Introduction
Sentinel lymph node biopsy (SLNB) was introduced in
the 1990s (1). Sentinel lymph nodes
(SLNs) are the first lymph nodes that receive lymphatic drainage
from the breast (2–4). Due to the relative novelty of SLNB in
surgical operations, definitive standards are not available. The
number of removed SLNs that can accurately predict lymph node
status remains controversial. Yi et al (5) observed that the removal of five SLNs
was sufficient to identify the presence of metastatic lymph nodes
for >99% of patients. Kim et al (6) reported that the removal of at least two
lymph nodes during SLNB may be acceptable. Boughey et al
(7) and Boileau et al
(8) reported that the removal of at
least two sentinel nodes could lower the false-negative rate of
SLNs in post-neoadjuvant chemotherapy. Yi et al (5) and Zakaria et al (9) reported that positive nodes were usually
identified following the resection of the first four or five nodes.
The German Gynecological Oncology Group recommends removal of at
least two SLNs to reduce false negative rates (10). In order to reduce false negative
rates and increase the detection rate, several surgeons may remove
additional lymph nodes that are not true SLNs (11,12).
Sugie et al (11) and Inoue
et al (12) have published
data on the removal of enlarged lymph nodes that are hard, palpable
and suspicious for metastases, and can therefore be considered
SLNs.
SLNs are theoretically the first lymph nodes reached
by metastatic cancer cells that migrate from a primary tumor to the
breast (13). SLNs are detected by
injecting a dye into the breast and detecting the first draining
lymph node(s), which is/are subsequently sampled as pathological
specimens. In routine clinical practice, it has been reported that
blue-stained and/or fluorescent-stained lymph nodes are typically
defined as SLNs (12,14–17). The
number of stained lymph nodes is time-dependent. However, the time
intervals between observation and injection have not been uniformly
reported in the literature. The time interval varies between 2 and
15 min (12,16,18–22).
When SLNB was performed 2 min following injection, it was reported
that the median number of SLNs was 1.8 per patient (range, 1–4)
(19). The median number of SLNs was
2.4 (range, 1–7) when the time interval was 3.5–5.0 min (12). When SLNB was performed 5–15 min
following injection, the median number of SLNs ranged between 1 and
9 (16,22). Based on these studies, it is assumed
that the number of SLNs performed by different medical
practitioners may vary and that not all stained lymph nodes are
true SLNs.
In the present study, SLNB was performed with
methylene blue (MB) and the indocyanine green (ICG) double-tracer
technique, and all the stained lymph nodes were observed carefully.
The association between stained lymph nodes and the lymphatic
draining network was clarified. The current report aimed to
distinguish stained SNLs and stained non-SLNs. Furthermore, the
assessment of the identification and preservation of stained
resected non-SLNs during SLNB was performed in breast cancer.
Materials and methods
Patients
A total of 61 female participants were included in
the present study. The median age of the patients was 49.44 years
(range, 21–68 years). The patients were diagnosed with breast
cancer between September 2016 and September 2018 at the Qilu
Hospital, Shandong University (Jinan, China). Patients with
clinical T1-T3N0M0 breast cancer (23) were considered eligible. The exclusion
criteria included inflammatory breast cancer and stage IV disease
(23). The axillary lymph node
status was evaluated prior to surgery. All the patients received
precise SLNB. Written informed consent was obtained from all
patients, and the present study was approved by the Ethics
Committee on the Scientific Research of Shandong University Qilu
Hospital (Jinan, China; approval no. KYLL-2016-231).
Precise SLNB
Precise SLNB was performed for all the patients as
previously described (24).
Initially, MB and ICG were injected into the periareolar tissue.
Subsequently, the lymphatic ducts were marked on the skin and the
lymphatic channels were dissected precisely. All the stained lymph
nodes were identified. All the first lymph nodes that received
lymphatic drainage of the breast were designated as SLNs. The
remaining stained lymph nodes were denoted as stained non-SLNs. The
stained lymph nodes that directly followed the output lymphatic
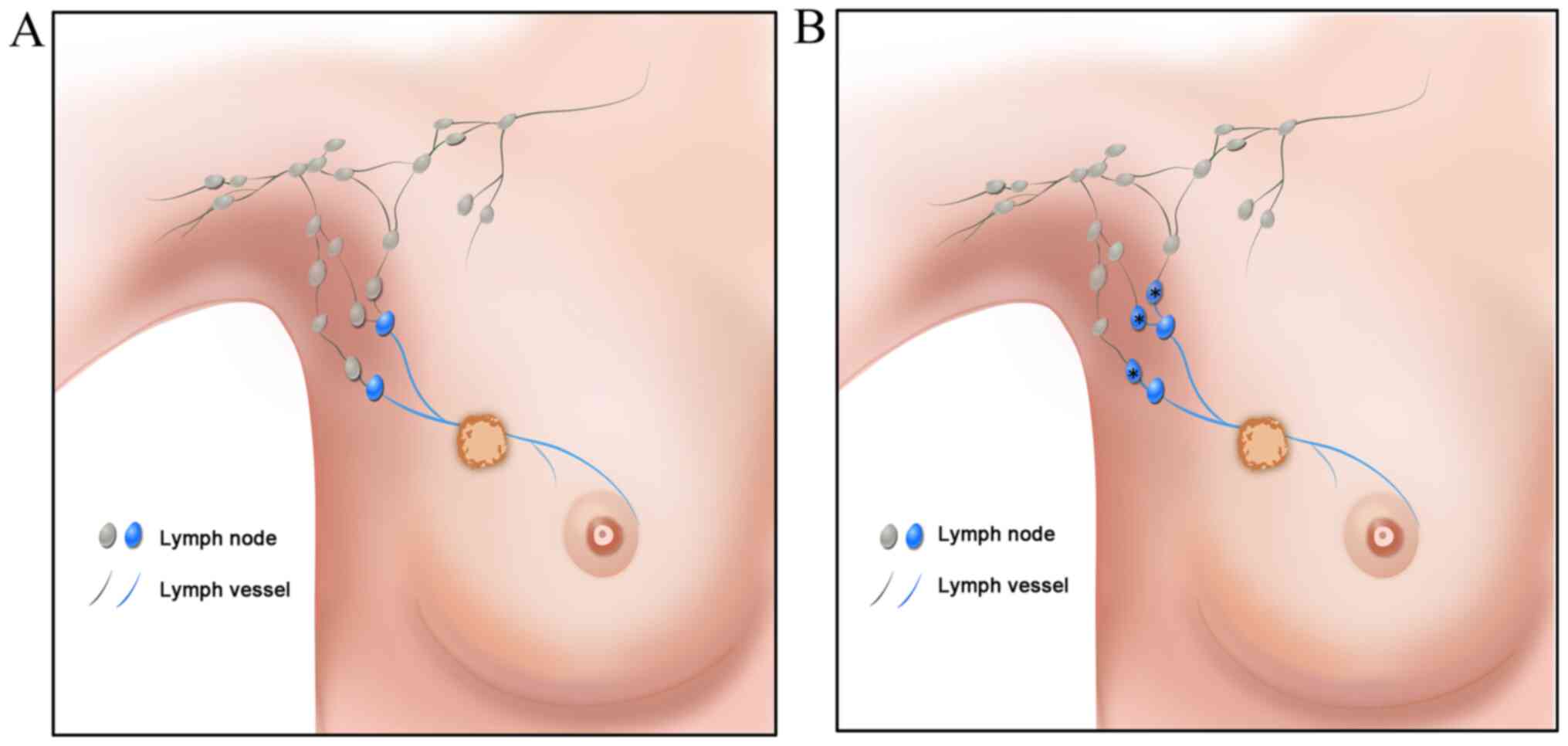
duct of the SLNs were defined as post-SLNs (poSLNs). True SLNs were
stained during SLNB (Fig. 1A). In
addition, the poSLNs could be stained if the time intervals were
longer (Fig. 1B). The time from the
injection to the dissection of the SLN and poSLN was ~5–10 min and
the SLNB was guided by the ICG fluorescence real-time dynamic
imaging system (Jinan Smart Technology Co., Ltd.). The lymphatic
vessels were observed transcutaneously in real time on the monitor
screen.
Data collection
The SLNs and poSLNs were marked separately prior to
histopathological examination, performed as previously described
(25,26). Intraoperative, frozen-section
staining was performed with hematoxylin and eosin according to
routine hospital procedures. The remaining SLN and poSLN tissues
were formalin-fixed and paraffin-embedded for routine
histopathological examination. Briefly, the tissues were harvested
and fixed in 10% formalin at 24°C for 12 h. The paraffin-embedded
specimens were cut into 4-µm-thick sections and heated at 60°C for
60 min. The status of SLNs and poSLNs was calculated by comparison
of the results of the intraoperative frozen-section staining and by
routine histopathological examination. If there were no tumor cells
in lymph nodes, the status was defined as negative. If there were
tumor cells in lymph nodes, it was defined as positive.
Results
Patient information
Between September 2016 and September 2018, a total
of 61 patients with breast cancer were enrolled. A total of 51
patients presented with invasive ductal cancer, 7 exhibited ductal
carcinoma in situ, 1 had invasive lobular carcinoma and 2
exhibited another type of carcinoma. A total of 28 patients
received axillary lymph node dissection (ALND) and the other 33
patients underwent mastectomy and SLNB.
SLNB with poSLN dissection in patients
with breast cancer
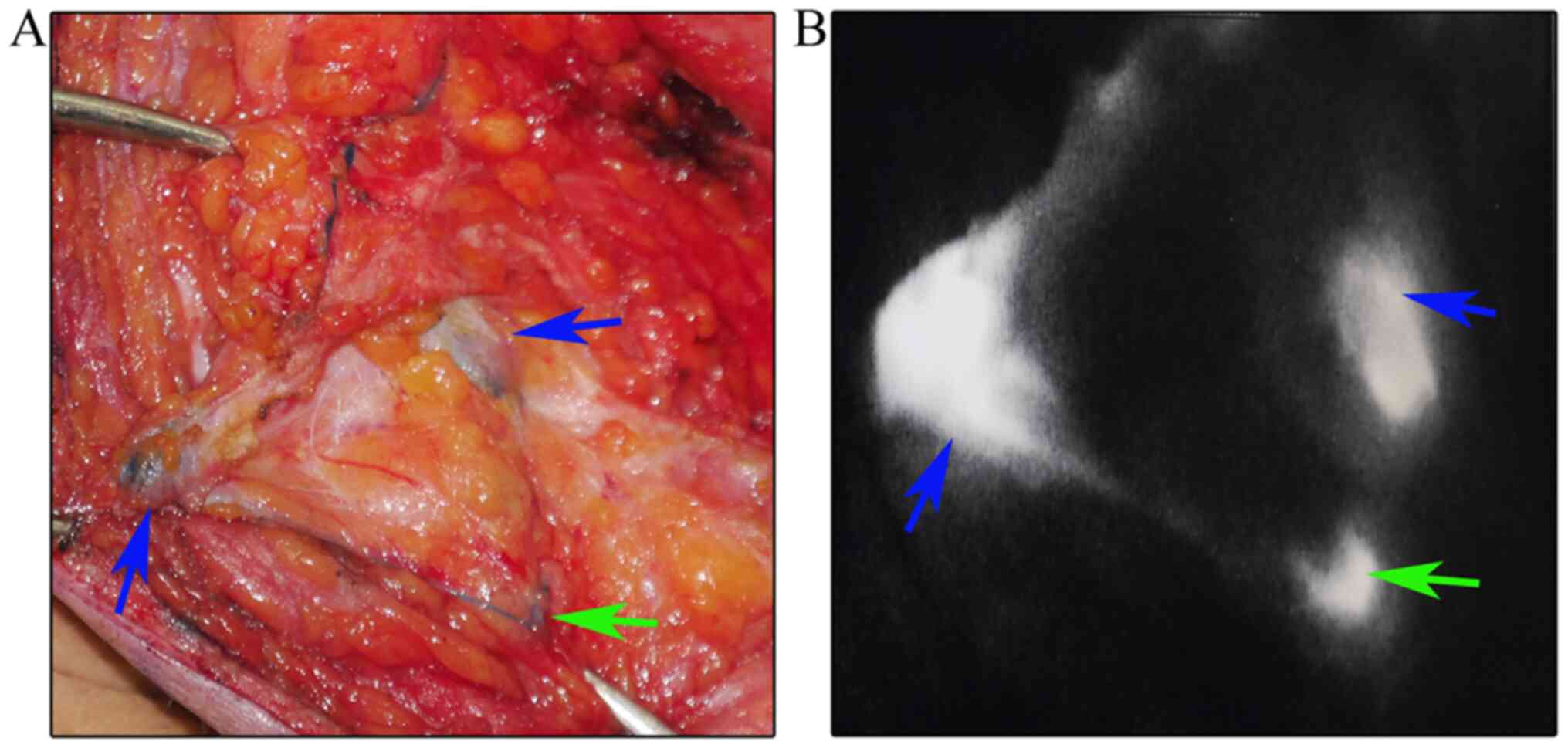
The data demonstrated staining of certain lymph
nodes that were identified subsequently to the SLNs. This staining
occurred during precise SLNB and this type of lymph node was
defined as a stained non-SLN. The lymph nodes that directly
followed the SLNs were designated as poSLNs (Fig. 2). It has been suggested that all
blue-stained nodes and/or fluorescence-positive lymph nodes can be
removed and defined as SLNs (27).
However, it was identified that the poSLNs could also be stained
during the operation if the time interval following MB and ICG
injection was too long. The exact methodology of SLNB and the
decisions as to whether poSLNs should be dissected remain
controversial. The present study was conducted to determine whether
poSLNs should be dissected during SLNB.
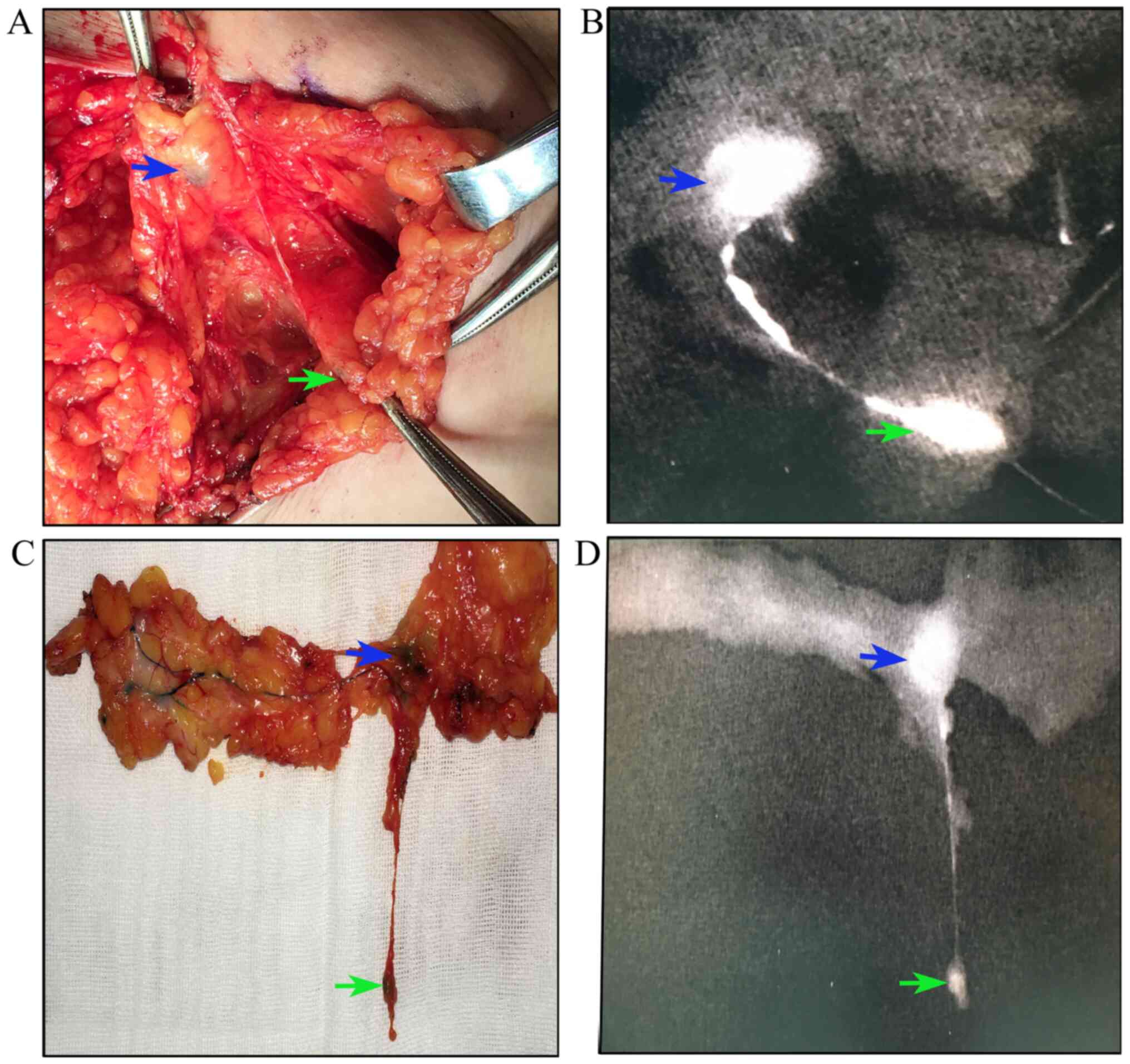
Initially, a total of 61 patients with breast cancer
were enrolled. The characteristics of the patients are presented in
Table I. Each patient received
precise SLNB guided by the lymphatic drainage pathway. All lymph
nodes that received the first lymphatic drainage were designated as
SLNs. All SLNs could be stained. In addition, the lymphatic duct
and poSLNs could be stained (Fig.
3). The results of the present study revealed that 30 patients
exhibited 1 SLN, 17 patients 2 SLNs, 10 patients 3 SLNs, 1 patient
4 SLNs and 3 patients 5 SLNs. The median number of SLNs was 1.85. A
median of 2.28 poSLNs were resected. A total of 1, 2, 3, 4, 5 and 6
poSLNs were identified in 23, 21, 6, 7, 3 and 1 patient(s),
respectively (data not shown).
 | Table I.Characteristics of the patients with
breast cancer (n=61). |
Table I.
Characteristics of the patients with
breast cancer (n=61).
| Variable | Total, n |
|---|
| Age, years |
|
|
≤45 | 20 |
|
>45 | 41 |
| Number of
tumors |
|
| 1 | 55 |
| 2 | 4 |
| 3 | 1 |
| 4 | 1 |
| Tumor size, cm |
|
| ≤2 | 38 |
|
>2 | 23 |
| ER status |
|
|
Negative | 13 |
|
Positive | 48 |
| PR status |
|
|
Negative | 12 |
|
Positive | 49 |
| HER-2 status |
|
|
Negative | 49 |
|
Positive | 12 |
PoSLN dissection is not necessary in
patients undergoing SLNB
The results of the present study revealed that 16
patients exhibited positive SLNs and 45 patients indicated negative
SLNs. In the SLN-negative patients, all poSLNs were negative.
Furthermore, no additional metastases were present in any of the
axillary lymph nodes. In the 16 SLN-positive patients, 11 exhibited
no additional lymph node metastasis, 2 presented with both positive
SLNs and poSLNs, and 3 had additional axillary lymph node
metastasis (data not shown).
Discussion
Breast cancer is a frequently encountered cancer and
is the second-leading cause of cancer-associated mortality
worldwide in 2019 (28). James et
al (29) reported 268,670
expected breast cancer cases and 41,400 expected deaths due to
breast cancer in 2018 in the USA. Recurrence and metastasis are
major causes of breast cancer-associated death (30). The assessment of the regional lymph
node status in patients with breast cancer is important for the
treatment and prediction of prognosis (31). ALND is used to assess regional lymph
node status in patients with breast cancer. However, it appears to
be associated with increased morbidity of complications including
arm lymphedema and pain, seroma formation and vascular and brachial
plexus injuries (32,33). SLNB has rapidly been replacing
standard axillary lymph dissection as the choice of several
surgeons, and it is considered the standard procedure for axillary
evaluation in early-stage breast cancer (2,34).
With regard to the development of SLNB, less
aggressive axillary surgery is applied in breast cancer; however,
surgeon bias may exist during the procedure (35). Therefore, it is vital to conduct SLNB
precisely. In the present study, a novel method was developed for
intraoperative, precise SLNB in patients with breast cancer. The
data indicated that the current method exhibited high sensitivity.
To the best of our knowledge, the present study is the first to
introduce the concept of poSLNs. This novel method can precisely
identify true SLNs and stained non-SLNs. The number of SLNs was
time-dependent, and the results were consistent with those reported
in previous studies (12,16,18–22).
Therefore, it is not appropriate to regard all blue stained or
fluorescence-positive lymph nodes as SLNs. In addition, as the
number of SLNs for patients is uncertain, it is not appropriate to
remove at least 2 or 4–5 lymph nodes during SLNB. Therefore, the
identification and preservation of stained non-SLNs in breast
cancer is important.
The clinical studies that have been performed on
SLNB have mostly focused on accuracy, detection rates and false
negative rates (8,11,12).
Therefore, surgeons may remove a higher number of non-SLNs and
designate them as SLNs. This approach may reduce the false negative
rate, while increasing potential complications. Previous studies
have reported that the number of SLNs varies between 1 and 12
(21,36,37). In
addition, the number of SLNs identified in a combined group (ICG
and MB) has been shown to be higher than that found in the blue dye
or RI alone groups (38,39). This finding demonstrated a risk in
detecting specific non-SLNs. In the present study, all patients
were negative for poSLNs in the SLN-negative group. No additional
metastases were noted in the axillary lymph nodes. The data
confirmed that the duration of time following ICG and MB injection
was highly significant for SLNB. The present study strongly
suggested that the resection of poSLNs was not necessary when SLNB
was conducted. In addition, two patients exhibited no additional
lymph node metastases, with the exception of poSLNs and SLNs. The
Z0011 trial suggested that avoidance of an ALND may be permitted,
even for SLN-positive patients (37). This finding suggests that a
proportion of patients with positive SLNs may avoid ALND if poSLNs
are removed in breast cancer patients undergoing SLNB. The status
of both SLNs and poSLNs may be superior to that of SLNs alone
during the assessment of the axillary lymph node status. The
combination of SLNB and poSLNB may provide a substitute for ALND in
certain SLN-positive patients, which should be tested in future
studies.
The present study contains certain limitations.
Firstly, the patient number was relatively small. Secondly, the
medical information lacked data on disease prognosis and associated
complications of these patients. Therefore, a large randomized,
controlled, multicenter trial should be conducted in the future in
order to address these limitations.
In conclusion, the present study demonstrated that
intraoperative dissection of the lymphatic network can distinguish
true SLNs and stained non-SLNs, such as poSLNs. The data confirmed
that not all stained lymph nodes were true SLNs. Furthermore, it
was shown that identification and preservation of stained non-SLNs
is possible during SLNB in breast cancer. The current study
provides important clinical applications for precision
medicine.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81672613 and 81874119), the
Special Foundation for Taishan Scholars (grant no. ts20190971), the
Special Support Plan for National High Level Talents (Ten Thousand
Talents Program W01020103), the National Key Research and
Development Program (grant no. 2018YFC0114705) and the Qilu
Hospital Clinical New Technology Developing Foundation (grant nos.
2018-7 and 2019-3).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QY and XL designed the study and drafted the
manuscript. XL, SC and YD were involved in acquisition of data,
drafting and revising the manuscript. HG, LJ, XK and TM edited the
manuscript and were involved in the conception and design of the
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study were
in accordance with the ethical standards of the institutional
and/or national research committee and with the 1964 Helsinki
Declaration and its later amendments or comparable ethical
standards. The study was approved by the Ethics Committee on
Scientific Research of Shandong University Qilu Hospital (Jinan,
China; approval no. KYLL-2016-231). Written informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Manca G, Tardelli E, Rubello D, Gennaro M,
Marzola MC, Cook GJ and Volterrani D: Sentinel lymph node biopsy in
breast cancer: A technical and clinical appraisal. Nucl Med Commun.
37:570–576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lyman GH, Temin S, Edge SB, Newman LA,
Turner RR, Weaver DL, Benson AB III, Bosserman LD, Burstein HJ,
Cody H III, et al: Sentinel lymph node biopsy for patients with
early-stage breast cancer: American society of clinical oncology
clinical practice guideline update. J Clin Oncol. 32:1365–1383.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vuthaluru S and Srivastava A: Axillary vs.
sentinel lymph node dissection for invasive breast cancer. JAMA.
305:22902011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lyman GH, Somerfield MR, Bosserman LD,
Perkins CL, Weaver DL and Giuliano AE: Sentinel lymph node biopsy
for patients with early-stage breast cancer: American society of
clinical oncology clinical practice guideline update. J Clin Oncol.
35:561–564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yi M, Meric-Bernstam F, Ross MI, Akins JS,
Hwang RF, Lucci A, Kuerer HM, Babiera GV, Gilcrease MZ and Hunt KK:
How many sentinel lymph nodes are enough during sentinel lymph node
dissection for breast cancer? Cancer. 113:30–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim MK, Park HS, Kim JY, Kim S, Nam S,
Park S and Kim SI: The clinical implication of the number of lymph
nodes harvested during sentinel lymph node biopsy and its effects
on survival outcome in patients with node-negative breast cancer.
Am J Surg. 214:726–732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boughey JC, Ballman KV, Hunt KK, McCall
LM, Mittendorf EA, Ahrendt GM, Wilke LG and Le-Petross HT: Axillary
ultrasound after neoadjuvant chemotherapy and its impact on
sentinel lymph node surgery: Results from the American college of
surgeons oncology group Z1071 trial (alliance). J Clin Oncol.
33:3386–3393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boileau JF, Poirier B, Basik M, Holloway
CMB, Gaboury L, Sideris L, Meterissian S, Arnaout A, Brackstone M,
McCready DR, et al: Sentinel node biopsy after neoadjuvant
chemotherapy in biopsy-proven node-positive breast cancer: The SN
FNAC study. J Clin Oncol. 33:258–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zakaria S, Degnim AC, Kleer CG, Diehl KA,
Cimmino VM, Chang AE, Newman LA and Sabel MS: Sentinel lymph node
biopsy for breast cancer: How many nodes are enough? J Surg Oncol.
96:554–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liedtke C, Jackisch C, Thill M, Thomssen
C, Müller V and Janni W; AGO Breast Committee, : AGO
recommendations for the diagnosis and treatment of patients with
early breast cancer: Update 2018. Breast Care (Basel). 13:196–208.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugie T, Kinoshita T, Masuda N, Sawada T,
Yamauchi A, Kuroi K, Taguchi T, Bando H, Yamashiro H, Lee T, et al:
Evaluation of the clinical utility of the ICG fluorescence method
compared with the radioisotope method for sentinel lymph node
biopsy in breast cancer. Ann Surg Oncol. 23:44–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inoue T, Nishi T, Nakano Y, Nishimae A,
Sawai Y, Yamasaki M and Inaji H: Axillary lymph node recurrence
after sentinel lymph node biopsy performed using a combination of
indocyanine green fluorescence and the blue dye method in early
breast cancer. Breast Cancer. 23:295–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lorek A, Boratyn-Nowicka A and
Szlachta-Światkowska E: Sentinel lymph node (sln) in breast cancer.
Review of current identification methods. Wiad Lek. 70:85–91.
2017.PubMed/NCBI
|
|
14
|
Percy DB, Pao JS, McKevitt E, Dingee C,
Kuusk U and Warburton R: Number of nodes in sentinel lymph node
biopsy for breast cancer: Are surgeons still biased? J Surg Oncol.
117:1487–1492. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen S, Xu Q, Zhou Y, Mao F, Guan J and
Sun Q: Comparison of sentinel lymph node biopsy guided by blue dye
with or without indocyanine green in early breast cancer. J Surg
Oncol. 117:1841–1847. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tong M, Guo W and Gao W: Use of
fluorescence imaging in combination with patent blue dye versus
patent blue dye alone in sentinel lymph node biopsy in breast
cancer. J Breast Cancer. 17:250–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirano A, Kamimura M, Ogura K, Kim N,
Hattori A, Setoguchi Y, Okubo F, Inoue H, Miyamoto R, Kinoshita J,
et al: A comparison of indocyanine green fluorescence imaging plus
blue dye and blue dye alone for sentinel node navigation surgery in
breast cancer patients. Ann Surg Oncol. 19:4112–4116. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papadia A, Gasparri ML, Buda A and Mueller
MD: Sentinel lymph node mapping in endometrial cancer: Comparison
of fluorescence dye with traditional radiocolloid and blue. J
Cancer Res Clin Oncol. 143:2039–2048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pitsinis V, Provenzano E, Kaklamanis L,
Wishart GC and Benson JR: Indocyanine green fluorescence mapping
for sentinel lymph node biopsy in early breast cancer. Surg Oncol.
24:375–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van der Vorst JR, Schaafsma BE, Verbeek
FP, Hutteman M, Mieog JSD, Lowik CWG, Liefers GJ, Frangioni JV, van
de Velde CJH and Vahrmeijer AL: Randomized comparison of
near-infrared fluorescence imaging using indocyanine green and
99(m) technetium with or without patent blue for the sentinel lymph
node procedure in breast cancer patients. Ann Surg Oncol.
19:4104–4111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hirche C, Murawa D, Mohr Z, Kneif S and
Hunerbein M: ICG fluorescence-guided sentinel node biopsy for
axillary nodal staging in breast cancer. Breast Cancer Res Treat.
121:373–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Toh U, Iwakuma N, Mishima M, Okabe M,
Nakagawa S and Akagi Y: Navigation surgery for intraoperative
sentinel lymph node detection using Indocyanine green (ICG)
fluorescence real-time imaging in breast cancer. Breast Cancer Res
Treat. 153:337–344. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cserni G, Chmielik E, Cserni B and Tot T:
The new TNM-based staging of breast cancer. Virchows Arch.
472:697–703. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Chen S, Jiang L, Kong X, Ma T, Xu H
and Yang Q: Precise intraoperative sentinel lymph node biopsies
guided by lymphatic drainage in breast cancer. Oncotarget.
8:63064–63072. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su P, Zhang Q and Yang Q:
Immunohistochemical analysis of Metadherin in proliferative and
cancerous breast tissue. Diagn Pathol. 5:382010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang N, Li X, Tao K, Jiang L, Ma T, Yan
S, Yuan C, Moran MS, Liang F, Haffty BG and Yang Q: BCL-2 (−938C
> A) polymorphism is associated with breast cancer
susceptibility. BMC Med Genet. 12:482011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Li Y, Zhou Y, Mao F, Lin Y, Guan
J and Sun Q: Diagnostic performance of indocyanine green-guided
sentinel lymph node biopsy in breast cancer: A meta-analysis. PLoS
One. 11:e01555972016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Sauer AG, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
James TA, Coffman AR, Chagpar AB, Boughey
JC, Klimberg VS, Morrow M, Giuliano AE and Harlow SP:
Troubleshooting sentinel lymph node biopsy in breast cancer
surgery. Ann Surg Oncol. 23:3459–3466. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gerber B, Heintze K, Stubert J, Dieterich
M, Hartmann S, Stachs A and Reimer T: Axillary lymph node
dissection in early-stage invasive breast cancer: Is it still
standard today? Breast Cancer Res Treat. 128:613–624. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mansel RE, Fallowfield L, Kissin M, Goyal
A, Newcombe RG, Dixon JM, Yiangou C, Horgan K, Bundred N, Monypenny
I, et al: Randomized multicenter trial of sentinel node biopsy
versus standard axillary treatment in operable breast cancer: The
ALMANAC trial. J Natl Cancer Inst. 98:599–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim T, Giuliano AE and Lyman GH: Lymphatic
mapping and sentinel lymph node biopsy in early-stage breast
carcinoma: A metaanalysis. Cancer. 106:4–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giuliano AE, Ballman KV, McCall L, Beitsch
PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW,
Blumencranz PW, et al: Effect of axillary dissection vs. no
axillary dissection on 10-year overall survival among women with
invasive breast cancer and sentinel node metastasis: The ACOSOG
Z0011 (alliance) randomized clinical trial. JAMA. 318:918–926.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dixon JM, Grewar J, Twelves D, Graham A,
Martinez-Perez C and Turnbull A: Factors affecting the number of
sentinel lymph nodes removed in patients having surgery for breast
cancer. Breast Cancer Res Treat. Aug 18–2020.(Online ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aoyama K, Kamio T, Ohchi T, Nishizawa M
and Kameoka S: Sentinel lymph node biopsy for breast cancer
patients using fluorescence navigation with indocyanine green.
World J Surg Oncol. 9:1572011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Giuliano AE, Hunt KK, Ballman KV, Beitsch
PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM and
Morrow M: Axillary dissection vs. no axillary dissection in women
with invasive breast cancer and sentinel node metastasis: A
randomized clinical trial. JAMA. 305:569–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ji Y, Luo N, Jiang Y, Li Q, Wei W, Yang H
and Liu J: Clinical utility of the additional use of blue dye for
indocyanine green for sentinel node biopsy in breast cancer. J Surg
Res. 215:88–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sugie T, Sawada T, Tagaya N, Kinoshita T,
Yamagami K, Suwa H, Ikeda T, Yoshimura K, Niimi M, Shimizu A and
Toi M: Comparison of the indocyanine green fluorescence and blue
dye methods in detection of sentinel lymph nodes in early-stage
breast cancer. Ann Surg Oncol. 20:2213–2218. 2013. View Article : Google Scholar : PubMed/NCBI
|