Introduction
Human epidermal growth factor receptor-2 (HER2) is
overexpressed in 20–25% of patients with breast cancer, and
anti-HER2 targeted therapy is extensively used in patients with
HER2+ breast cancer. However, there is a need for
alternative treatments, as the majority of patients with
HER2+ metastatic breast cancer (MBC) eventually develop
resistance to trastuzumab (1). As a
prognostic and predictive factor, HER2 amplification/overexpression
has been proven to contribute to the development of central nervous
system (CNS) metastases (2–4). Chemotherapy is not routinely
administered to patients with CNS metastases from breast cancer, as
the majority of anticancer agents do not cross the blood-brain
barrier. Patients with breast cancer who develop CNS disease have a
poor prognosis, with a 1-year survival rate of ~20%, which makes
CNS metastasis a significant consideration for oncologists with
regards to treatment (5).
As an oral small molecule and dual tyrosine kinase
inhibitor of epidermal growth factor receptor and HER2, lapatinib
interferes with signal transduction pathways involved in tumour
cell proliferation and growth to exert an antitumor effect on
HER2+ breast cancer (6,7).
Lapatinib in combination with capecitabine was approved on March
13, 2007 by the U.S. Food and Drug Administration for the treatment
of patients with HER2+ MBC who had received prior
treatment that included an anthracycline, a taxane and trastuzumab
(8). In addition, certain studies
have reported the efficacy and safety of lapatinib plus
chemotherapy or endocrine therapy as first-line treatment in
patients with previously untreated brain metastases from
HER2+ MBC and newly diagnosed HER2+ MBC
(9–11). Brain metastases develop in 30–50% of
patients with HER2+ MBC. The combination of lapatinib
and capecitabine has confirmed CNS antitumor activity in
HER2+ breast cancer, which may promote the design of
novel targeted approaches to treatment in selected patients
(9,12,13). The
present real-world clinical study was designed to evaluate the
efficacy and tolerability of a lapatinib-based regimen in patients
with HER2+ MBC.
Patients and methods
Study population
The medical records of adult patients with
HER2+ breast cancer, who had received lapatinib-based
treatment after being diagnosed with MBC, were retrospectively
analyzed at Beijing Cancer Hospital between January 2014 and
November 2019. The inclusion criteria were as follows: i)
HER2+ recurrent/MBC following surgery, or initial-stage
IV inoperable breast cancer diagnosed by pathological biopsy. HER2
positivity was determined in the primary or metastatic focus and
was defined as immunohistochemical staining of 3+ or 2+ with
evidence of gene amplification determined by fluorescence in
situ hybridization; ii) patient age of 18–80 years; iii)
presence of ≥1 measurable lesions, according to the Response
Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (14); iv) Eastern Cooperative Oncology Group
performance status score of ≤2; v) life expectancy of >3 months;
and vi) no treatment contraindications. The patients received 1,250
mg lapatinib once daily at approximately the same time every
morning, with permitted dose reductions and delays for
lapatinib-related toxic effects. Data on demographics, clinical
outcomes and toxicity were collected based on retrospective
evaluation of medical records for the descriptive analyses.
Evaluation of efficacy
All measurable lesions were recorded before
lapatinib-based treatment, and imaging examination and measurements
were carried out every two cycles. According to RECIST version 1.1,
the evaluation of efficacy was divided into complete response (CR),
partial response (PR), stable disease (SD) and progressive disease
(PD). Objective response rate (ORR)=(CR + PR)/total cases; disease
control rate (DCR) = (CR + PR + SD)/total cases; and clinical
benefit rate (CBR) = CR + PR + SD ≥6 months/total cases.
Progression-free survival (PFS) was defined as the time period from
the date of lapatinib-based treatment initiation until the date of
first evidence of disease progression, or the date of death in the
absence of disease progression. Overall survival (OS) was defined
as the time period from the first day of treatment until the date
of death or the date of the last follow-up. Patient follow-up
generally consisted of regular physical examinations and laboratory
assessments (haematological tests and serum biochemistry), left
ventricular ejection fraction (LVEF) evaluation and computed
tomography (CT) scans. Trastuzumab resistance was defined as
recurrence/metastasis within 1 year after adjuvant therapy with
trastuzumab or disease progression following the use of trastuzumab
for <6 months in patients with MBC.
Evaluation of safety
Patients were monitored for adverse events (AEs).
According to the National Cancer Institute's Common Terminology
Criteria for Adverse Events (version 4.0) (15), the severity of AEs was determined as
mild (grade 1), moderate (grade 2), severe (grade 3),
life-threatening or disabling (grade 4), or fatal (grade 5). A
cardiac event was defined as a decline in the LVEF that was
symptomatic, regardless of the degree of decline, or was
asymptomatic but with a relative decrease of ≥20% from baseline to
a level below the institution's lower limit of the normal range.
Lapatinib was discontinued if the patients presented with
symptomatic cardiac AEs.
Statistical analysis
Data were analyzed using SPSS version 15.0 (SPSS
Inc.), ‘survMISC’ package in R version 3.2.5 (http://www.r-project.org) (16) and SAS macro Renyi developed by Davis
in SAS 9.4 (SAS Institute, Inc.) (17). Categorical parameters were expressed
as number (percentage) of patients. Differences between groups were
calculated using the χ2 test, where appropriate.
P<0.05 was considered to indicate a statistically significant
difference. Survival was estimated by the Kaplan-Meier method.
P<0.05 (two-sided test) was considered to indicate statistical
significance. Kaplan-Meier estimates of PFS and OS were completed
using a log-rank test or the Renyi method (when survival curve
crossover between the groups was observed). ORR and CBR were
analyzed using exact methods, and a two-sided Fisher's exact test
was used to compare the groups. The Cox proportional hazards model
was used for multivariate analysis. P<0.05 (two-sided test) was
considered to indicate statistical significance.
Results
Patient characteristics
According to the inclusion criteria, 92 patients who
had received a lapatinib-based regimen after being diagnosed with
HER2+ MBC were retrospectively analyzed in the present
study. The patient demographics and baseline clinical
characteristics are summarized in Table
I. The mean patient age was 50.5 years (range, 26–73 years).
The patients were all pathologically diagnosed with
HER2+ breast cancer, with the histological types
including invasive ductal carcinoma (92.4%) and invasive lobular
carcinoma (2.2%). HER2 positivity was detected in the primary and
metastatic lesions of 86 and 6 patients, respectively. Among the 42
patients who underwent biopsy of the metastatic lesions, 31
patients (73.8%) were HER2+ in both the primary and
metastatic lesions, 5 patients (11.9%) were HER2− in the
primary but HER2+ in the metastatic lesions, 5 patients
(11.9%) were HER2+ in the primary but HER2−
in the metastatic lesions and the HER2 status of 1 patient (2.4%)
was unknown in the primary and HER2+ in the metastatic
lesions. Hormone receptor positivity was defined as oestrogen
and/or progesterone receptor positivity. In the present study, the
hormone receptor status was positive in 48 (52.2%) and negative in
44 (47.8%) cases. The most common first metastatic sites were the
lymph nodes (43.5%), lung (41.3%), bone (30.4%) and liver (29.3%),
with brain as the first metastatic site in 13.0% of the patients. A
total of 47 patients had 11 metastatic sites when first diagnosed
with MBC, 25 patients had 2 and 20 patients had ≥3.
 | Table I.Demographic and baseline clinical
characteristics of patients with human epidermal growth factor
receptor-2-positive metastatic breast cancer who received
lapatinib-based treatment (n=92). |
Table I.
Demographic and baseline clinical
characteristics of patients with human epidermal growth factor
receptor-2-positive metastatic breast cancer who received
lapatinib-based treatment (n=92).
| Characteristics | No. (%) |
|---|
| Median age (range),
years | 50.5 (26–73) |
| Histological
type |
|
| Invasive
ductal carcinoma | 85 (92.4) |
| Invasive
lobular carcinoma | 2
(2.2) |
|
Others | 5
(5.4) |
| Hormone receptor
status |
|
|
Positive | 48 (52.2) |
|
Negative | 44 (47.8) |
| Metastatic
sites |
|
| Lymph
nodes | 40 (43.5) |
|
Lung | 38 (41.3) |
|
Bone | 30 (32.6) |
|
Liver | 28 (30.4) |
|
Brain | 14 (15.2) |
| Chest
wall | 13 (14.1) |
| Trastuzumab
pretreatment for breast cancer |
|
|
Yes | 78 (84.8) |
|
Adjuvant setting | 68 (73.9) |
|
Metastatic setting | 10 (10.9) |
| No | 14 (15.2) |
| Current line of
treatment for metastatic disease |
|
| 1 | 5
(5.4) |
| 2 | 11 (12.0) |
| ≥3 | 76 (82.6) |
Treatment
Among the 92 patients with HER2+ MBC, 78
(84.8%) were previously treated with trastuzumab in the adjuvant or
recurrent/metastatic setting, 10 patients (10.9%) received
trastuzumab in the recurrent/metastatic setting after receiving a
lapatinib-based regimen, and 4 patients (4.3%) had not received
trastuzumab. Different combinations of lapatinib were used: i) 68
patients (73.9%) received lapatinib combined with chemotherapy,
including capecitabine in 38 patients, gemcitabine in 9, docetaxel
in 7, paclitaxel in 5, oral etoposide in 3, vinorelbine in 3,
cyclophosphamide in 2 and albumin-bound paclitaxel in 1; ii) 6
patients (6.5%) received lapatinib combined with endocrine therapy,
including 3 patients with letrozole, 2 patients with exemestane and
1 patient with fulvestrant; iii) 4 patients (4.3%) received
lapatinib combined with trastuzumab; iv) 10 patients (10.9%)
received lapatinib combined with chemotherapy and trastuzumab, with
capecitabine used in 4 patients, paclitaxel in 2, vinorelbine in 2,
gemcitabine in 1 and docetaxel in 1; v) 2 patients (2.2%) received
lapatinib combined with chemotherapy followed by endocrine therapy,
with docetaxel and then exemestane used in 1 case, and liposomal
doxorubicin followed by fulvestrant in the other case; and vi) 2
patients (2.2%) received lapatinib combined with endocrine therapy
and trastuzumab, with letrozole used in 1 case and exemestane in
the other case. Until November 30, 2019, disease progression was
observed in 79 patients, and 13 patients were still receiving
lapatinib treatment.
Treatment efficacy
The 92 patients were divided into three groups
according to the line of lapatinib-based treatment, with 18 (19.6%)
receiving first-line, 30 (32.6%) second-line and 44 (47.8%)
third/later-line treatment. The efficacy rate, median PFS and ORR
were calculated (Table II). No CR
was observed among the 92 patients, whereas PR was observed in 20
(21.7%), SD in 60 (65.2%) and PD in 12 (13.0%) patients, resulting
in an ORR of 21.7% (20/92), DCR of 87.0% (80/92) and CBR of 47.8%
(44/92). The median PFS was 5.8 months [95% confidence interval
(CI): 4.9–6.8] and the median OS 21.5 months (95% CI:
19.7–23.3).
 | Table II.Efficacy of lapatinib-based treatment
in patients with human epidermal growth factor receptor-2-positive
breast cancer (n=92). |
Table II.
Efficacy of lapatinib-based treatment
in patients with human epidermal growth factor receptor-2-positive
breast cancer (n=92).
| Efficacy
measures | First-line | Second-line |
Third/later-line | Total |
|---|
| Median PFS (95%
CI), months | 10.4
(7.9–12.8) | 5.2 (2.8–7.6) | 5.1 (4.3–5.9) | 5.8 (4.9–6.8) |
| Median OS (95% CI),
months | 32.9
(6.3–59.4) | 29.1
(18.0–40.3) | 13.0
(1.3–24.7) | 21.5
(19.7–23.3) |
| Objective response
rate, % | 38.9 | 23.3 | 13.6 | 21.7 |
| Disease control
rate, % | 100 | 83.3 | 84.1 | 87.0 |
| Partial response,
n | 7 | 7 | 6 | 20 |
| Stable disease,
n | 11 | 18 | 31 | 60 |
| Progressive
disease, n | 0 | 5 | 7 | 12 |
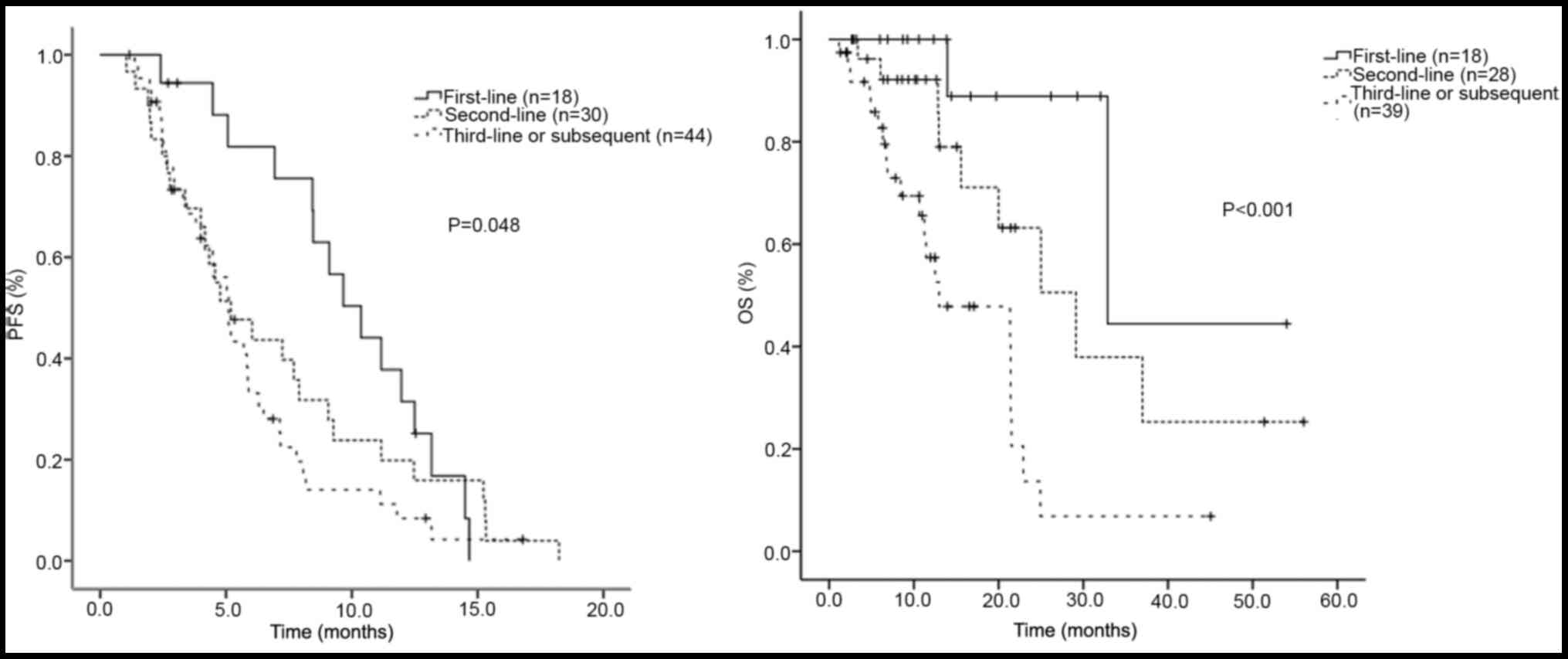
In the three groups of patients receiving
lapatinib-based regimen as first-, second- and third/later-line
treatment, the median PFS was 10.4, 5.2 and 5.1 months, the median
OS was 32.9, 29.1 and 13.0 months, the ORR was 38.9, 23.3 and
13.60%, and the DCR was 100, 83.3 and 84.1%, respectively.
Differences in the median PFS among the three groups were
calculated and found to be statistically significant (P=0.048).
Differences in the median OS among the three groups were also found
to be statistically significant. The χ2 test was used to
compare the distribution of therapeutic efficacy in the three
groups, with the results exhibiting no statistically significant
difference. A survival curve was drawn using the Kaplan-Meier PFS
estimates (Fig. 1). Among the 92
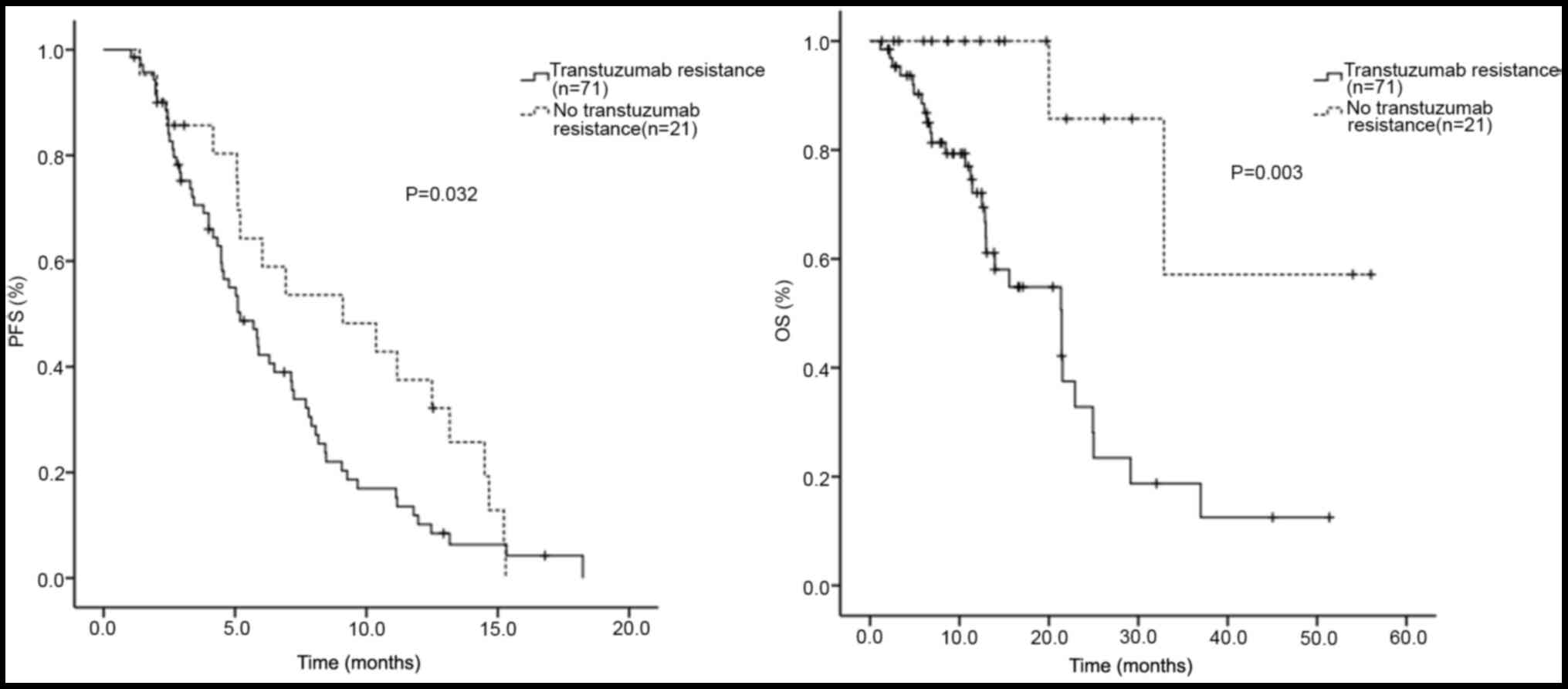
patients with HER2+ MBC, 71 were trastuzumab-resistant.
In the trastuzumab-resistant (n=71) and trastuzumab-sensitive
(n=21) patients, the median PFS was 5.2 and 9.1 months, and the
median OS was 21.4 and 44.3 months, respectively. Differences in
the median PFS (P=0.032) and OS (P=0.003) between the two groups
were statistically significant. A survival curve was drawn using
the Kaplan-Meier PFS and OS estimates (Fig. 2).
Different treatment combinations
The 92 patients were divided into several groups
according to the different agents combined with lapatinib: i) The
median PFS of the groups of patients receiving lapatinib combined
with chemotherapy (n=70), endocrine therapy (n=6) and targeted
therapy (n=16) was 5.9 (95% CI: 4.1–7.7), 5.1 (95% CI: 4.1–6.1) and
5.9 (95% CI: 1.5–10.2) months, respectively. The P-value was 0.878
when comparing the median PFS among these groups. ii) The median
PFS of the two groups of patients receiving lapatinib combined with
targeted (n=16) and non-targeted (n=76) therapy was 5.9 (95% CI:
1.5–10.2) and 5.7 (95% CI: 4.7–6.7) months, respectively (P=0.903).
iii) The median PFS of the two groups of patients receiving
lapatinib combined with chemotherapy (n=76) and chemotherapy plus
trastuzumab (n=10) was 5.9 (95% CI: 4.5–7.3) and 5.9 (95% CI:
2.2–9.5) months, respectively (P=0.239). Among the 92 patients, 68
received lapatinib in combination with chemotherapy alone. The
median PFS of these 68 patients was 5.9 (95% CI: 4.5–7.3) months,
while the median PFS of the three groups was 10.4 (95% CI:
7.9–12.8) months in the first-line (n=17), 4.6 months (95% CI:
3.5–5.6) months in the second-line (n=20) and 5.0 (95% CI: 3.5–6.6)
months in the third/later-line (n=31) setting. The median PFS
differed significantly among these three groups (P=0.027).
Capecitabine vs. other chemotherapy
agents
Among the 68 patients who received lapatinib plus
chemotherapy alone, 38 received capecitabine (12 cases in the
first-line, 13 cases in the second-line and 13 cases in the
third/later-line setting), and 30 cases received other chemotherapy
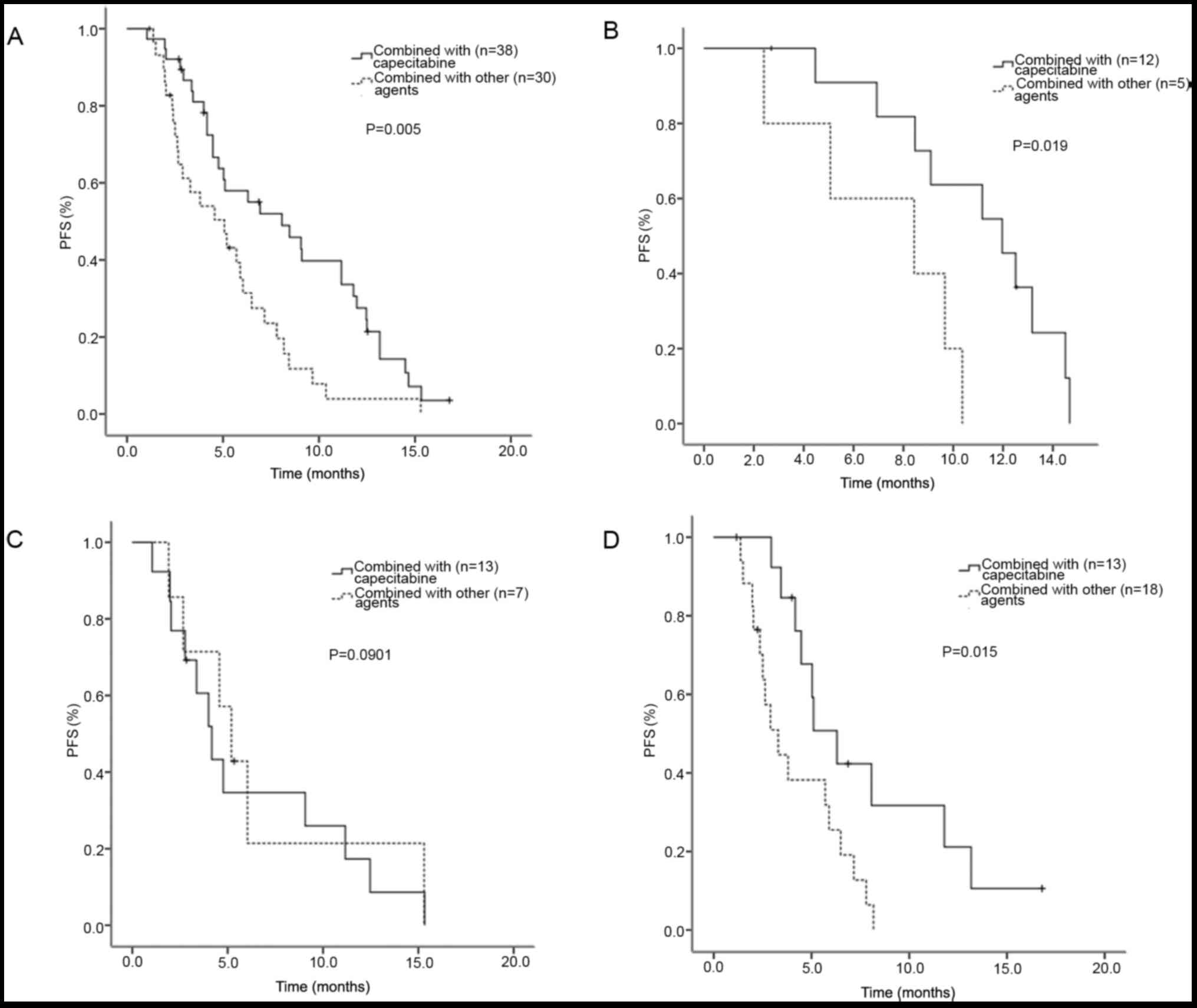
agents. The patients receiving lapatinib plus capecitabine (n=38)
had a prolonged PFS of 8.1 (95% CI: 4.3–11.8) months, compared with
patients receiving lapatinib plus other chemotherapy agents (n=30)
with a median PFS of 5.1 months (95% CI: 2.6–7.5 months; P=0.005;
Fig. 3A). In the first-line setting,
the median PFS of the patients receiving lapatinib combined with
capecitabine (n=12) and other chemotherapy agents (n=5) was 12.0
(95% CI: 8.3–15.6) and 8.4 (95% CI: 1.2–15.7) months, respectively.
The patients receiving lapatinib combined with capecitabine
exhibited longer PFS compared with patients receiving lapatinib
combined with other chemotherapy agents (P=0.019; Fig. 3B). In the second-line setting, the
median PFS of the patients receiving lapatinib combined with
capecitabine (n=13) and other chemotherapy agents (n=7) was 4.2
(95% CI: 2.9–5.5) and 5.2 (95% CI: 3.6–6.8) months, respectively.
The median PFS did not differ significantly between these two
groups (P=0.901; Fig. 3C). In the
third/later-line setting, the median PFS of the patients receiving
lapatinib combined with capecitabine (n=13) and other chemotherapy
agents (n=18) was 6.3 (95% CI: 4.2–8.4) and 3.3 (95% CI: 2.0–4.6)
months, respectively. The patients receiving lapatinib combined
with capecitabine had a longer PFS compared with the patients
receiving lapatinib combined with other chemotherapy agents
(P=0.015; Fig. 3D). Survival curves
were drawn by Kaplan-Meier PFS estimates in these different groups
(Fig. 3).
Of the 38 patients who received lapatinib plus
capecitabine, 12 had received capecitabine prior to lapatinib, and
the remaining 26 patients had received capecitabine first. The
median PFS of these two groups of patients was 6.3 (95% CI:
2.1–10.5) and 9.1 (95% CI: 4.3–13.8) months, respectively
(P=0.945).
Brain metastasis
In the present study, 14 patients with brain
metastasis from HER2+ MBC were treated with lapatinib at
the recurrence and metastasis stage. Among the 14 patients with
brain metastasis who had received lapatinib-based treatment, 6 had
received lapatinib as first-line, 5 as second-line, 2 as third-line
and 1 as fourth-line treatment. Lapatinib was combined with
capecitabine (n=5), docetaxel (n=3), capecitabine plus trastuzumab
(n=2), trastuzumab (n=1), paclitaxel (n=1), capecitabine plus
gemcitabine (n=1), and vinorelbine plus trastuzumab (n=1). All
patients with brain metastasis received radiotherapy. The follow-up
results revealed a median PFS of 8.4 (95% CI: 2.2–14.7) months in
14 patients with brain metastases. The number of patients with PR,
SD and PD was 5, 7 and 2, respectively, with an ORR of 35.7% and a
DCR of 85.7%. When comparing the median PFS between patients with
(n=14) and without (n=78) brain metastasis, the difference was not
statistically significant (P=0.569).
Multivariate analysis
Multivariate logistic regression analysis was
performed to evaluate the independent prognostic factors of median
PFS in patients with HER2+ MBC receiving lapatinib-based
treatment, and revealed that the line of lapatinib-based treatment
[hazard ratio (HR)=1.55; 95% CI: 1.1–2.3; P=0.024) and its
combination with capecitabine (HR=2.288; 95% CI: 1.2–4.2; P=0.009)
were strong predictors of the median PFS of patients with
HER2+ MBC (Table
III).
 | Table III.Multivariate analysis of the
independent prognostic factors of median progression-free survival
of patients with human epidermal growth factor receptor-2-positive
metastatic breast cancer receiving lapatinib-based treatment
(n=92). |
Table III.
Multivariate analysis of the
independent prognostic factors of median progression-free survival
of patients with human epidermal growth factor receptor-2-positive
metastatic breast cancer receiving lapatinib-based treatment
(n=92).
| Prognostic
factors | P-value | HR | 95% CI |
|---|
| Line of
lapatinib-based treatment | 0.024a | 1.55 | 1.06–2.268 |
| Combination with
capecitabine | 0.009a | 2.288 | 1.234–4.245 |
| Presence of liver
metastases | 0.29 | 1.427 | 0.739–2.758 |
| Presence of brain
metastases | 0.392 | 1.437 | 0.626–3.3 |
| Presence of
visceral metastases | 0.462 | 0.764 | 0.373–1.564 |
| Number of
metastatic sites | 0.341 | 0.82 | 0.545–1.234 |
| Hormone receptor
status | 0.251 | 1.396 | 0.79–2.468 |
AEs
The lapatinib-based combination treatment was
generally well-tolerated. The most common lapatinib-related AEs
were diarrhoea, rash and hand-foot syndrome. Diarrhoea was recorded
in 16 patients (4 cases of grade 1, 11 cases of grade 2 and 1 case
of grade 3). Rash was recorded in 9 patients (6 cases of grade 1
and 3 cases of grade 2). Hand-foot syndrome was recorded in 4
patients (2 cases of grade 1, 1 case of grade 2 and 1 case of grade
3). There was no episode of febrile neutropenia or symptomatic
cardiac events (Table IV). The dose
of lapatinib was reduced in 14 patients, of whom 12 had diarrhoea
and 2 had hand-foot syndrome (grades 2–3).
 | Table IV.Lapatinib-related adverse effects and
dose reduction. |
Table IV.
Lapatinib-related adverse effects and
dose reduction.
| Adverse
effects | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|
| Diarrhoea | 4 | 11a | 1a | 0 | 0 |
| Rash | 6 | 3 | 0 | 0 | 0 |
| Hand-foot
syndrome | 2 | 1a | 1a | 0 | 0 |
| Dose reduction |
| 12a | 2a |
|
|
Discussion
Anti-HER2 agents are supposed to be used soon after
the diagnosis of HER2+ MBC. Novel and emerging agents
targeting HER2 and its pathway are associated with notable clinical
benefits in patients having received treatment with trastuzumab.
Lapatinib was approved for use in combination with capecitabine in
patients with HER2+ MBC who had received prior
treatment, including treatment with an anthracycline, a taxane and
trastuzumab, in 2007 (8). Subsequent
research explored the efficacy and tolerability of lapatinib in
different treatment phases of HER2+ MBC, and
demonstrated that lapatinib-based combination regimens may be
beneficial for heavily pretreated patients with HER2+
MBC (18).
The present study is a real-world study that aimed
to determine the efficacy and tolerability of the lapatinib-based
treatment in a Chinese HER2+ MBC patient population.
According to the findings, lapatinib-based combination treatment
was effective and well-tolerated, with a median PFS of 5.8 months,
a median OS of 21.5 months, an ORR of 21.7%, a DCR of 87.0% and
limited AEs. A lapatinib-based regimen was used as first-line
treatment in 18 patients who progressed following treatment with
trastuzumab as adjuvant therapy. The median PFS was 10.4 months,
the median OS was 32.9 months, the ORR was 38.9% and the DCR was
100%, which clearly demonstrated the benefits of lapatinib, and
supported its use combined with chemotherapy as a first-line
alternative for patients with trastuzumab-resistant
HER2+ MBC. Similarly, the EMILIA trial (10) randomized patients with
HER+ MBC who had been pretreated with trastuzumab and
taxane to receive trastuzumab emtansine (T-DM1) or lapatinib plus
capecitabine. The results revealed a median PFS of 9.6 months with
T-DM1, compared with 6.4 months with lapatinib plus capecitabine
(HR=0.65; 95% CI: 0.55–0.77; P<0.001). Trastuzumab plus
chemotherapy or endocrine therapy is commonly used at present as
first-line treatment for HER2+ MBC. However, lapatinib
combined with chemotherapy may be another option for
trastuzumab-pretreated patients, since T-DM1 was not approved until
January, 2020 in China.
In the present study, a lapatinib-based regimen was
used in 30 patients as second-line treatment (median PFS, 5.2
months; median OS, 29.1 months; ORR, 23.3%; DCR, 83.3%) and in 44
patients as third/later-line treatment (median PFS, 5.1 months;
median OS, 13.0 months; ORR, 13.6%; DCR, 84.1%), which confirmed
the survival benefits of lapatinib when used as second/later-line
treatment. Previous studies reported similar results following
treatment with lapatinib plus capecitabine or trastuzumab. Bian
et al (19) compared
lapatinib plus capecitabine with trastuzumab plus capecitabine in
trastuzumab-resistant and taxane-pretreated patients with
HER2+ MBC, and revealed a significantly improved PFS
(6.0 vs. 4.5 months) and higher proportion of patients with a PFS
of ≥6 months (55 vs. 30%; P=0.005). In the EGF100151 study
(20,21), lapatinib plus capecitabine
significantly prolonged median PFS, and reduced the risk of disease
progression and death, when compared with capecitabine alone, with
a controllable toxicity and good tolerance. Furthermore, the risk
of disease progression was lower when using lapatinib plus
capecitabin as second-line treatment, as compared to
third/later-line treatment. The EGF104900 study (22) demonstrated that trastuzumab combined
with lapatinib may achieve a better PFS (prolonged by 3.9 months)
and OS compared with lapatinib alone in patients with
HER2+ MBC. Baez-Vallecillo et al (23) analyzed 520 patients with
HER2+ MBC who had received prior treatment with
trastuzumab, pertuzumab and/or ado-T-DM1 and then lapatinib, and
revealed a median treatment duration of lapatinib of 5.0 months, a
median time-to-progression (TTP) of 6.0 months, and a CBR of 28%.
The present study demonstrated that the median PFS of the
trastuzumab-resistant (n=71) and trastuzumab-sensitive (n=21)
patients was 5.2 and 9.1 months, and the median OS was 21.4 and
44.3 months, respectively. Based on the present findings and the
aforementioned research, a lapatinib-based combination (including
lapatinib combined with chemotherapy or trastuzumab) appears to be
a superior alternative, particularly in trastuzumab-pretreated
patients with HER2+ MBC. The results of the present
study also suggested that the earlier use of the lapatinib
combination was correlated with a better PFS (24).
The patients were divided into different groups
according to the different combination treatments with lapatinib,
and no significant difference in the median PFS was observed among
the patients receiving lapatinib combined with chemotherapy,
endocrine therapy and targeted therapy, lapatinib combined with
targeted and non-targeted therapy, and lapatinib combined with
chemotherapy and chemotherapy plus trastuzumab. Thus, various
combinations may be taken into consideration when using lapatinib.
Based on our experience, lapatinib plus endocrine therapy or
trastuzumab may also be considered for older patients (>65 years
old) who are in a relatively poor condition and unable to tolerate
chemotherapy.
In the present study, of the 68 patients who
received lapatinib plus chemotherapy alone, 38 received
capecitabine and 30 other chemotherapy agents. A total of 23/30
(76.7%) patients received lapatinib combined with other
chemotherapy drugs, since they had developed PD on capecitabine
before, and 3/30 (10%) patients received lapatinib combined with
other chemotherapy drugs, which had shown good efficacy as previous
treatments. A significant difference was observed in the whole
group, and the first-line and third/later-line subgroups, with
patients receiving lapatinib combined with capecitabine exhibiting
a significantly improved PFS compared with those receiving
lapatinib combined with other chemotherapy agents. The results were
consistent with the evidence on lapatinib combined with
capecitabine in HER2+ MBC reported by several clinical
trials. Capecitabine in combination with lapatinib is an acceptable
treatment option for patients, since the agents may be taken orally
at home, are associated with limited AEs and a small effect on
immune function, contributing to an optimal quality of life for
these patients. In addition, the pharmacological mechanisms
underlying the positive interaction between lapatinib and
capecitabine were investigated in human breast cancer models, and
it was observed that lapatinib clearly downregulated thymidylate
synthase (TS) activity, thereby improving the efficacy of
capecitabine, and that capecitabine optimized the downregulation of
p-AKT and p-P42/44 expression by lapatinib. Specifically, lapatinib
and capecitabine modulated each other's molecular determinants of
response, and concomitant dosing appeared to be the optimal method
for combining these agents, which suggested that the association
between lapatinib and capecitabine has the potential to overcome
breast cancer resistance associated with TS overexpression
(25). Therefore, lapatinib plus
capecitabine may be considered as a superior combination for
patients with HER2+ MBC due to its efficacy, convenience
and tolerability. It was hypothesized that the reason for the lack
of significant differences in the second-line subgroup was due to
the limited number of cases. In addition, the prior use of
capecitabine did not affect the median PFS.
Among patients with MBC, 6–16% will develop
metastatic CNS disease (2,5). However, brain metastasis may be
underdiagnosed without routine screening, due to the lack of
clinical symptoms. Lapatinib is able to cross the blood-brain
barrier and concentrate in HER2+ MBC, but not in normal
brain tissue, due to HER2 selectivity (26–28).
Patients with brain metastasis must receive appropriate
radiotherapy and systemic treatment. In the present study, the
median PFS of the 14 patients with brain metastases from
HER2+ MBC was 8.4 months, the ORR was 35.7% and the DCR
was 85.7%, which suggested that lapatinib may be a viable
therapeutic option for such patients. In the phase II LANDSCAPE
study (9), lapatinib plus
capecitabine was effective in patients with previously untreated
brain metastases from HER2+ MBC, with an objective CNS
response of 65.9% (95% CI: 50.1–79.5) and a median TTP of 5.5
months (95% CI: 4.3–6.0), which supported the use of lapatinib plus
capecitabine as first-line treatment for patients with brain
metastases from HER2+ breast cancer. A study from Turkey
(29) also demonstrated that
lapatinib plus capecitabine treatment conferred a significant
survival benefit to patients with brain metastasis from breast
cancer, as compared with trastuzumab-based treatment. The present
analysis confirmed the benefits of lapatinib in patients with brain
metastases from HER2+ breast cancer.
Multivariate logistic regression analysis revealed
that the line of lapatinib-based treatment and its combination with
capecitabine were independent prognostic factors for the median PFS
of patients with HER2+ MBC, which supports the earlier
use of lapatinib and its combination with capecitabine.
There were certain limitations to the present study.
The included patients represented a highly selected population
referred to tertiary care cancer centres, limiting the ability to
generalize from the present results. Large-scale prospective
clinical trials are required to elucidate the therapeutic effect
and safety of lapatinib plus capecitabine in HER2+
MBC.
In conclusion, lapatinib-based treatment was found
to be effective and well-tolerated by patients with
HER2+ MBC (even trastuzumab-pretreated patients),
particularly when combined with capecitabine.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All the datasets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
XG, HL, YY and RL contributed to the study
conception and design. Material preparation and data collection
were performed by XG and YY, and XG, RZ and HL analyzed and
interpreted the patient data. The draft of the manuscript was
written by XG and revised by PL. XG, HL, YY and RL commented on
previous versions of the manuscript. All the authors have read and
approved the final manuscript. Each of the authors has sufficiently
participated in the work to take public responsibility for
appropriate parts of the content and agrees to be accountable for
all aspects of the work to ensure that questions regarding the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by the
Ethics Committee of Peking University Cancer Hospital and
Institute.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Lim B, Murthy RK, Lee J, Jackson SA, Iwase
T, Davis DW, Willey JS, Wu J, Shen Y, Tripathy D, et al: A phase Ib
study of entinostat plus lapatinib with or without trastuzumab in
patients with HER2-positive metastatic breast cancer that
progressed during trastuzumab treatment. Br J Cancer.
120:1105–1112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leyland-Jones B: Human epidermal growth
factor receptor 2-positive breast cancer and central nervous system
metastases. J Clin Oncol. 27:5278–5286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaal EC and Vecht CJ: CNS complications of
breast cancer: Current and emerging treatment options. CNS Drugs.
21:559–579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gabos Z, Sinha R, Hanson J, Chauhan N,
Hugh J, Mackey JR and Abdulkarim B: Prognostic significance of
human epidermal growth factor receptor positivity for the
development of brain metastasis after newly diagnosed breast
cancer. J Clin Oncol. 24:5658–5663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pieńkowski T and Zielinski CC: Trastuzumab
treatment in patients with breast cancer and metastatic CNS
disease. Ann Oncol. 21:917–924. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moy B and Goss PE: Lapatinib-associated
toxicity and practical management recommendations. Oncologist.
12:756–765. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murphy CG and Modi S: HER2 breast cancer
therapies: A review. Biologics. 3:289–301. 2009.PubMed/NCBI
|
|
8
|
Ryan Q, Ibrahim A, Cohen MH, Johnson J, Ko
CW, Sridhara R, Justice R and Pazdur R: FDA drug approval summary:
Lapatinib in combination with capecitabine for previously treated
metastatic breast cancer that overexpresses HER-2. Oncologist.
13:1114–1119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bachelot T, Romieu G, Campone M, Diéras V,
Cropet C, Dalenc F, Jimenez M, Le Rhun E, Pierga JY, Gonçalves A,
et al: Lapatinib plus capecitabine in patients with previously
untreated brain metastases from HER2-positive metastatic breast
cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol.
14:64–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verma S, Miles D, Gianni L, Krop IE,
Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E, et al:
Trastuzumab emtansine for HER2-positive advanced breast cancer. N
Engl J Med. 367:1783–1791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnston S, Pippen J Jr, Pivot X,
Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A,
Kennedy MJ, et al: Lapatinib combined with letrozole versus
letrozole and placebo as first-line therapy for postmenopausal
hormone receptor-positive metastatic breast cancer. J Clin Oncol.
27:5538–5546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin NU, Diéras V, Paul D, Lossignol D,
Christodoulou C, Stemmler HJ, Roché H, Liu MC, Greil R, Ciruelos E,
et al: Multicenter phase II study of lapatinib in patients with
brain metastases from HER2-positive breast cancer. Clin Cancer Res.
15:1452–1459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bachelot T, Le Rhun E, Labidi-Gally I,
Heudel P, Gilabert M, Bonneterre J, Pierga JY and Gonçalves A:
Systemic treatment of brain metastases from breast cancer:
Cytotoxic chemotherapy and targeted therapies. Bull Cancer.
100:7–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
NCI, . CTCAE. simplehttp://evs.nci.nih.gov.cmich.idm.oclc.org/ftp1/CTCAE/about.htmlMay
17–2010
|
|
16
|
Royston P: Explained variation for
survival models. Stata J. 6:83–96. 2006. View Article : Google Scholar
|
|
17
|
Davis M and Xie SX: Caution: Hazards
crossing! Using the Renyi test statistic in survival analysis.
PharmaSUG2011-Paper SP06, 2011. simplehttps://www.pharmasug.org/proceedings/2011/SP/PharmaSUG-2011-SP06.pdf
|
|
18
|
Greil R, Borštnar S, Petráková K, Marcou
Y, Pikiel J, Wojtukiewicz MZ, Koza I, Steger GG, Linn M, Das Gupta
A and Cwiertka K: Combination therapy of lapatinib and capecitabine
for ErbB2-positive metastatic or locally advanced breast cancer:
Results from the lapatinib expanded access program (LEAP) in
central and Eastern Europe. Onkologie. 34:233–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bian L, Wang T, Zhang S and Jiang Z:
Trastuzumab plus capecitabine vs lapatinib plus capecitabine in
patients with trastuzumab resistance and taxane-pretreated
metastatic breast cancer. Tumour Biol. 34:3153–3158. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cameron D, Casey M, Press M, Lindquist D,
Pienkowski T, Romieu CG, Chan S, Jagiello-Gruszfeld A, Kaufman B,
Crown J, et al: A phase III randomized comparison of lapatinib plus
capecitabine versus capecitabine alone in women with advanced
breast cancer that has progressed on trastuzumab: Updated efficacy
and biomarker analyses. Breast Cancer Res Treat. 112:533–543. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geyer CE, Forster J, Lindquist D, Chan S,
Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A,
Kaufman B, et al: Lapatinib plus capecitabine for HER2-positive
advanced breast cancer. N Engl J Med. 355:2733–2743. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blackwell KL, Burstein HJ, Storniolo AM,
Rugo HS, Sledge G, Aktan G, Ellis C, Florance A, Vukelja S,
Bischoff J, et al: Overall survival benefit with lapatinib in
combination with trastuzumab for patients with human epidermal
growth factor receptor 2-positive metastatic breast cancer: Final
results from the EGF104900 study. J Clin Oncol. 30:2585–2592. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baez-Vallecillo L, Raghavendra AS, Hess
KR, Barcenas CH, Moulder SL, Tripathy D, Valero V and Murthy RK:
Lapatinib activity in metastatic human epidermal growth factor
receptor 2-positive breast cancers that received prior therapy with
trastuzumab, pertuzumab, and/or ado-trastuzumab emtansine (T-DM1).
Breast Cancer Res Treat. 176:227–234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Untch M and Lück HJ: Lapatinib-member of a
new generation of ErbB-targeting drugs. Breast Care (Basel).
5:8–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chefrour M, Milano G, Formento P,
Giacometti S, Denden A, Renée N, Iliadis A, Fischel JL and
Ciccolini J: Positive interaction between lapatinib and
capecitabine in human breast cancer models: Study of molecular
determinants. Fundam Clin Pharmacol. 26:530–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gril B, Palmieri D, Bronder JL, Herring
JM, Vega-Valle E, Feigenbaum L, Liewehr DJ, Steinberg SM, Merino
MJ, Rubin SD and Steeg PS: Effect of lapatinib on the outgrowth of
metastatic breast cancer cells to the brain. J Natl Cancer Inst.
100:1092–1103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saleem A, Searle GE, Kenny LM, Huiban M,
Kozlowski K, Waldman AD, Woodley L, Palmieri C, Lowdell C, Kaneko
T, et al: Lapatinib access into normal brain and brain metastases
in patients with Her-2 overexpressing breast cancer. EJNMMI Res.
5:302015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morikawa A, Peereboom DM, Thorsheim HR,
Samala R, Balyan R, Murphy CG, Lockman PR, Simmons A, Weil RJ,
Tabar V, et al: Capecitabine and lapatinib uptake in surgically
resected brain metastases from metastatic breast cancer patients: A
prospective study. Neuro Oncol. 17:289–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaplan MA, Isikdogan A, Koca D, Kucukoner
M, Gumusay O, Yildiz R, Dayan A, Demir L, Geredeli C, Kocer M, et
al: Clinical outcomes in patients who received lapatinib plus
capecitabine combination therapy for HER2-positive breast cancer
with brain metastasis and a comparison of survival with those who
received trastuzumab-based therapy: A study by the Anatolian
society of medical oncology. Breast Cancer. 21:677–683. 2014.
View Article : Google Scholar : PubMed/NCBI
|