Introduction
The incidence of esophageal cancer has been
exhibiting annual increases (1,2) and it
is among the top five causes of cancer-associated death worldwide
(1–3). In Asia, the majority of esophageal
cancer cases are diagnosed pathologically as esophageal squamous
cell carcinoma (ESCC) (1,3,4).
Multidisciplinary interventions, including surgery, chemotherapy
and/or radiotherapy, are recommended for ESCC according to the
tumor stage (5). Despite the rapid
progression in therapeutic methods, less than one in five ESCC
patients survived after five years (1,4–6). Increasing evidence demonstrates that
several genetic and epigenetic alterations contribute to the
carcinogenesis of ESCC (5,7,8). The
identification of novel targets to develop therapeutic strategies
is of vital importance to improve the survival of ESCC
patients.
Several studies focusing on human malignancies have
indicated dysregulation of the recently discovered long non-coding
RNAs (lncRNA), which do not give rise to functional proteins
(9–11). LncRNAs were indicated to exhibit
multifaced functions in important biological processes, including
cell growth, apoptosis, embryonic development and tumorigenesis, by
downregulating or upregulating protein expression at the chromatin
organization, epigenetic control, and transcriptional and
posttranscriptional levels (9,12–16).
Numerous lncRNAs have been indicated to be significantly
dysregulated in human tumors and may be ideal candidates for
prognostic markers (17–20). Li et al (21) proposed a three-lncRNA panel composed
of ENST00000435885.1, XLOC_013014 and ENST00000547963.1 that may be
used to predict the survival of patients with esophageal cancer
patients. Upregulated metastasis-associated lung adenocarcinoma
transcript 1 predicts poor prognosis in several human cancer types,
including ESCC (21–24).
Family with sequence similarity 83 member H
antisense 1 (FAM83H) is an lncRNA of 12,198 nucleotides in length
whose encoding gene is located in the antisense region of FAM83H
and which has been indicated to promote tumor progression. Several
studies have identified FAM83H-AS1 as an oncogenic factor in
bladder cancer (25), intestinal
malignancies (26,27), lung adenocarcinoma (28) and pancreatic ductal adenocarcinoma
(29). Zhang et al (28) indicated that upregulated FAM83H-AS1
promoted proliferation by activating the MET/EGFR signaling pathway
in lung cancer. Furthermore, by blocking the NOTCH pathway,
knockdown of FAM83H-AS1 suppressed the proliferation of colorectal
cancer cells (26). In a recent
study using several datasets, FAM83H-AS1 was indicated to be
involved in the regulation of the transcriptional profile of
pancreatic tumor samples and cell lines (29). However, the expression levels and
roles of FAM83H-AS1 in ESCC have so far remained to be determined.
Considering its crucial role in tumor pathogenesis, the present
study aimed to explore associations of FAM83H-AS1 with ESCC.
The present study suggested that, in comparison with
paired para-tumorous tissues or normal esophageal epithelial cells,
FAM83H-AS1 expression in cancer tissues and cells was markedly
upregulated. High expression of FAM83H-AS1 was observed in patients
with the presence of metastasis to the lymph nodes and an advanced
tumor stage. Furthermore, high FAM83H-AS1 expression was indicated
to predict poor overall and disease-free survival in patients with
ESCC. Cell proliferation and Transwell migration assays confirmed
that FAM83H-AS1 facilitated the malignant progression of ESCC.
These results indicated that FAM83H-AS1 may be used as a prognostic
marker and a novel therapeutic target in ESCC.
Materials and methods
Tissue samples and clinical data
collection
In the present study, 134 patients with ESCC who did
not undergo any neoadjuvant anti-tumor therapy were enrolled.
Surgery of enrolled subjects was performed at the First Affiliated
Hospital of Anhui Medical University (Hefei, China) between March
2007 and August 2010. All diagnoses were confirmed by professional
pathological evaluation. The clinicopathological characteristics of
the patients with ESCC retrieved from their medical records are
presented in Table I. Dissected
tissue samples were immediately soaked in RNAlater®
solution (Beyotime Institute of Biotechnology) prior to careful
preservation in an ultra-low temperature freezer (−80°C) following
a standard procedure.
 | Table I.Association between
clinicopathological parameters and FAM83H-AS1 expression. |
Table I.
Association between
clinicopathological parameters and FAM83H-AS1 expression.
|
| FAM83H-AS1
expression |
|
|---|
|
|
|
|
|---|
| Variable | Low | High | P-value |
|---|
| Age (years) |
|
| 1.000 |
|
<60 | 37 (55.2) | 37 (55.2) |
|
|
≥60 | 30 (44.8) | 30 (44.8) |
|
| Sex |
|
| 0.836 |
|
Male | 51 (76.1) | 53 (79.1) |
|
|
Female | 16 (23.9) | 14 (20.9) |
|
| Tumor size
(cm) |
|
| 0.342 |
|
<5 | 59 (88.1) | 54 (80.6) |
|
| ≥5 | 8 (11.9) | 13 (19.4) |
|
| Degree of
differentiation |
|
| 0.146 |
| Well or
moderate | 56 (83.6) | 48 (71.6) |
|
|
Poor | 11 (16.4) | 19 (28.4) |
|
| Lymph node
metastasis |
|
| <0.001 |
|
Present | 25 (37.3) | 48 (71.6) |
|
|
Absent | 42 (62.7) | 19 (28.4) |
|
| TNM stage |
|
| <0.001 |
|
I–II | 49 (73.1) | 22 (32.8) |
|
|
III | 18 (26.9) | 45 (67.2) |
|
Cell culture
A total of 4 ESCC cell lines (KYSE410, KYSE510,
KYSE520 and KYSE30) were purchased from Deutsche Sammlung von
Mikroorganismen und Zellkulturen (DSMZ, Braunschweig, Germany,
http://www.leibniz-gemeinschaft.de/institute/leibniz-institute-alle-listen/leibniz-institut-dsmz-deutsche-sammlung-von-mikroorganismen-und-zellkulturen-gmbh.html).
All cells were cultured according to a previous protocol (30) and tested for mycoplasma infection
prior to use. The medium for NE1 esophageal epithelial cells (DSMZ)
was mixed with an equal amount of defined keratinocyte serum-free
medium (cat. no. 10744019; Gibco, Thermo Fisher Scientific, Inc.)
with growth supplements and EpLife medium with 60 µM calcium (cat.
no. MEPI500CA; Gibco; Thermo Fisher Scientific, Inc.).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
RNA extraction was performed according to standard
protocols with TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The extracted products (1 µg) were then
subjected to RT in order to obtain complementary DNA using random
primers with the Prime Script RT Reagent Kit (Takara Bio, Inc.).
The SYBR Premix Taq (Promega Corp.) kit was used to determine the
expression levels of specific genes in the qPCR assays. The
2−ΔΔCq method (30) was
used to quantify the relative expression of FAM83H-AS1. The primers
had the following sequences: FAM83H-AS1 forward,
5′-TCCTCAAGCAAAGCACTC-3′ and reverse, 5′-TACGGCAGAAAGAACCAA-3′;
GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′.
Small interfering (si)RNA
transfection
The cells (4×105 cells/well) were seeded
in the 6-well plate on the day prior to transfection with
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.). In brief,
siRNA (5 µl/well) and Lipofectamine 2000 (5 µl/well) were
individually incubated with 125 µl Opti-Minimum Essential Medium
(MEM; Gibco; Thermo Fisher Scientific, Inc.) at room temperature
for 5 min. They were then mixed and incubated at room temperature.
After 20 min, the mixture was added into the culture medium
according to the manufacturer's protocol. At two days after
transfection, the cells were collected for RT-qPCR, MTS and
Transwell assays. The sequences of siRNAs targeting FAM83H-AS1
(si#FAM83H-AS1) and the scrambled control siRNAs (NC) were as
follows: si#FAM83H-AS1: 5′-GCTGAATCACGTCAAGTAT-3′, and NC:
5′-TTCTCCGAACGTGTCACGT-3′.
MTS assay
The cell proliferation was measured with an MTS kit
(Promega Corp.). In brief, 100 µl suspension containing 500 cells
was added to each well of a 96-well plate. At the indicated
time-points, 10 µl MTS was added to each well, followed by
additional incubation for 2 h at 37°C. The absorbance was measured
at 450 nm (SpectraMax® M5 Multi-Mode Microplate Reader;
Molecular Devices LLC) following gentle agitation for 10 min. All
assays were performed in three independent experiments and each
condition was assessed in triplicate.
Transwell migration assay
To evaluate the migration capability, ESCC cells in
the exponential growth phase were collected, washed with PBS,
resuspended in serum-free medium and 200 µl of this suspension
containing 1×105 cells was added to the top of the 8 µm
chamber (BD Biosciences). The lower compartment was filled with 500
µl fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.).
After 24 h of culture at 37°C, the migrated cells were fixed with
methanol for 15 min at room temperature, stained with 0.1% crystal
violet for 30 min at room temperature and counted under a
microscope. All assays were performed in triplicate.
Statistical analysis
SPSS (version 19.0; IBM Corp.) and GraphPad Prism
6.0 (GraphPad, Inc.) were used to perform statistical analysis and
graph plotting, respectively. The Kaplan-Meier method was utilized
to produce survival curves, while log-rank tests were used to
calculate the P-values. Univariate and multivariate Cox models
including hazard ratios and 95% confidence intervals were
constructed in SPSS. An unpaired Student's t-test and the
Chi-squared test were used to determine the significance of the
differences between groups. P<0.05 was considered to indicate a
significant difference.
Results
General patient characteristics
In the present cohort, the percentage of male
patients with ESCC was 77.6% and the median age was 60.0 years (age
range, 36.0–88.0 years). The number of subjects diagnosed as having
stage I, II and III ESCC was 5 (3.7%), 66 (49.3%) and 63 (47.0%),
respectively. More than half of the patients (73/134) had lymph
node metastasis (Table I).
Upregulation of FAM83H-AS1 in ESCC
cancer tissues and cells
The expression of FAM83H-AS1 in ESCC samples was
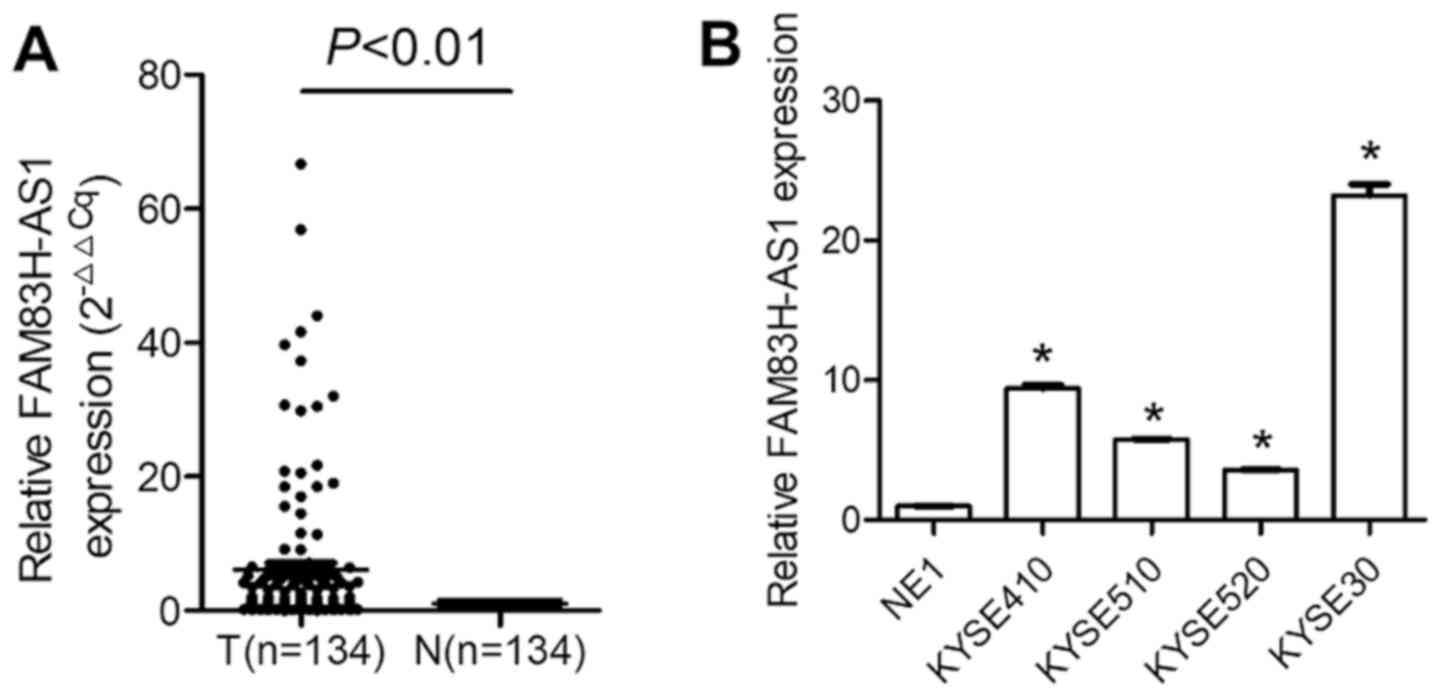
first detected. As presented in Fig.
1A, FAM83H-AS1 was significantly upregulated in ESCC vs.
adjacent epithelial tissues (P=0.0007). FAM83H-AS1 expression
levels were then evaluated in ESCC cell lines and the results
demonstrated that in KYSE410, KYSE510, KYSE520 and KYSE30 cells,
FAM83H-AS1 was significantly upregulated compared with that in NE1
(Fig. 1B; P<0.05).
Associations between FAM83H-AS1
expression levels and clinicopathological parameters
To characterize FAM83H-AS1 expression in ESCC, the
fold change value, which indicates differences in expression
ratios, was utilized. The median fold change value was considered
as the cutoff. The association of FAM83H-AS1 with
clinicopathological indices was analyzed (Table I). Patients with metastasis to lymph
nodes or advanced TNM stage tended to have high expression of
FAM83H-AS1 (Table I). However, no
obvious associations of FAM83H-AS1 with other clinicopathological
characteristics, including age, gender and degree of
differentiation, were observed (Table
I).
Association between FAM83H-AS1
expression and survival in ESCC
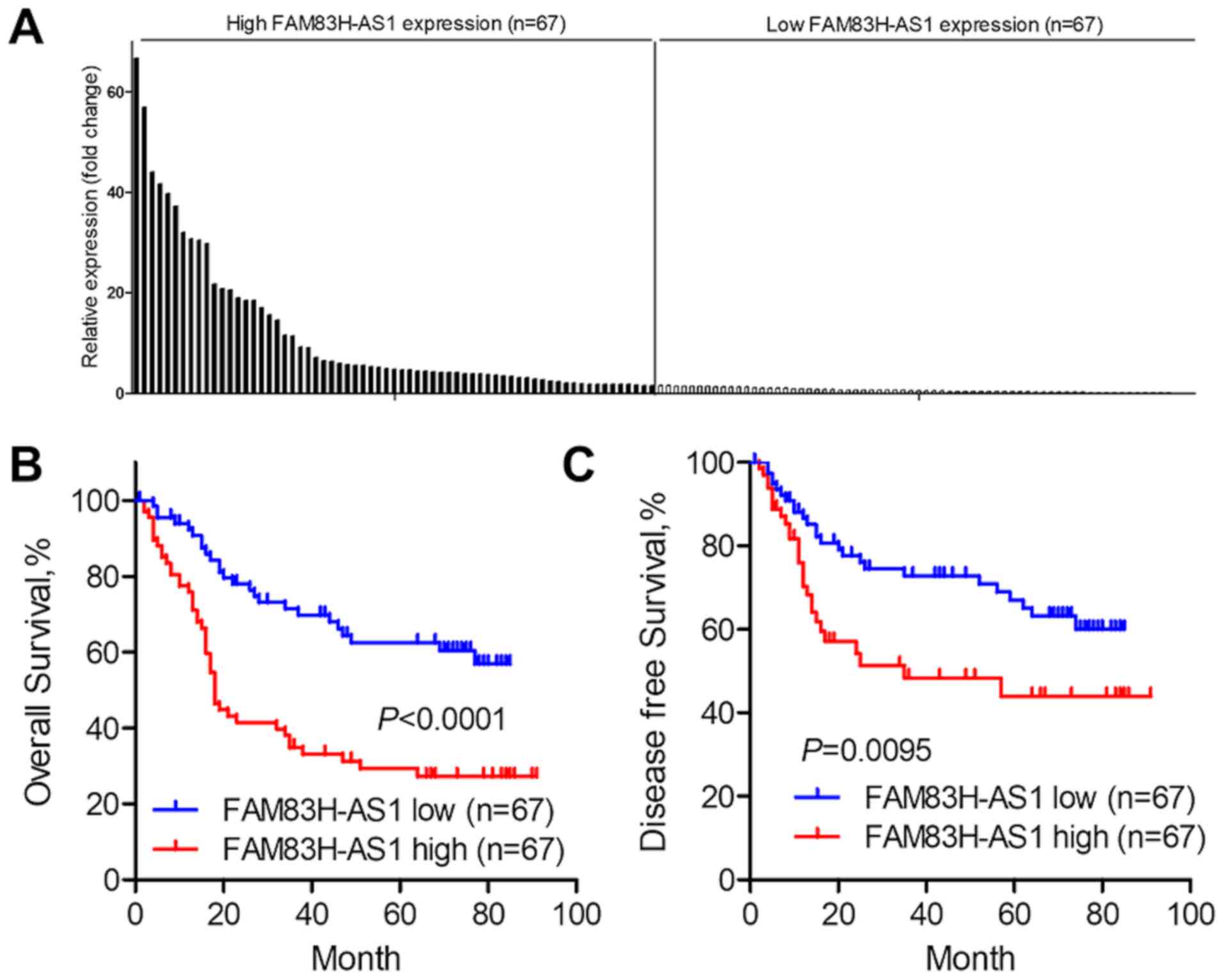
It was then analyzed whether FAM83H-AS1 may be used
as a prognostic predictor in ESCC. The expression of FAM83H-AS1 in
the cancer tissues was profiled and the median fold change was 1.55
(Fig. 2A). The Kaplan-Meier method
and the log-rank test were used for further survival analysis. As
presented in Fig. 2B, the overall
survival of patients with high FAM83H-AS1 expression was
significantly worse than that of patients with low FAM83H-AS1
expression (P<0.0001). Furthermore, high expression of
FAM83H-AS1 indicated poor disease-free survival of patients with
ESCC (P=0.0095; Fig. 2C). Univariate
survival analyses suggested that a larger tumor size (P=0.030),
presence of lymph node metastasis (P<0.001), advanced TNM stage
(P<0.001) and upregulated FAM83H-AS1 expression (P<0.001)
influenced the prognosis of patients with ESCC (Table II). Further multivariate analyses
indicated that only the TNM stage (P<0.001) and FAM83H-AS1
expression (P=0.014) were independent predictors of overall
(Table II).
 | Table II.Univariate and multivariate analyses
of overall survival prognostic factors in patients with esophageal
squamous cell carcinoma. |
Table II.
Univariate and multivariate analyses
of overall survival prognostic factors in patients with esophageal
squamous cell carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≥60/<60
years) | 1.46
(0.91–2.33) | 0.117 | – | – |
| Sex
(male/female) | 0.93
(0.54–1.61) | 0.799 | – | – |
| Tumor size
(≥5/<5 cm) | 1.92
(1.06–3.46) | 0.030 | 1.36
(0.74–2.48) | 0.321 |
| Differentiation
(poor/well, moderate) | 1.57
(0.93–2.64) | 0.091 | – | – |
| Lymph node
metastasis (present/absent) | 2.90
(1.73–4.86) | <0.001 | 1.19
(0.47–2.97) | 0.714 |
| TNM stage
(III/I–II) | 3.38
(2.05–5.58) | <0.001 | 2.71
(1.59–4.61) | <0.001 |
| FAM83H-AS1
(high/low) | 2.68
(1.64–4.39) | <0.001 | 1.93
(1.14–3.25) | 0.014 |
Oncogenic roles of FAM83H-AS1 in
vitro
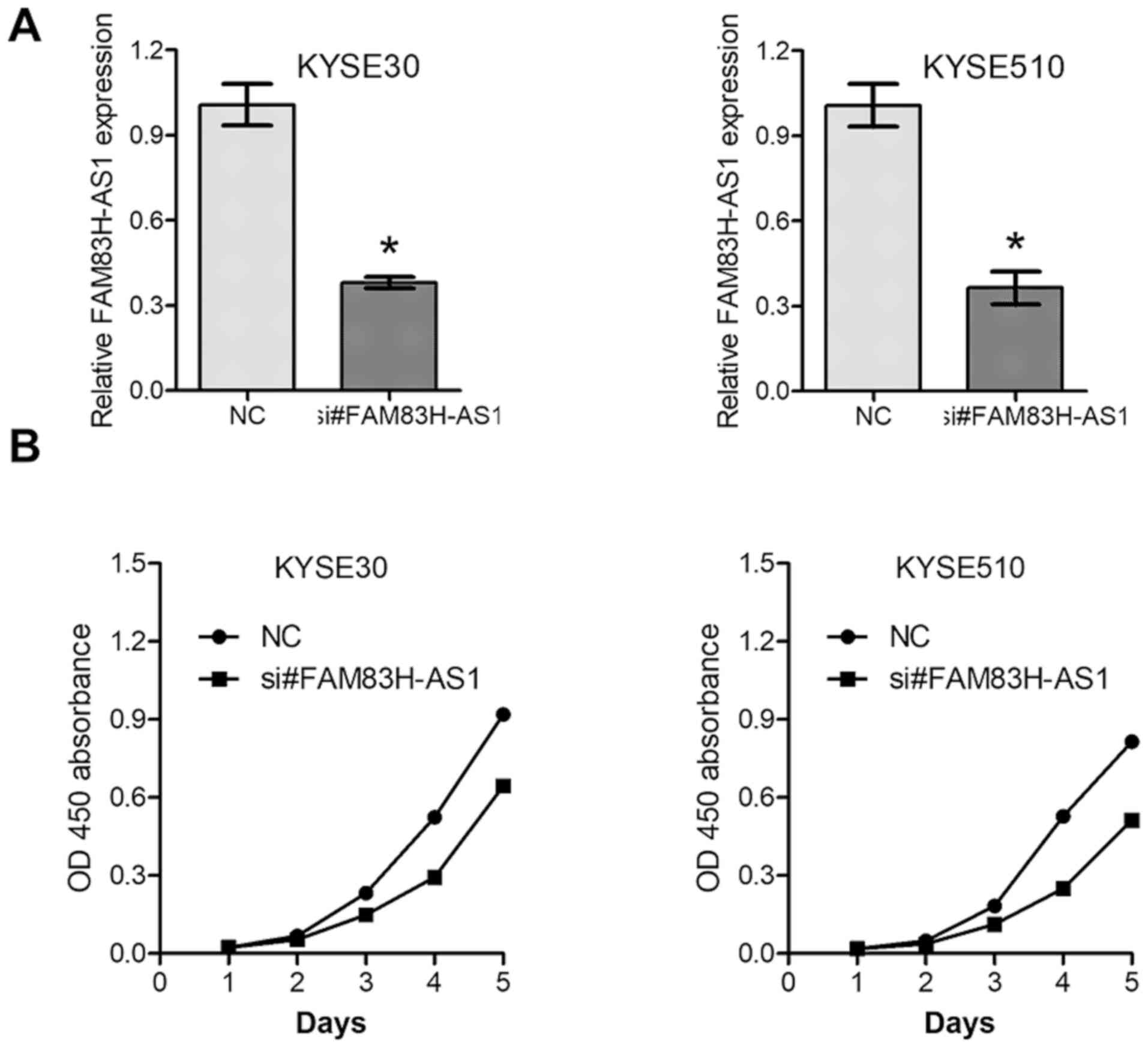
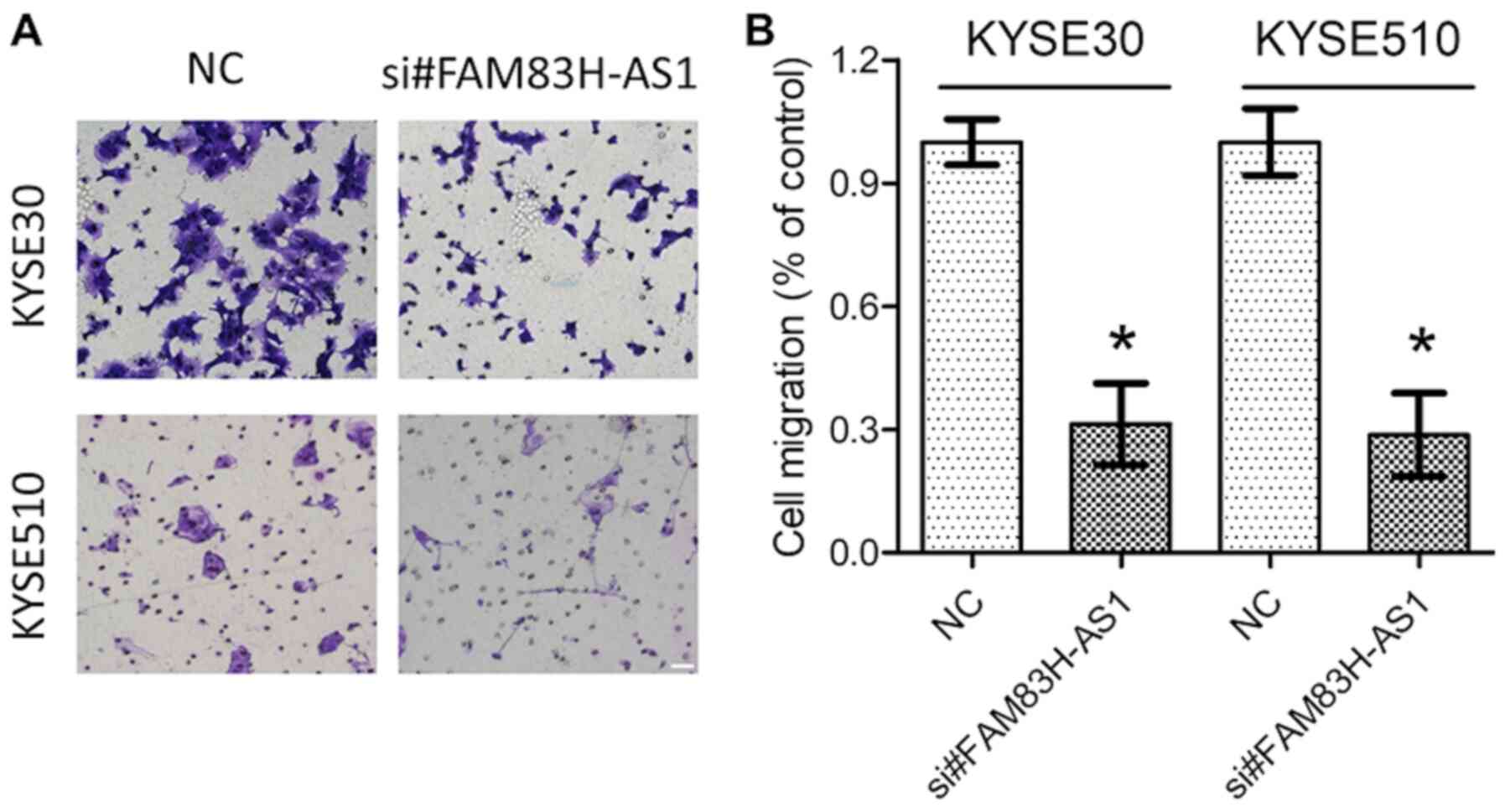
The above results prompted us to further explore the
function of dysregulated FAM83H-AS1 in ESCC. si#FAM83H-AS1, as well
as the NC were introduced into KYSE30 and KYSE510 cells with
relatively high expression levels of FAM83H-AS1. The knockdown
efficiency was confirmed by RT-qPCR analysis (Fig. 3A). MTS and Transwell assays revealed
that knockdown of FAM83H-AS1 significantly inhibited cell
proliferation (Fig. 3B) and
decreased the migration capacity (Fig.
4A and B) of the ESCC cell lines KYSE30 and KYSE510. These data
further suggested oncogenic roles of FAM83H-AS1 in ESCC.
Discussion
ESCC ranks among the top five causes of
cancer-associated death in Eastern Asia, including China (1,3,6). Therefore, identification of novel
molecular candidates that may be used for the precise diagnosis and
prognosis prediction remains an urgent requirement. The present
study demonstrated marked overexpression of FAM83H-AS1 in ESCC
compared with paired normal tissues. In addition, its expression in
ESCC cell lines was significantly upregulated compared with that in
the normal NE1 cell line. Furthermore, FAM83H-AS1 expression was
associated with lymph node metastasis and the TNM stage. Of note,
overexpression of FAM83H-AS1 was and independent predictor of poor
overall survival of patients with ESCC and is thus an ideal
candidate for a prognostic biomarker.
As evolutionally conserved molecules with
tissue-specific expression (13,15,16),
lncRNAs have been reported to be involved in the pathophysiology of
several human diseases, including cancer (10,17,19,21,24).
Dysregulation of lncRNA may exert tumor-suppressive or oncogenic
characteristics and may be detected throughout the entire process
of cancer development. For instance, Qi et al (31) indicated that by downregulating
cyclin-dependent kinase-interacting protein 1 and cadherin 1,
ArfGAP with GTPase domain, ankyrin repeat and PH domain 2-AS1
promotes stomach cancer progression. Chen et al (20) suggested that overexpressed HOTTIP in
ESCC predicts poor prognosis. Silencing of HOTTIP attenuated
malignant phenotypes including proliferation and invasion (20).
In the present study, a positive association between
the upregulation of FAM83H-AS1 and metastasis to the lymph nodes
was observed. Previous studies have indicated that downregulation
of Notch1 signaling in tumor tissues contributed to lymph node
metastasis and tumor-induced lymphangiogenesis of ESCC (32). Furthermore, overexpression of
FAM83H-AS1 promoted the migration of SW480 and HT29 cells through
modulation of the Notch pathway (26). In addition, microRNA (miR)-136, which
was reported as a direct target of FAM83H-AS1 in breast cancer
(33), was indicated to be
associated with lymph node metastasis in ESCC (34). However, whether and how Notch
signaling, miR-136 or other pathways mediate the oncogenic roles of
FAM83H-AS1 in ESCC warrants further exploration.
Several lines of evidence have suggested that
upregulated FAM83H-AS1 predicted poor prognosis of malignant
diseases, including bladder cancer (25), gastrointestinal cancer (26,27,29),
ovarian cancer (35) and lung cancer
(28). The present results indicated
that aberrant upregulation of FAM83H-AS1 was associated with poor
prognosis of patients with ESCC. These studies indicated that
FAM83H-AS1 may be widely overexpressed in human cancers and have
crucial roles in the pathogenesis of cancers.
To date, only a limited number of studies have
explored the oncogenic characteristics of FAM83H-AS1 in human
malignancies. Silencing of FAM83H-AS1 decreased the malignant
behavior of bladder cancer cells, including proliferation,
migration, invasion and cycle arrest progression (25), while activation of MET/EGFR signaling
underlined the pro-tumorous roles of FAM83H-AS1 in lung cancer
(28). In the present study, genetic
interference assays uncovered that inhibition of FAM83H-AS1
expression significantly suppressed the proliferation and migration
of ESCC cells, which provided further evidence for the oncogenic
role of FAM83H-AS1 in human malignancies. The mechanisms underlying
the oncogenic role of FAM83H-AS1 may be complex and tumor
type-specific, and the downstream targets modulated by FAM83H-AS1
require further investigation.
In conclusion, the present study demonstrated
upregulation of FAM83H-AS1 in ESCC. Of note, elevated FAM83H-AS1
expression was associated with shortened survival. However, further
in-depth research is warranted to uncover the molecular mechanisms
of the proliferative and metastasis-enhancing effects of FAM83H-AS1
in ESCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JD contributed to the conception of the present
study. LB and RW performed the experiments and data analysis. PL
contributed to data collection and interpretation, and LB, RW and
JD drafted the initial manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with The 1964
Declaration of Helsinki and its later amendments or comparable
ethical standards. All cases had complete follow-up information and
provided written informed consent. The present study was approved
by the Research Ethics Committee of The First Affiliated Hospital
of Anhui Medical University (Hefei, China; approval no. Quick-PJ
2020-08-11).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oh CM, Won YJ, Jung KW, Kong HJ, Cho H,
Lee JK, Lee DH and Lee KH; Community of Population-Based Regional
Cancer Registries, : Cancer statistics in Korea: Incidence,
mortality, survival, and prevalence in 2013. Cancer Res Treat.
48:436–450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeng H, Zheng R, Zhang S, Zuo T, Xia C,
Zou X and Chen W: Esophageal cancer statistics in China, 2011:
Estimates based on 177 cancer registries. Thorac Cancer. 7:232–237.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Essadi I, Lalya I and Mansouri H:
Esophageal carcinoma. N Engl J Med. 372:1470–1471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao YB, Chen ZL, Li JG, Hu XD, Shi XJ, Sun
ZM, Zhang F, Zhao ZR, Li ZT, Liu ZY, et al: Genetic landscape of
esophageal squamous cell carcinoma. Nat Genet. 46:1097–1102. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang SD, Yuan Y, Zhuang CW, Li BL, Gong
DJ, Wang SG, Zeng ZY and Cheng HZ: MicroRNA-98 and microRNA-214
post-transcriptionally regulate enhancer of zeste homolog 2 and
inhibit migration and invasion in human esophageal squamous cell
carcinoma. Mol Cancer. 11:512012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gutschner T and Diederichs S: The
hallmarks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun M and Kraus WL: From discovery to
function: The expanding roles of long noncoding RNAs in physiology
and disease. Endocr Rev. 36:25–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lalevee S and Feil R: Long noncoding RNAs
in human disease: Emerging mechanisms and therapeutic strategies.
Epigenomics. 7:877–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Batista PJ and Chang HY: Long noncoding
RNAs: Cellular address codes in development and disease. Cell.
152:1298–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ge XS, Ma HJ, Zheng XH, Ruan HL, Liao XY,
Xue WQ, Chen YB, Zhang Y and Jia WH: HOTAIR, a prognostic factor in
esophageal squamous cell carcinoma, inhibits WIF-1 expression and
activates Wnt pathway. Cancer Sci. 104:1675–1682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren K, Li Y, Lu H, Li Z, Li Z, Wu K, Li Z
and Han X: Long noncoding RNA HOTAIR controls cell cycle by
functioning as a competing endogenous RNA in esophageal squamous
cell carcinoma. Transl Oncol. 9:489–497. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu C, Yang L, Chen H and Shan Z:
Upregulated long non-coding RNA BC032469 enhances carcinogenesis
and metastasis of esophageal squamous cell carcinoma through
regulating hTERT expression. Tumour Biol. Oct 10–2016.(Epub ahead
of print). doi: 10.1007/s13277-016-5428-9. View Article : Google Scholar
|
|
20
|
Chen X, Han H, Li Y, Zhang Q, Mo K and
Chen S: Upregulation of long noncoding RNA HOTTIP promotes
metastasis of esophageal squamous cell carcinoma via induction of
EMT. Oncotarget. 7:84480–84485. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J, Chen Z, Tian L, Zhou C, He MY, Gao
Y, Wang S, Zhou F, Shi S, Feng X, et al: LncRNA profile study
reveals a three-lncRNA signature associated with the survival of
patients with oesophageal squamous cell carcinoma. Gut.
63:1700–1710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu L, Wu Y, Tan D, Meng H, Wang K, Bai Y
and Yang K: Up-regulation of long noncoding RNA MALAT1 contributes
to proliferation and metastasis in esophageal squamous cell
carcinoma. J Exp Clin Cancer Res. 34:72015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thum T and Fiedler J: LINCing MALAT1 and
angiogenesis. Circ Res. 114:1366–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gutschner T, Hammerle M, Eissmann M, Hsu
J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al:
The noncoding RNA MALAT1 is a critical regulator of the metastasis
phenotype of lung cancer cells. Cancer Res. 73:1180–1189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shan H, Yang Y, Zhu X, Han X, Zhang P and
Zhang X: FAM83H-AS1 is associated with clinical progression and
modulates cell proliferation, migration, and invasion in bladder
cancer. J Cell Biochem. 120:4687–4693. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu S, Dong W, Zhao P and Liu Z: lncRNA
FAM83H-AS1 is associated with the prognosis of colorectal carcinoma
and promotes cell proliferation by targeting the Notch signaling
pathway. Oncol Lett. 15:1861–1868. 2018.PubMed/NCBI
|
|
27
|
Yang L, Xu L, Wang Q, Wang M and An G:
Dysregulation of long non-coding RNA profiles in human colorectal
cancer and its association with overall survival. Oncol Lett.
12:4068–4074. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang J, Feng S, Su W, Bai S, Xiao L, Wang
L, Thomas DG, Lin J, Reddy RM, Carrott PW, et al: Overexpression of
FAM83H-AS1 indicates poor patient survival and knockdown impairs
cell proliferation and invasion via MET/EGFR signaling in lung
cancer. Sci Rep. 7:428192017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arnes L, Liu Z, Wang J, Maurer C,
Sagalovskiy I, Sanchez-Martin M, Bommakanti N, Garofalo DC,
Balderes DA, Sussel L, et al: Comprehensive characterisation of
compartment-specific long non-coding RNAs associated with
pancreatic ductal adenocarcinoma. Gut. 68:499–511. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang F, Wang Y, Sun P, Wang ZQ, Wang DS,
Zhang DS, Wang FH, Fu JH, Xu RH and Li YH: Fibrinogen promotes
malignant biological tumor behavior involving
epithelial-mesenchymal transition via the p-AKT/p-mTOR pathway in
esophageal squamous cell carcinoma. J Cancer Res Clin Oncol.
143:2413–2424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qi F, Liu X, Wu H, Yu X, Wei C, Huang X,
Ji G, Nie F and Wang K: Long noncoding AGAP2-AS1 is activated by
SP1 and promotes cell proliferation and invasion in gastric cancer.
J Hematol Oncol. 10:482017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su C, Chen Z, Luo H, Su Y, Liu W, Cai L,
Wang T, Lei Y and Zhong B: Different patterns of NF-KB and Notch1
signaling contribute to tumor-induced lymphangiogenesis of
esophageal squamous cell carcinoma. J Exp Clin Cancer Res.
30:852011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han C, Fu Y, Zeng N, Yin J and Li Q:
LncRNA FAM83H-AS1 promotes triple-negative breast cancer
progression by regulating the miR-136-5p/metadherin axis. Aging
(Albany NY). 12:3594–3616. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang HZ, Yin YF, Wan WJ, Xia D, Wang R
and Shen XM: Up-regulation of microRNA-136 induces apoptosis and
radiosensitivity of esophageal squamous cell carcinoma cells by
inhibiting the expression of MUC1. Exp Mol Pathol. 110:1042782019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gong YB and Zou YF: Clinical significance
of lncRNA FAM83H-AS1 in ovarian cancer. Eur Rev Med Pharmacol Sci.
23:4656–4662. 2019.PubMed/NCBI
|