Introduction
Mesenchymal stem cells are pluripotent cells that
possess the potential to differentiate into myocytic, osteocytic,
chondrocytic and adipocytic cells (1–4). Several
types of sarcoma are considered to be induced by differentiation
defects in mesenchymal stem cells (5). Normal differentiation is accompanied by
growth arrest, whereas sarcoma cells retain their proliferative
capacity via the suppression of terminal differentiation (6). Thus, the induction of terminal
differentiation appears to be an attractive therapeutic strategy
for sarcomas. Differentiation therapies would be expected to
suppress tumor proliferation and avoid the morbidity induced by
current chemotherapies.
Liposarcoma is one of the most common sarcomas in
adults (7). Myxoid liposarcoma
(MLS), characterized by the chimeric oncoproteins translocated in
liposarcoma (TLS)-CCAAT/enhancer-binding protein homologous protein
(CHOP) in most cases or Ewing's sarcoma-CHOP in rare cases, is a
major subtype of liposarcoma (8,9). TLS is
also known as fused in sarcoma, and CHOP has the alternative names
DNA-damage-inducible transcript 3 and growth arrest- and DNA
damage-inducible gene 153. Previous studies have shown that the
antitumor compound trabectedin (also termed ET-743) promotes the
adipocytic differentiation of MLS (10,11).
Furthermore, in our previous study, it was demonstrated that the
knockdown of TLS-CHOP or its downstream molecule proteoglycan 4
promoted adipogenesis in an MLS-derived cell line under adipogenic
conditions (12). However, the
degree of adipocytic differentiation induced in these previous
studies appeared to be insufficient to provide comprehensive
recovery from MLS. Therefore, further studies are needed to
establish an effective strategy for the differentiation therapy of
MLS.
Megakaryoblastic leukemia 1 (MKL1), also known as
myocardin-related transcription factor-A, is a transcriptional
coactivator affecting various biological mechanisms, including
epithelial-mesenchymal transition (13), epidermal cell fate decisions
(14), skeletal myogenic
differentiation (15), circadian
rhythm (16) and plasticity of the
nervous system (17,18). Moreover, MKL1 is involved in the
regulation of the expression of approximately one thousand genes
(16). Previous studies have
revealed that the knockdown of MKL1 in mouse preadipocyte cell
lines drives adipocytic differentiation (19,20).
Thus, the knockdown of MKL1 in MLS cells is also expected to
stimulate adipogenesis. Conversely, MKL1 was initially identified
as part of the fusion protein RNA-binding motif protein-15
(RBM15)-MKL1 in acute megakaryoblastic leukemia (21,22).
Notably, RBM15-MKL1 is thought to play an important role in
oncogenesis. Thus, the aberrant control of MKL1 expression may have
oncogenic effects in other types of cells.
In the present study, the effects of MKL1 knockdown
on the differentiation and growth of MLS cells were examined. The
results may suggest possibilities for the development of a novel
differentiation therapy targeting MKL1.
Materials and methods
Cell line
Human MLS-derived 1955/91 cells, as previously
described (12), were maintained in
Dulbecco's modified Eagle's medium (DMEM; D5796; Sigma-Aldrich;
Merck KGaA) containing 10% fetal bovine serum (FBS; Biowest;
http://www.biowest.net) at 37°C in a 5%
CO2 environment. The cells were provided by Professor
Masahiko Kuroda (Tokyo Medical University).
Cell quantification was performed as previously
described (23). For each
experiment, cell numbers were calculated from four independent
counts using a hemocytometer.
Small interfering RNA (siRNA)
transfection and adipocytic differentiation assay
For siRNA transfection, cells were transfected with
siRNA (20 nM final concentration) using Lipofectamine RNAiMAX
Transfection Reagent and OPTI-MEM I Reduced Serum Medium (both
Thermo Fisher Scientific, Inc.) as previously described (12). TLS-CHOP and negative control
siRNAs were as previously described (24), and the target sequence of the
MKL1 siRNA was 5′-CATGGAGCTGGTGGAGAAGAA-3′, as previously
designed by Varney et al (25).
For the adipocytic differentiation assay, a
MilliporeSigma Chemicon Adipogenesis Assay Kit (ECM950; Thermo
Fisher Scientific, Inc.) and calcium (+)-pantothenate (FUJIFILM
Wako Pure Chemical Corporation) were used. One-day preconfluent
1955/91 cells grown in growth medium (DMEM containing 10% FBS) were
transfected with MKL1 siRNA or negative control siRNA. The
next day, the cells reached confluence, and the medium was replaced
with initiating medium (DMEM containing 10% FBS, 100 µM
insulin, 250 µM 3-isobutyl-1-methylxanthine, 1 µM
dexamethasone and 8.5 µM calcium (+)-pantothenate). After 4
days, the medium was changed to maintenance medium (initiating
medium without 3-isobutyl-1-methylxanthine) and the cells were
incubated for a further 6 days. These culture media were replaced
with fresh media every second day. The cells were then washed with
phosphate-buffered saline (PBS) twice, incubated in Oil Red O
Solution at room temperature for 15 min, washed three times with
wash solution, and observed using an inverted microscope (ECLIPSE
TS100; Nikon Corporation) and a Microscope Camera Control Unit
(DS-L3; Nikon Corporation).
Western blot analysis
Protein samples were prepared as previously
described (26) and quantified using
the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.).
Samples containing equal amounts of protein (10 µg/lane)
were separated by SDS-PAGE (10% gel) and transferred to Hybond ECL
nitrocellulose membranes (Amersham; GE Healthcare). To confirm
equal sample loading, the membranes were stained with Ponceau S
solution (Sigma-Aldrich; Merck KGaA) for 1–2 min at room
temperature. After blocking with 5% skimmed milk powder (FUJIFILM
Wako Pure Chemical Corporation) in PBS at room temperature for 1 h,
the membranes were probed with specific primary antibodies at room
temperature overnight. The primary antibodies and dilutions used
were as follows: Anti-TLS-CHOP antibody [1:2,500; clone 14;
generated previously (27)]; MKL1
antibody (1:2,000; cat. no. A302-201A; Bethyl Laboratories, Inc.);
peroxisome proliferator-activated receptor (PPAR γ (81B8) rabbit
monoclonal antibody (1:1,000; cat. no. 2443; Cell Signaling
Technology, Inc.); cleaved caspase-3 (Asp175) antibody (1:1,000;
cat. no. 9661; Cell Signaling Technology, Inc.); poly (ADP-ribose)
polymerase (PARP) antibody (1:1,000; cat. no. 9542; Cell Signaling
Technology, Inc.); CCAAT/enhancer-binding protein (C/EBP)β antibody
(H-7) (1:500; cat. no. sc-7962; Santa Cruz Biotechnology, Inc.);
proliferating cell nuclear antigen (PCNA) antibody (FL-261)
(1:5,000; cat. no. sc-7907; Santa Cruz Biotechnology, Inc.);
minichromosome maintenance 2 (MCM2) antibody (E-8) (1:1,000; cat.
no. sc-373702; Santa Cruz Biotechnology, Inc.); Ki67 antibody
(1:1,000; cat. no. sc-23900; Santa Cruz Biotechnology, Inc.);
monoclonal anti-α-tubulin antibody (1:2,500; cat. no. T5168;
Sigma-Aldrich; Merck KGaA); and anti-glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) antibody (1:20,000; cat. no. G8795;
Sigma-Aldrich; Merck KGaA). The membranes were then washed with PBS
three times, incubated in 5% skimmed milk powder in PBS with goat
anti-mouse IgG H&L (ab205719; Abcam) or goat anti-rabbit IgG
H&L (ab205718; Abcam) at room temperature for 1 h, and washed
with PBS three times. The signals were visualized using ECL Prime
Western Blotting Detection Reagent (Amersham; GE Healthcare) and
detected with a Chemiluminescence CCD Imaging System (AE9300
Ez-Capture MG; ATTO Corporation).
Statistical analysis
Groups were compared using one-way analysis of
variance followed by Turkey-Kramer's test. P<0.05 was considered
to indicate a statistically significant difference. Excel 2010
software (Microsoft Corporation), with the add-in software Statcel
3 (OMS Publishing Inc.; http://www.oms-publ.co.jp/), was used to perform the
statistical analysis.
Results
MKL1 knockdown promotes adipocytic
differentiation of MLS cells
Our previous study demonstrated that the human
MLS-derived cell line 1955/91 can be induced to differentiate into
adipocytes by the knockdown of certain MLS
sarcomagenesis-associated proteins (12). Thus, 1955/91 cells were used in the
present study to examine whether MKL1 knockdown stimulates the
adipocytic differentiation of MLS cells. One day before the cells
reached confluence, the 1955/91 cells cultured in growth medium
were transfected with MKL1-specific siRNA or negative
control siRNA; the day of transfection was arbitrarily set as day
0. On the next day (day 1), the cells appeared to be confluent as
expected, and adipogenic stimulation of the cells was initiated by
the addition of adipogenic agents in accordance with the adipocytic
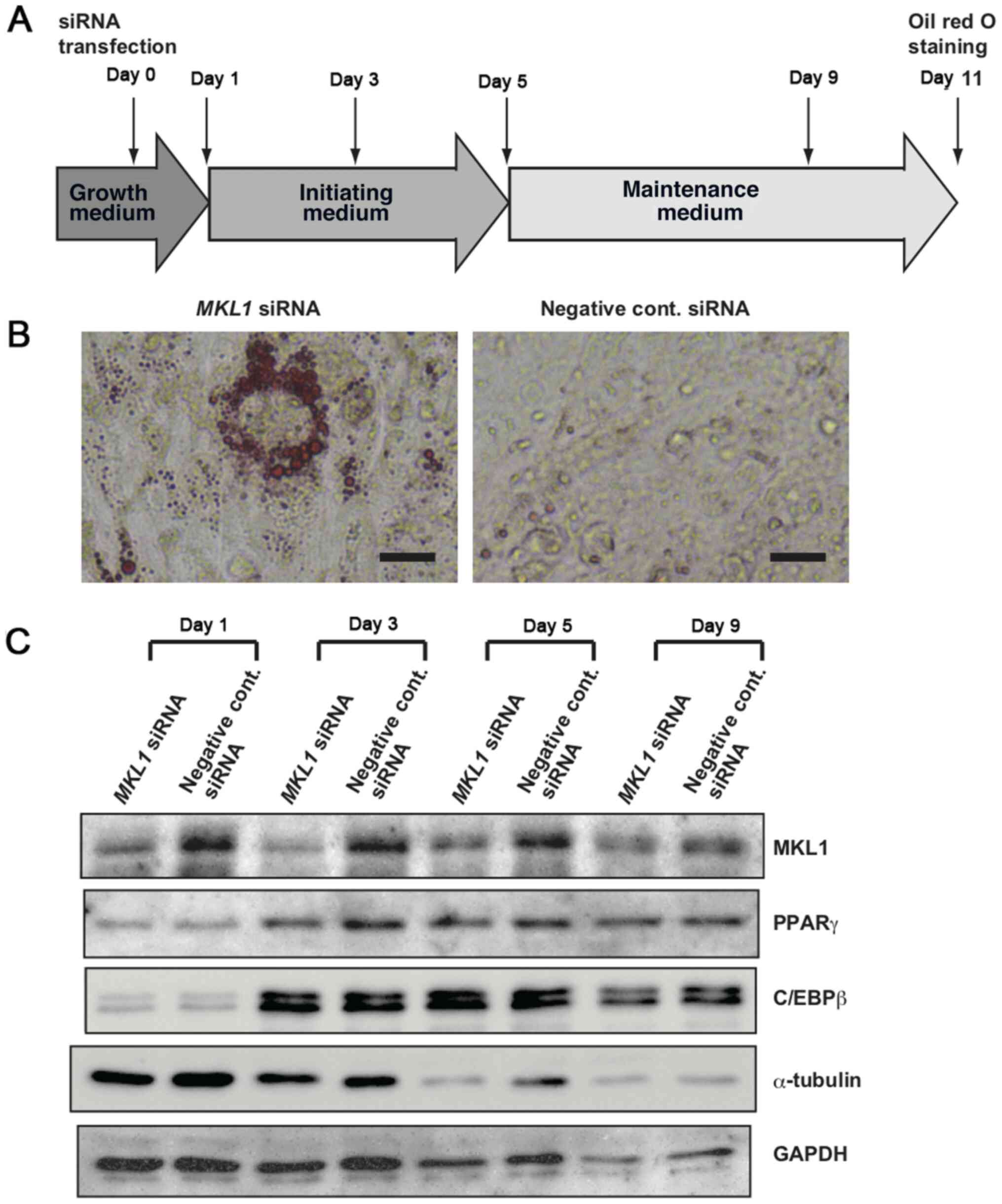
differentiation protocol illustrated in Fig. 1A. On day 11, the accumulation of
lipid droplets in the cells was evaluated by Oil Red O staining,
which stains neutral lipids. Oil Red O-positive cells were observed
in the MKL1 siRNA-transfected cells, although the positive
cells were sporadically distributed (Fig. 1B). However, most of the cells
transfected with negative control siRNA did not exhibit any Oil Red
O staining (Fig. 1B). Western blot
analysis confirmed that MKL1 siRNA markedly decreased MKL1
expression during adipocytic differentiation (Fig. 1C). Previous studies have shown that
the expression of PPARγ promotes the adipogenesis of preadipocyte
cells (28,29), and that the knockdown of MKL1
increases PPARγ expression, as well as the transcriptional activity
of PPARγ target genes (19,20). Notably, PPARγ expression was observed
to be induced by adipogenic agents during the adipocytic
differentiation process in the present study (Fig. 1C). However, the induced levels of
PPARγ in the MKL1-knockdown cells were lower, not higher, than
those in the control cells on day 3. Furthermore, C/EBPβ expression
was examined in the present study (Fig.
1C). C/EBPβ plays multiple essential roles during adipogenesis
(30). C/EBPβ expression was also
induced by adipogenic agents, with no marked difference observed in
C/EBPβ expression levels between the MKL1-knockdown cells and
control cells. GAPDH and α-tubulin expression levels were also
examined as controls. Interestingly, a remarkable reduction in the
α-tubulin level was observed in the MKL1-knockdown cells on day 5
when compared with the control cells (Fig. 1C).
Effect of TLS-CHOP on MKL1
expression
Previous studies have reported that one of the
oncogenic functions of the MLS-specific fusion oncoprotein,
TLS-CHOP, is the inhibition of adipocytic differentiation (31,32).
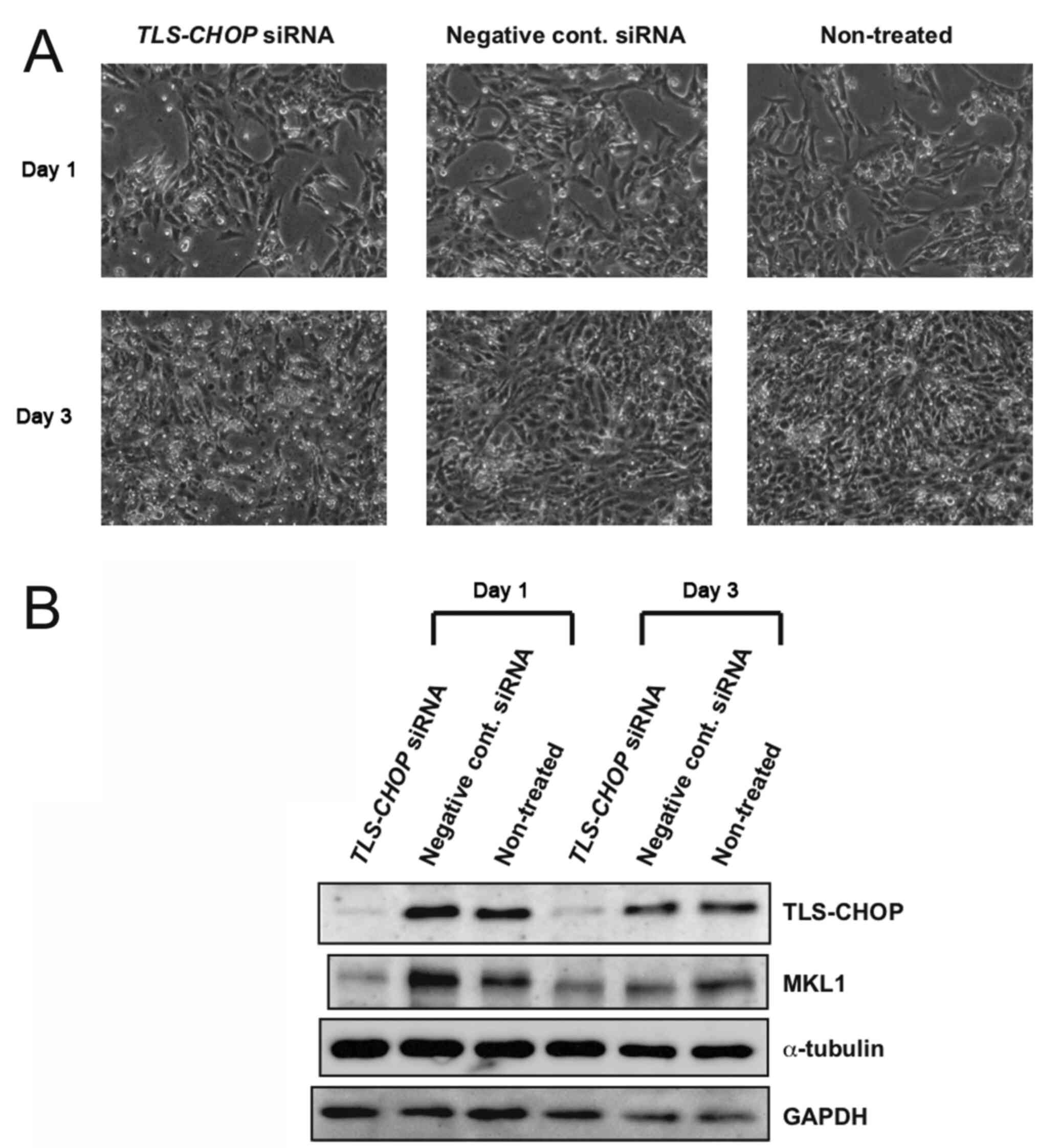
This prompted an investigation of whether MKL1 expression is
induced by TLS-CHOP. The MLS-derived 1955/91 cells at ~20%
confluence were transfected with TLS-CHOP siRNA or negative
control siRNA. As previously reported, the knockdown of TLS-CHOP by
specific siRNA inhibited the growth of MLS cells (Fig. 2A) (24). The cells were harvested on days 1 and
3 post-transfection and protein samples prepared from the cells
were examined by western blotting. Although MKL1 expression was
inhibited by TLS-CHOP knockdown on day 1 after siRNA transfection,
no notable reduction in MKL1 expression was observed in the
TLS-CHOP-knockdown cells when compared with the control cells on
day 3 after siRNA transfection (Fig.
2B). Thus, TLS-CHOP may have limited effects on MKL1 expression
in MLS cells.
MKL1 knockdown reduces the
proliferation of MLS cells
In the adipocytic differentiation assay illustrated
schematically in Fig. 1, cells
transfected with MKL1 siRNA and those transfected with
negative control siRNA appeared to reach confluence on day 1 after
transfection. However, it was suspected that the densities of these
‘confluent’ cell cultures moderately differed. Thus, the effect of
MKL1 knockdown on the proliferation of MLS-derived 1955/91 cells
was examined. As shown in Fig. 3A,
MKL1 siRNA reduced the growth of MLS cells under normal
growth conditions. Using western blot analysis, MKL1 knockdown in
MKL1 siRNA-transfected cells was confirmed (Fig. 3B). On day 3 post siRNA transfection,
TLS-CHOP expression appeared to be marginally decreased in the
MKL1-knockdown cells compared with the control cells (Fig. 3B), suggesting that MKL1 knockdown
failed to affect TLS-CHOP expression. Furthermore, MKL1 knockdown
did not induce PPARγ and C/EBPβ expression (Fig. 3B).
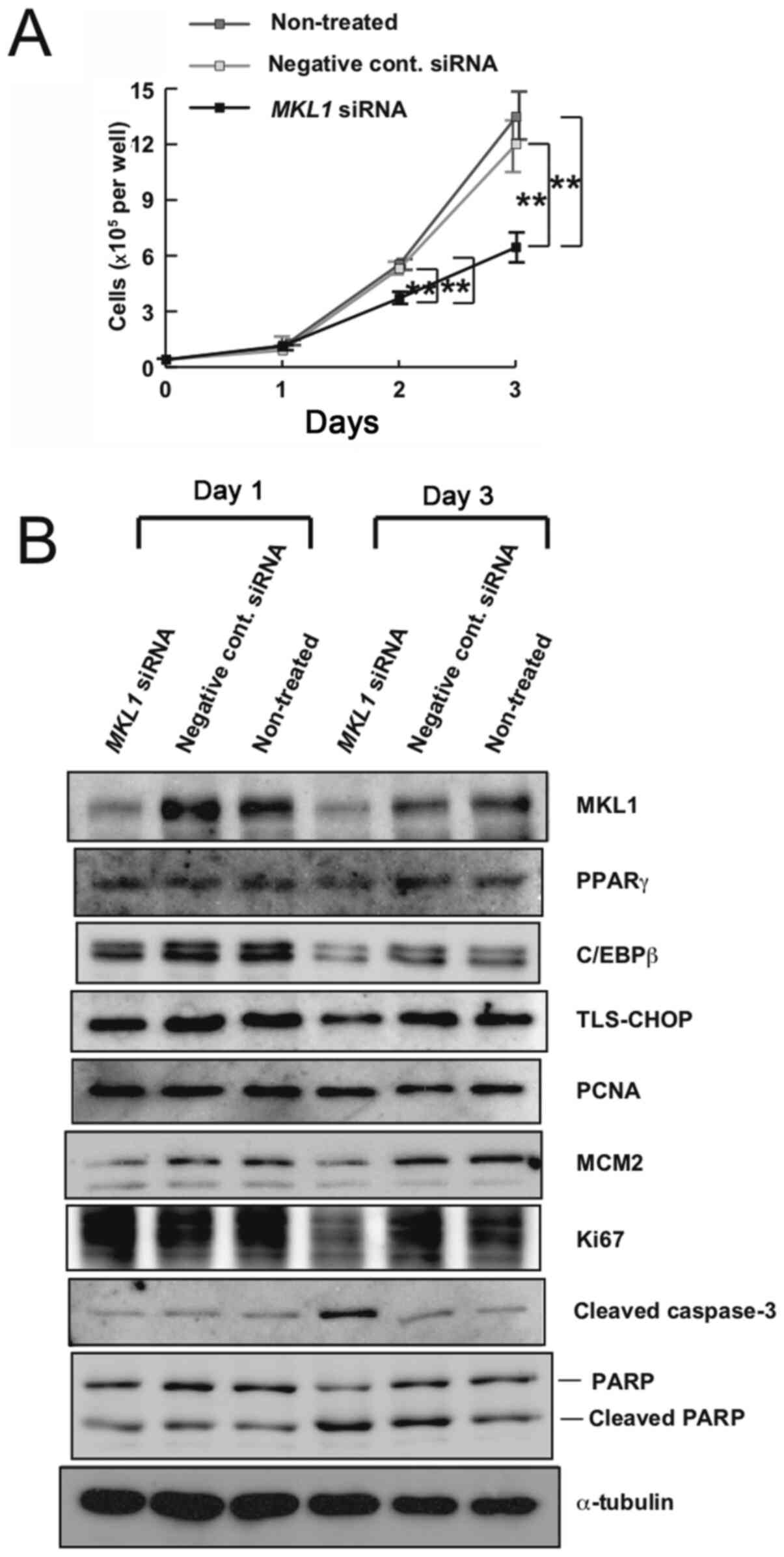 | Figure 3.Effect of MKL1 knockdown on the
growth of 1955/91 cells. (A) Growth curves of 1955/91 cells. After
siRNA transfection (day 0), the cells in 12-well culture plates
were quantified at several time points. Bars represent standard
deviation. **P<0.01. (B) Western blot analysis of MKL1, PPARγ,
C/EBPβ, TLS-CHOP, PCNA, MCM2, Ki67, cleaved caspase-3, PARP and
cleaved PARP in 1955/91 cells on days 1 and 3 post siRNA
transfection. α-tubulin is shown as a loading control. Results
shown are representative of three independent experiments. MKL1,
megakaryoblastic leukemia 1; siRNA, small interfering RNA; PPARγ,
peroxisome proliferator-activated receptor γ; C/EBPβ,
CCAAT/enhancer-binding protein β; TLS-CHOP, translocated in
liposarcoma-CCAAT/enhancer-binding protein homologous protein;
PCNA, proliferating cell nuclear antigen; MCM2, minichromosome
maintenance 2; PARP, poly (ADP-ribose) polymerase. |
Additionally, the expression levels of several cell
proliferation markers, namely PCNA, MCM2 and Ki67, were explored
(Fig. 3B). PCNA levels were not
reduced in the MKL1-knockdown cells compared with the control
cells. However, MKL1 knockdown suppressed the expression of MCM2
and Ki67.
Furthermore, the expression of some apoptosis
markers, namely cleaved caspase-3 and cleaved PARP, were
investigated (Fig. 3B).
Interestingly, the presence of cleaved caspase-3 and cleaved PARP
signals was detected even in control cells, suggesting that
apoptotic pathways may be innately induced to a certain extent in
MLS-derived 1955/91 cells. Although notable differences in the
levels of cleaved PARP between the MKL1-knockdown and control cells
were not observed, cleaved caspase-3 levels were increased in the
MKL1-knockdown cells compared with the control cells on day 3 after
siRNA transfection (Fig. 3B).
Discussion
The present study demonstrated that the knockdown of
MKL1 promoted the adipocytic differentiation of MLS cells. This
result suggests that MKL1 may be a promising target for
differentiation therapy in MLS. However, adipogenesis occurred
sporadically under the experimental conditions used in the present
study. This could be attributed to MKL1 being insufficiently
knocked down, and thus failing to induce the complete
differentiation of MLS cells. Therefore, the selection of more
effective siRNA target sequences in the MKL1 gene may be
crucial. Conversely, MKL1 knockdown was observed to reduce the
proliferation of MLS cells under normal growth conditions. In
vitro adipogenesis requires non-proliferating progenitors to be
induced by confluent contact inhibition before the cells are
exposed to adipogenic agents (33,34).
Hence, MKL1 knockdown may have opposing effects on the induction of
adipocytic differentiation. Thus, although MKL1 is an attractive
target molecule for the development of a differentiation therapy
for MLS, further investigations into the molecular functions of
MKL1 are required.
To elucidate the cause of the reduced proliferation
of MKL1-knockdown MLS cells observed under normal growth
conditions, the expression levels of the proliferation markers
MCM2, Ki67 and PCNA were examined. It was observed that MCM2 and
Ki67 expression levels were reduced in MKL1-knockdown cells.
However, PCNA expression levels did not show a marked difference
between the MKL1-knockdown and control cells. The family of MCM
proteins, including MCM2, are key molecules for the regulation of
DNA replication. MCM proteins are stably expressed throughout the
cell cycle. Ki67 is expressed in all proliferating cells, and its
expression level fluctuates during the cell cycle. However, the
function of Ki67 during the cell cycle is unclear. PCNA is
expressed in proliferating cells and its expression increases
markedly during the S phase. PCNA has important roles in DNA
replication, DNA repair and cell cycle control. Owing to its role
in DNA repair, PCNA is a less specific proliferation marker. PCNA
expression may be detected not only in actively proliferating cells
but also in DNA-damaged cells (35).
Thus, the reduced proliferation of MKL1-knockdown cells may be
explained by the reduced expression of MCM2 and Ki67. Additionally,
the observation that PCNA protein expression was not reduced in the
MKL1-knockdown cells could imply the existence of the cells
undergoing DNA repair. The expression levels of apoptotic markers,
namely cleaved caspase-3 and cleaved PARP, were also examined in
the present study. Activated PARP promotes DNA repair, whereas
cleaved caspase-3 cleaves and inactivates PARP and induces
apoptosis (36). Three days after
MKL1 siRNA transfection, it was observed that cleaved
caspase-3 levels were increased in MKL1-knockdown cells. This
suggests that MKL1 knockdown may promote apoptotic pathways in MLS
cells. However, both cleaved caspase-3 and cleaved PARP were
detected in all the cells examined, suggesting that apoptotic
pathways may be activated to a certain extent in the cells. In MLS
cells, the TLS-CHOP fusion oncoprotein comprises the N-terminal
half of TLS fused to the full-length CHOP protein (24). CHOP is normally expressed at
extremely low levels but is strongly induced by endoplasmic
reticulum stress, and it cleaves and activates caspase-3 (37). Thus, the typically expressed TLS-CHOP
may consistently activate caspase-3 to a certain degree in the
MLS-derived cell line. Nevertheless, DNA damage and the promotion
of apoptotic pathways appear to be involved in the reduced
proliferation of MKL1-knockdown MLS cells. However, further studies
are required to determine the relevant details.
In a previous study, Nobusue et al (19) demonstrated that the loss of MKL1
induces PPARγ expression sufficiently to drive the adipocytic
differentiation of mouse preadipocyte cells. However, although MKL1
knockdown was required in addition to treatment with adipogenic
agents to promote the adipogenesis of MLS cells under the
experimental conditions of the present study, no further induction
of PPARγ expression was observed in MKL1-knockdown cells compared
with control cells at each time point during the adipogenesis
induction process. These results suggest that MKL1 knockdown does
not further increase the induction of PPARγ expression by
adipogenic agents, at least in MLS cells. Adipocytic
differentiation is regulated by a molecular cascade involving
numerous transcription factors. C/EBPβ plays a central role in the
cascade and induces the expression of two dominant transcription
factors, C/EBPα and PPARγ, for terminal adipocytic differentiation
(30). In the present study, C/EBPβ
expression was induced only by adipogenic agents, and MKL1
knockdown failed to demonstrate an additional inductive effect on
C/EBPβ during the adipocytic differentiation process. Thus, we
hypothesize that MKL1 knockdown may stimulate another novel and
critical mechanism associated with adipocytic differentiation in
MLS cells. In this case, MKL1 would become increasingly important
for the promotion and inhibition of adipogenesis in MLS cells.
In the adipocytic differentiation assay, a marked
reduction in the α-tubulin level in MKL1-knockdown cells was
observed on day 5 when compared with that in control cells. The
biosynthetic rate for cytoskeletal proteins, including α-tubulin,
has been reported to be greatly reduced during adipocytic
differentiation (38). Thus, the
reduced level of α-tubulin in MKL1-knockdown cells on day 5 may
suggest the progression of adipocytic differentiation. Conversely,
it was observed that α-tubulin expression was reduced even in the
negative control cells on day 9. The biosynthetic alteration of
cytoskeletal proteins occurs at a very early stage during
adipocytic differentiation (38),
and the induction of PPARγ and C/EBPβ in both MKL1-knockdown cells
and control cells was observed. Thus, we consider that the process
of adipocytic differentiation also progressed to a certain extent
in control cells. Furthermore, the expression level of GAPDH was
reduced in both MKL1-knockdown and control cells on day 9. Previous
studies have shown that the expression of GAPDH increases during
adipocytic differentiation (39,40). By
contrast, the adipogenesis inhibitor berberine decreases GAPDH
expression during adipocytic differentiation (40). Thus, some factors that inhibit
adipogenesis in MLS cells may decrease GAPDH expression. Several
previous studies have suggested suitable reference genes for
quantitative real-time polymerase chain reaction during adipocytic
differentiation (39–41). However, to the best of our knowledge,
appropriate loading control proteins for western blot analysis
during adipocytic differentiation have not yet been identified.
The MLS-specific fusion oncoprotein TLS-CHOP is
considered an abnormal transcription factor associated with
sarcomagenesis, tumor maintenance and the inhibition of
adipogenesis (31,32,42–44).
Thus, we hypothesized that TLS-CHOP would induce MKL1 expression
and consequently suppress adipocytic differentiation in MLS cells.
However, the western blotting results suggest that TLS-CHOP has
limited effects on MKL1 expression in MLS cells, at least, under
the experimental conditions used in the present study.
Additionally, MKL1 knockdown exhibited almost no effect on TLS-CHOP
expression. Thus, the inhibition of adipogenesis by TLS-CHOP
appears to have little impact on MKL1 expression. By contrast, a
previous study reported that the downregulation of TLS-CHOP
expression does not increase PPARγ expression, but PPARγ agonists
enhance adipocytic differentiation via the downregulation of
TLS-CHOP in endogenous mesenchymal stem cells in a mouse model of
MLS (11). Based on these results,
TLS-CHOP and MKL1 may inhibit adipogenesis by different mechanisms.
Furthermore, TLS-CHOP may repress some downstream genes of PPARγ.
However, the results of the present study suggest that the
mechanisms by which TLS-CHOP and MKL1 inhibit the adipocytic
differentiation of MLS are complex.
Acknowledgements
The authors of the present study would like to thank
Professor Masahiko Kuroda (Tokyo Medical University) for providing
the MLS-derived 1955/91 cells.
Funding
The present study was supported by a Grant-in-Aid
for Scientific Research (C) from the Japan Society for the
Promotion of Science (grant no. 17K08768).
Availability of data and materials
The datasets shown and/or analyzed in the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YK, KY, KO and YM designed the study. YK, KY and KO
performed the experiments. YK, KY, KO and FS analyzed the data. KO
and YM drafted the initial manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
He BC, Chen L, Zuo GW, Zhang W, Bi Y,
Huang J, Wang Y, Jiang W, Luo Q, Shi Q, et al: Synergistic
antitumor effect of the activated PPARgamma and retinoid receptors
on human osteosarcoma. Clin Cancer Res. 16:2235–2245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aubin JE: Regulation of osteoblast
formation and function. Rev Endocr Metab Disord. 2:81–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luu HH, Song WX, Luo X, Manning D, Luo J,
Deng ZL, Sharff KA, Montag AG, Haydon RC and He TC: Distinct roles
of bone morphogenetic proteins in osteogenic differentiation of
mesenchymal stem cells. J Orthop Res. 25:665–677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deng ZL, Sharff KA, Tang N, Song WX, Luo
J, Luo X, Chen J, Bennett E, Reid R, Manning D, et al: Regulation
of osteogenic differentiation during skeletal development. Front
Biosci. 13:2001–2021. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luther G, Rames R, Wagner ER, Zhu G, Luo
Q, Bi Y, Kim SH, Gao JL, Huang E, Yang K, et al: Molecular basis of
differentiation therapy for soft tissue sarcomas. Trends Cancer
Res. 6:69–90. 2010.PubMed/NCBI
|
|
7
|
Fritchie KJ, Goldblum JR, Tubbs RR, Sun Y,
Carver P, Billings SD and Rubin BP: The expanded histologic
spectrum of myxoid liposarcoma with an emphasis on newly described
patterns: Implications for diagnosis on small biopsy specimens. Am
J Clin Pathol. 137:229–239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dal Cin P, Sciot R, Panagopoulos I, Aman
P, Samson I, Mandahl N, Mitelman F, Van den Berghe H and Fletcher
CD: Additional evidence of a variant translocation t(12;22) with
EWS-CHOP fusion in myxoid liposarcoma: Clinicopathologic features.
J Pathol. 182:437–441. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loubignac F, Bourtoul C and Chapel F:
Myxoid liposarcoma: A rare soft tissue tumor with a misleading
benign appearance. World J Surg Oncol. 7:422009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Forni C, Minuzzo M, Virdis E, Tamborini E,
Simone M, Tavecchio M, Erba E, Grosso F, Gronchi A, Aman P, et al:
Trabectedin (ET-743) promotes differentiation in myxoid liposarcoma
tumors. Mol Cancer Ther. 8:449–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Charytonowicz E, Terry M, Coakley K, Telis
L, Remotti F, Cordon-Cardo C, Taub RN and Matushansky I: PPARγ
agonists enhance ET-743-induced adipogenic differentiation in a
transgenic mouse model of myxoid round cell liposarcoma. J Clin
Invest. 122:886–898. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oikawa K, Mizusaki A, Takanashi M, Ozaki
T, Sato F, Kuroda M and Muragaki Y: PRG4 expression in myxoid
liposarcoma maintains tumor cell growth through suppression of an
antitumor cytokine IL-24. Biochem Biophys Res Commun. 485:209–214.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gomez EW, Chen QK, Gjorevski N and Nelson
CM: Tissue geometry patterns epithelial-mesenchymal transition via
intercellular mechanotransduction. J Cell Biochem. 110:44–51.
2010.PubMed/NCBI
|
|
14
|
Connelly JT, Gautrot JE, Trappmann B, Tan
DW, Donati G, Huck WT and Watt FM: Actin and serum response factor
transduce physical cues from the microenvironment to regulate
epidermal stem cell fate decisions. Nat Cell Biol. 12:711–718.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Selvaraj A and Prywes R: Megakaryoblastic
leukemia-1/2, a transcriptional co-activator of serum response
factor, is required for skeletal myogenic differentiation. J Biol
Chem. 278:41977–41987. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esnault C, Stewart A, Gualdrini F, East P,
Horswell S, Matthews N and Treisman R: Rho-actin signaling to the
MRTF coactivators dominates the immediate transcriptional response
to serum in fibroblasts. Genes Dev. 28:943–958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kalita K, Kharebava G, Zheng JJ and Hetman
M: Role of megakaryoblastic acute leukemia-1 in ERK1/2-dependent
stimulation of serum response factor-driven transcription by BDNF
or increased synaptic activity. J Neurosci. 26:10020–10032. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kalita K, Kuzniewska B and Kaczmarek L:
MKLs: Co-factors of serum response factor (SRF) in neuronal
responses. Int J Biochem Cell Biol. 44:1444–1447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nobusue H, Onishi N, Shimizu T, Sugihara
E, Oki Y, Sumikawa Y, Chiyoda T, Akashi K, Saya H and Kano K:
Regulation of MKL1 via actin cytoskeleton dynamics drives adipocyte
differentiation. Nat commun. 5:33682014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosenwald M, Efthymiou V, Opitz L and
Wolfrum C: SRF and MKL1 independently inhibit brown adipogenesis.
PLoS One. 12:e01706432017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma Z, Morris SW, Valentine V, Li M,
Herbrick JA, Cui X, Bouman D, Li Y, Mehta PK, Nizetic D, et al:
Fusion of two novel genes, RBM15 and MKL1, in the t(1;22)(p13;q13)
of acute megakaryoblastic leukemia. Nat Genet. 28:220–221. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mercher T, Coniat MB, Monni R, Mauchauffe
M, Nguyen Khac F, Gressin L, Mugneret F, Leblanc T, Dastugue N,
Berger R and Bernard OA: Involvement of a human gene related to the
drosophila spen gene in the recurrent t(1;22) translocation of
acute megakaryocytic leukemia. Proc Natl Acad Sci USA.
98:5776–5779. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oikawa K, Ohbayashi T, Kiyono T, Nishi H,
Isaka K, Umezawa A, Kuroda M and Mukai K: Expression of a novel
human gene, human wings apart-like (hWAPL), is associated with
cervical carcinogenesis and tumor progression. Cancer Res.
64:3545–3549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oikawa K, Tanaka M, Itoh S, Takanashi M,
Ozaki T, Muragaki Y and Kuroda M: A novel oncogenic pathway by
TLS-CHOP involving repression of MDA-7/IL-24 expression. Br J
Cancer. 106:1976–1979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Varney SD, Betts CB, Zheng R, Wu L, Hinz
B, Zhou J and Van De Water L: Hic-5 is required for myofibroblast
differentiation by regulating mechanically dependent MRTF-A nuclear
accumulation. J Cell Sci. 129:774–787. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oikawa K, Ohbayashi T, Mimura J,
Fujii-Kuriyama Y, Teshima S, Rokutan K, Mukai K and Kuroda M:
Dioxin stimulates synthesis and secretion of IgE-dependent
histamine-releasing factor. Biochem Biophys Res Commun.
290:984–987. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oikawa K, Ishida T, Imamura T, Yoshida K,
Takanashi M, Hattori H, Ishikawa A, Fujita K, Yamamoto K,
Matsubayashi J, et al: Generation of the novel monoclonal antibody
against TLS/EWS-CHOP chimeric oncoproteins that is applicable to
one of the most sensitive assays for myxoid and round cell
liposarcomas. Am J Surg Pathol. 30:351–356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tontonoz P, Hu E, Graves RA, Budavari AI
and Spiegelman BM: mPPAR gamma 2: tissue-specific regulator of an
adipocyte enhancer. Genes Dev. 8:1224–1234. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chawla A, Schwarz EJ, Dimaculangan DD and
Lazar MA: Peroxisome proliferator-activated receptor (PPAR) gamma:
Adipose-predominant expression and induction early in adipocyte
differentiation. Endocrinology. 135:798–800. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo L, Li X and Tang QQ: Transcriptional
regulation of adipocyte differentiation: A central role for
CCAAT/enhancer-binding protein (C/EBP) β. J Biol Chem. 290:755–761.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Batchvarova N, Wang XZ and Ron D:
Inhibition of adipogenesis by the stressinduced protein CHOP
(Gadd153). EMBO J. 14:4654–4661. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuroda M, Ishida T, Takanashi M, Satoh M,
Machinami R and Watanabe T: Oncogenic transformation and inhibition
of adipocytic conversion of preadipocytes by TLS/FUS-CHOP type II
chimeric protein. Am J Pathol. 151:735–744. 1997.PubMed/NCBI
|
|
33
|
Rosen ED and Spiegelman BM: Molecular
regulation of adipogenesis. Annu Rev Cell Dev Biol. 16:145–171.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gregoire FM, Smas CM and Sul HS:
Understanding adipocyte differentiation. Physiol Rev. 78:783–809.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Juríková M, Danihel Ľ, Polák Š and Varga
I: Ki67, PCNA, and MCM proteins: Markers of proliferation in the
diagnosis of breast cancer. Acta Histochem. 118:544–552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Malhotra U, Zaidi AH, Kosovec JE, Kasi PM,
Komatsu Y, Rotoloni CL, Davison JM, Irvin CR, Hoppo T, Nason KS, et
al: Prognostic value and targeted inhibition of survivin expression
in esophageal adenocarcinoma and cancer-adjacent squamous
epithelium. PLoS One. 8:e783432013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Spiegelman BM and Farmer SR: Decreases in
tubulin and actin gene expression prior to morphological
differentiation of 3T3 adipocytes. Cell. 29:53–60. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arsenijevic T, Gre´goire F, Delforge V,
Delporte C and Perret J: Murine 3T3-L1 adipocyte cell
differentiation model: Validated reference genes for qPCR gene
expression analysis. PLoS One. 7:e375172012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang J, Tang H, Zhang Y, Deng R, Shao L,
Liu Y, Li F, Wang X and Zhou L: Identification of suitable
reference genes for quantitative RT-PCR during 3T3-L1 adipocyte
differentiation. Int J Mol Med. 33:1209–1218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gentile AM, Lhamyani S, Coín-Aragüez L,
Oliva-Olivera W, Zayed H, Vega-Rioja A, Monteseirin J, Romero-Zerbo
SY, Tinahones FJ, Bermúdez-Silva FJ and Bekay RE: RPL13A and EEF1A1
are suitable reference genes for qPCR during adipocyte
differentiation of vascular stromal cells from patients with
different BMI and HOMA-IR. PLoS One. 11:e01570022016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kuroda M, Wang X, Sok J, Yin Y, Chung P,
Giannotti JW, Jacobs KA, Fitz LJ, Murtha-Riel P, Turner KJ and Ron
D: Induction of a secreted protein by the myxoid liposarcoma
oncogene. Proc Natl Acad Sci USA. 96:5025–5030. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Perez-Mancera PA, Bermejo-Rodrıguez C,
Sanchez-Martin M, Abollo-Jimenez F, Pintado B and Sanchez-Garcıa I:
FUS-DDIT3 prevents the development of adipocytic precursors in
liposarcoma by repressing PPARgamma and C/EBPalpha and activating
eIF4E. PLoS One. 3:e25692008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Andersson MK, Goransson M, Olofsson A,
Andersson C and Aman P: Nuclear expression of FLT1 and its ligand
PGF in FUS-DDIT3 carrying myxoid liposarcomas suggests the
existence of an intracrine signaling loop. BMC Cancer. 10:2492010.
View Article : Google Scholar : PubMed/NCBI
|