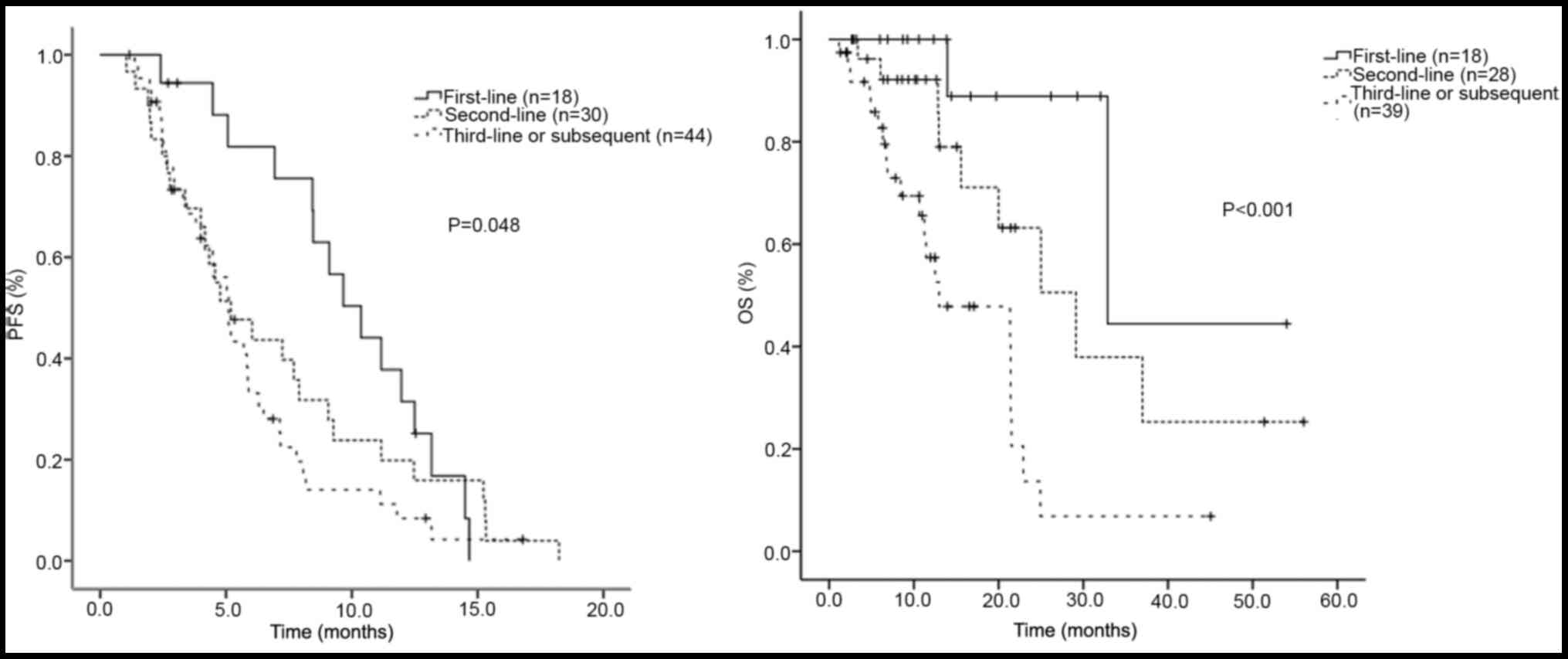
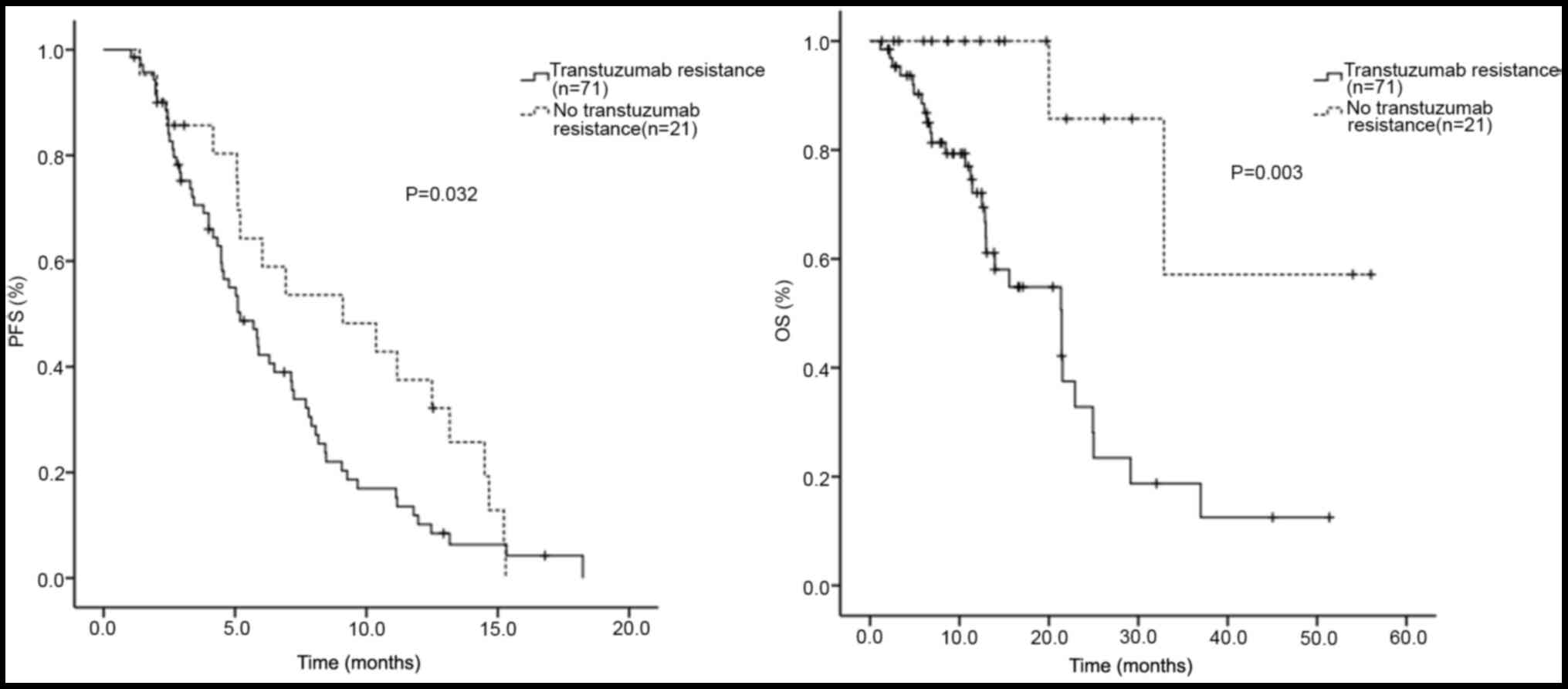
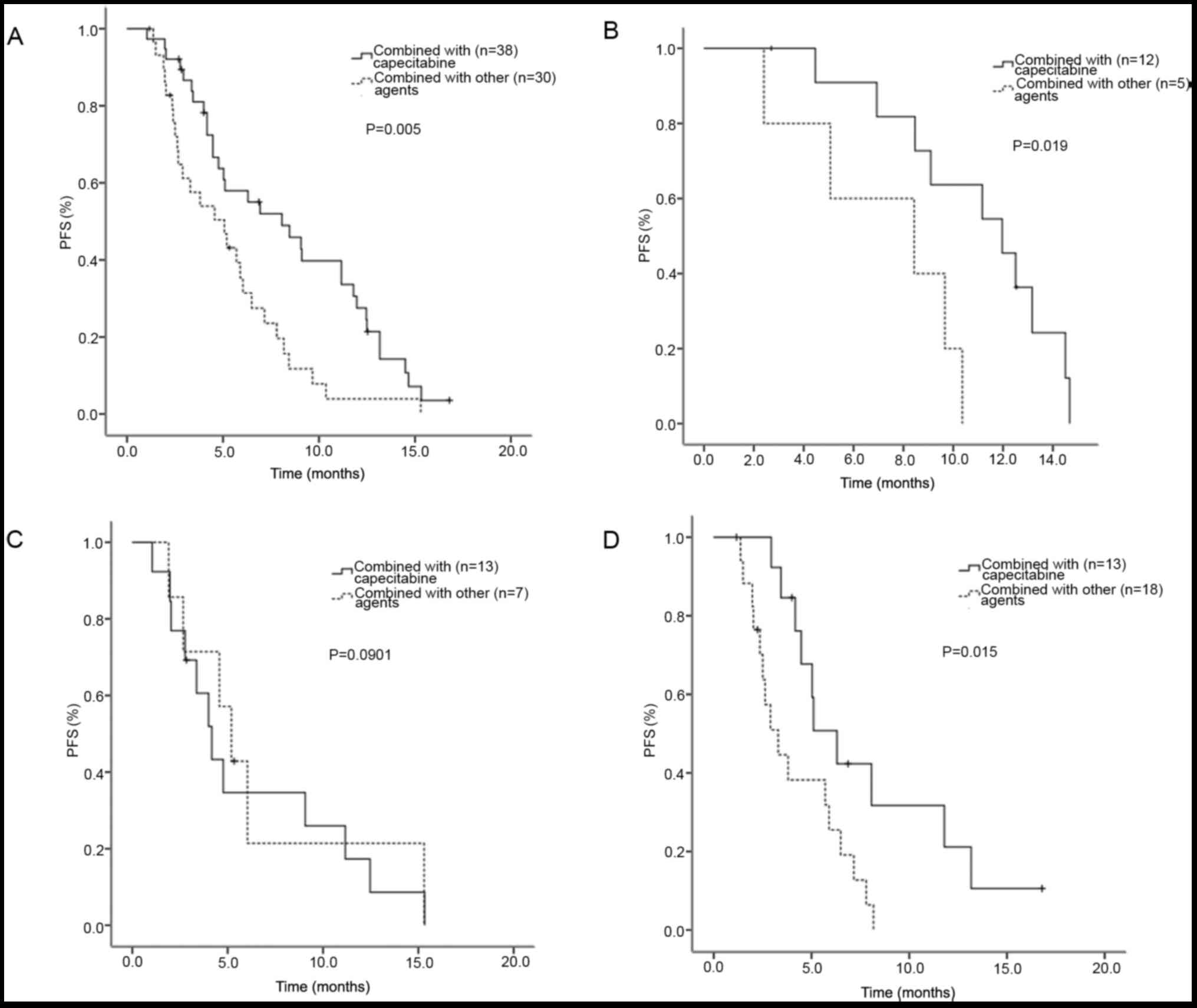
|
1
|
Lim B, Murthy RK, Lee J, Jackson SA, Iwase
T, Davis DW, Willey JS, Wu J, Shen Y, Tripathy D, et al: A phase Ib
study of entinostat plus lapatinib with or without trastuzumab in
patients with HER2-positive metastatic breast cancer that
progressed during trastuzumab treatment. Br J Cancer.
120:1105–1112. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leyland-Jones B: Human epidermal growth
factor receptor 2-positive breast cancer and central nervous system
metastases. J Clin Oncol. 27:5278–5286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaal EC and Vecht CJ: CNS complications of
breast cancer: Current and emerging treatment options. CNS Drugs.
21:559–579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gabos Z, Sinha R, Hanson J, Chauhan N,
Hugh J, Mackey JR and Abdulkarim B: Prognostic significance of
human epidermal growth factor receptor positivity for the
development of brain metastasis after newly diagnosed breast
cancer. J Clin Oncol. 24:5658–5663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pieńkowski T and Zielinski CC: Trastuzumab
treatment in patients with breast cancer and metastatic CNS
disease. Ann Oncol. 21:917–924. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moy B and Goss PE: Lapatinib-associated
toxicity and practical management recommendations. Oncologist.
12:756–765. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murphy CG and Modi S: HER2 breast cancer
therapies: A review. Biologics. 3:289–301. 2009.PubMed/NCBI
|
|
8
|
Ryan Q, Ibrahim A, Cohen MH, Johnson J, Ko
CW, Sridhara R, Justice R and Pazdur R: FDA drug approval summary:
Lapatinib in combination with capecitabine for previously treated
metastatic breast cancer that overexpresses HER-2. Oncologist.
13:1114–1119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bachelot T, Romieu G, Campone M, Diéras V,
Cropet C, Dalenc F, Jimenez M, Le Rhun E, Pierga JY, Gonçalves A,
et al: Lapatinib plus capecitabine in patients with previously
untreated brain metastases from HER2-positive metastatic breast
cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol.
14:64–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verma S, Miles D, Gianni L, Krop IE,
Welslau M, Baselga J, Pegram M, Oh DY, Diéras V, Guardino E, et al:
Trastuzumab emtansine for HER2-positive advanced breast cancer. N
Engl J Med. 367:1783–1791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnston S, Pippen J Jr, Pivot X,
Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A,
Kennedy MJ, et al: Lapatinib combined with letrozole versus
letrozole and placebo as first-line therapy for postmenopausal
hormone receptor-positive metastatic breast cancer. J Clin Oncol.
27:5538–5546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin NU, Diéras V, Paul D, Lossignol D,
Christodoulou C, Stemmler HJ, Roché H, Liu MC, Greil R, Ciruelos E,
et al: Multicenter phase II study of lapatinib in patients with
brain metastases from HER2-positive breast cancer. Clin Cancer Res.
15:1452–1459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bachelot T, Le Rhun E, Labidi-Gally I,
Heudel P, Gilabert M, Bonneterre J, Pierga JY and Gonçalves A:
Systemic treatment of brain metastases from breast cancer:
Cytotoxic chemotherapy and targeted therapies. Bull Cancer.
100:7–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
NCI, . CTCAE. simplehttp://evs.nci.nih.gov.cmich.idm.oclc.org/ftp1/CTCAE/about.htmlMay
17–2010
|
|
16
|
Royston P: Explained variation for
survival models. Stata J. 6:83–96. 2006. View Article : Google Scholar
|
|
17
|
Davis M and Xie SX: Caution: Hazards
crossing! Using the Renyi test statistic in survival analysis.
PharmaSUG2011-Paper SP06, 2011. simplehttps://www.pharmasug.org/proceedings/2011/SP/PharmaSUG-2011-SP06.pdf
|
|
18
|
Greil R, Borštnar S, Petráková K, Marcou
Y, Pikiel J, Wojtukiewicz MZ, Koza I, Steger GG, Linn M, Das Gupta
A and Cwiertka K: Combination therapy of lapatinib and capecitabine
for ErbB2-positive metastatic or locally advanced breast cancer:
Results from the lapatinib expanded access program (LEAP) in
central and Eastern Europe. Onkologie. 34:233–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bian L, Wang T, Zhang S and Jiang Z:
Trastuzumab plus capecitabine vs lapatinib plus capecitabine in
patients with trastuzumab resistance and taxane-pretreated
metastatic breast cancer. Tumour Biol. 34:3153–3158. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cameron D, Casey M, Press M, Lindquist D,
Pienkowski T, Romieu CG, Chan S, Jagiello-Gruszfeld A, Kaufman B,
Crown J, et al: A phase III randomized comparison of lapatinib plus
capecitabine versus capecitabine alone in women with advanced
breast cancer that has progressed on trastuzumab: Updated efficacy
and biomarker analyses. Breast Cancer Res Treat. 112:533–543. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geyer CE, Forster J, Lindquist D, Chan S,
Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A,
Kaufman B, et al: Lapatinib plus capecitabine for HER2-positive
advanced breast cancer. N Engl J Med. 355:2733–2743. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blackwell KL, Burstein HJ, Storniolo AM,
Rugo HS, Sledge G, Aktan G, Ellis C, Florance A, Vukelja S,
Bischoff J, et al: Overall survival benefit with lapatinib in
combination with trastuzumab for patients with human epidermal
growth factor receptor 2-positive metastatic breast cancer: Final
results from the EGF104900 study. J Clin Oncol. 30:2585–2592. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baez-Vallecillo L, Raghavendra AS, Hess
KR, Barcenas CH, Moulder SL, Tripathy D, Valero V and Murthy RK:
Lapatinib activity in metastatic human epidermal growth factor
receptor 2-positive breast cancers that received prior therapy with
trastuzumab, pertuzumab, and/or ado-trastuzumab emtansine (T-DM1).
Breast Cancer Res Treat. 176:227–234. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Untch M and Lück HJ: Lapatinib-member of a
new generation of ErbB-targeting drugs. Breast Care (Basel).
5:8–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chefrour M, Milano G, Formento P,
Giacometti S, Denden A, Renée N, Iliadis A, Fischel JL and
Ciccolini J: Positive interaction between lapatinib and
capecitabine in human breast cancer models: Study of molecular
determinants. Fundam Clin Pharmacol. 26:530–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gril B, Palmieri D, Bronder JL, Herring
JM, Vega-Valle E, Feigenbaum L, Liewehr DJ, Steinberg SM, Merino
MJ, Rubin SD and Steeg PS: Effect of lapatinib on the outgrowth of
metastatic breast cancer cells to the brain. J Natl Cancer Inst.
100:1092–1103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saleem A, Searle GE, Kenny LM, Huiban M,
Kozlowski K, Waldman AD, Woodley L, Palmieri C, Lowdell C, Kaneko
T, et al: Lapatinib access into normal brain and brain metastases
in patients with Her-2 overexpressing breast cancer. EJNMMI Res.
5:302015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morikawa A, Peereboom DM, Thorsheim HR,
Samala R, Balyan R, Murphy CG, Lockman PR, Simmons A, Weil RJ,
Tabar V, et al: Capecitabine and lapatinib uptake in surgically
resected brain metastases from metastatic breast cancer patients: A
prospective study. Neuro Oncol. 17:289–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaplan MA, Isikdogan A, Koca D, Kucukoner
M, Gumusay O, Yildiz R, Dayan A, Demir L, Geredeli C, Kocer M, et
al: Clinical outcomes in patients who received lapatinib plus
capecitabine combination therapy for HER2-positive breast cancer
with brain metastasis and a comparison of survival with those who
received trastuzumab-based therapy: A study by the Anatolian
society of medical oncology. Breast Cancer. 21:677–683. 2014.
View Article : Google Scholar : PubMed/NCBI
|