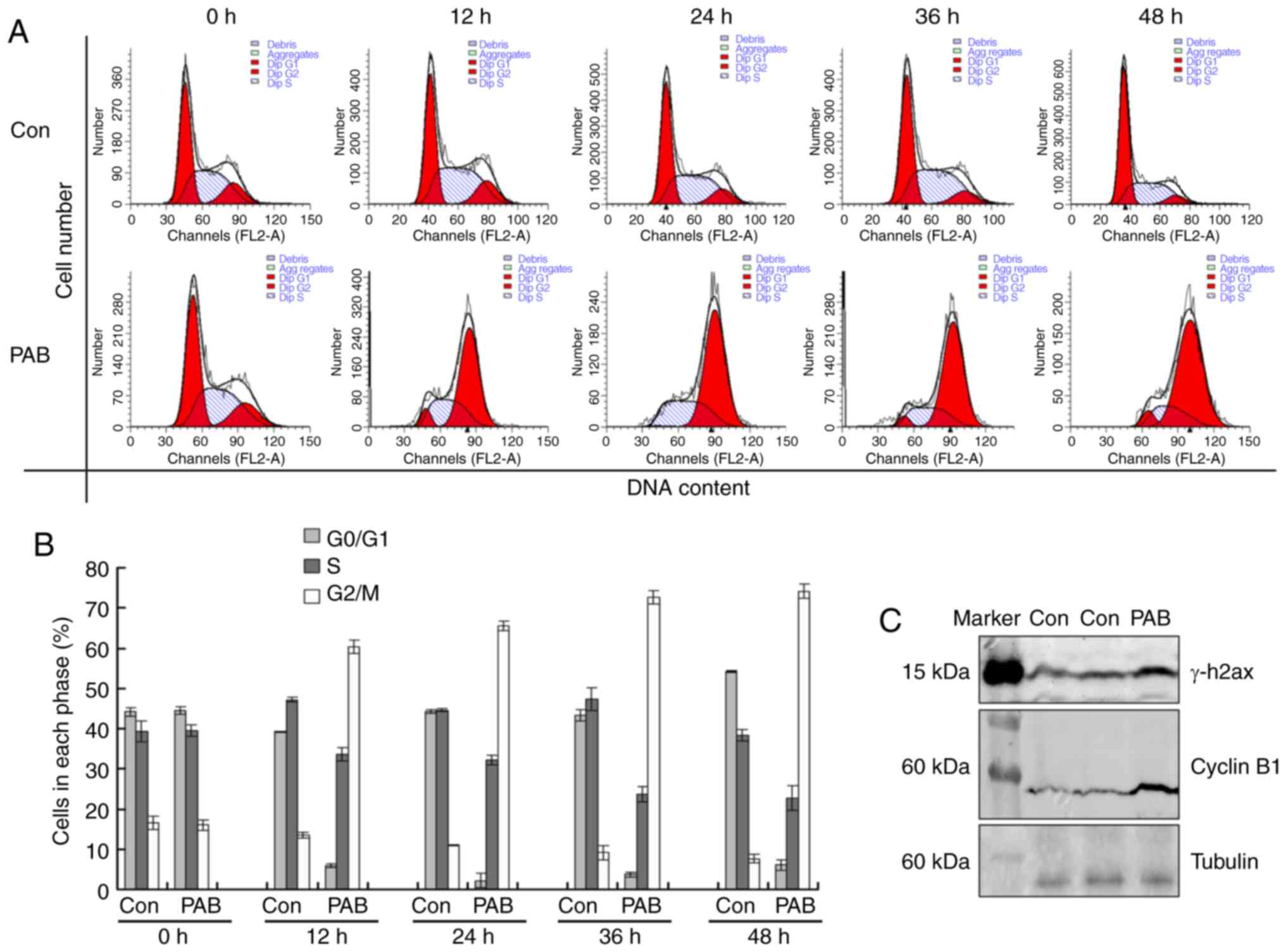
PAB induces G2/M cell cycle
arrest
To investigate the mechanism of cell proliferation
inhibition mediated by PAB, flow cytometry was used for cell cycle
analysis. Following 4 µM PAB treatment for 12, 24, 36 or 48 h, the
cell number of tetraploid cells was increased compared with that in
the control group (Fig. 5A and B),
indicating that the PAB-treated cells were arrested at the
G2/M phase. Western blot analysis results demonstrated
that PAB treatment upregulated the expression levels of γ-H2AX and
cyclin B1 at 24 h post-treatment (Fig.
5C). Therefore, PAB induced cell cycle arrest, which was likely
in the M phase.
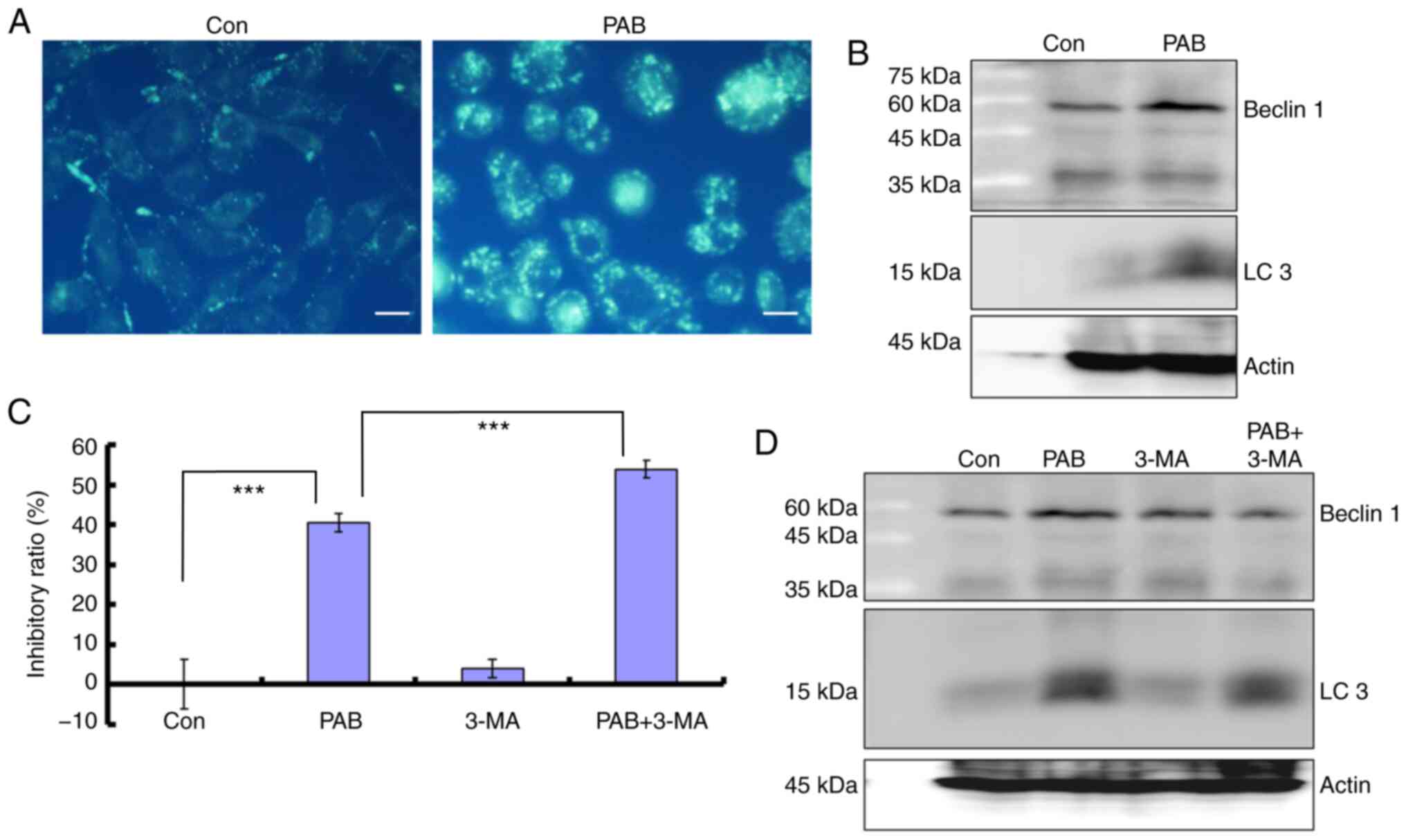
PAB induces autophagy
To further confirm the role of PAB in inhibiting
cell proliferation, the current study examined whether PAB induced
autophagy in RB cells. The results demonstrated that 4 µM PAB
increased the MDC-positive points, which are considered to be
markers of autophagy (6,7) (Fig. 6A).
The present study further demonstrated that at 24 h, the expression
levels of autophagy markers beclin 1 and LC3 were increased in RB
cells treated with PAB compared with those in the control group
(Fig. 6B). In the presence of the
autophagy inhibitor 3-MA (2 mM), an increased proliferation
inhibition ratio was observed in PAB-treated cells compared with
control treatment (Fig. 6C), and
comparing to PAB treatment group, the expression level of beclin 1
was decreased in PAB together with 3-MA treatment group, and LC3
had a decreased trend (Fig. 6D).
Therefore, inhibiting autophagy promotes cell death in PAB-treated
RD cells.
Discussion
PAB exhibits potent antitumor effects on human
breast cancer MCF-7, cervical cancer HeLa and melanoma A375 cells
by inducing apoptosis (1–5), and on murine fibrosarcoma L929, human
thyroid squamous cell carcinoma SW579 cells and human lung
fibroblasts MRC5 by inducing autophagy (6–8). In the
present study, the effects of PAB were examined on human
rhabdomyosarcoma RD cell proliferation. The present study provided
novel information that may aid further translation of new candidate
drugs into improved clinical treatments.
The results of the present study demonstrated that
PAB inhibited RD cell proliferation. PAB inhibits the proliferation
of several types of cancer cells, and the present study broadens
the antineoplastic spectrum of PAB through confirming PAB
inhibiting Rhabdomyosarcoma RD cell growth and cell migration and
inducing apoptosis and cell cycle arrest.
Previous studies have demonstrated that PAB induces
apoptosis alone (1–5), apoptosis and autophagy (22) or autophagy alone (6–8) to exert
its inhibitory role in tumor cells. The results of the present
study confirmed that PAB induced apoptosis in RD cells, which was
consistent with the data reported in previous studies (1–5). In
addition, in the present study, apoptosis was accompanied by
autophagy, which was consistent with the results of a previous
study in human breast cancer MCF-7 cells (22). Currently, the mechanism by which PAB
induces apoptosis or autophagy in specific types of cells is
unknown. It was speculated that in various types of cells,
different apoptotic or autophagic factors may determine the cell
fate following PAB treatment. Different apoptotic or autophagic
factors will be investigated in future studies.
PAB has been reported to exert its antitumor effects
via disrupting tubulin function (20). The ability of PAB to disrupt tubulin
formation in RD cells was examined in the present study, and the
results demonstrated that PAB treatment resulted in the aggregation
of microtubule fibers, which was consistent with a previous study
(20). Human rhabdomyosarcoma can
spread locally, regionally or distantly, depending on the
aggressiveness of the tumor cells (9). In the present study PAB treatment
appeared to inhibit cell migration. In addition, PAB induced cell
cycle arrest at the G2/M phase following 12-h treatment, whereas
the expression levels of γ-H2AX and cyclin B1 were upregulated at
24 h post-treatment compared with those in the control group. These
results suggested that PAB induced M phase arrest. Therefore, PAB
exerted its antitumor effects through multiple mechanisms of
action.
In conclusion, the results of the present study
demonstrated that PAB exerted its antitumor roles by inducing
apoptosis, autophagy and cell cycle arrest in human
rhabdomyosarcoma RD cells. Therefore, PAB may be considered a
potential treatment agent for human rhabdomyosarcoma.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81871634 and 81301416), the
Postdoctoral Science Foundation of China (grant nos. 2014M561302
and 2015T80299), the Norman Bethune Program of Jilin University
(grant no. 2015202), the Jilin Provincial Science and Technology
Department (grant nos. 20140204004YY, 20160414025GH and
20190304064YY) and the Department of Human Resources and Social
Security of Jilin Province (grant no. 2016014).
Availability of data and materials
All data generated or analyzed during the study are
included in this published article.
Authors' contributions
JY, CL and FW designed the experiments; BW, TW, YW,
WH performed the experiments; JY, CL, FW, BW, TW, YW, WH, SZ, YS,
JL and YL analyzed the data. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pan DJ, Li ZL, Hu CQ, Chen K, Chang JJ and
Lee KH: The cytotoxic principles of Pseudolarix kaempferi:
Pseudolaric acid-A and -B and related derivatives. Planta Med.
56:383–385. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gong XF, Wang MW, Tashiro S, Onodera S and
Ikejima T: Pseudolaric acid B induces apoptosis through p53 and
Bax/Bcl-2 pathways in human melanoma A375-S2 cells. Arch Pharm Res.
28:68–72. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gong X, Wang M, Tashiro S, Onodera S and
Ikejima T: Involvement of JNK-initiated p53 accumulation and
phosphorylation of p53 in pseudolaric acid B induced cell death.
Exp Mol Med. 38:428–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu JH, Cui Q, Jiang YY, Yang W, Tashiro S,
Onodera S and Ikejima T: Pseudolaric acid B induces apoptosis,
senescence, and mitotic arrest in human breast cancer MCF-7. Acta
Pharmacol Sin. 28:1975–1983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu JH, Wang HJ, Li XR, Tashiro S, Onodera
S and Ikejima T: Protein tyrosine kinase, JNK, and ERK involvement
in pseudolaric acid B-induced apoptosis of human breast cancer
MCF-7 cells. Acta Pharmacol Sin. 29:1069–1076. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu J, Li X, Tashiro S, Onodera S and
Ikejima T: Bcl-2 family proteins were involved in pseudolaric acid
B-induced autophagy in murine fibrosarcoma L929 cells. J Pharmacol
Sci. 107:295–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu J, Ren P, Zhong T, Wang Y, Yan M, Xue
B, Li R, Dai C, Liu C, Chen G and Yu XF: Pseudolaric acid B
inhibits proliferation in SW579 human thyroid squamous cell
carcinoma. Mol Med Rep. 12:7195–7202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Gao H, Wu T, Wang Z, Song F, Chen
A, Zhang J, Zhang W, Zhang H and Yu J: Pseudolaric acid B induced
autophagy, but not apoptosis, in MRC5 human fibroblast cells. Oncol
Lett. 15:863–870. 2018.PubMed/NCBI
|
|
9
|
Dziuba I, Kurzawa P, Dopierala M, Larque
AB and Januszkiewicz-Lewandowska D: Rhabdomyosarcoma in
children-current pathologic and molecular classfication. PoI J
Pathol. 69:20–32. 2018. View Article : Google Scholar
|
|
10
|
Li FF, Yi S, Wen L, He J, Yang LJ, Zhao J,
Zhang BP, Cui GH and Chen Y: Oridonin induces NPM mutant protein
translocation and apoptosis in NPM1c+ acute myeloid leukemia cells
in vitro. Acta Pharmacol Sin. 35:806–813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi M, Yao G, Fan S, Cheng W, Tashiro S,
Onodera S and Ikejima T: Pseudolaric acid B induces mitotic
catastrophe followed by apoptotic cell death in murine fibrosarcoma
L929 cells. Eur J Pharmacol. 683:16–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahn JH, Lee YW, Ahn SK and Lee M:
Oncogenic BRAF inhibitor UAI-201 induces cell cycle arrest and
autophagy in BRAF mutant glioma cells. Life Sci. 104:38–46. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang R, Xiao X, Wang PY, Wang L, Guan Q,
Du C and Wang XJ: Stimulation of autophagic activity in human
glioma cells by anti-proliferative ardipusilloside I isolated from
Ardisia pusilla. Life Sci. 110:15–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Yu J, Liu L, Wei Z, Ehrlich ES, Liu
G, Li J, Liu X, Wang H, Yu XF and Zhang W: Coxsackievirus A16
infection induces neural cell and non-neural cell apoptosis in
vitro. PLoS One. 9:e1111742014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee YJ, Won AJ, Lee J, Jung JH, Yoon S,
Lee BM and Kim HS: Molecular mechanism of SAHA on regulation of
autophagic cell death in tamoxifen-resistant MCF-7 breast cancer
cells. Int J Med Sci. 9:881–893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee YZ, Yang CW, Chang HY, Hsu HY, Chen
IS, Chang HS, Lee CH, Lee JC, Kumar CR, Qiu YQ, et al: Discovery of
selective inhibitors of Glutaminase-2, which inhibit mTORC1,
activate autophagy and inhibit proliferation in cancer cells.
Oncotarget. 5:6087–6101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He H, Feng YS, Zang LH, Liu WW, Ding LQ,
Chen LX, Kang N, Hayashi T, Tashiro S, Onodera S, et al: Nitric
oxide induces apoptosis and autophagy; autophagy down-regulates NO
synthesis in physalin A-treated A375-S2 human melanoma cells. Food
Chem Toxicol. 71:128–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han Y, Yang YN, Yuan HH, Zhang TT, Sui H,
Wei XL, Liu L, Huang P, Zhang WJ and Bai YX: UCA1, a long
non-coding RNA up-regulated in colorectal cancer influences cell
proliferation, apoptosis and cell cycle distribution. Pathology.
46:396–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee H, Chin H, Kim K and Lee D: ERBB3
knockdown induces cell cycle arrest and activation of Bak and
Bax-dependent apoptosis in colon cancer cells. Oncotarget.
5:5138–5152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong VK, Chiu P, Chung SS, Chow LM, Zhao
YZ, Yang BB and Ko BC: Pseudolaric acid B, a novel
microtubule-destabilizing agent that circumvents multidrug
resistance phenotype and exhibits antitumor activity in vivo. Clin
Cancer Res. 11:6002–6011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song F, Yu X, Zhang H, Wang Z, Wang Y,
Meng X and Yu J: Pseudolaric acid B inhibits neuroglioma cell
proliferation through DNA damage response. Oncol Rep. 38:2211–2218.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu J, Chen C, Xu T, Yan M, Xue B, Wang Y,
Liu C, Zhong T, Wang Z, Meng X, et al: Pseudolaric acid B activates
autophagy in MCF-7 human breast cancer cells to prevent cell death.
Oncol Lett. 11:1731–1737. 2016. View Article : Google Scholar : PubMed/NCBI
|