Introduction
Prostate cancer is a prevalent type of cancer in
older men and is one of the leading causes of cancer-associated
mortality among men worldwide (1).
Hormone therapy, or androgen ablation therapy, is considered the
first-line clinical treatment for patients with metastatic disease;
however, in the majority of cases, the effect of androgen
deprivation is temporary (2).
Subsequently, these patients ultimately become insensitive to
androgen ablation, and hormone-refractory prostate cancer (HRPC)
develops after 18–24 months of conventional treatment (3). HRPC is a common type of malignant,
metastatic tumor with poor prognosis, common recurrence and a high
fatality rate (4). Therefore, the
inevitable progression of HRPC presents the urgent need for novel
therapeutic approaches (5).
The cancer stem cell theory proposes that tumor
tissue harbors its own cancer stem cells and regards these cells as
the key to regeneration, metastasis and recurrence (6). A number of reports have demonstrated
that tumor malignancy and oncogenesis are associated with the
expression of stemness genes, such as sex determining region Y-box
2 (SOX2), OCT-4, Kruppel like factor 4 and c-Myc (7,8).
Malignant tumors are associated with characteristics of tumor-like
stem cells (9). The high expression
of stemness proteins causes malignant tumor cells to be insensitive
to drugs and enhances drug resistance (10). Resistant malignant cancers exhibit
abnormal expression of stemness genes, which are capable of
inducing self-renewal and differentiation programs (11). The expression of stemness genes is
closely associated with cancer stem cell resistance (12,13). The
transcription factor SOX2, a stem-like cell marker is associated
with the expression of numerous gene products involved in cell
proliferation, growth, differentiation and apoptosis (14). SOX2 maintains the self-renewal
activity of undifferentiated embryonic stem cells and is expressed
abnormally in a variety of tumors (15). In squamous cell carcinoma, SOX2
serves an important role in regulating tumor growth and maintaining
stem cell qualities (16). SOX2 can
improve the self-renewal ability of lung cancer stem cells, which
upregulate SOX2 (17). Furthermore,
in neuroblastoma stem cells, SOX2 promotes biological processes,
such as proliferation, clonal formation and tumorigenesis, and
exerts an inhibitory effect on differentiation (18). Downregulation of SOX2 can arrest the
cell cycle and promote apoptosis to inhibit the growth of gastric
cancer cells (19). Therefore,
identifying the key regulatory targets for stemness genes should be
a key focus for future research in order to decrease cancer stem
cell resistance.
Chloride voltage-gated channels (CLCs) form
permeable cell membrane channels for chloride ions or other anions
and can be classified into seven subtypes: CLC-1, CLC-2, CLC-3,
CLC-4, CLC-5, CLC-6 and CLC-7 (20).
CLC expression has a direct influence on the proliferation,
migration and cell cycle of cancer cells (21). CLC-1 regulates the metastasis and
invasion of gastric cancer cells (22), and CLC-3 is relevant to the
proliferation of the nasopharyngeal carcinoma CNE-2Z cell line
(23). CLC-3 affects the cell cycle
by altering the expression levels of cyclin D1, which, in turn,
regulates CLC-3 via CDK4/6 phosphorylation (24). However, CLC proteins are both ion
transport channels and signaling proteins in vivo (25,26). The
present study demonstrated that CLCs serve important roles in cell
proliferation, cell cycle and apoptosis. It was hypothesized that
CLCs are also involved in regulating stemness genes, and the
results may provide novel insights into identifying therapeutic
targets.
The present study aimed to investigate the ability
of the CLC inhibitor DIDS to inhibit the cell cycle, and to
investigate the associations between CLC-3 and SOX2 in the HRPC
DU145 cell line. The effects of CLC-3- and SOX2-knockdown on cell
cycle progression, and the associations between CLC-3 and SOX2 were
observed. In addition, the present study aimed to identify
potential targets for HRPC therapy from the perspective of CLC
proteins and SOX2.
Materials and methods
Cell culture
The DU145 and PC-3 cell lines were purchased from
the Shanghai Institute of Biochemistry. HFF-1 was purchased from
the American Type Culture Collection. DU145 and HFF-1 cells were
maintained in DMEM (Thermo Fisher Scientific, Inc.) and PC-3 cells
were maintained in RPMI 1640 (Thermo Fisher Scientific, Inc.). The
media were supplemented with 10% FBS (Thermo Fisher Scientific,
Inc.) and 1% penicillin/streptomycin (Thermo Fisher Scientific,
Inc.) in a 5% CO2 incubator at 37°C.
Cell viability assays
For MTT assays, 5,000 cells/well were seeded in
96-well culture plates, incubated for 24 h and treated with 0, 25,
50, 100, 200 or 400 µM CLC inhibitor DIDS. After 48 h, the cells
were incubated with 0.5 mg/ml MTT and the plates were cultured for
4 h at 37°C. The culture medium was removed and formazan crystals
were dissolved in 100 µl DMSO. The absorbance of each well was
determined at 490 nm using an iMark microplate reader (Bio-Rad
Laboratories, Inc.). Each condition was evaluated in six wells, and
all experiments were repeated at least three times.
Flow cytometry
Cell apoptosis was analyzed using an Annexin V/PI
kit (BD Biosciences) and flow cytometry. DU145 cells were collected
by trypsinization, washed twice in PBS, fixed with 500 µl binding
buffer, and incubated with 5 µl Annexin V-EGFP and the DNA binding
dye PI (50 mg/ml) for 5–15 min at 37°C in the dark. Finally, the
cells were analyzed using an Elite flow cytometer (CytoFLEX S;
Beckman Coulter, Inc.) with a peak fluorescence gate to distinguish
aggregates after 1 h. Next, the cell cycle distribution was
assessed. Briefly, following treatment with 100 µM DIDS for 48 h,
the cells were collected by trypsinization, washed in PBS and fixed
in 70% ethanol for 30 min at 4°C. After being washed with PBS, the
cells were incubated with PI (50 mg/ml) and RNase (1.0 mg/ml) for
30 min at 37°C in the dark. Finally, the cells were washed, and red
fluorescence was analyzed using an Elite flow cytometer (CytoFLEX
S, Beckman Coulter, Inc.) with a peak fluorescence gate to
distinguish aggregates. All data were analyzed by ModFit LT 4.1
(Verity Software House, Inc.).
Reverse transcription-quantitative
PCR
Total RNA in DU145 cells was extracted using
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and
reverse transcribed to cDNA using oligo(dT), reverse transcriptase,
5X reaction buffer and dNTPs (all Thermo Fisher Scientific, Inc.).
cDNA and oligo(dT) were incubated at 65°C for 5 min and then
reverse transcriptase, 5X reaction buffer and dNTPs were added and
incubated for 60 min at 42°C. The reaction was terminated by
heating at 70°C for 10 min. Relative gene expression was analyzed
using the 2−∆∆Cq method (27) with GAPDH as the internal control
gene, and was quantified using SuperReal PreMix SYBR Green
(FP204-02; Tiangen Biotech Co., Ltd.) on an Applied Biosystems 7500
Fast Real-Time PCR system (Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: Initial denaturation at
95°C for 5 min, followed by 40 cycles at 95°C for 30 sec, 58°C for
30 sec and 72°C for 40 sec, then a final extension at 72°C for 10
min. The gene-specific primer pairs were as follows: CLC-1 forward,
5′-CAGCATCTGTGCC-3′ and reverse, 5′-GTGCTTAGCAAGAAACTGGC-3′; CLC-2
forward, 5′-AGACAATCCCTACACCCTTCAA-3′ and reverse,
5′-TGTCGGTAGAACACCTTGTCAC-3′; CLC-3 forward,
5′-CAAUGGAUUUCCUGUCAUATT−3′ and reverse,
5′-UAUGACAGGAAAUCCAUUGTA−3; CLC-4 forward, 5′-GCGTCTCATCGGGTTTGC−3′
and reverse, 5′-TTGCTCACAATGCCCTCTTTG−3′; CLC-5 forward,
5′-CTGTGCCACTGCTTCAAC−3′ and reverse, 5′-CTGAGGGCAAATCCCACTAA-3′;
CLC-6 forward, 5′-GTCGCGCAAGACTGTAACCA-3′ and reverse,
5′-CGGCGAAATTCCATACCTG-3′; CLC-7 forward,
5′-GAAAGGAAGGGCCAATGATC-3′ and reverse, 5′-CAGGAACTGATYCCAGAAGG-3′;
and GAPDH forward, 5′-CTCATGACCACAGTCCATGC-3′ and reverse,
5′-CACATTGGGGGTAGGAACAC-3′.
Antibodies and western blotting
The cells were lysed in M-PER mammalian protein
extraction reagent (Thermo Fisher Scientific, Inc.), Protein
concentrations were measured using a BCA protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Equal amounts (10 µg) of protein
were resolved by 10% SDS-PAGE and transferred to a PVDF membrane
(Merck KGaA). After blocking with 5% skimmed dried milk at room
temperature for 1 h, the PVDF membrane was probed with the
indicated primary antibody at 4°C overnight. The following
antibodies were used in the present study: Tubulin (1:10,000; cat.
no. T5168; Sigma-Aldrich; Merck KGaA), CLC-3 (1:1,000; cat. no.
ab28736; Abcam), cyclin D1 (1:1,000; cat. no. 2922; Cell Signaling
Technology, Inc.), P27 (1:1,000; cat. no. 3686; Cell Signaling
Technology, Inc.) and SOX2 (1:1,000; cat. no. 3579; Cell Signaling
Technology, Inc.). The next day, the PVDF membrane was blocked with
HRP-conjugated secondary antibody (goat anti-rabbit; cat. no.
ARG65351; or goat anti-mouse; cat. no. ARG65350; 1:5,000; Arigo
Biolaboratories Corp.) at room temperature for 1 h and then results
were detected by electrochemiluminescence using Immobilon Western
Chemiluminescent HRP Substrate (cat. no. WBKLS0500; Merck
Millipore). ImageJ software (v.1.48; National Institutes of Health)
was used to semi-quantify the bands.
Cell transfection
CLC-3 and SOX2 small interfering RNAs (siRNAs) were
purchased from Guangzhou RiboBio Co., Ltd. The siRNA target
sequences (5′-3′) were as follows: siCLC-3-#1, CGACGCAAGTCCACGAAAT;
siCLC-3-#2, GCAGGCATTGGAGTATATT; siCLC-3-#3, CAATAGAAAGTGCCAGGAA;
siSOX2-#1, CCAAGACGCTCATGAAGAA; siSOX2-#2, CCACCTACAGCATGTCCTA; and
siSOX2-#3, GCTCGCAGACCTACATGAA. Cells were incubated with RPMI 1640
containing 2.5% FBS (without penicillin/streptomycin). siRNAs (25
nM) were transfected using Lipofectamine RNAiMAX (13778-150; Thermo
Fisher Scientific, Inc.) with OPTI-MEM (31985070; Thermo Fisher
Scientific, Inc.) for 24 h. All experiments were performed
according to the manufacturers' protocols.
Immunofluorescence staining
A total of 1×105 cells/well were seeded
in 6-well culture plates and exposed to different treatments. After
fixing with 4% (v/v) paraformaldehyde in room temperature for 30
min, they were permeabilized with 0.1% (v/v) Triton X-100/PBS and
blocked with 5% (v/v) BSA (Guangzhou Weijia Technology Co., Ltd.)
or PBS for 10 min. Primary antibodies (SOX2; cat. no. 3579;
dilution, 1:500 in 0.1% Triton X-100; Cell Signaling Technology,
Inc.) were used and the cells placed in a wet box at 4°C overnight.
Secondary antibodies (cat. no. 31460; FITC-conjugated goat
anti-rabbit IgG; dilution, 1:150 in PBS; Thermo Fisher Scientific,
Inc.) were used and placed in a wet box at room temperature for 1 h
to detect and visualize SOX2. Hoechst 33342 (DAPI; Molecular
Probes; Thermo Fisher Scientific, Inc.) was used to label DNA.
Images were captured using fluorescence microscopy (Olympus
Corporation).
Coimmunoprecipitation
Cells were collected by trypsinization, washed three
times with PBS, and lysed in 400 µl NETN lysis buffer [100 mM NaCl,
0.5 mM EDTA, 20 mM Tris-Cl (pH 8.0) and 0.5% (v/v) nonidet P-40]
for 20 min. Protein A/G beads (C600694; Sangon Biotech Co., Ltd.)
that had been washed in ice-cold PBS were mixed with the target
antibody and cell lysates under rotation for 2–4 h at 4°C. The
mixture was centrifuged at 4,000 × g for 1 min at 4°C, exposed to a
protease inhibitor and PMSF for 24 h, and washed with NETN2.
Western blotting, performed as aforementioned, was used to detect
the presence of specific proteins: SOX2 (cat. no. 3579; Cell
Signaling Technology, Inc.) or CLC-3 (cat. no. ab28736; Abcam).
Gene chip technology
Total RNA was extracted using TRIzol reagent (Thermo
Fisher Scientific, Inc.) and further purified using a Qiagen RNeasy
Mini kit (74106) according to the manufacturer's protocol. RNA
quality was assessed by 1% formaldehyde agarose gel electrophoresis
and quantitated spectrophotometrically. For the microarray
analysis, 0.1 µg total RNA was used to synthesize double-stranded,
biotin-tagged cDNA using a MessageAmp™ Premier RNA Amplification
kit (cat. no. 4385821; Thermo Fisher Scientific, Inc.) according to
the manufacturers' protocols. The resulting biotin-tagged cRNA was
fragmented to 35–200 bases according to the Affymetrix protocols.
Hybridization was performed at 45°C with rotation for 16 h
(Affymetrix Gene Chip Hybridization Oven 640; Thermo Fisher
Scientific, Inc.). The Gene Chip arrays were washed, stained
(streptavidin-phycoerythrin) on an Affymetrix Fluidics Station 450
(Thermo Fisher Scientific, Inc.) and scanned on a Gene Chip Scanner
3000 (Thermo Fisher Scientific, Inc.).
Normalization
The hybridization data were analyzed using Gene Chip
Operating software (GCOS v 1.4; Thermo Fisher Scientific, Inc.).
The scanned images were first assessed visually and subsequently
analyzed to generate raw data files (CEL files) using the default
settings in GCOS 1.4. An invariant set normalization procedure was
performed to normalize the different arrays using a DNA-chip
analyzer (dChip).
Analysis of two factors
In a comparison analysis, the present study applied
a two-class unpaired method in the Significant Analysis of
Microarray software (v.4.01; Standford University.) to identify
significantly differentially expressed genes between the test and
control groups. Stemness-associated genes were determined to be
significantly differentially expressed with a selection threshold
of false discovery rate (FDR) <5% and fold-change >2.0 in the
SAM output.
Tissue specificity analysis
Microarray data were preliminarily screened with a
selection threshold of FDR <5% using a multiclass method in SAM.
The resulting data were then screened for a >2-fold-change in
expression in the tissue of interest compared with other tissues
and a Wilcoxon Rank-Sum test significance level of 0.05
(P<0.05).
In vivo studies
Mice were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. The resuspension solution of
DU145 cancer cells (3×106 cells/mouse) in PBS was
inoculated subcutaneously into the hind-flank of 4-week-old male
BALB/c-nu/nu mice weighing 18–20 g. A total of 20 mice were used.
Animals were housed at 26°C, with a relative humidity of 50–60% and
a room wind speed of 0.1–0.2 m/sec. Fresh air was used for
ventilation at a frequency of 15 times/h in a barrier environment.
The animal room used fluorescent light, with a 12-h light and 12-h
dark cycle. The animals were allowed free intake of water and food.
Tumors developed after 1 week, and the mice were randomly divided
into three groups. The treatment group was intravenously injected
with 45 mg/kg DIDS (D3514-250 MG; Sigma-Aldrich; Merck KGaA) in a
total volume of 100 µl. Intraperitoneal injection with DMSO in a
total volume of 100 µl (1.3 or 4%) was performed for the control
group. Animals were monitored and tumor length and width were
measured every other day, and tumor volume was calculated according
to the following formula: Length × width2/2.
Measurements were performed in a manner blinded to group
allocation. All animals were euthanized by cervical dislocation
within 4 weeks after inoculating cancer cells. The animal study was
designed and carried out according to the principles of Sun Yat-sen
University Institutional Animal Care and Use Committee. Animals
were sacrificed due to progressive disease if tumor burden was
>2,500 mm3 according to the Animal Care Guidelines of
Sun Yat-sen University Institutional Animal Care and Use Committee.
All tumors were <2,500 mm3 in size. A total of 10
mice were used in each group and no animals died during the
experiment until the animals were euthanized by cervical
dislocation. Animal health and behavior were monitored every day.
Death was verified by observing the animals until no spontaneous
breathing was noted for 2–3 min and no blinking reflex was
observed.
Statistical analysis
All experiments were repeated at least three times.
The data are presented as the mean ± standard deviation and were
analyzed using SPSS software (v.20.0; IBM Corp.). Differences
between two groups were analyzed using an unpaired Student's
t-test, and differences among multiple groups were analyzed using
one-way ANOVA followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
CLC-3 is a target of cell cycle
regulators in prostate cancer cells
CLCs have been demonstrated to be key factors in the
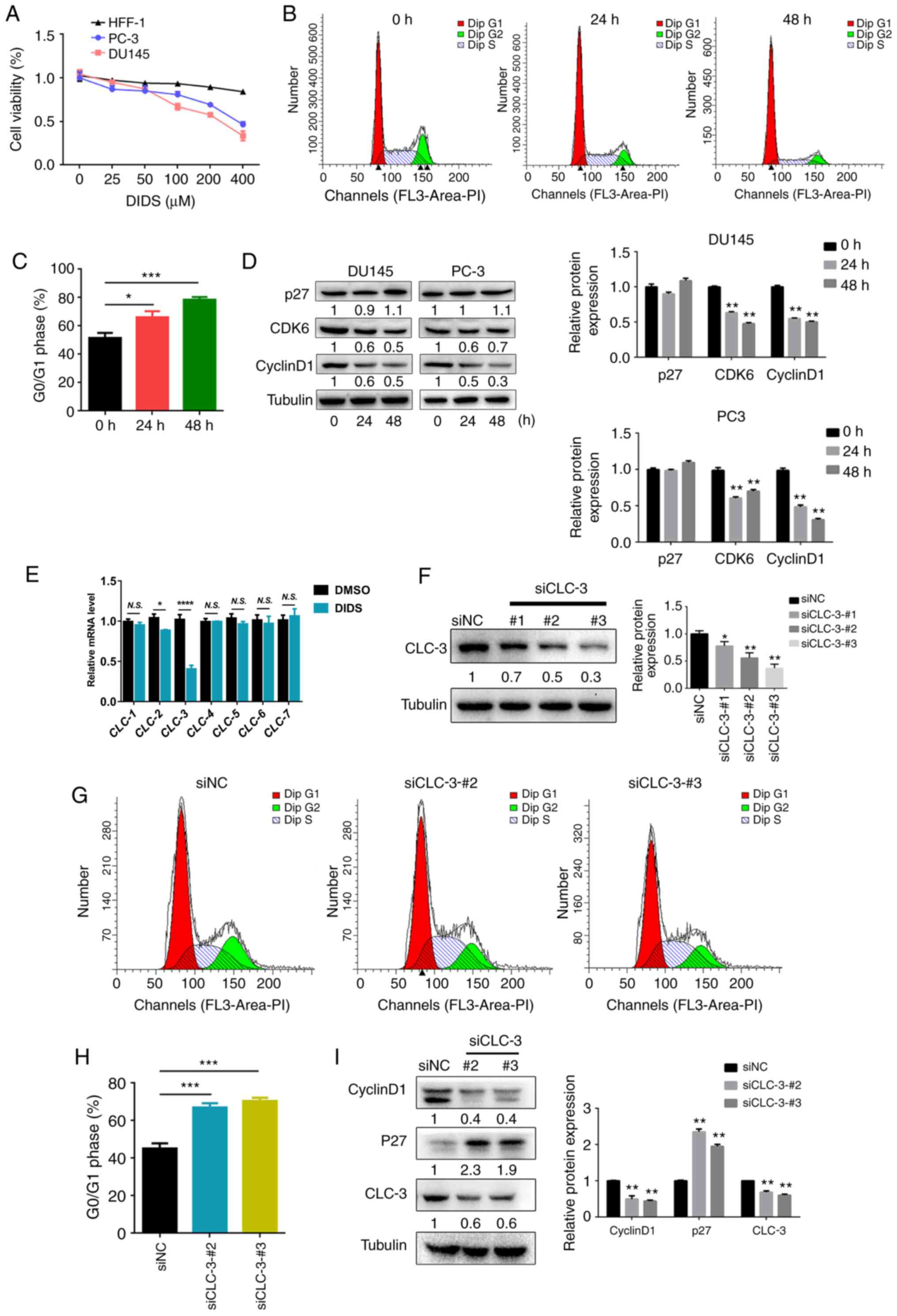
regulation of the cell cycle and cell proliferation (28). DIDS is a classic CLC blocker
(29). The present study first
examined whether DIDS affects the rate of cell viability. DU145,
PC-3 and HFF-1 cells were treated with various concentrations of
DIDS for 48 h, and cell viability was assessed using an MTT assay.
Following treatment with DIDS for 48 h, cell viability was
suppressed in DU145 and PC-3 cells, but DIDS treatment had no
significant effects on HFF-1 cells (Fig.
1A). The results revealed that inhibition of CLCs suppressed
cell viability, suggesting that DIDS may suppress cell
proliferation. In order to determine whether the decline in
viability of DIDS was caused by cell cycle arrest, cell cycle
analysis was performed using flow cytometry. DU145 cells were
treated with DIDS for 24 and 48 h. DIDS induced
G0/G1 phase arrest compared with cells at 0 h
(Fig. 1B and C). Cyclin D1 is an
important regulator of the transition from
G0/G1 phase to S phase (30). P16, P27 and P53 can bind to
cyclin/CDablK (cyclin-dependent kinase) complexes and regulate the
G1-S transition by inhibiting the activity of these
complexes (31). Western blotting of
the cell cycle proteins cyclin D1, P27 and CDK6 demonstrated that
DIDS significantly decreased cyclin D1 and CDK6 protein levels, and
modestly (P>0.05) increased P27 protein levels in DU145 cells
(Fig. 1D). These data suggest that
DIDS blocked cell cycle progression at the
G0/G1 phase, thereby inhibiting DU145 cell
proliferation. In order to investigate the role of specific CLC
subtypes in regulating the cell cycle in prostate cancer cells, the
effects of CLCs on cell cycle progression were evaluated. CLC-3
expression was decreased in DU145 cells following treatment with
DIDS (Fig. 1E). To determine the
role of endogenous CLC-3 in cell cycle regulation, the effects of
CLC-3-knockdown using specific siRNAs on cell cycle progression
were determined. The present study screened three siRNA-liposome
mixtures via western blotting: siCLC-3-#1, siCLC-3-#2 and
siCLC-3-#3. CLC-3 expression levels were most clearly decreased by
siCLC-3-#2 and siCLC-3-#3 (Fig. 1F).
Cell cycle distribution was analyzed via flow cytometry.
CLC-3-knockdown inhibited the progression of cells from
G1 phase to S phase (Fig. 1G
and H). Additionally, western blotting of the cell cycle
proteins cyclin D1 and P27 revealed that CLC-3 knockdown
significantly decreased cyclin D1 protein expression and increased
P27 protein expression in DU145 cells (Fig. 1I). The present study demonstrated
that CLC-3 was involved in cell cycle regulation in DU145 cells,
and that knockdown of this protein arrested cells at
G0/G1 phase.
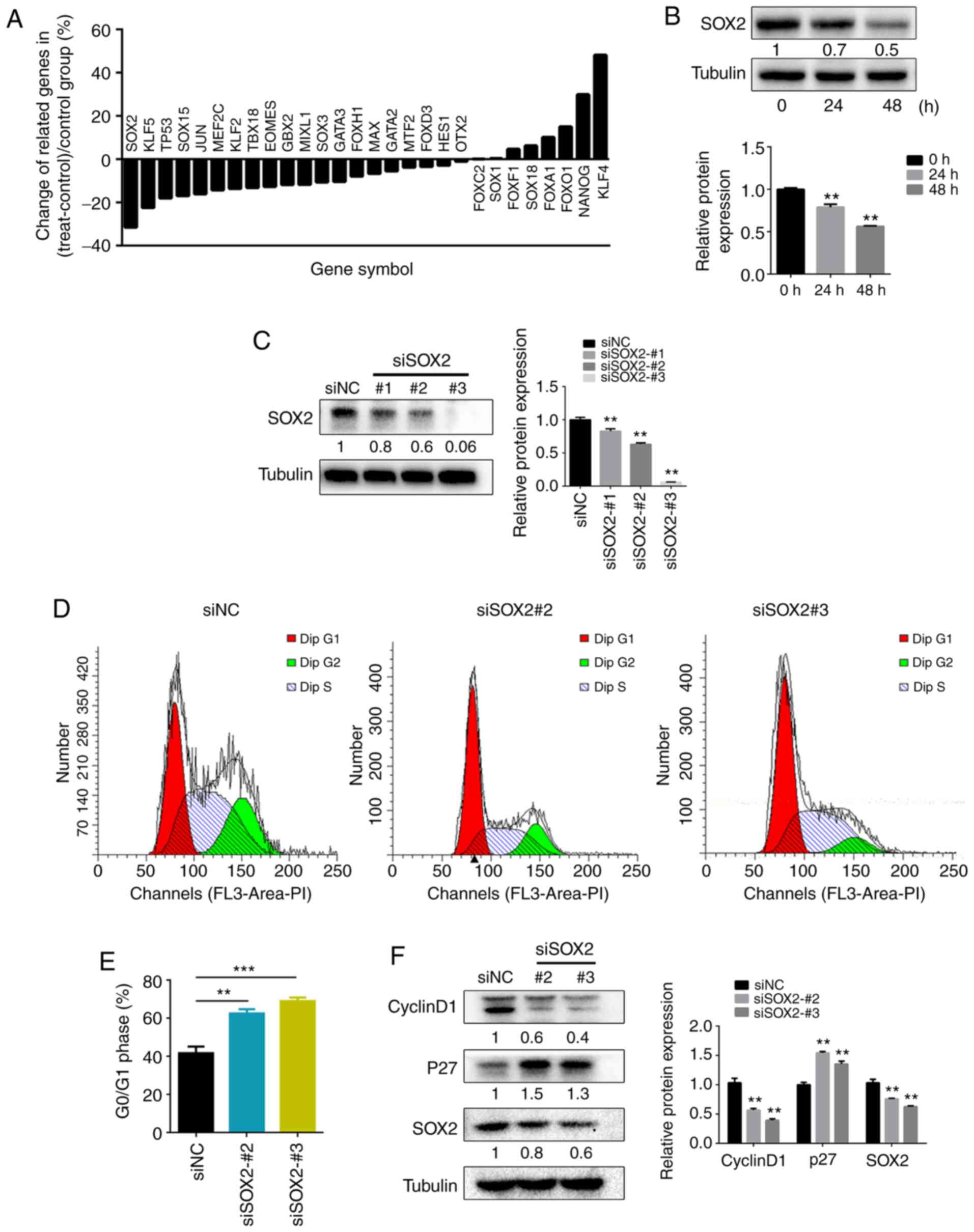
SOX2 arrests DU145 cells at the
G0/G1 phase
In order to investigate the upstream molecular
targets in the effects on cell cycle, DU145 cells were treated with
DIDS, and whole genome expression analysis was performed using a
gene chip in the present study. It was revealed that SOX2
expression was decreased to the greatest extent (Fig. 2A). Subsequently, western blotting was
used to examine the protein expression levels of SOX2 following
DIDS treatment. SOX2 expression was markedly decreased in the DIDS
treatment group compared with cells at 0 h (Fig. 2B). In order to verify the role of
SOX2 in the regulation of the cell cycle, the effects of knockdown
of SOX2 expression with SOX2 siRNA on cell cycle progression were
evaluated. To determine the efficiency of the SOX2 siRNA, the
present study used western blotting to detect SOX2 protein
expression, which was decreased after 48 h of treatment with
SOX2-#1, SOX2-#2 and SOX2-#3 siRNA (Fig.
2C). Since SOX2-#1 did not display a significant interference
efficiency, SOX2-#2 and SOX2-#3 siRNA were used to decrease SOX2
expression and detect cell cycle changes via flow cytometry. The
results indicated that SOX2 siRNA inhibited cell cycle progression
by arresting the cells in the G0/G1 phase
(Fig. 2D and E), which was
consistent with the results that occurred after inhibiting CLCs or
exposing cells to CLC-3 siRNA. Next, western blot analysis of the
cell cycle proteins cyclin D1 and P27 demonstrated that SOX2
knockdown also significantly decreased cyclin D1 protein levels and
increased P27 protein levels in DU145 cells (Fig. 2F). Therefore, the present study
concluded that SOX2 was involved in the cell cycle changes
following inhibition of CLCs. However, the interaction mechanism
between SOX2 and CLCs remains unclear.
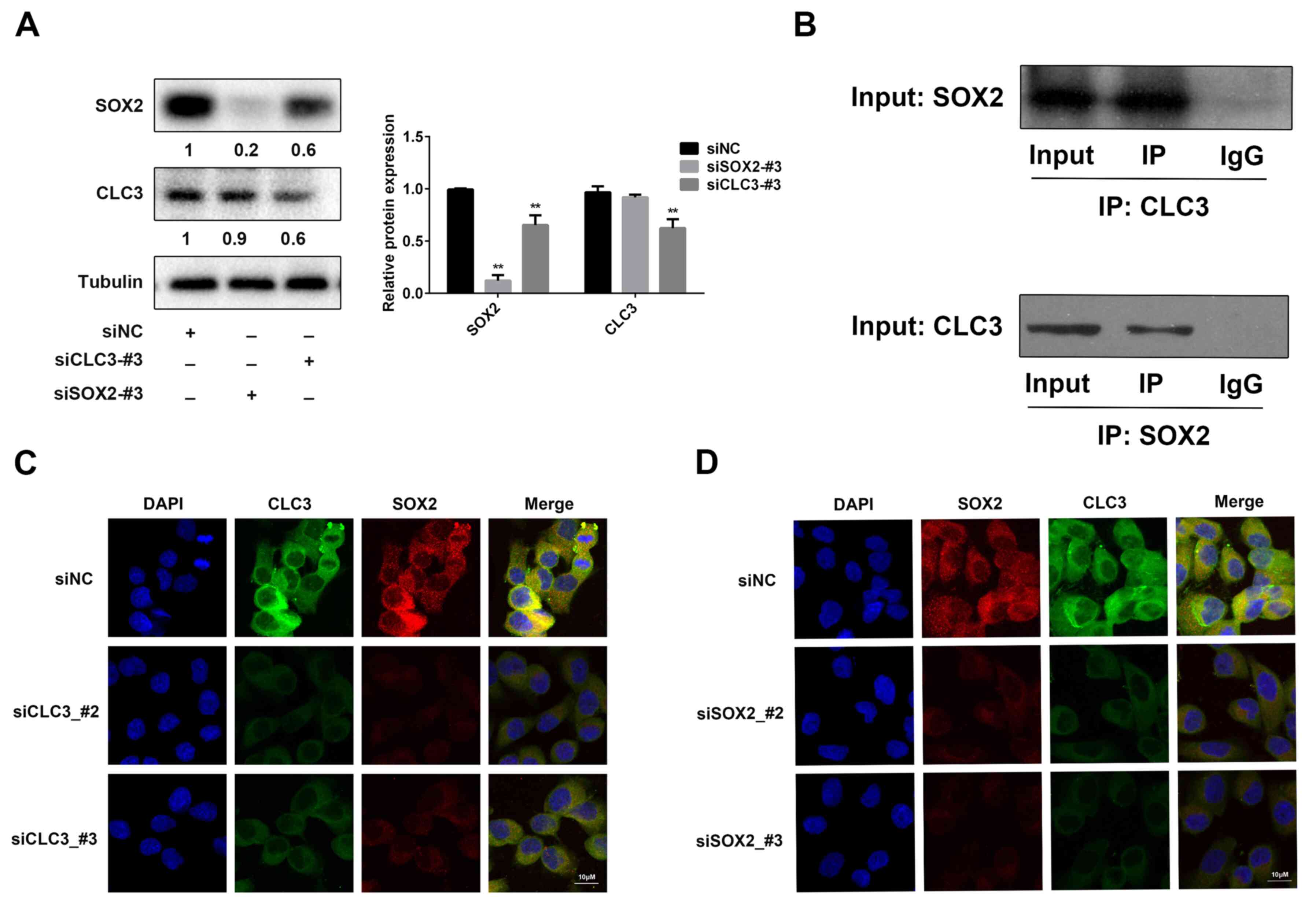
CLC-3 and SOX2 co-regulate the cell
cycle in prostate cancer cells
The results suggested that SOX2 and CLC-3 exhibit
the same cell cycle regulatory behavior. The present study assessed
whether endogenous SOX2 and CLC-3 interact with each other. Western
blot analysis demonstrated the association between SOX2 and CLC-3.
Knockdown of endogenous CLC-3 expression decreased SOX2 expression
(Fig. 3A). Subsequently,
coimmunoprecipitation experiments analyzed the interaction between
CLC-3 and SOX2. SOX2 coimmunoprecipitated CLC-3, and CLC-3
coimmunoprecipitated SOX2 (Fig. 3B).
Next, to further investigate the association between SOX and CLC-3,
the expression of these proteins was knocked down, and the effects
were analyzed using immunofluorescence. The fluorescence intensity
of CLC-3 and SOX2 was decreased in the siCLC-3 groups compared with
the control group (Fig. 3C). The
fluorescence intensity of SOX2 and CLC-3 was decreased in the
siSOX2 groups compared with in the control group (Fig. 3D). Based on these findings, it was
suggested that SOX2 can bind to CLC-3, and that CLC-3 can also
individually bind to SOX2, and co-regulate the cell cycle.
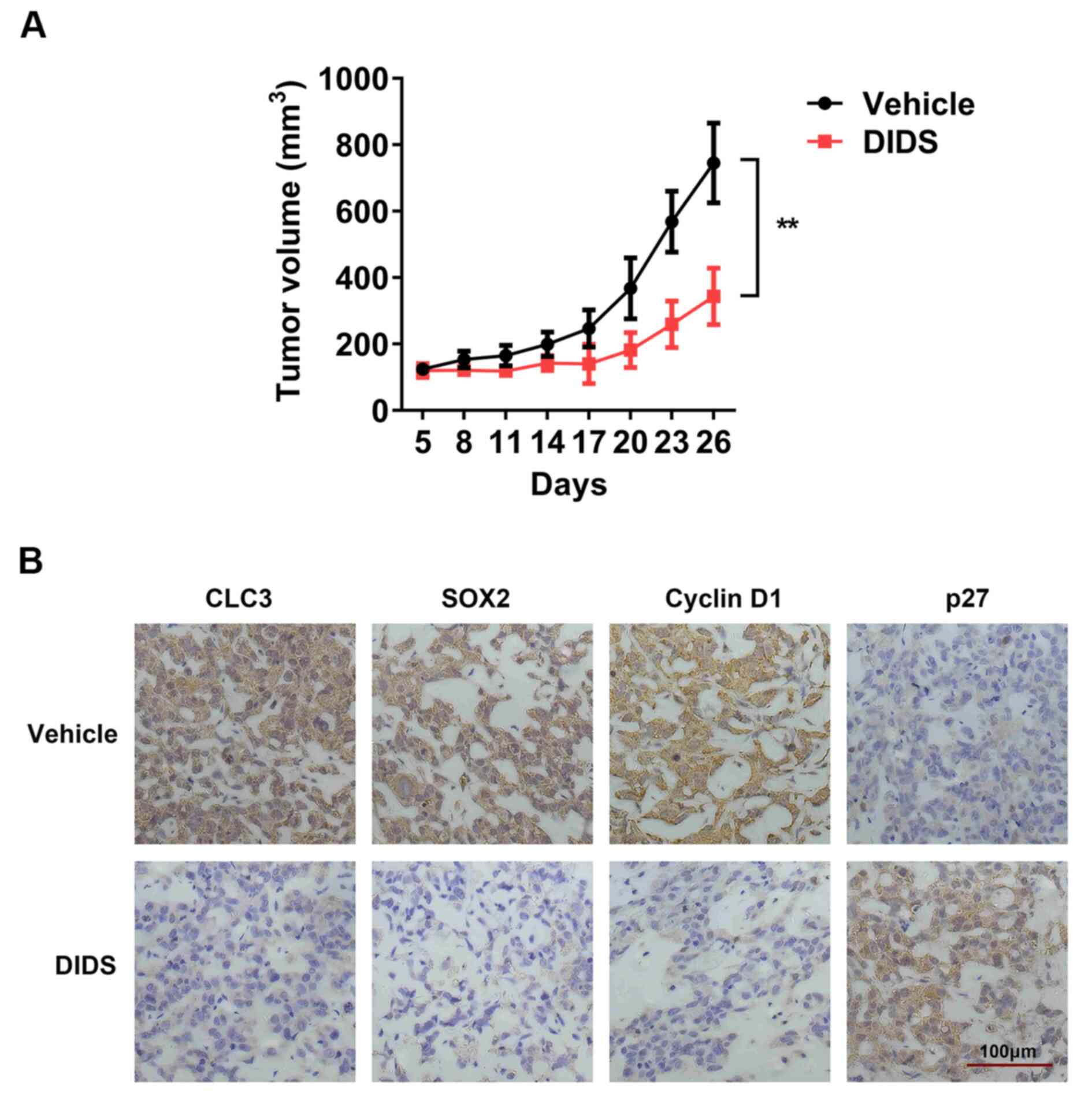
DIDS restricts tumor growth
Given the concerns regarding the DIDS efficacy in
vitro, the present study aimed to evaluate this influence in a
subcutaneous xenograft model. A DU145 subcutaneous xenograft model
was developed in nude mice. The present study performed
subcutaneous injections of DIDS. The tumor volume in the DIDS (45
mg/kg) group remained at ~0.4 cm3, but the tumor volume
was increased to 0.8 cm3 in the control group (Table SI). Tumor growth was markedly
restricted in the DIDS group compared with in the control group
(Fig. 4A; Table SI). In addition, the present study
analyzed CLC-3, SOX2, P27 and cyclin D1 expression in DU145
subcutaneous xenograft tumor sections by immunohistochemistry
staining across DIDS groups. Blue staining indicated the nucleus,
and brown staining indicated the target protein. In the DIDS group,
the expression levels of CLC-3, SOX2 and cyclin D1 were decreased,
whereas P27 expression was increased (Fig. 4B). It was suggested that DIDS blocked
the tumor cell cycle and inhibited tumor growth in vivo.
Discussion
Cancer stem cells are a minor fraction of cancer
cells but can enable tumor heterogeneity and initiate tumor
formation. Accumulating evidence has demonstrated the existence of
cancer stem cells and supports their role in conferring therapeutic
resistance (32). Malignant tumors
are characterized by poor prognosis, increased invasiveness and
high recurrence rates. Tumors are difficult to cure due to the
development of therapeutic resistance (33). Malignant tumor cells exhibit
cancer-like stem cell properties with high expression levels of
stemness genes (34,35). The abnormal expression of CLCs is
closely associated with the occurrence and development of malignant
tumors (36). Studying malignant
tumor resistance and CLCs has become popular in international and
domestic research (37–39). However, there is little proof
regarding the association between CLCs and stemness.
CLCs have various important roles in cellular
functions, ranging between control of cell excitability and
regulation of cell volume (40).
CLC-3 are members of the voltage-gated CLC superfamily and are
upregulated in numerous cancer cells. PCR demonstrated a marked
change in CLC-3 expression compared with other CLC family members
following treatment with DIDS in prostate cancer cells. In the
plasma membrane, CLC-3 functions as a CLC and is associated with
cell proliferation and apoptosis (41). CLC-3 is also located in intracellular
compartments, contributing to cellular acidity, which increases
drug sequestration and leads to chemotherapy drug resistance
(20). The present study suggested
that CLC-3 serves a role in androgen resistance in DU145 cells.
DIDS treatment or transfection with CLC-3 siRNA inhibited cell
proliferation by arresting cells in the G0/G1
phase via the downregulation of cyclin D1 and the upregulation of
P27.
Gene chip analysis demonstrated marked changes in
stemness-associated factors following DIDS treatment and a
significant change in SOX2. Therefore, the present study focused on
SOX2. SOX2 is associated with numerous biological processes,
including cell cycle, migration, DNA damage and apoptosis, and is
essential during mammalian embryogenesis and later in life;
however, abnormal SOX2 expression can be pernicious (42). Several previous studies have
demonstrated that exogenous elevation of SOX2 levels can promote
resistance to clinical treatment (43–45).
Notably, reductions in SOX2 levels have been demonstrated to
significantly decrease cell viability, clonal growth, sphere
formation and tumorigenicity in a number of different types of
malignant cancer (46). Stable
overexpression of SOX2 has been reported to increase growth in the
gastric tumor N87 cell line both in vitro and in vivo
(47), while another study has
demonstrated that SOX2 regulates the cell cycle proteins cyclin D1
and P27, and that overexpression of SOX2 confers a poor prognosis
in terms of malignancy (48). SOX2
can regulate P27 and cyclin D1 to promote the
G0/G1 phase transition in Ewing's sarcoma,
which expresses high levels of SOX2 (49). In the present study, knockdown of
SOX2 arrested the cell cycle at the G0/G1
phase in DU145 cells. It was suggested that SOX2 may be a target of
CLC-3 as it has the same effect as CLC-3 on cell cycle in DU145
cells. Western blotting and immunoprecipitation assays revealed the
association between SOX2 and CLC-3. SOX2 coimmunoprecipitated
CLC-3, and conversely, CLC-3 individually coimmunoprecipitated
SOX2, indicating that there is bi-directional regulation between
SOX2 and CLC-3.
The present study provided novel insights into the
treatment of malignant prostate cancer. To the best of our
knowledge, the present study was the first to demonstrate that the
CLC protein CLC-3 is closely associated with the stemness gene
SOX2, and it was suggested that CLCs could be the potential targets
of stemness genes. Recognizing and focusing on the role of this
association in therapeutic resistance could improve the treatment
options for patients with various types of cancer, particularly
those with refractory tumors, and it will support the development
of novel strategies to more effectively treat some of the deadliest
cancers.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Planning Project of Guangdong Province (grant no.
2014A020211022), and the Science and Technology Planning Project of
Guangzhou Canton (grant no. 201510010074).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC, FW and XL were primarily involved in the
experimental design. JC and FW wrote the manuscript. JC, FW and YH
reviewed, collected and analyzed the data. YL, SY, XC and YH
performed the experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Sun
Yat-sen University Institutional Animal Care and Use Committee and
the Animal Ethical and Welfare Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Summers N, Vanderpuye-Orgle J, Reinhart M,
Gallagher M and Sartor O: Efficacy and safety of post-docetaxel
therapies in metastatic castration-resistant prostate cancer: A
systematic review of the literature. Curr Med Res Opin.
33:1995–2008. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Best CJ, Gillespie JW, Yi YJ, Chandramouli
GV, Perlmutter MA, Gathright Y, Erickson HS, Georgevich L, Tangrea
MA, Duray PH, et al: Molecular alterations in primary prostate
cancer after androgen ablation therapy. Clin Cancer Res.
11:6823–6834. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dicitore A, Grassi ES, Borghi MO, Gelmini
G, Cantone MC, Gaudenzi G, Persani L, Caraglia M and Vitale G:
Antitumor activity of interferon-β1a in hormone refractory prostate
cancer with neuroendocrine differentiation. J Endocrinol Invest.
40:761–770. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katzenwadel A and Wolf P: Androgen
deprivation of prostate cancer: Leading to a therapeutic dead end.
Cancer Lett. 367:12–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boccellino M, Alaia C, Misso G, Cossu AM,
Facchini G, Piscitelli R, Quagliuolo L and Caraglia M: Gene
interference strategies as a new tool for the treatment of prostate
cancer. Endocrine. 49:588–605. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao TT: The progress of cancer stem cells
in gynecology oncology. J Int Obstetrics Gynecol. 2010.
|
|
7
|
Farhana L, Antaki F, Anees MR,
Nangia-Makker P, Judd S, Hadden T, Levi E, Murshed F, Yu Y, Van
Buren E, et al: Role of cancer stem cells in racial disparity in
colorectal cancer. Cancer Med. 5:1268–1278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin F, Lin P, Zhao D, Chen Y, Xiao L, Qin
W, Li D, Chen H, Zhao B, Zou H, et al: Sox2 targets cyclinE, p27
and survivin to regulate androgen-independent human prostate cancer
cell proliferation and apoptosis. Cell Prolif. 45:207–216. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z, Zhang W, Phillips JB, Arora R,
McClellan S, Li J, Kim JH, Sobol RW and Tan M: Immunoregulatory
protein B7-H3 regulates cancer stem cell enrichment and drug
resistance through MVP-mediated MEK activation. Oncogene.
38:88–102. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Najafi M, Farhood B and Mortezaee K:
Cancer stem cells (CSCs) in cancer progression and therapy. J Cell
Physiol. 234:8381–8395. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J, Ding P, Li L, Gu H, Zhang X, Zhang
L, Wang N, Gan L, Wang Q, Zhang W and Hu W: CD59 regulation by SOX2
Is required for epithelial cancer stem cells to evade complement
surveillance. Stem Cell Reports. 8:140–151. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amini S, Fathi F, Mobalegi J,
Sofimajidpour H and Ghadimi T: The expressions of stem cell
markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3,
Dppa4, and Esrrb in bladder, colon, and prostate cancer, and
certain cancer cell lines. Anat Cell Biol. 47:1–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balça-Silva J, Matias D, Dubois LG,
Carneiro B, do Carmo A, Girão H, Ferreira F, Ferrer VP, Chimelli L,
Filho PN, et al: The expression of connexins and SOX2 reflects the
plasticity of glioma stem-like cells. Transl Oncol. 10:555–569.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Basu-Roy U, Seo E, Ramanathapuram L, Rapp
TB, Perry JA, Orkin SH, Mansukhani A and Basilico C: Sox2 maintains
self renewal of tumor-initiating cells in osteosarcomas. Oncogene.
31:2270–2282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boumahdi S, Driessens G, Lapouge G, Rorive
S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E,
et al: SOX2 controls tumour initiation and cancer stem-cell
functions in squamous-cell carcinoma. Nature. 511:246–250. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao D, Pan C, Sun J, Gilbert C,
Drews-Elger K, Azzam DJ, Picon-Ruiz M, Kim M, Ullmer W, El-Ashry D,
et al: VEGF drives cancer-initiating stem cells through
VEGFR-2/Stat3 signaling to upregulate Myc and Sox2. Oncogene.
34:3107–3119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang S, Zheng J, Xiao X, Xu T, Tang W, Zhu
H, Yang L, Zheng S, Dong K, Zhou G and Wang Y: SOX2 promotes
tumorigenicity and inhibits the differentiation of I-type
neuroblastoma cells. Int J Oncol. 46:317–323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsuoka J, Yashiro M, Sakurai K, Kubo N,
Tanaka H, Muguruma K, Sawada T, Ohira M and Hirakawa K: Role of the
stemness factors sox2, oct3/4, and nanog in gastric carcinoma. J
Surg Res. 174:130–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuoka J, Yashiro M, Sakurai K, Kubo N,
Tanaka H, Muguruma K, Sawada T, Ohira M and Hirakawa K: Research
and progress on ClC2 (Review). Mol Med Rep. 16:11–22. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abeyrathne PD, Chami M and Stahlberg H:
Biochemical and biophysical approaches to study the structure and
function of the chloride channel (ClC) family of proteins.
Biochimie 128–129. 154–162. 2016. View Article : Google Scholar
|
|
22
|
Zhao W, Lu M and Zhang Q: Chloride
intracellular channel 1 regulates migration and invasion in gastric
cancer by triggering the ROS-mediated p38 MAPK signaling pathway.
Mol Med Rep. 13:37112016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu B, Mao J, Wang L, Zhu L, Li H, Wang W,
Jin X, Zhu J and Chen L: ClC-3 chloride channels are essential for
cell proliferation and cell cycle progression in nasopharyngeal
carcinoma cells. Acta Biochim Biophys Sin (Shanghai). 42:370–380.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Wang T, Zhao Z and Weinman SA: The
ClC-3 chloride channel promotes acidification of lysosomes in
CHO-K1 and Huh-7 cells. Am J Physiol Cell Physiol. 282:C1483–C1491.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suh KS, Malik M, Shukla A and Yuspa SH:
CLIC4, skin homeostasis and cutaneous cancer: Surprising
connections. Mol Carcinog. 46:599–604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ponsioen B, van Zeijl L, Langeslag M,
Berryman M, Littler D, Jalink K and Moolenaar WH: Spatiotemporal
regulation of chloride intracellular channel protein CLIC4 by RhoA.
Mol Biol Cell. 20:4664–4672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang W, Liu M, Zhu L, Liu S, Luo H, Ma L,
Wang H, Lu R, Sun X, Chen L and Wang L: Functional expression of
chloride channels and their roles in the cell cycle and cell
proliferation in highly differentiated nasopharyngeal carcinoma
cells. Physiol Rep. 2:e121372014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang GL, Wang XR, Lin MJ, He H, Lan XJ and
Guan YY: Deficiency in ClC-3 chloride channels prevents rat aortic
smooth muscle cell proliferation. Circ Res. 91:E28–E32. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee S, Kwon MC, Jang JP, Sohng JK and Jung
HJ: The ginsenoside metabolite compound K inhibits growth,
migration and stemness of glioblastoma cells. Int J Oncol.
51:414–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye D, Luo H, Lai Z, Zou L, Zhu L, Mao J,
Jacob T, Ye W, Wang L and Chen L: ClC-3 chloride channel proteins
regulate the cell cycle by Up-regulating cyclin D1-CDK4/6 through
suppressing p21/p27 expression in nasopharyngeal carcinoma cells.
Sci Rep. 6:302762016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hatano Y, Fukuda S, Hisamatsu K, Hirata A,
Hara A and Tomita H: Multifaceted interpretation of colon cancer
stem cells. Int J Mol Sci. 18:14462017. View Article : Google Scholar
|
|
33
|
Franceschi E, Minichillo S and Brandes AA:
Pharmacotherapy of glioblastoma: Established treatments and
emerging concepts. CNS Drugs. 31:675–684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang H, Zhu L, Zuo W, Luo H, Mao J, Ye D,
Li Y, Liu S, Wei Y, Ye W, et al: The ClC-3 chloride channel protein
is a downstream target of cyclin D1 in nasopharyngeal carcinoma
cells. Int J Biochem Cell Biol. 45:672–683. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tian Y, Guan Y, Jia Y, Meng Q and Yang J:
Chloride intracellular channel 1 regulates prostate cancer cell
proliferation and migration through the MAPK/ERK pathway. Cancer
Biother Radiopharm. 29:339–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klumpp L, Sezgin EC, Eckert F and Huber
SM: Ion channels in brain metastasis. Int J Mol Sci. 17:15132016.
View Article : Google Scholar
|
|
37
|
Chen CD, Wang CS, Huang YH, Chien KY,
Liang Y, Chen WJ and Lin KH: Overexpression of CLIC1 in human
gastric carcinoma and its clinicopathological significance.
Proteomics. 7:155–1567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stühmer W and Pardo LA: (+) channels as
therapeutic targets in oncology. Future Med Chem. 2:745–755. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McCalmont WF, Heady TN, Patterson JR,
Lindenmuth MA, Haverstick DM, Gray LS and Macdonald TL: Design,
synthesis, and biological evaluation of novel T-Type calcium
channel antagonists. Bioorg Med Chem Lett. 14:3691–3695. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hong S, Bi M, Wang L, Kang Z, Ling L and
Zhao C: CLC-3 channels in cancer (Review). Oncol Rep. 33:507–514.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guan YT, Xie Y, Zhou H, Shi HY, Zhu YY,
Zhang XL, Luan Y, Shen XM, Chen YP, Xu LJ, et al: Overexpression of
chloride channel-3 (ClC-3) is associated with human cervical
carcinoma development and prognosis. Cancer Cell Int. 19:82019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wuebben EL and Rizzino A: The dark side of
SOX2: Cancer-a comprehensive overview. Oncotarget. 8:44917–44943.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bareiss PM, Paczulla A, Wang H, Schairer
R, Wiehr S, Kohlhofer U, Rothfuss OC, Fischer A, Perner S, Staebler
A, et al: SOX2 expression associates with stem cell state in human
ovarian carcinoma. Cancer Res. 73:5544–5555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li D, Zhao LN, Zheng XL, Lin P, Lin F, Li
Y, Zou HF, Cui RJ, Chen H and Yu XG: Sox2 is involved in paclitaxel
resistance of the prostate cancer cell line PC-3 via the PI3K/Akt
pathway. Mol Med Rep. 10:3169–3176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Piva M, Domenici G, Iriondo O, Rábano M,
Simões BM, Comaills V, Barredo I, López-Ruiz JA, Zabalza I, Kypta R
and Vivanco MD: Sox2 promotes tamoxifen resistance in breast cancer
cells. EMBO Mol Med. 6:66–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tian Y, Jia X, Wang S, Li Y, Zhao P, Cai
D, Zhou Z, Wang J, Luo Y and Dong M: SOX2 oncogenes amplified and
operate to activate AKT signaling in gastric cancer and predict
immunotherapy responsiveness. J Cancer Res Clin Oncol.
140:1117–1124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Szaryńska M, Olejniczak A and Kmieć Z: The
role of cancer stem cells in pathogenesis of colorectal cancer.
Postepy Hig Med Dosw (Online). 70:1469–1482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Stivarou T, Cipolleschi MG, D'Amico M,
Mannini A, Mini E, Rovida E, Dello Sbarba P, Olivotto M and Marzi
I: The complex metabolic network gearing the G1/S transition in
leukemic stem cells: Hints to a rational use of antineoplastic
agents. Oncotarget. 6:31985–31996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yamawaki K, Ishiguro T, Mori Y, Yoshihara
K, Suda K, Tamura R, Yamaguchi M, Sekine M, Kashima K, Higuchi M,
et al: Sox2-dependent inhibition of p21 is associated with poor
prognosis of endometrial cancer. Cancer Sci. 108:632–640. 2017.
View Article : Google Scholar : PubMed/NCBI
|