Introduction
Osteosarcoma (OS) is the most common bone tumor in
children, adolescents and young adults worldwide; its incidence
rate among adolescents (15–19 years old) is as high as 8–11 million
per year, and the long-term survival rate of metastatic or
recurrent patients is <20% (1–3).
Previous epidemiological studies of OS revealed a higher prevalence
in males, with a peak at 15 years of age (4). Although treatment has improved, its
efficacy in patients with metastatic and recurrent OS remains poor
(1). Therefore, the identification
of new biomarkers and targeted anticancer therapy for OS are
required. MicroRNAs (miRNAs/miRs) are endogenous RNAs with ~22
nucleotides (5) that pair with
specific bases, such as 3′-untranslated regions (UTRs), to induce
RNA degradation or translation inhibition, thereby regulating
differentiation, proliferation, apoptosis and other biological
processes (6,7).
miR-1236-3p is located within Chr6p21.33 and is
embedded within the intron of negative elongation factor E
(8). Previous studies have reported
that aberrant miR-1236-3p expression is involved in the tumor
progression of various types of human cancer, including
hepatocellular carcinoma, ovarian carcinoma and renal cell
carcinoma (9–11). Moreover, Poos et al (12) reported the importance of miRNAs in
OS, demonstrating that downregulation of miR-9-5p, miR-138 and
miR-214 promoted OS cell proliferation and migration. However, the
significance of miR-1236-3p in OS cells is not completely
understood. Therefore, the present study aimed to assess the
potential use of miR-1236-3p for the diagnosis and treatment of
OS.
Materials and methods
Clinical samples
A total of 20 OS tissue and 20 chondroma tissue
samples were obtained from 40 patients (including 24 men and 16
women; n=20 per group). The samples were collected between February
2017 and February 2020 at the Second Affiliated Hospital of
Zhejiang University (Hangzhou, China) and Zhejiang Coastal Police
Corps Hospital (Jiaxing, China). The OS tissue samples were
obtained from patients before the administration of neoadjuvant
chemotherapy. All patients or the legal guardians of patients
<18 years old provided written informed consent. The present
study was approved by the Ethics Committee of Sir Run Run Shaw
Hospital. The present study included 40 patients with an average
age of 26.8 years (age range, 11–35 years). All OS and chondroma
were present in the bones of the patients' limbs. None of the
patients with OS displayed metastasis, and pathologists
characterized OS and chondroma biopsy samples to the standards
defined by the World Health Organization (13). All biopsied specimens were
immediately placed in liquid nitrogen and stored at −80°C.
Cell lines and culture condition
The human osteoblast cell line hFOB1.19 and six
human OS cell lines (HOS, 143B, MG63, U2OS, SJSA-1 and SAOS-2) were
obtained from Shanghai FuHeng Biological Technology Co., Ltd. The
Venor GeM mycoplasma detection kit (Minerva Biolabs GmbH) was used
to confirm that all cell lines were free of mycoplasma. All cells
were maintained in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
at 37°C with 5% CO2.
Cell transfection
At 50–60% confluence, HOS and U2OS cells were
transfected with miR-1236-3p mimic, negative control (NC) mimic,
inhibitor NC, miR-1236-3p inhibitor, Wnt3a-small interfering
(si)RNA or NC siRNA (10 nM final concentration; Shanghai GenePharma
Co., Ltd.) using Lipofectamine® 3000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Subsequent experiments were
performed 48 h after cell transfection.
Fluorescence in situ hybridization
(FISH)
FAM (488)-labeled, locked nucleic acid miR-1236-3p
probes were designed and synthesized by Wuhan Servicebio Technology
Co., Ltd. The miR-1236-3p probe signals were detected using a
commercial FISH kit (Wuhan Servicebio Technology Co., Ltd.)
according to the manufacturer's instructions. The images were
acquired using an ECLIPSE CI positive fluorescence microscope
(Nikon Corporation). Details of the FISH experiment are presented
in Data S1.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from OS cells using TRIzol
Reagent (CoWin Biosciences) according to the manufacturer's
instructions. Total RNA was reverse transcribed into cDNA using the
HiFiScipt cDNA Synthesis kit (CoWin Biosciences) at 85°C for 15 min
and 42°C for 5 min. Subsequently, human miR-1236-3p, Wnt10a,
lymphoid enhancer-binding factor 1 (LEF1), Wnt3a, β-catenin, c-Myc
and cyclin D1 were amplified via qPCR using the following primers:
miR-1236-3p forward, 5′-CCTCTTCCCCTTGTCTCTCCAG-3′ and reverse,
5′-TATGGTTGTTCACGACTCCTTCAC-3′; Wnt10a forward,
5′-TGCTCCTGTTCTTCCTACTGC-3′ and reverse,
5′-GGGGATCTTGTTGCGAGTCT-3′; LEF1 forward,
5′-TGCATCAGGTACAGGTCCAAG-3′ and reverse,
5′-ACGTTGGGAATGAGCTTCGT-3′; Wnt3a forward,
5′-AGTTTGGTGGGATGGTGTCTC-3′ and reverse,
5′-CTTGAGGTGCATGTGGCTGG-3′; c-Myc forward, 5′-TCCTCGGATTCTCTGCTC-3′
and reverse, 5′-GCTGCGTAGTTGTGCTGATG-3′; and cyclin D1 forward,
5′-TGACCCCGCACGATTTCATT-3′ and reverse, 5′-CATGGAGGGCGGATTGGAAA-3′.
qPCR was performed using SYBR® Premix Ex Taq (Takara
Bio, Inc.) with StepOnePlus™ Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The following thermocycling conditions
were used for qPCR: Initial denaturation at 95°C for 5 min;
followed by 40 cycles of denaturation at 95°C for 10 sec, annealing
at 60°C for 10 sec and extension at 72°C for 20 sec. GAPDH and U6
were used as endogenous controls using the following primers: GAPDH
forward, 5′-AGCCACATCGCTCAGACAC-3′ and reverse,
5′-GCCCAATACGACCAAATCC-3′; and U6 forward,
5′-AGGGCTGTCTCTGGGAGAAT-3′ and reverse, 5′-CTCATGGTTGTGGCTCCCTT-3′.
miRNA and mRNA expression levels were calculated using the
2−ΔΔCq method (14).
Cell function experiments
For the colony formation assays, following
transfection, cells were seeded into 6-well plates
(1×103 cells/well) and cultured for 10 days without
disturbance. The colonies were then fixed with 4% paraformaldehyde
for 15 min and stained with 1% crystal violet (Sigma-Aldrich; Merck
KGaA) for 30 min at room temperature. A colony was defined as
>50 cells and the average number of colonies in 3 separate wells
(each group contained 3 wells) was calculated using ImageJ
(v2.1.4.7; National Institutes of Health) to assess the colony
formation ability.
For the Cell Counting Kit-8 (CCK-8) assays,
transfected cells were seeded into 96-well plates (1×103
cells/well). Cell proliferation ability was determined using CCK-8
reagent (Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. Briefly, cell proliferation was measured at 0, 24, 48 and
72 h post-transfection. Cells were incubated with 10 µl/well of
CCK-8 solution at 37°C during the last 1 h of the culture. A
microplate reader was then used to detect the absorbance of each
well at 0, 24, 48 and 72 h (wavelength, 450 nm).
A Transwell migration assay was conducted to assess
OS cell migration. The Transwell system was a 24-well Boyden
chamber with a polycarbonate membrane (pore size, 8 mm). A total of
5×103 transfected cells suspended in 200 µl serum-free
DMEM were plated into the upper chamber. The lower chamber was
filled with DMEM supplemented with 10% FBS. The Transwell system
was incubated at 37°C with 5% CO2. Following incubation
for 24 h, migratory cells were fixed with 4% paraformaldehyde for
15 min and stained with 0.5% crystal violet for 30 min at room
temperature. Migratory cells were counted by inverted light
microscopy in three randomly selected fields of view and analyzed
(magnification, ×100).
For the wound healing assay, transfected cells were
starved using serum-free medium for 24 h and then seeded into
6-well plates and cultured in serum-free DMEM. At 90–100%
confluence, a linear scratch was created using a 200 µl pipette tip
and cells were washed three times with PBS. An inverted light
microscope was used to visualize the wounds at 0 and 24 h
(magnification, ×40). All the aforementioned cell function
experiments were performed three times.
Flow cytometry for cell cycle
analysis
HOS and U2OS cells were starved using serum-free
medium for 24 h prior to transfection with miR-1236-3p mimic or
Wnt3a siRNA. At 48 h post-transfection, 2×106 cells were
fixed with 75% ice-cold ethanol overnight. Subsequently, cells were
stained with PI working solution for 15 min at room temperature
using the Annexin V-FITC/PI Apoptosis Detection kit (BD
Biosciences) according to the manufacturer's protocol. Data were
collected on BD FACSCanto (BD Biosciences) and analyzed using
FlowJo 10 software (FlowJo LLC). The assay was performed three
times.
Protein extraction and western
blotting
Total protein was extracted from cells using
radioimmunoprecipitation assay lysis buffer (EMD Millipore) and the
protein concentration was quantified using a bicinchoninic acid
protein assay kit (EMD Millipore). The same amount of protein (30
µg/lane) was separated via 5–10% SDS-PAGE and transferred to PVDF
membranes (EMD Millipore) using a semi-dry blotting apparatus
(Bio-Rad Laboratories, Inc.). The membranes were blocked with 5%
(w/v) non-fat milk in Tris-buffered saline with Tween-20 (100 mM
NaCl, 50 mM Tris and 0.1% Tween-20) at room temperature for 1 h.
The membranes were incubated at 4°C overnight with the following
primary antibodies: Anti-Wnt3a (cat. no. 2391; 1:1,000; Cell
Signaling Technology, Inc.), anti-β-catenin (cat. no. 51067–2-AP;
1:5,000), anti-cyclin D1 (cat. no. 60186-1-lg; 1:1,000;),
anti-c-Myc (cat. no. 10828-1-AP; 1:2,000), anti-vimentin (cat. no.
10366-1-AP; 1:2,000), anti-N-cadherin (cat. no. 22018-1-AP;
1:2,000) and anti-GAPDH (cat. no. 10494-1-AP; 1:2,000) (purchased
from ProteinTech Group, Inc.). Following primary antibody
incubation, the membranes were incubated with HRP-conjugated
secondary antibodies (cat. no. SA00001-1 and SA00001-2; 1:5,000;
ProteinTech Group, Inc.) for 1 h at 37°C. ECL Luminous Liquid (EMD
Millipore) was used to visualize the bands. GAPDH expression was
used as the internal control.
miR-1236-3p target gene prediction and
pathway enrichment analysis
TargetScan (version 3.1; http://www.targetscan.org/mamm_31/) and miRanda
(http://www.microrna.org/microrna/home.do) were used to
predict the target genes of miR-1236-3p. Venny (version 2.1.0;
http://bioinfogp.cnb.csic.es/tools/venny/index.html)
was used to screen overlapping target genes between TargetScan and
miRanda. The Database for Annotation, Visualization and Integrated
Discovery (https://david.ncifcrf.gov/) was then
used to perform Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway enrichment analyses for the overlapping target genes. KEGG
was used to predict the main pathways affected by miR-1236-3p.
P<0.05 was considered as the cut-off value.
Dual luciferase reporter assay
The putative target genes of miR-1236-3p were
predicted using TargetScan. Firefly luciferase (FL) and
Renilla luciferase (RL) reporter vectors were synthesized by
Shanghai GeneChem Co., Ltd. 293T cells (Shanghai FuHeng Biological
Technology Co., Ltd.) were seeded into 96-well plates and grown to
30% confluence 24 h before transfection. Subsequently, miR-1236-3p
mimic or NC mimic (10 nM final concentration) was co-transfected
with 50 ng RL reporter vector and 5 ng FL reporter vector with or
without the Wnt3a 3′-UTR using Lipofectamine 3000. At 48 h
post-transfection, the Dual-Luciferase Reporter Assay System
(Promega Corporation) was used to measure firefly and
Renilla luciferase activities according to the
manufacturer's instructions. The assays were performed three
times.
Immunohistochemical (IHC)
analysis
Paraffin-embedded samples were dewaxed with xylene
and rehydrated with graded ethanol. The samples were incubated
overnight at 4°C with the following primary antibodies (all 1:100;
ProteinTech Group, Inc.): Anti-β-catenin (cat. no. 51067-2-AP),
c-Myc (cat. no. 10828-1-AP), cyclin D1 (cat. no. 60186-1-lg), Wnt3a
(cat. no. HZ-1296) and vimentin (cat. no. 10366-1-AP). Following
primary incubation, samples were incubated with universal secondary
antibodies (HRP-labeled). Finally, the positive detection rate was
used to score the IHC results and staining intensity. Further
details of the IHC analysis are presented in Data S1.
Tumor xenograft model in nude
mice
The miR-1236-3p sponge, empty vector and packing
plasmids (pSPAX2 and pMD2G) were obtained from Shanghai GeneChem
Co., Ltd. Packaging plasmids (1.5 µg pSPAX2 and 0.5 µg pMD2G) were
co-transfected with 2 µg viral vectors (miR-1236-3p sponge or empty
vector) into 7×106 293T cells. At 24 h
post-transfection, the transfected 293T cell medium was used to
infect HOS cells according to the manufacturer's protocol. After 48
h, 1 µg/ml puromycin was used to select the infected cells. After
72 h, transfected HOS cells (4×106) were suspended in
200 µl PBS and, following anesthetization via isoflurane inhalation
(3%), they were subcutaneously injected into the junction of the
right hindlimb and the hip of nude mice (8 male mice; age, 3–4
weeks; Animal Experimental Center of Sir Run Run Shaw Hospital of
Zhejiang University). Control mice were injected with
vector-transfected HOS cells and experimental mice were injected
with miR-1236-3p sponge-transfected HOS cells. All mice were fed by
the animal room staff and monitored every 7 days for the
measurement of tumor volume (mm3; length ×
width2 ×1/2) (15). After
5 weeks, three nude mice presented tumor ulcers on the site where
the tumor cells had been implanted. At this point, the experiment
was immediately terminated and the mice were sacrificed via
cervical dislocation. After confirming the onset of rigor mortis,
the tumors were harvested and weighed. Each group included 4 mice.
The humane endpoints established in the present study were as
follows: i) Loss of appetite, when nude mice completely lost
appetite for 24 h; ii) weakness, unable to eat and drink by
themselves, unable to stand for up to 24 h or extremely reluctant
to stand; iii) tumor, the tumor volume was >1,500
mm3, the tumor metastasized or the tumor grew rapidly to
ulceration, causing infection or necrosis; iv) musculoskeletal
system, muscle damage, bone damage and limbs unable to move; v)
nervous system, abnormal central nervous response (twitching,
trembling, paralysis and crooked head); and vi) death, when the
animal was mentally depressed without hypoesthesia or sedation,
accompanied by hypothermia (<37°C).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 5; GraphPad Software, Inc.). Data are
presented as the mean ± SD (n=3). The unpaired Student's t-test was
used to analyze comparisons between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
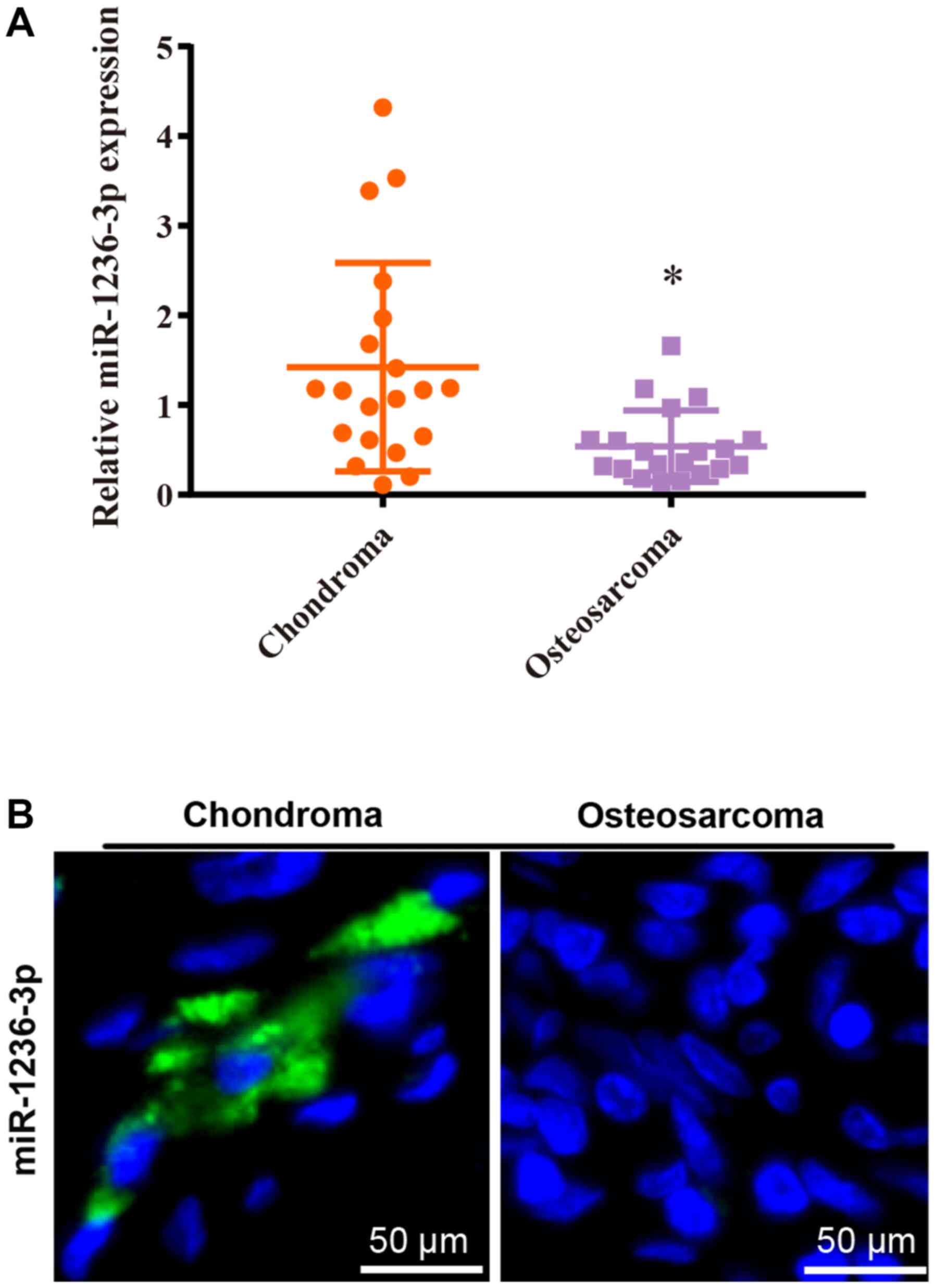
Reduced miR-1236-3p in OS tissues and
cell lines
The RT-qPCR results indicated that miR-1236-3p
expression levels were significantly decreased in OS tissues
compared with chondroma tissues (Fig.
1A), which was consistent with the FISH assay results (Fig. 1B).
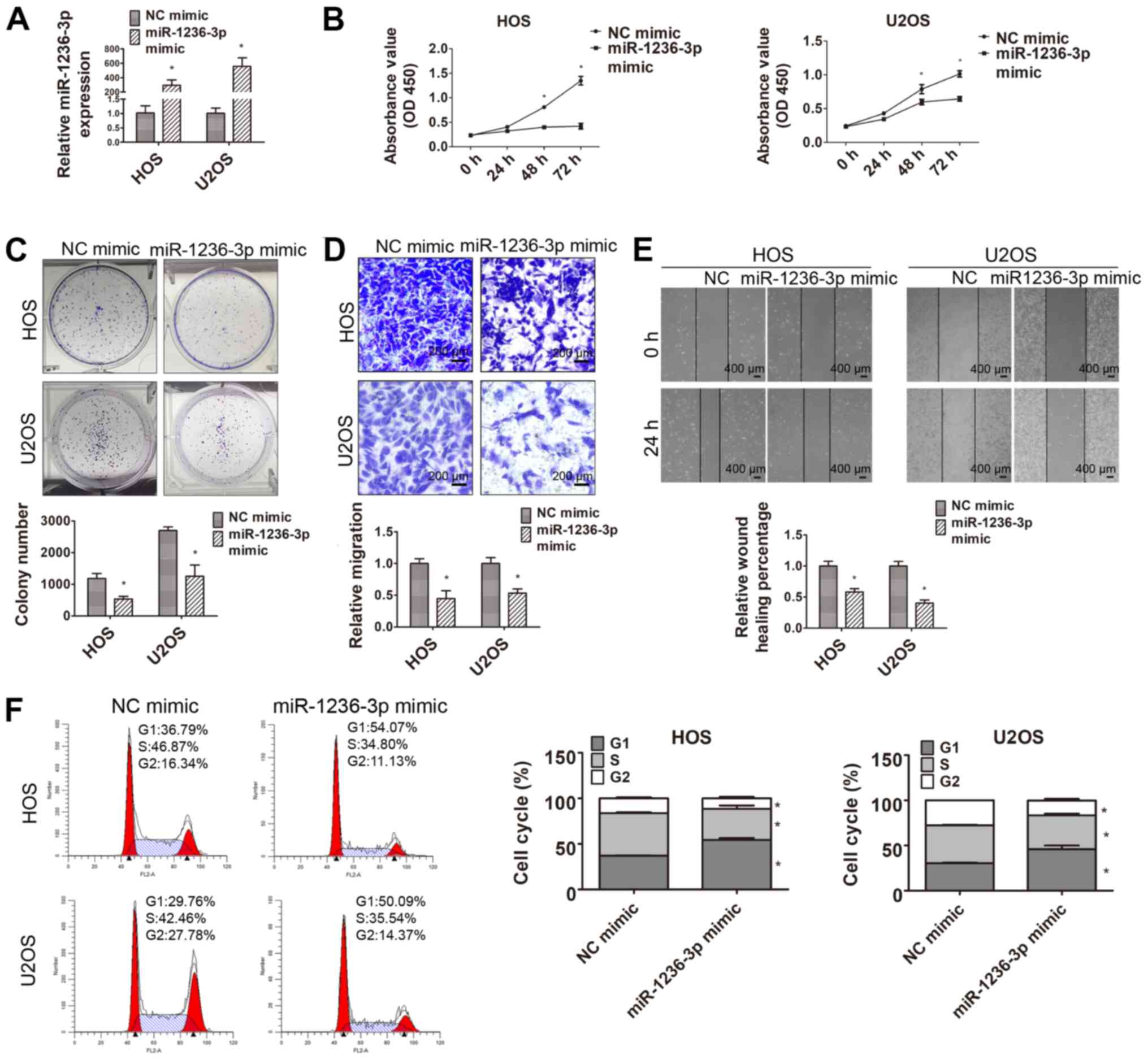
miR-1236-3p inhibits HOS and U2OS cell
proliferation and migration
Aberrant miR-1236-3p expression prompted further
investigation of its role in OS. miR-1236-3p mimic was transfected
into HOS and U2OS OS cell lines to investigate the function of
miR-1236-3p in OS. The RT-qPCR results suggested that miR-1236-3p
mimic significantly increased miR-1236-3p expression in HOS and
U2OS cells compared with NC mimic (Fig.
2A). The CCK-8 and colony formation assays were conducted to
evaluate HOS and U2OS cell proliferation following transfection
with miR-1236-3p mimic (Fig. 2B and
C). The results indicated an inhibitory role of miR-1236-3p in
OS cell proliferation, as demonstrated by significantly decreased
cell proliferation and colony formation in the miR-1236-3p mimic
group compared with the NC mimic group. As shown in Fig. 2D and E, miR-1236-3p mimic
significantly inhibited HOS and U2OS cell migration compared with
NC mimic. The flow cytometry results also suggested that
miR-1236-3p overexpression arrested the cell cycle at the
G1 phase in both cell lines (Fig. 2F).
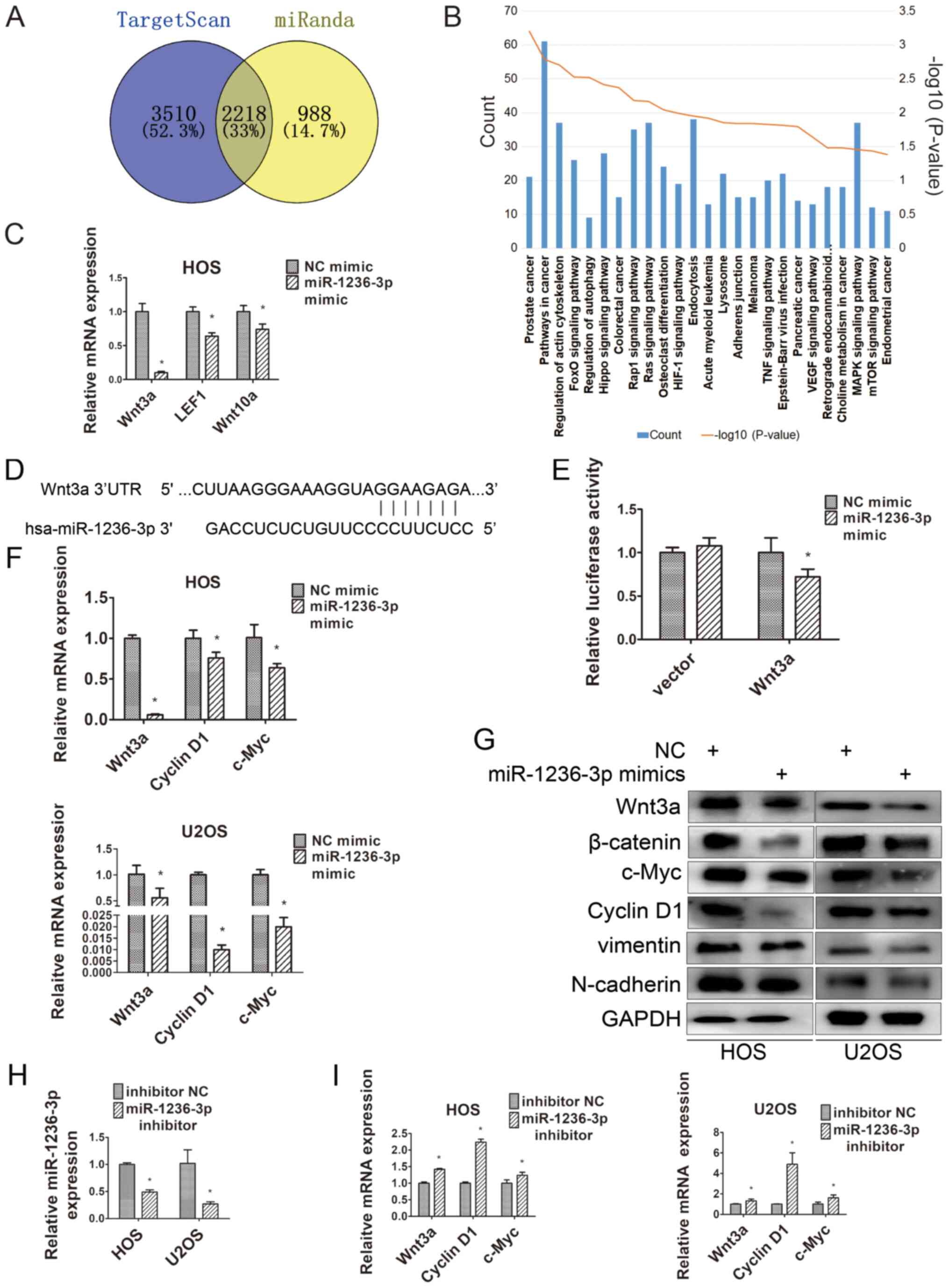
Wnt3a is the target of miR-1236-3p in
OS cells and miR-1236-3p inhibits Wnt3a expression
miR-1236-3p overexpression significantly decreased
OS cell migration and proliferation and blocked the cell cycle,
prompting the exploration of the target genes associated with the
regulation of OS cell progression. TargetScan and miRanda were used
to predict 5,728 and 3,206 miR-1236-3p target genes, respectively,
with 2,218 overlapping genes (Fig.
3A). By conducting pathway enrichment analysis, ‘pathways in
cancer’ was identified as the most likely pathway targeted by
miR-1236-3p (Fig. 3B). Numerous
literature reports on the role of the Wnt signaling pathway in
osteosarcoma have been published (16–18);
therefore, the genes in the signaling Wnt pathway (Wnt3a, Wnt10a
and LEF1) were selected for further investigation. Compared with NC
mimic, miR-1236-3p overexpression significantly decreased LEF1,
Wnt3a and Wnt10a expression levels, with Wnt3a expression levels
being decreased to the lowest levels among the three genes
(Fig. 3C). The base pairing between
the 3′UTR of Wnt3a and miR-1236-3p was predicted using TargetScan
(Fig. 3D). To test the targeting
effect of miR-1236-3p on Wnt3a, dual luciferase reporter assays
were performed to assess the association between miR-1236-3p and
the potential binding site. Compared with NC mimic, miR-1236-3p
overexpression significantly decreased the relative luciferase
activity of Wnt3a (Fig. 3E). Western
blotting and RT-qPCR were performed to determine the expression of
Wnt3a and its downstream genes in HOS and U2OS cells. The
downstream genes of Wnt3a include c-Myc, cyclin D1, β-catenin,
N-cadherin and vimentin (15). c-Myc
and cyclin D1 are closely related to cell cycle regulation, whereas
N-cadherin and vimentin are key molecular markers of
epithelial-mesenchymal transition (EMT) (19,20). As
presented in Fig. 3F and G,
miR-1236-3p overexpression decreased the expression levels of Wnt3a
and its downstream genes compared with the NC mimic group. In
addition, the RT-qPCR results suggested that miR-1236-3p inhibitor
significantly downregulated miR-1236-3p expression compared with
inhibitor NC (Fig. 3H), which
significantly increased the expression of Wnt3a and its downstream
genes, including cyclin D1 and c-Myc (Fig. 3I).
Wnt3a knockdown decreases OS cell
migration and proliferation
Based on the finding that the 3′UTR of Wnt3a
contained a binding site for miR-1236-3p, the biological role of
Wnt3a in OS cells was explored. The IHC results indicated that
Wnt3a, β-catenin and downstream genes (c-Myc and cyclin D1) were
upregulated in OS tissues compared with control chondroma tissues
(Fig. 4A). The RT-qPCR results also
indicated that Wnt3a was significantly upregulated in OS tissues
compared with control chondroma tissues (Fig. 4B). Moreover, compared with the
control hFOB1.19 cell line, Wnt3a expression levels were
significantly increased in the OS cell lines (HOS, U2OS, MG63,
143B, SJSA-1 and SAOS-2; Fig. 4C).
Due to the relatively strong proliferation ability of HOS cells and
the relatively high level of Wnt3a expression in U2OS cells, HOS
and U2OS cells were selected for subsequent experiments. To
investigate the role of Wnt3a in OS cell migration and
proliferation, OS cells were transfected with Wnt3a-siRNA or NC,
followed by cell function experiments. RT-qPCR and western blotting
were performed to verify the transfection efficiency of Wnt3a-siRNA
(Fig. 4D and E). The CCK-8 and
colony formation assays were conducted to evaluate HOS and U2OS
cell proliferation following transfection with Wnt3a-siRNA
(Fig. 4F and G). The results
indicated that Wnt3a knockdown significantly decreased OS cell
proliferation compared with NC. In addition, Wnt3a knockdown
significantly inhibited HOS and U2OS cell migration compared with
NC (Fig. 4H and I). The flow
cytometry results suggested that Wnt3a knockdown also arrested the
cell cycle at the G1 phase for both HOS and U2OS cells
(Fig. 4J).
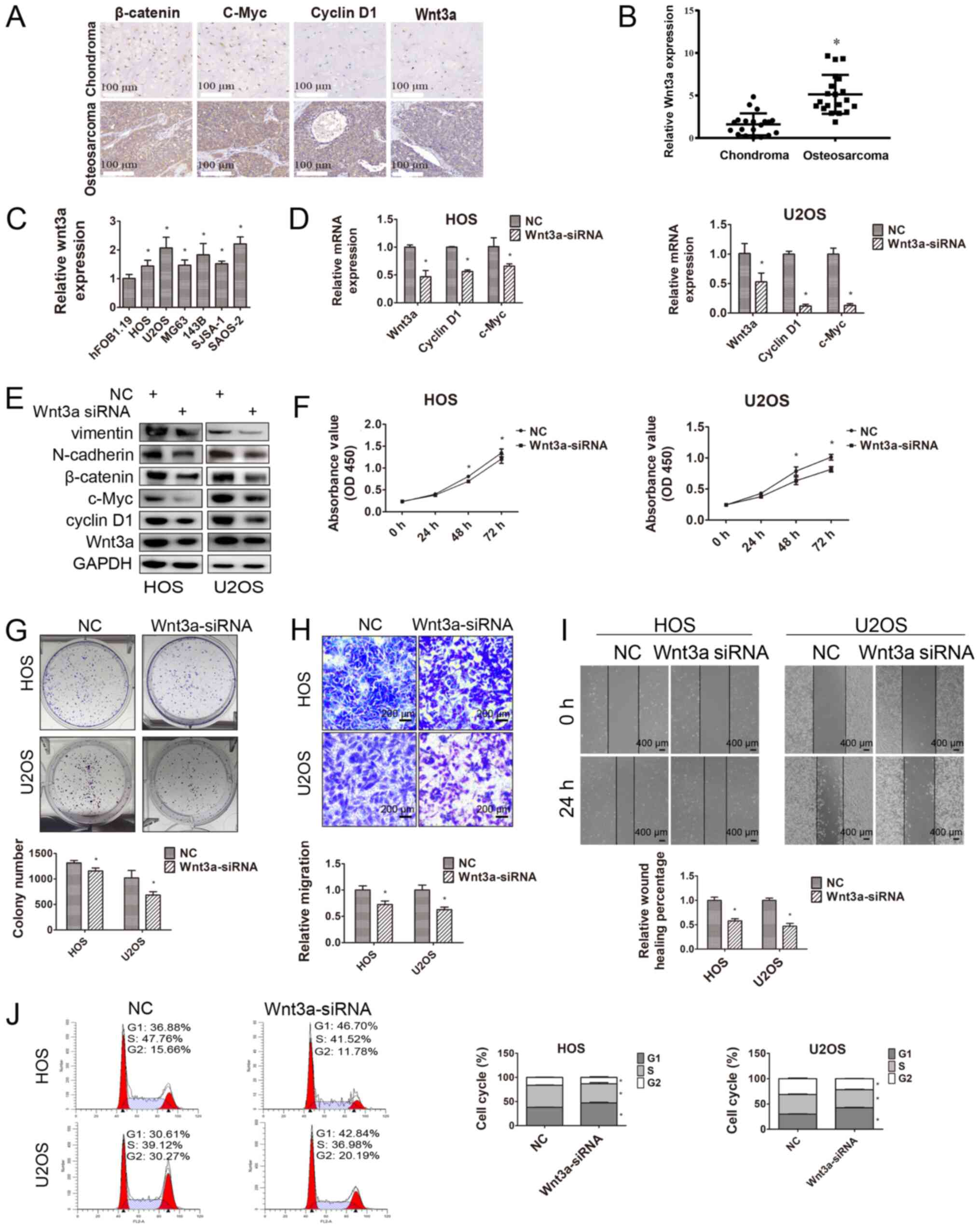 | Figure 4.Effects of Wnt3a on OS cells. (A)
Wnt3a, β-catenin, c-Myc and cyclin D1 expression levels in OS and
chondroma tissues were measured via immunohistochemistry (scale
bar, 100 µm). (B) Relative Wnt3a expression in OS tissues (n=20)
and chondroma tissues (n=20). *P<0.05 vs. chondroma. (C)
Relative Wnt3a expression in hFOB1.19 and OS cell lines (HOS, U2OS,
MG63, 143B, SJSA-1 and SAOS-2). Each Student's t-test was evaluated
separately for each set of 2 comparisons (hFOB1.19 vs. each of the
6 osteosarcoma cell lines). Wnt3a knockdown decreased the (D) mRNA
and (E) protein expression levels of its downstream genes. (F) The
Cell Counting Kit-8 assay was performed to assess cell
proliferation following transfection with NC or Wnt3a-siRNA at 0,
24, 48 and 72 h. (G) Relative long-term cell proliferation was
evaluated by conducting colony formation assays following
transfection with NC or Wnt3a-siRNA. (H) Relative HOS and U2OS cell
migration was evaluated by performing Transwell migration assays
following transfection with NC or Wnt3a-siRNA (scale bar, 200 µm).
(I) The effect of Wnt3a on HOS and U2OS cell migration was
determined by performing the wound healing assay (scale bar, 400
µm). (J) The cell cycle distribution in HOS and U2OS cell lines
following transfection with NC or Wnt3a-siRNA was analyzed via flow
cytometry. *P<0.05 vs. NC. OS, osteosarcoma; NC, negative
control; siRNA, small interfering RNA; miR, microRNA; OD, optical
density. |
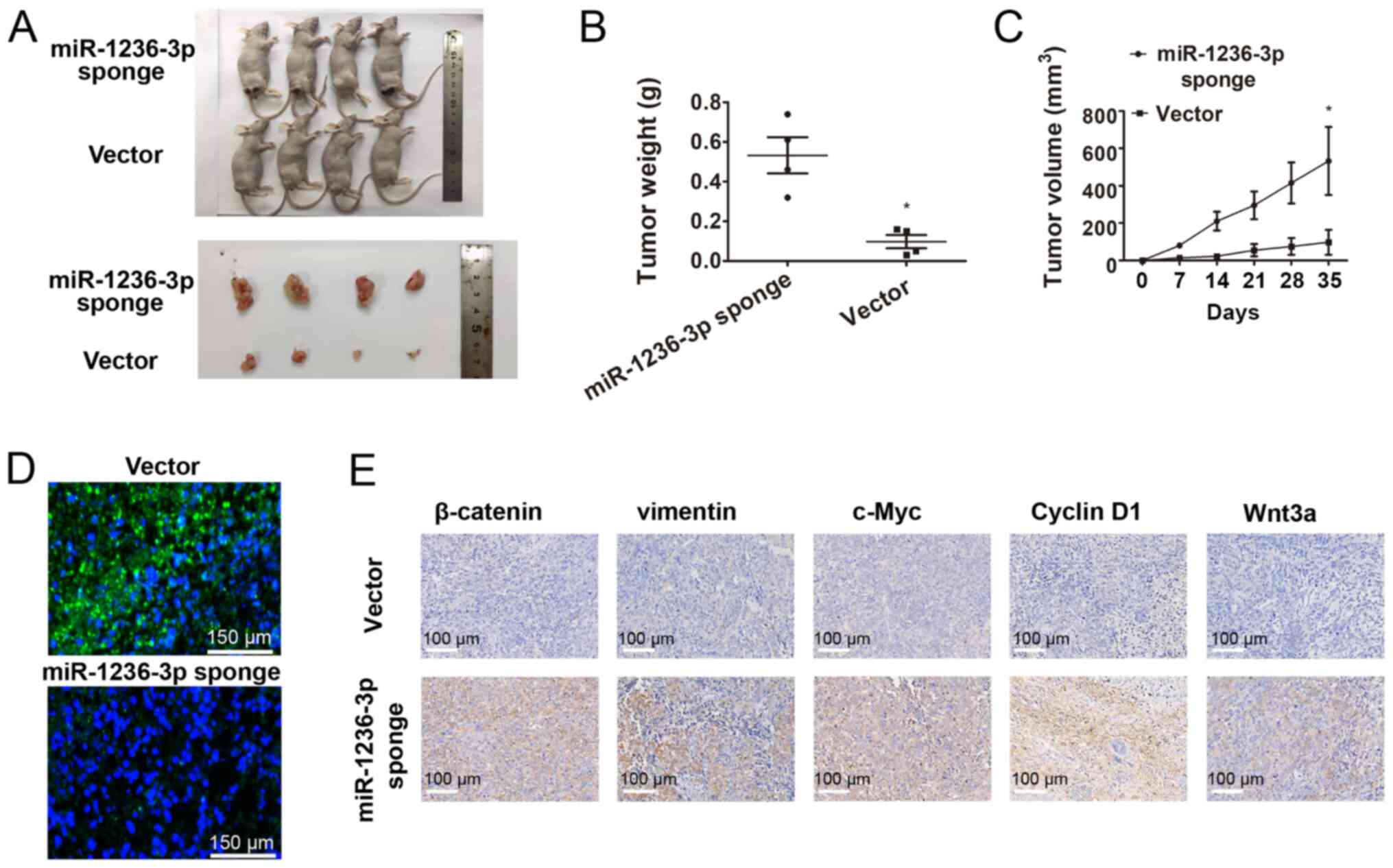
miR-1236-3p overexpression decreases
OS cell proliferation in vivo
To further investigate the inhibitory effect of
miR-1236-3p on OS cell proliferation, nude mice were subcutaneously
injected with HOS cells transfected with miR-1236-3p sponge or
vector. Tumor growth was faster in the miR-1236-3p sponge group
compared with the vector group, with the longest width of the
largest tumor being 1.85 (1.00 cm in control group); Fig. 5A-C). In addition, the FISH assay
results indicated that miR-1236-3p sponge markedly reduced
miR-1236-3p expression in tumor tissues compared with the vector
group (Fig. 5D). miR-1236-3p sponge
also increased the mean positive area for Wnt3a, β-catenin,
vimentin, c-Myc and cyclin D1 in tumor tissues compared with the
vector group, as determined via IHC analysis (Fig. 5E). Detailed records of the tumor
sizes throughout the experience are presented in Table SI.
Discussion
OS is a common malignant orthopedic tumor (3). Neoadjuvant chemotherapy with
intercalated surgery is an effective treatment strategy for
patients with resectable OS who are aged <40 years. However, the
prognosis is often poor in patients with metastasis or recurrence,
and in patients aged >40 years (21). Low miR-1236-3p expression is
associated with gastric cancer (8),
ovarian carcinoma (11) and lung
adenocarcinoma (22). However, the
effect of miR-1236-3p on OS cell invasion and proliferation has not
yet been studied.
In the present study, the FISH results indicated
markedly lower miR-1236-3p expression levels in OS tissues compared
with chondroma tissue. Therefore, to investigate whether low
miR-1236-3p expression was related to OS cell proliferation and
migration, rescue experiments were performed by transfecting OS
cells with miR-1236-3p mimic, which resulted in increased
miR-1236-3p expression compared with the NC mimic group.
Consequently, the cell function experiment results suggested that
miR-1236-3p overexpression significantly decreased OS cell
proliferation and migration compared with NC mimic. In addition,
the flow cytometry results indicated that miR-1236-3p
overexpression inhibited OS cell proliferation by inducing cell
cycle arrest at the G1 phase.
The functional role of the Wnt signaling pathway in
OS is not completely understood (23). A number of studies support the idea
that the Wnt signaling pathway serves an anti-tumorigenic effect in
OS (24–26), whereas other studies suggest that
aberrant activation of the canonical Wnt signaling pathway serves
an important role in OS initiation, maintenance and resistance to
chemotherapy (27,28). In addition, a large number of studies
have demonstrated that miRNA has an effect on the development of OS
by targeting the Wnt signaling pathway. For example, miR-130b
enhanced OS cell proliferation by targeting naked cuticle homology
2, a negative regulator of the Wnt signaling pathway (29,30).
Yang et al (31) reported
that miR-425-5p inhibited tumorigenesis via the Wnt/β-catenin
signaling pathway in OS. The canonical Wnt pathway is initiated by
the binding of appropriate Wnt ligands to the Frizzled and
low-density lipoprotein receptor-related protein 5/6 co-receptor.
Under normal conditions, without proper Wnt ligands (Wnt-1, Wnt-2,
Wnt-3, Wnt-3a, Wnt-4, Wnt-8 and Wnt-10b), excess β-catenin in the
cytoplasm is degraded by a multi-protein complex comprised of
glycogen synthase kinase 3β, adenomatous polyposis coli and axin
(32). When the Wnt signaling
pathway is activated, β-catenin can no longer be degraded, and
instead it accumulates in the cytosol and enters the nucleus to
form a transcriptional complex with the T-cell factor/lymphoid
enhancer-binding factor family, activating the expression of a wide
range of downstream genes, including c-Myc, cyclin D1, vimentin and
N-cadherin (32–34). c-Myc and cyclin D1 are cell
cycle-related proteins that can induce cell cycle arrest at the
G1 phase (19). In
addition, EMT is closely associated with the migratory ability of
tumor cells and oncogenic EMT has been reported to upregulate
vimentin and N-cadherin expression levels (20).
Compared with the human osteoblast cell line
(hFOB1.19), the expression of Wnt3a was significantly increased in
OS cell lines. Furthermore, the base pairing between the 3′UTR of
Wnt3a and miR-1236-3p was identified, and miR-1236-3p
overexpression inhibited Wnt3a expression compared with NC mimic,
as indicated by the dual luciferase reporter assay results.
Subsequently, the results indicated that miR-1236-3p overexpression
and knockdown decreased and increased the expression levels of
Wnt3a and its downstream genes, respectively, compared with the
corresponding NC groups. In conclusion, it was hypothesized that
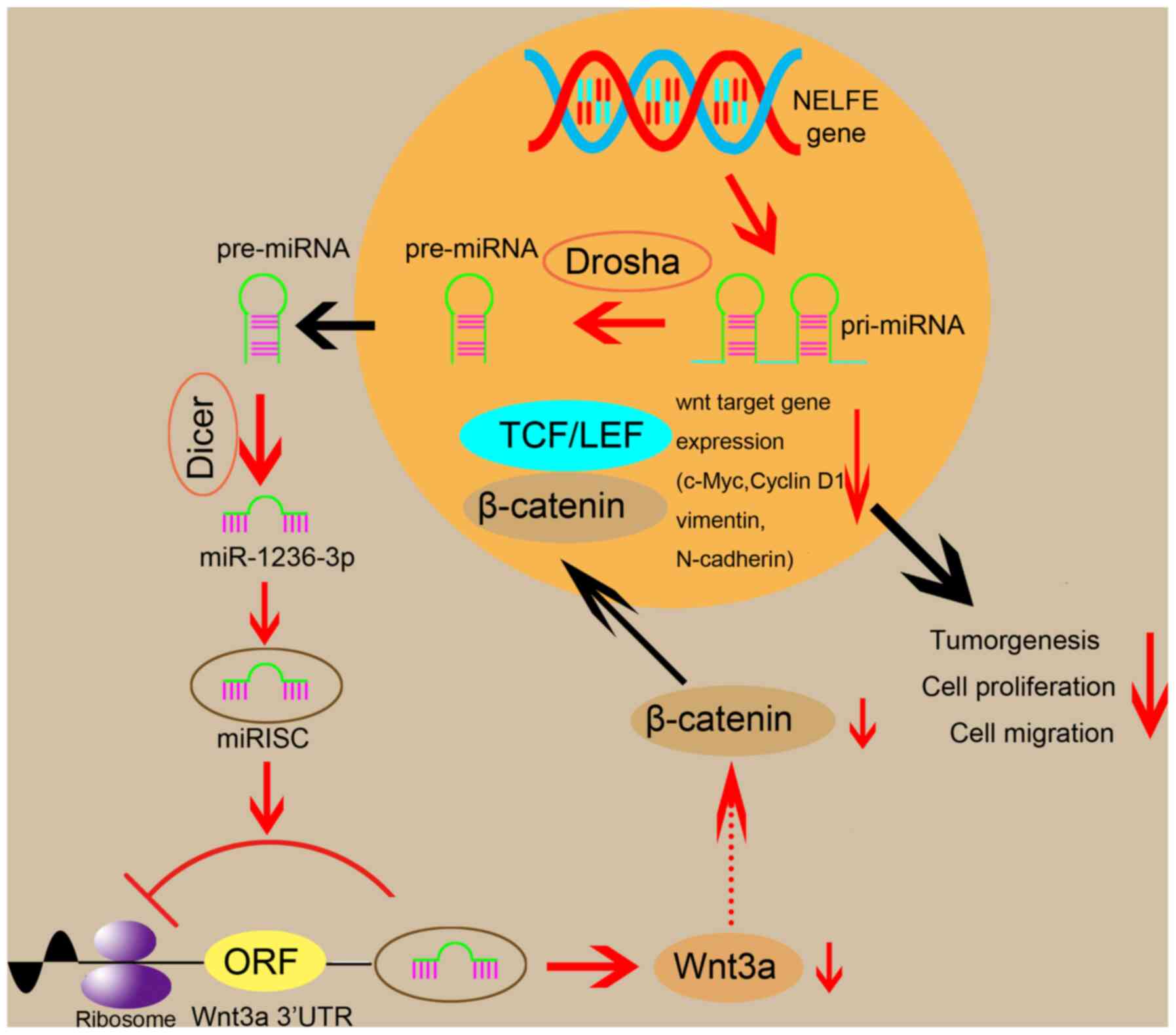
miR-1236-3p regulated the Wnt signaling pathway by acting on Wnt3a,
a Wnt protein reported to activate the signaling pathway (32).
To further investigate whether miR-1236-3p affected
OS cell proliferation and migration via targeting Wnt3a, OS cells
were transfected with Wnt3a-siRNA to downregulate Wnt3a expression.
The cell function experiments, cell cycle experiments, RT-qPCR and
western blotting results displayed consistent results between Wnt3a
knockdown and miR-1236-3p overexpression. The results indicated
that miR-1236-3p affected the Wnt signaling pathway by acting on
Wnt3a, ultimately affecting OS cell proliferation and migration
(Fig. 6). However, the present study
had a number of limitations. First, it could not be determined
whether miR-1236-3p affected the Wnt signaling pathway via other
target genes. Thus, additional studies are required to assess other
links between miR-1236-3p and the Wnt signaling pathway. Second,
when constructing the nude mice tumor xenograft models, nude mice
were not weighed before and after the experiment, which prevented
the analysis of the effect of tumor growth on the body weight of
nude mice.
To the best of our knowledge, the present study was
the first to explore the relationship between miR-1236-3p and OS.
miR-1236-3p inhibited OS cell proliferation and migration by
inhibiting the G1/S transition and EMT. The present
study may provide novel insights into the treatment of OS, which
may have the potential to be applied in the clinical setting
following further investigation.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Zhe Gong (The
Second Affiliated Hospital of Zhejiang University, Hangzhou, China)
and Mr Jun Li (Zhejiang Coastal Police Corps Hospital, Jiaxing,
China) for their assistance with the experiments.
Funding
The present study was supported by the National Key
R&D Program of China (grant no. 2018YFC1105200), the Key
Research and Development Plan in Zhejiang Province (grant no.
2018C03060), the National Nature Science Fund of China (grant nos.
81871797, 81874015, 81871796, 81873985, 81802680 and 81802147), the
Natural Science Fund of Zhejiang Province (grant nos. Z15H060002,
LY16H060004, LY19H160058 and LY16H060002) and the Medical Science
and Technology Project of Zhejiang Province of China (grant no.
2017179447).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WX and ZH designed the present study. JL and JC
conducted the experiments and carried out the statistical analysis.
JL wrote the paper and WX revised the paper. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent. The
present study was approved by the Ethics Committee of Sir Run Run
Shaw Hospital. All animal experiments were approved by the Animal
Care Committee of Sir Run Run Shaw Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
FISH
|
fluorescence in situ
hybridization
|
|
IHC
|
immunohistochemical analysis
|
|
miRNA/miR
|
microRNA
|
|
NC
|
negative control
|
|
OS
|
osteosarcoma
|
|
UTR
|
untranslated region
|
References
|
1
|
Li Z, Dou P, Liu T and He S: Application
of long noncoding RNAs in osteosarcoma: Biomarkers and therapeutic
targets. Cell Physiol Biochem. 42:1407–1419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ritter J and Bielack SS: Osteosarcoma. Ann
Oncol. 21 (Suppl 7):vii320–vii325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stiller CA, Bielack SS, Jundt G and
Steliarova-Foucher E: Bone tumours in European children and
adolescents, 1978–1997. Report from the automated childhood cancer
information system project. Eur J Cancer. 42:2124–2135. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim VN and Nam JW: Genomics of microRNA.
Trends Genet. 22:165–173. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pasquinelli AE, Hunter S and Bracht J:
MicroRNAs: A developing story. Curr Opin Genet Dev. 15:200–205.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
An JX, Ma MH, Zhang CD, Shao S, Zhou NM
and Dai DQ: miR-1236-3p inhibits invasion and metastasis in gastric
cancer by targeting MTA2. Cancer Cell Int. 18:662018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao R, Cai C, Gan J, Yang X, Shuang Z, Liu
M, Li S and Tang H: miR-1236 down-regulates alpha-fetoprotein, thus
causing PTEN accumulation, which inhibits the PI3K/Akt pathway and
malignant phenotype in hepatoma cells. Oncotarget. 6:6014–6028.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang C, Tang K, Li Z, Chen Z, Xu H and Ye
Z: Targeted p21(WAF1/CIP1) activation by miR-1236 inhibits cell
proliferation and correlates with favorable survival in renal cell
carcinoma. Urol Oncol. 34:59.e23–e34. 2016. View Article : Google Scholar
|
|
11
|
Wang Y, Yan S, Liu X, Zhang W, Li Y, Dong
R, Zhang Q, Yang Q, Yuan C, Shen K and Kong B: miR-1236-3p
represses the cell migration and invasion abilities by targeting
ZEB1 in high-grade serous ovarian carcinoma. Oncol Rep.
31:1905–1910. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poos K, Smida J, Nathrath M, Maugg D,
Baumhoer D and Korsching E: How microRNA and transcription factor
co-regulatory networks affect osteosarcoma cell proliferation. PLoS
Comput Biol. 9:e10032102013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jundt G: Updates to the WHO classification
of bone tumours. Pathologe. 39:107–116. 2018.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Liu G, Wu Y, Ma J, Wu H, Xie Z,
Chen S, Yang Y, Wang S, Shen P, et al: CircMYO10 promotes
osteosarcoma progression by regulating miR-370-3p/RUVBL1 axis to
enhance the transcriptional activity of β-catenin/LEF1 complex via
effects on chromatin remodeling. Mol Cancer. 18:1502019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martins-Neves SR, Paiva-Oliveira DI,
Fontes-Ribeiro C, Bovée JVMG, Cleton-Jansen A-M and Gomes CMF:
IWR-1, a tankyrase inhibitor, attenuates Wnt/β-catenin signaling in
cancer stem-like cells and inhibits in vivo the growth of a
subcutaneous human osteosarcoma xenograft. Cancer Lett. 414:1–15.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mu Y, Zhang L, Chen X, Chen S, Shi Y and
Li J: Silencing microRNA-27a inhibits proliferation and invasion of
human osteosarcoma cells through the SFRP1-dependent Wnt/β-catenin
signaling pathway. Biosci Rep. 39:BSR201823662019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Lu Q, Xie W, Wang Y and Wang G:
Anti-tumor effects of triptolide on angiogenesis and cell apoptosis
in osteosarcoma cells by inducing autophagy via repressing
Wnt/β-catenin signaling. Biochem Biophys Res Commun. 496:443–449.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kaldis P and Pagano M: Wnt signaling in
mitosis. Dev Cell. 17:749–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Whelan JS and Davis LE: Osteosarcoma,
chondrosarcoma, and chordoma. J Clin Oncol. 36:188–193. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bian T, Jiang D, Liu J, Yuan X, Feng J, Li
Q, Zhang Q, Li X, Liu Y and Zhang J: miR-1236-3p suppresses the
migration and invasion by targeting KLF8 in lung adenocarcinoma
A549 cells. Biochem Biophys Res Commun. 492:461–467. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao SJ, Jiang YQ, Xu NW, Zhang Q, Wang
SY, Li J, Wang YH, Zhang YL, Jiang SH, Wang YJ, et al: SPARCL1
suppresses osteosarcoma metastasis and recruits macrophages by
activation of canonical WNT/β-catenin signaling through
stabilization of the WNT-receptor complex. Oncogene. 37:1049–1061.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matushansky I, Hernando E, Socci ND, Mills
JE, Matos TA, Edgar MA, Singer S, Maki RG and Cordon-Cardo C:
Derivation of sarcomas from mesenchymal stem cells via inactivation
of the Wnt pathway. J Clin Invest. 117:3248–3257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cai Y, Mohseny AB, Karperien M, Hogendoorn
PC, Zhou G and Cleton-Jansen AM: Inactive Wnt/beta-catenin pathway
in conventional high-grade osteosarcoma. J Pathol. 220:24–33. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Basu-Roy U, Seo E, Ramanathapuram L, Rapp
TB, Perry JA, Orkin SH, Mansukhani A and Basilico C: Sox2 maintains
self renewal of tumor-initiating cells in osteosarcomas. Oncogene.
31:2270–2282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pridgeon MG, Grohar PJ, Steensma MR and
Williams BO: Wnt signaling in ewing sarcoma, osteosarcoma, and
malignant peripheral nerve sheath tumors. Curr Osteoporos Rep.
15:239–246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li C, Shi X, Zhou G, Liu X, Wu S and Zhao
J: The canonical Wnt-beta-catenin pathway in development and
chemotherapy of osteosarcoma. Front Biosci (Landmark Ed).
18:1384–1391. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Li Y, Wang N, Yang L, Zhao W and
Zeng X: miR-130b targets NKD2 and regulates the Wnt signaling to
promote proliferation and inhibit apoptosis in osteosarcoma cells.
Biochem Biophys Res Commun. 471:479–485. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao S, Kurenbekova L, Gao Y, Roos A,
Creighton CJ, Rao P, Hicks J, Man TK, Lau C, Brown AM, et al: NKD2,
a negative regulator of Wnt signaling, suppresses tumor growth and
metastasis in osteosarcoma. Oncogene. 34:5069–5079. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang G, Zhang C, Wang N and Chen J:
miR-425-5p decreases LncRNA MALAT1 and TUG1 expressions and
suppresses tumorigenesis in osteosarcoma via Wnt/β-catenin
signaling pathway. Int J Biochem Cell Biol. 111:42–51. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xi Y and Chen Y: Wnt signaling pathway:
Implications for therapy in lung cancer and bone metastasis. Cancer
Lett. 353:8–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng Z, Wu T, Li Y, Xu Z, Zhang S, Liu B,
Chen Q and Tian D: MicroRNA-370-3p inhibits human glioma cell
proliferation and induces cell cycle arrest by directly targeting
β-catenin. Brain Res. 1644:53–61. 2016. View Article : Google Scholar : PubMed/NCBI
|