Introduction
Acute myeloid leukemia (AML) is a malignant clonal
disease of hematopoietic stem cells; chromosomal aberrations have
been recognized as the most important prognostic marker in patients
with AML (1). Of all cases of AML,
40–50% have a normal karyotype (2).
Recent technological advances in gene expression profiling have
allowed for the identification of more aberrantly expressed genes
and the assessment of their predictive value for survival of
patients with AML with normal karyotype (NK-AML). The expression
levels of genes such as neurocaldin delta, MAPK binding protein 1,
copine 3 and RUNX family transcription factor 1 provide further
references for predicting the prognosis of patients with NK-AML
(3–6). Ahn et al (7) reported that in patients with NK-AML,
the presence of mutated chromatin, RNA-splicing genes or both was
associated with poor survival. In general, these studies (3–7) have
indicated high genetic heterogeneity in NK-AML. Patients with
NK-AML exhibit marked differences in terms of prognosis (8). While the significance of single gene
expression in NK-AML progression remains restrictive, gene
expression signature-derived scoring systems have a promising
prognostic value in AML (9).
Previous studies have demonstrated that the immune
system, consisting of immune-related genes (IRGs),
tumor-infiltrating lymphocytes and cytokines, is vital during the
initiation and progression of cancer. The immunological
surveillance and immune escape in bone marrow microenvironment have
an impact on the clinical outcome of patients (10). IRGs actively participate in the
process of immune activity, involving antigen presentation,
cytokine signaling or immune-checkpoint modulation (11). Several IRGs in the
immune/inflammatory response, such as interleukin (IL)-10, IL-15,
Toll-like receptor (TLR)8 and TRAIL, have been indicated as being
involved in the proliferation and differentiation of AML cells
(12–14). Increased soluble IL-2 receptor levels
in adult AML are associated with reduced chemotherapy response and
reduced overall survival time (15).
In addition, clinical benefits have been observed with immune
checkpoint therapies in patients with AML. Programmed cell death
protein 1 and cytotoxic T-lymphocyte antigen 4 inhibitors may be
effective in elderly patients with AML (16). CD163 has been identified as a
potential therapeutic target (17).
However, to the best of our knowledge, the clinical role,
particularly the prognostic role of IRGs in NK-AML, has remained to
be determined.
Based on the aforementioned studies on the influence
of immunity factors on the prognosis of AML, the present study
aimed to identify differentially expressed IRGs in patients with
NK-AML and assess their utility as survival predictors. For this
purpose, public data from The Cancer Genome Atlas (TCGA) and Gene
Expression Omnibus (GEO) databases were subjected to bioinformatics
analyses. Furthermore, given that integrated prognostic analysis of
multiple immune genes is more meaningful compared with the
predictive value of a single gene, the present study aimed to
propose an integrative risk score model consisting of the IRGs
associated with prognosis. Data of the immune status of patients
with NK-AML enrolled in the present study from obtained from two
databases and further investigated.
Materials and methods
Study design
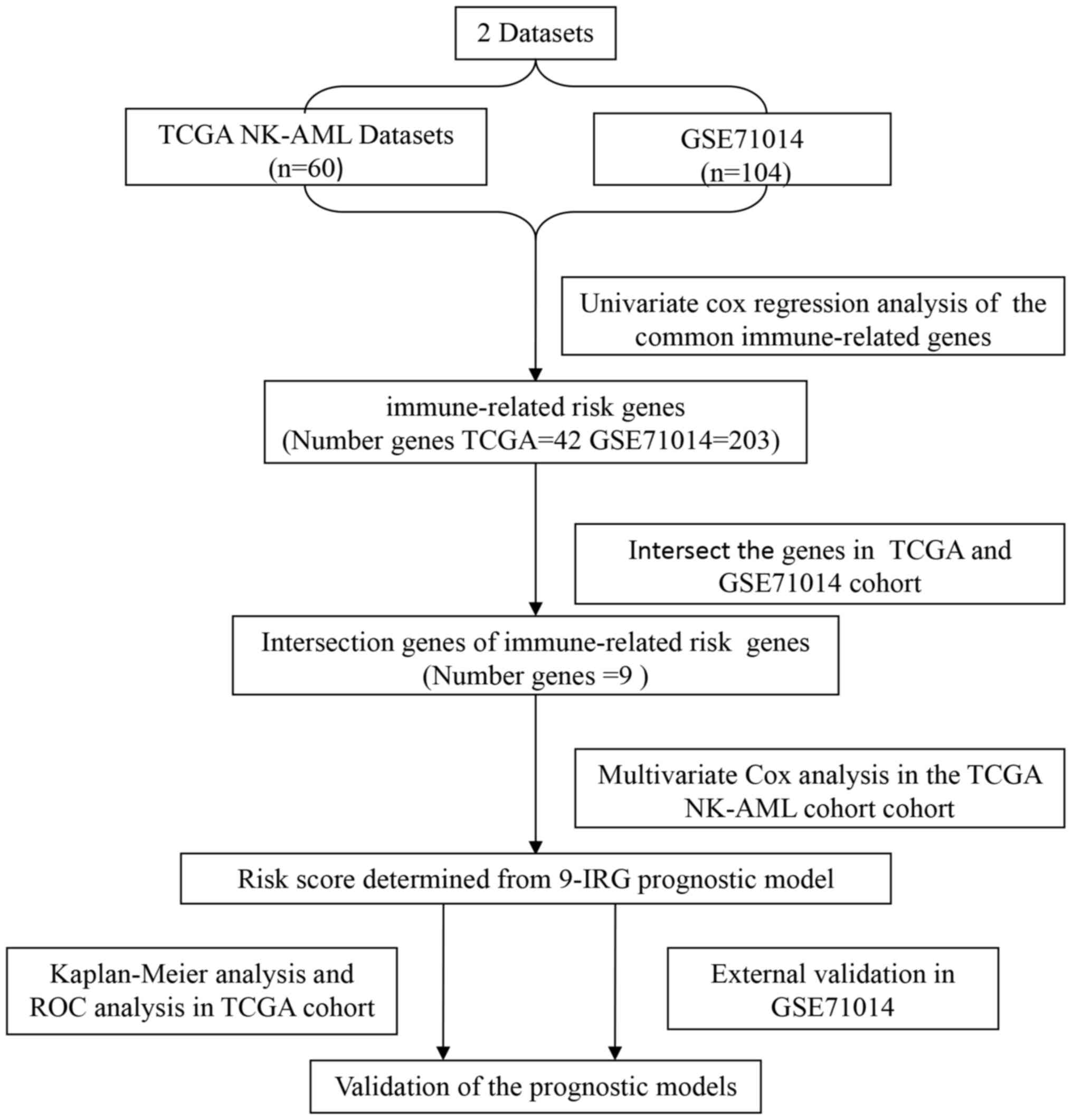
A flowchart illustrating the process of establishing
the IRG risk score (IRGRS) model is presented in Fig. 1. The gene expression profiles and
clinical data of patients with NK-AML from TCGA (https://tcga-data.nci.nih.gov/tcga/) and the GEO
datasets (GSE71014) (https://ncbi.nlm.nih) were retrospectively analyzed.
The expression levels of the common IRGs were screened from all
protein-coding genes of the two datasets. Subsequently, the
prognostic genes in TCGA and GEO datasets were identified by
univariate Cox analysis. The survival-associated genes intersection
of TCGA and GEO data were further analyzed to construct the
prognostic model in the TCGA cohort. The GEO cohort (GSE71014) was
employed to validate the immune-associated risk score model.
Statistical analyses were performed using R software (version
3.5.3; http://www.r-project.org/) and
Bioconductor (version 3.5.3; http://www.bioconductor.org/) was utilized for the
bioinformatics analysis.
Patient data
The RNA-sequencing (RNA-seq) datasets of patients
with AML, which had been experimentally determined using the
Illumina HiSeq 2000 RNA Sequencing platform, were obtained from
TCGA database (18). The
corresponding clinical information was downloaded from the
University of California Santa Cruz (UCSC) Xena site (https://xenabrowser.net/), including age, sex,
leukemia French American British morphology code, the molecular
analysis of nucleophosmin 1 (NPM1) and fms related receptor
tyrosine kinase 3 (FLT3) mutations, survival time and outcome.
Patients with normal karyotype were included and patients with
acute promyelocytic leukemia were excluded. A total of 60 adult
patients were selected from TCGA. There were 30 males (50.0%) and
30 females (50.0%) with a median age of 53.1 (range, 21–88) years.
All gene expression levels were detected using the RNA-seq method
in bone marrow cells of patients with NK-AML. The present study
also included a total of 104 adult patients with NK-AML with a
median age of 58 (range, 16–90) years from the GSE71014 dataset
(19). To generate this dataset,
cryopreserved bone marrow cells had been obtained from 104 patients
with de novo AML and each sample was analyzed using the
Illumina HumanHT-12 V4.0 expression beadchip (GPL10558). The gene
expression profiles were acquired from the matched probes and were
used for further analysis. In the present study, the IRGs were
acquired from the ImmPort database, comprising 2,498 genes
(https://immport.niaid.nih.gov) (20). The immune-associated genes that were
upregulated or downregulated in TCGA and GSE71014 datasets was
selected.
Construction and evaluation of the IRG
risk score
The immune-associated genes in two datasets that
were significantly associated with the overall survival of NK-AML
were identified using univariate Cox analysis respectively
(P≤0.005). The intersection of prognosis-associated IRGs in TCGA
and GSE71014 datasets were selected and displayed in a Venn diagram
(http://bioinformatics.psb.ugent.be/webtools/Venn/).
The prognosis-associated IRGs were then submitted for multivariate
analyses. Prognostic analysis was performed using the R package
‘survival’ (https://CRAN.R-project.org/package=survival; version
3.1–8). For the establishment of the prognostic score, the TCGA
dataset was adopted. The IRGRS model was constructed by multiplying
the gene expression levels of prognosis-associated IRGs with the
corresponding Cox coefficient (coe), as follows: Risk
score=∑ni IRGi × coei,
where i represents a certain specific IRG and n is the number of
all IRGs. This was accomplished using R package ‘states’
(https://github.com/andybega/states;
version 0.2.2).
The risk score of each patient in the TCGA cohort
was calculated according to this formula. The cut-off value was set
as the median risk score and all patients were divided into high-
and low-risk groups based on this value. Subsequently, it was
investigated whether the survival times were different between the
two groups. Kaplan-Meier curves were plotted for the OS of patients
with high-and low- risk scores. Survival outcomes were compared
using the log-rank test. The receiver operating characteristic
(ROC) analysis was used to evaluate the predictive performance of
the IRGRS model, making a distinction between the high-risk and
low-risk group. Areas under the curve (AUCs), optimal threshold
values, sensitivity and specificity, were determined using the R
package ‘survivalROC’ (https://CRAN.R-project.org/package=survivalROC;
version 1.0.3), to evaluate the significance of the survival
difference between the two groups. In order to demonstrate whether
the IRGRS may act as an independent risk factor associated with
survival, the IRGRS for patients with NK-AML were assessed using
univariate and multivariate Cox analyses which were adjusted for
age, sex and the mutation of NPM1 and FLT3. Among them, 60 years
was the cut-off for age grouping. In addition, the correlations
between IRGRS and Tregs were investigated were investigated, which
were assessed using Spearman's correlation.
Tumor immunity relevance of the
IRGRS
Regarding the close association between the IRGs and
immune cells of the bone marrow microenvironment, the immune
landscape was assessed in the present study. The abundance of 22
types of infiltrating immune cells of each patient with NK-AML in
the TCGA cohort was estimated by translating the expression levels
of genes downloaded from the TCGA cohort into the relative
proportion of immune cells. This was achieved with the CIBERSORT
algorithm based on the deconvolution, using the ‘CIBERSORT’ R
package (CIBERSORT R script v1.03; http://cibersort.stanford.edu/). This tool was
developed by Newman et al (21) and has been validated to quantify the
abundance of specific cell types successfully. Inaccurate samples
were eliminated (P≥0.05). The 22 types of immune cells contain B,
regulatory T cells (Tregs), CD4+T, CD8+ T,
natural killer, mast, plasma and dendritic cells, neutrophils,
eosinophils and macrophages. The association between IRGRS and
immune cell infiltration level in NK-AML was analyzed in the TCGA
dataset.
Validation of the immune-associated
risk score model for NK-AML
In order to evaluate the robustness of the IRGRS in
the prognostication of patients with NK-AML, this prognostic model
was tested in the GEO dataset GSE71014 as an independent validation
set, using the same analytical method used for the TCGA cohort.
Patients were divided into high- and low-risk groups with the
median risk score of the TCGA utilized as the cut-off value. The
GSE71014 dataset was not able to be used to validate the
association between the prognostic risk models and the clinical
characteristics due to a lack of clinical parameters.
Results
Construction of the IRGRS model and
internal validation
The aim of the present study was to construct an
immune-associated prognostic model of NK-AML. A total of 164
patients with NK-AML were included in the two cohort studies. In
the TCGA NK-AML cohort (n=60), 42 immune-associated prognostic
genes were identified. In the 103 NK-AML samples of the GSE71014
cohort, the expression of 203 IRGs was significantly associated
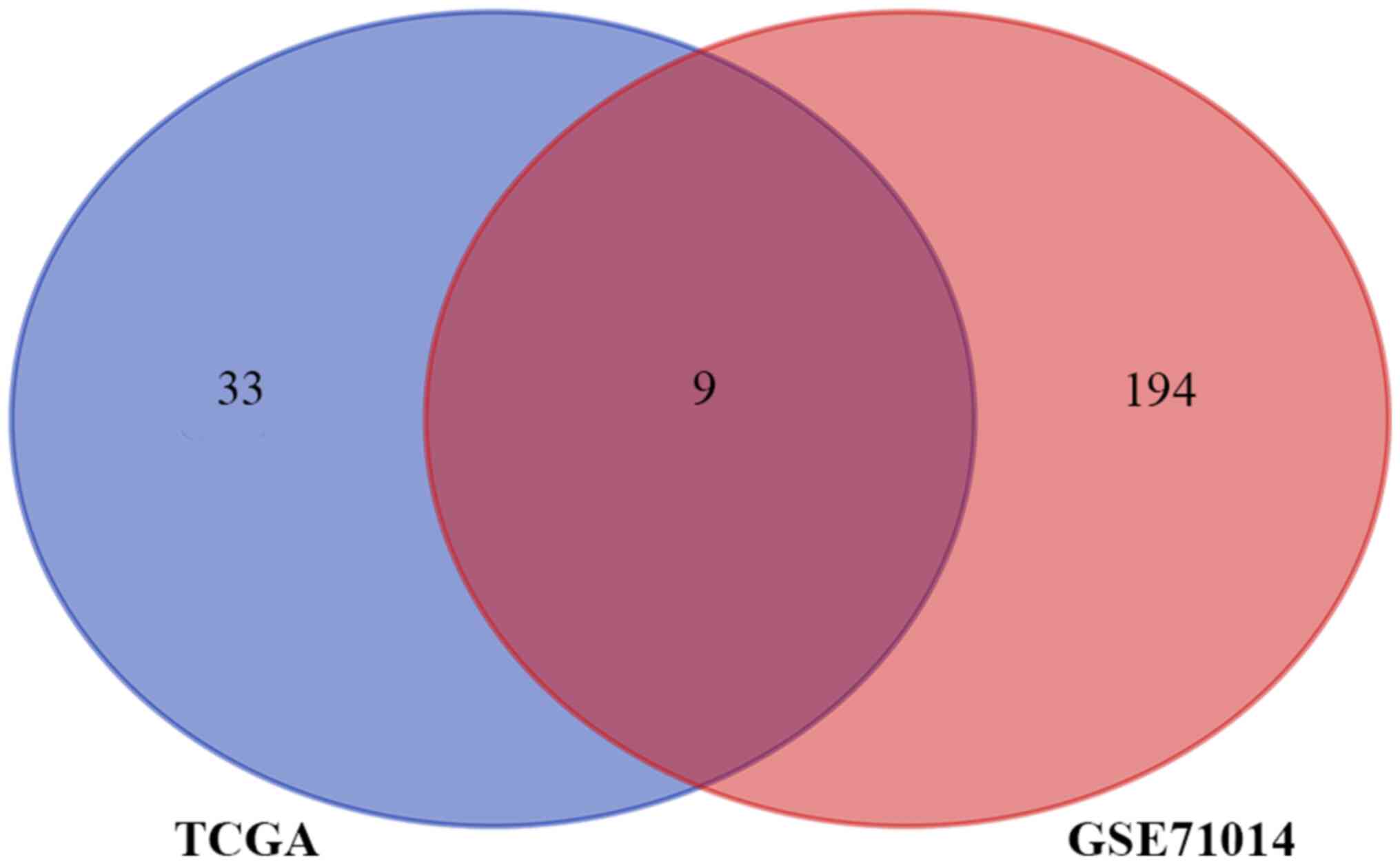
with OS (P<0.05). The Venn diagram represents the number of
overlapping IRGs between the two cohorts (n=9; Fig. 2). These nine genes were zinc finger
CCCH-type containing, antiviral 1 like (ZC3HAV1L), transferrin
receptor (TFRC), suppressor of cytokine signaling 1 (SOCS1), ELAV
like RNA binding protein 1 (ELAVL1), roundabout guidance receptor 3
(ROBO3), unc-93 homolog B1, TLR signaling regulator (UNC93B1),
protein tyrosine phosphatase non-receptor type 6 (PTPN6), IL-2
receptor subunit alpha (IL2RA) and IL3RA. The hazard ratio (HR),
95% CI and P-value of univariate analyses for these nine IRGs in
the TCGA cohort affecting OS are presented in Table I. In order to construct an IRG-based
prognostic model to predict the risk of NK-AML, the nine prognostic
IRGs were subjected to multivariate regression analysis using the
Cox proportional hazards model for OS in the TCGA cohort. According
to the corresponding coefficient value and expression value for
each gene, the formula to calculate the risk score was as follows:
IRGRS=[(−0.33298) × expression level of ZC3HAV1L] + [(−0.27376) ×
expression level of TFRC] + [(0.10993) × expression level of SOCS1]
+ [(−0.09726) × expression level of ELAVL1) + [(0.22322) ×
expression level of ROBO3] + [(−0.0229) × expression level of
UNC93B1] + [(0.246114) × expression level of PTPN6] + [0.227133 ×
expression level of IL2RA] + [(0.217204) × expression level of
IL3RA] (cut-off=1.006977).
 | Table I.Univariate Cox analysis for overall
survival of the nine immune-related genes in The Cancer Genome
Atlas cohort. |
Table I.
Univariate Cox analysis for overall
survival of the nine immune-related genes in The Cancer Genome
Atlas cohort.
| Gene name | HR | 95% CI | P-value |
|---|
| ZC3HAV1L | 0.736 | 0.545–0.994 | 0.045 |
| TFRC | 0.633 | 0.450–0.889 | 0.008 |
| SOCS1 | 1.621 | 1.217–2.159 | 0.001 |
| ELAVL1 | 0.400 | 0.165–0.972 | 0.043 |
| ROBO3 | 1.329 | 1.042–1.695 | 0.022 |
| UNC93B1 | 1.494 | 1.098–2.034 | 0.011 |
| PTPN6 | 1.557 | 1.022–2.371 | 0.039 |
| IL2RA | 1.354 | 1.141–1.606 | 0.001 |
| IL3RA | 1.593 | 1.105–2.296 | 0.013 |
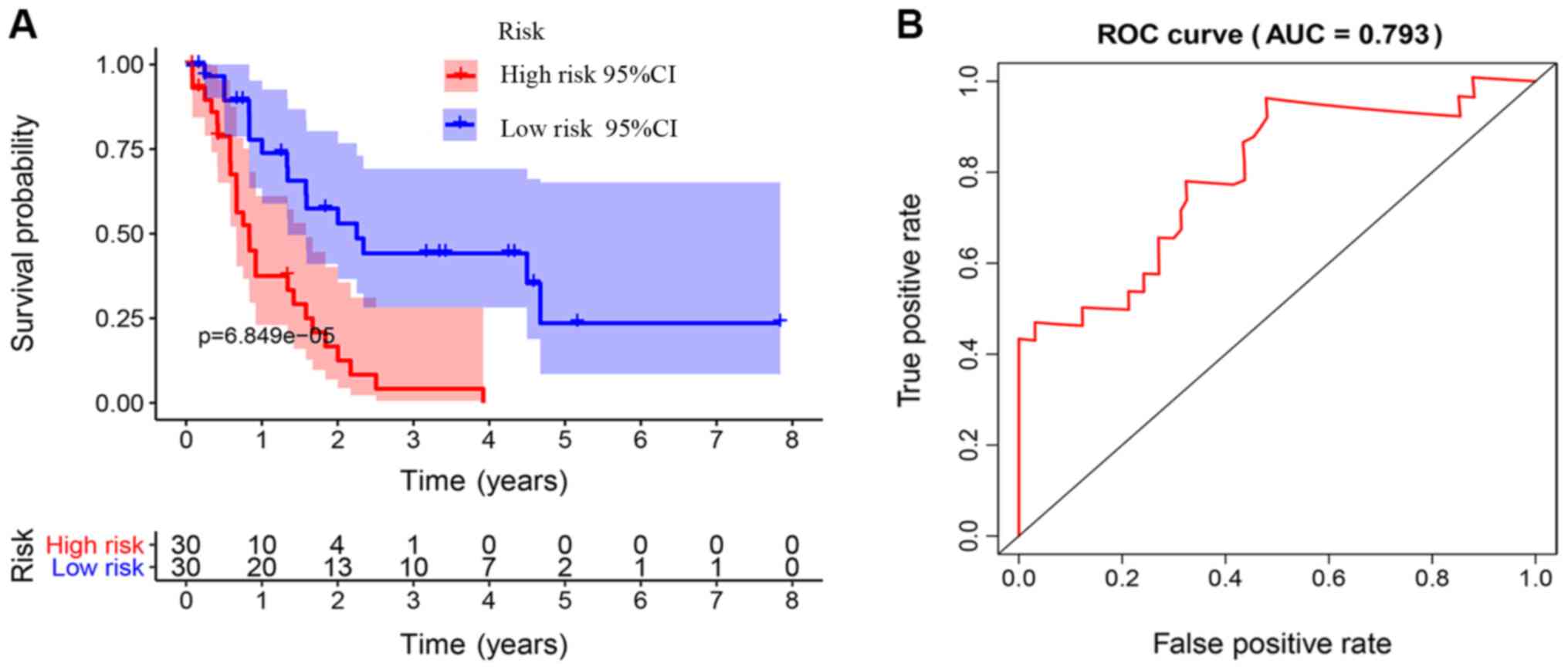
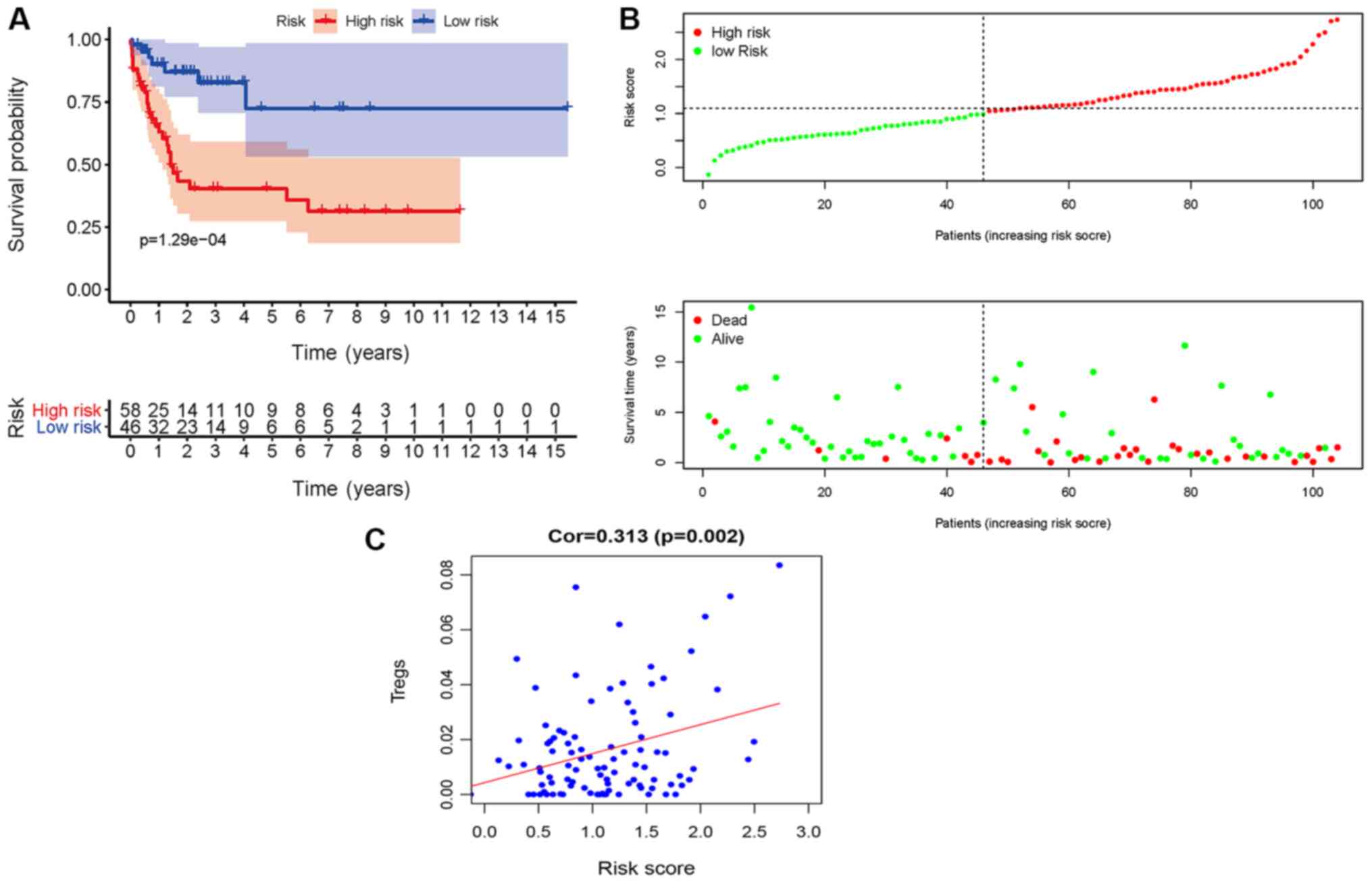
The Kaplan-Meier survival curves revealed that
patients in the high-risk group exhibited a significantly lower OS
rate than the low-risk group (P<0.01; Fig. 3A). The estimated 5-year OS rate was
8.0 vs. 54.9% for the high-risk and the low-risk group,
respectively. The AUC of the ROC curve for OS prediction was 0.793,
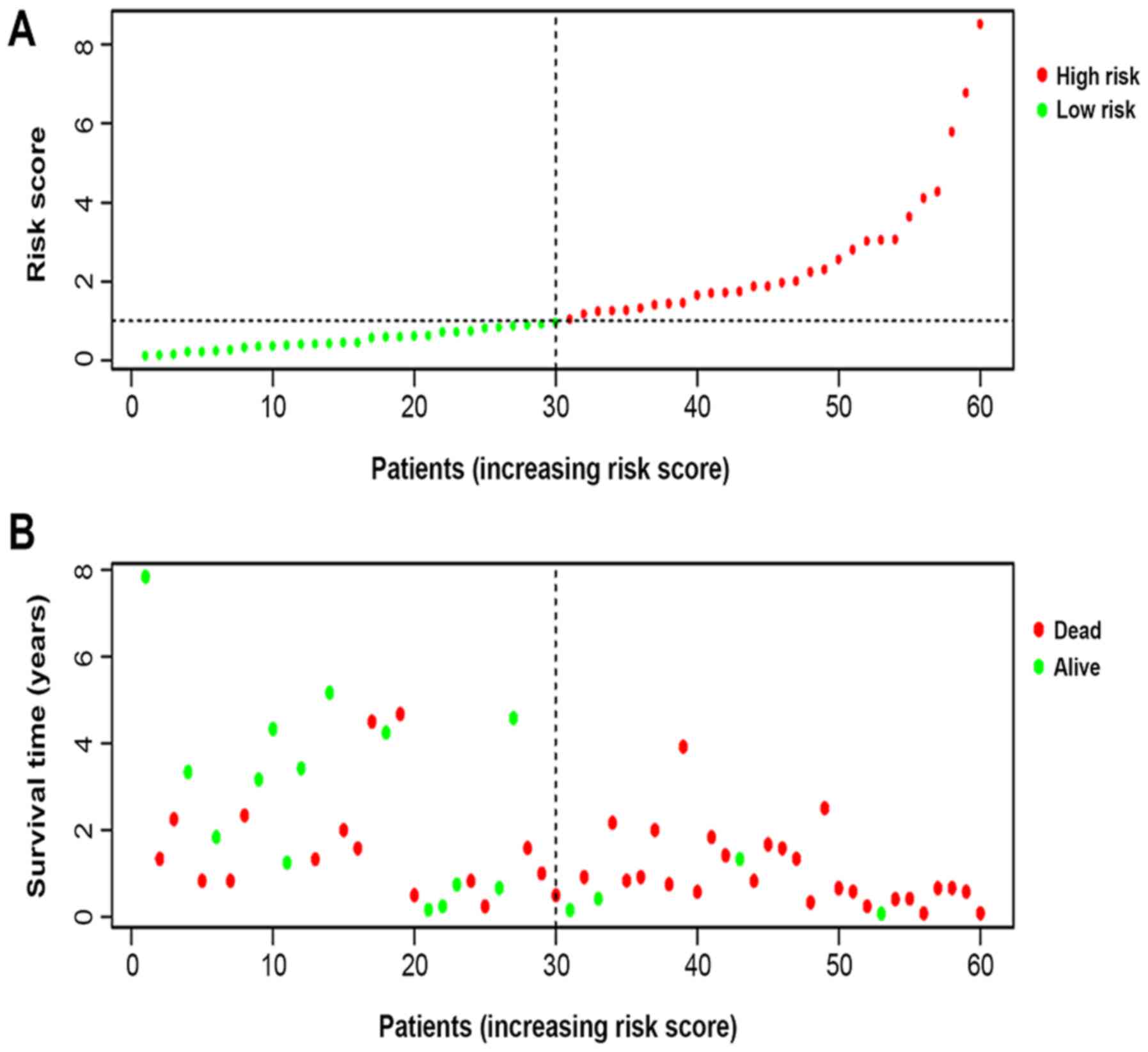
indicating a promising predictive value (Fig. 3B). In order to further investigate
the impact of the IRGRS on the clinical outcomes, a risk curve was
drawn. The prognostic model separates the NK-AML patients into two
groups with discrete clinical outcomes (Fig. 4A). It also revealed that as the risk
score increased, the survival time to decrease. (Fig. 4B). In the univariate regression
analysis, the IRGRS was significantly associated with OS (HR,
1.749; 95% CI, 1.440–2.124; P<0.001). This result was consistent
with that of the multivariate analysis (HR, 1.662; 95% CI,
1.321–2.091; P<0.001), which considered age, sex, the FAB
classification, the type of NPM1 and the FLT3 genotype as
co-effectors (Table II). These
results demonstrated that the IRGRS is an independent predictor of
OS in patients with NK-AML.
 | Table II.Univariate and multivariate Cox
analysis for overall survival of risk score and clinical parameters
in The Cancer Genome Atlas cohort. |
Table II.
Univariate and multivariate Cox
analysis for overall survival of risk score and clinical parameters
in The Cancer Genome Atlas cohort.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≥60
years) | 1.023 | 1.004–1.041 | 0.016 | 1.018 | 0.998–1.038 | 0.074 |
| Sex (male) | 0.738 | 0.400–1.362 | 0.331 | 0.497 | 0.243–1.018 | 0.056 |
| Grade (0–7) | 1.160 | 0.954–1.411 | 0.136 | 0.987 | 0.778–1.252 | 0.913 |
| FLT3
(positive) | 3.004 | 1.578–5.719 | 0.001 | 3.434 | 1.469–8.028 | 0.004 |
| NPM1
(positive) | 0.918 | 0.497–1.694 | 0.784 | 0.391 | 0.185–0.828 | 0.014 |
| Risk score
(>1.006977) | 1.749 | 1.440–2.125 | <0.001 | 1.662 | 1.321–2.091 | <0.001 |
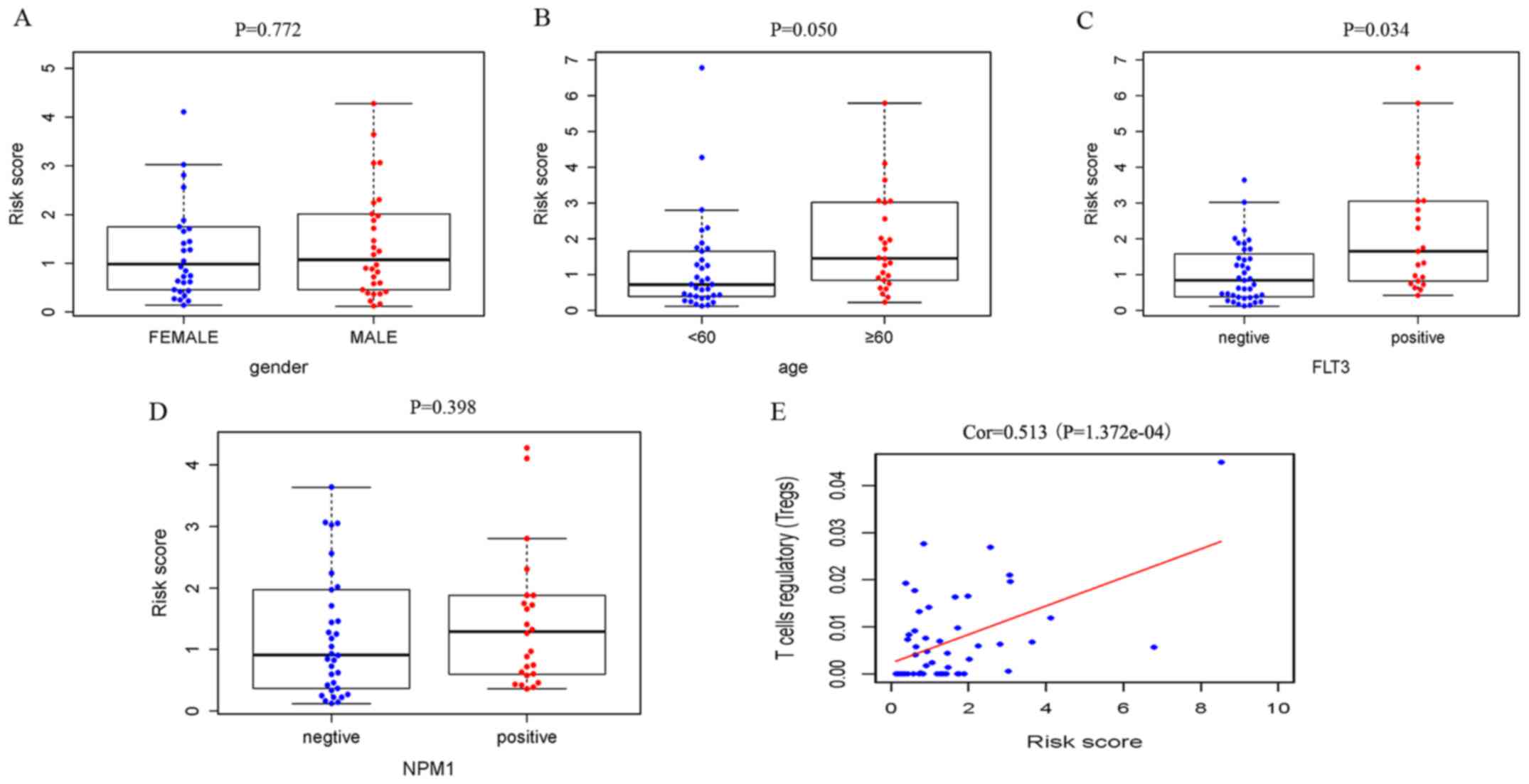
Correlation of the IRGRS with clinical
and immune characteristics
Relationships were analyzed between IRGRS model risk
score and clinical characteristics, including age, sex and the
mutations of NPM1 and FLT3. We also explored the relationships
between nine prognostic IRGs expression and this clinical
characteristics. (Table III). The
risk scores in the elderly group and FLT3 mutant group were
significantly higher compared with those in the non-elderly group
and FLT3 wild-type group. It was revealed that a high risk score
was associated with higher age (P=0.050) and a higher incidence of
FLT3 mutation (P=0.034), and there was no significant association
with sex (P=0.772) and NPM1 mutation (P=0.398; Fig. 5).
 | Table III.Association between immune indices
and demographic features and FLT3 and NPM1 mutation. |
Table III.
Association between immune indices
and demographic features and FLT3 and NPM1 mutation.
|
| Age, ≥60 vs. <60
years | Sex, male vs.
female | FLT3, mutant vs.
wildtype | NPM1, mutant vs.
wildtype |
|---|
|
|
|
|
|
|
|---|
| Gene | t | P-value | t | P-value | t | P-value | t | P-value |
|---|
| ZC3HAV1L | 1.879 | 0.067 | −0.355 | 0.724 | 0.721 | 0.475 | 1.359 | 0.180 |
| TFRC | 0.394 | 0.695 | 0.030 | 0.976 | 3.277 | 0.002 | 3.796 | <0.001 |
| SOCS1 | −2.86 | 0.006 | 0.752 | 0.455 | −1.579 | 0.123 | −0.678 | 0.501 |
| ELAVL1 | 1.332 | 0.191 | −1.349 | 0.183 | 1.790 | 0.080 | 1.595 | 0.116 |
| ROBO3 | −2.543 | 0.014 | −1.776 | 0.081 | −0.875 | 0.386 | 1.916 | 0.060 |
| UNC93B1 | −1.733 | 0.089 | −0.498 | 0.621 | −1.266 | 0.213 | −0.484 | 0.630 |
| PTPN6 | −1.341 | 0.186 | −0.046 | 0.964 | −1.611 | 0.116 | −1.736 | 0.089 |
| IL2RA | −1.142 | 0.258 | 0.373 | 0.710 | −2.494 | 0.018 | −0.68 | 0.500 |
| IL3RA | −0.851 | 0.399 | 0.604 | 0.549 | −0.526 | 0.601 | 1.019 | 0.312 |
| Risk score | −2.02 | 0.050 | 0.291 | 0.772 | −2.208 | 0.034 | −0.852 | 0.398 |
For the immunity relevance analysis of the IRGRS, 50
samples were available from the TCGA database, whose relative
content of immune cells had been precisely estimated. A correlation
analysis of the ratio of infiltrating immune cells and the IRGRS
was performed (Table IV). In TCGA,
significant correlations were identified between the IRGRS and
monocytes (P=0.014), NK resting cells (P=0.028) and Tregs
(P<0.001). As presented in Figs.
5E and 6C, a significantly
positive correlation was observed between the amount of Tregs and
the IRGRS in both TCGA and GEO databases (P<0.05).
 | Table IV.Correlation between immune scores and
immune cell fractions. |
Table IV.
Correlation between immune scores and
immune cell fractions.
|
| TCGA cohort | GEO cohort |
|---|
|
|
|
|
|---|
| Immune cell
type | Coef | P-value | Coef | P-value |
|---|
| B memory cells | −0.200 | 0.163 | 0.083 | 0.421 |
| Macrophages M0 | −0.166 | 0.250 | 0.006 | 0.957 |
| Macrophages M1 | −0.084 | 0.562 | −0.096 | 0.347 |
| Macrophages M2 | −0.040 | 0.780 | −0.105 | 0.306 |
| Monocytes | 0.345 | 0.014 | 0.086 | 0.403 |
| NK activated
cells | 0.050 | 0.729 | 0.138 | 0.179 |
| NK resting
cells | −0.311 | 0.028 | −0.001 | 0.993 |
| Plasma cells | −0.247 | 0.084 | −0.209 | 0.039 |
| Activated
CD4+ | −0.082 | 0.572 | 0.081 | 0.431 |
| T memory cells |
| Resting
CD4+ | −0.133 | 0.356 | −0.115 | 0.263 |
| T memory cells |
| CD4+
naïve T cells | −0.124 | 0.391 | −0.074 | 0.474 |
| CD8+ T
cells | −0.011 | 0.941 | 0.047 | 0.647 |
| T follicular helper
cells | −0.127 | 0.380 | −0.040 | 0.697 |
| T γ Δ cells | −0.084 | 0.561 | −0.308 | 0.002 |
| T regulatory
cells | 0.513 | <0.001 | 0.313 | 0.002 |
| B naïve cells | 0.009 | 0.952 | −0.003 | 0.978 |
| Activated dendritic
cells |
|
| −0.121 | 0.238 |
| Resting dendritic
cells |
|
| 0.268 | 0.008 |
| Eosinophils |
|
| 0.038 | 0.708 |
| Activated mast
cells |
|
| −0.165 | 0.105 |
| Resting mast
cells |
|
| −0.046 | 0.653 |
| Neutrophils |
|
| −0.009 | 0.932 |
Validation in the GEO database
Finally, the predictive model based on the IRGRS for
NK-AML was further validated in the GSE71014 cohort. Application of
the model to the validation cohort in a survival analysis was able
to distinctly discriminate patients with different survival time
according to their risk score value (Fig. 6A and B). Furthermore, in this cohort,
Tregs were positively correlated with the IRGRS (P=0.002; Fig. 6C). Plasma cells, T γ Δ cells and
resting dendritic cells were also correlated with the IRGRS. Taken
together, the data of the present study indicated that the
predictive IRGRS model was of great prognostic value in NK-AML.
Discussion
The significance of IRGs in AML progression and
immunotherapeutics have been reported previously (22,23).
However, to the best of our knowledge, no comprehensive,
genome-wide profiling analysis of the prognostic significance of
all IRGs in NK-AML has so far been performed. Thus, the expression
levels of IRGs in 164 patients with NK-AML were analyzed in the
present study and the prognostic value of IRGs was subsequently
assessed. A total of 42 and 203 IRGs with prognostic value were
identified from the TCGA and GEO cohort, respectively. Between the
two cohorts, nine overlapping IRGs with prognostic value in NK-AML
were identified. Due to the limited power of individual indicators
to predict prognosis, multivariate Cox regression analysis was used
to analyze the prognostic significance of nine IRGs and their
combination in NK-AML. The prognostic assessment model used in the
present study was combined based on the regression coefficient and
expression value of each of those nine IRGs. The AUC of the ROC
curve was 0.793, suggesting moderate potential for evaluating the
prognosis of patients with NK-AML based on the IRGRS model. The
present study then validated the predictive capability of the risk
model in another independent cohort, namely the 104 patients from
GSE71014. The subgroup of patients with NK-AML with high risk
scores exhibited poor survival. The IRGs-based risk score was
demonstrated to be of high prognostic value to predict the survival
of patients with NK-AML in the clinic and it provided valuable
biomarkers for this disease.
In addition, the present study also determined that
the nine IRGs constituting the model exhibited distinct
risk-associated patterns, including 6 relatively high-risk genes
(SOCS1, ROBO3, UNC93B1, PTPN6, IL2RA and IL3RA) and 3 relatively
low-risk genes (ZC3HAV1L, TFRC and UNC93B1). To the best of our
knowledge, the ZC3HAV1L gene has not been extensively investigated;
whereas its paralog ZC3HAV1 has been reported to participate in the
innate antiviral immune process (24). TFRC, a gene that takes part in iron
uptake, has the role of regulating the proliferation of T and B
cells. Although it is not considered relevant to the prognosis of
numerous different types of cancer, the prognostic value of TRFC in
AML remains controversial (25–27).
SOCS1 is involved in the negative regulation of cytokines in the
JAK/STAT3 signaling pathway. A study by Hou et al (28) revealed that its high expression was
indicative of unfavorable survival prognosis. ELAVL1, encoding
RNA-binding proteins, has been studied in a variety of tumor types.
It has been demonstrated to regulate the expression of tumor
protein 53 to mediate anti-proliferative activity and has an
important role in the differentiation of myeloid cells in AML
(29). ROBO3, an immunoglobulin
transmembrane receptor gene, is involved in the SLIT/ROBO pathway
associated with the development and progression of certain types of
cancer, where AML is also included (30,31).
UNC9381 was reported to be implicated in the innate and adaptive
immune response by regulating the TLR pathway, whose activation has
been observed in tumors (32,33). To
the best of our knowledge, the involvement of the UNC9381 gene in
AML has not been previously reported. PTPN6 is known to regulate
processes of cell proliferation and differentiation, the mitotic
cycle and oncogenic transformation. The implication of PTPN6 in AML
has gradually attracted increasing attention. It is primarily
expressed in hematopoietic cells and may have a marked effect on
the pathogenesis of leukemia (34).
IL2RA is a receptor for IL-2, which is involved in the regulation
of immune tolerance by controlling the activity of Tregs, whose
expression was recognized as a poor prognostic marker of leukemia
(15,35,36).
IL3RA, also named CD123, regulates the development of hematopoietic
and immune cells. Its high expression has been indicated to be
associated with poor prognosis in AML (37). However, the underlying molecular
mechanisms of the nine IRGs corresponding to their potential
clinical value in NK-AML require further investigation.
Poor prognosis in elderly patients and patients with
FLT3 mutations has been widely recognized (38). Of note, the results of the present
study revealed that the IRG model was associated with age and the
FLT3 mutation. An obviously higher proportion of older age over 60
and FLT3 mutation in patients with NK-AML was observed in the
higher risk score group. Furthermore, the IRG model may not only
serve as a prognostic indicator but also as an immune status
indicator. The present study investigated the associations between
the IRG model and immune cell infiltration in order to reflect the
status of the immune microenvironment of NK-AML. In TCGA cohort,
the levels of infiltrating monocytes and Tregs were positively
correlated with IRGRS, and NK resting cells were negatively
correlated with IRGRS. In the GEO cohort, resting dendritic cells,
T γ Δ cells and Tregs were positively correlated with IRGRS, whilst
plasma cells and T γ Δ cells were negatively correlated with IRGRS.
The level of infiltrating Tregs was significantly positively
correlated with IRGRS in two cohorts. The percentage of circulating
Tregs was higher in patients with AML compared with that in normal
controls and Tregs accumulating in the peripheral circulation
vigorously mediate immune suppression (38). Patients with lower Treg frequency at
diagnosis have a better response to induction chemotherapy
(16,39). It has been reported that an increased
amount of Tregs is associated with an elevated risk of relapse in
AML (40). The results of the
present study also suggested that the IRGRS model has the potential
to serve as a predictor for increased immune cell infiltration. The
role of immune cells in AML has remained to be fully investigated.
Radpour et al (41) indicated
that CD8+ T cells expand stem and progenitor cells in
low- but not high-risk AML. Immune cells with statistical
differences only in the TCGA or GEO cohort could not determine
their correlation with risk models. The role of immune cells in
acute myeloid leukemia still requires further studies with larger
samples. The preliminary observation of the present study may
provide a perspective for further investigation, which is required
in the future.
However, there were certain limitations to the
present study. First, the potential mechanism of these nine IRGs
and Treg cell-mediated immune infiltration in NK-AML progression
remains elusive and further in vitro or in vivo
experiments are required to clarify this. Furthermore,
transcriptome analysis is not able to reflect the global immune
status. In addition, the validity of the prognostic model based on
the nine IRGs identified in the present study requires to be
verified using more independent samples.
In conclusion, in the present study, the clinical
and prognostic value of IRGs in NK-AML was systematically analyzed.
An IRG-based prognostic evaluation model was constructed with a
moderate level of performance in the prognostication of patients
with NK-AML. The results of the present study provide novel
approaches for immunotherapy for patients with NK-AML.
Acknowledgements
Not applicable.
Funding
The present study was funded by Youth Support
Project of Luhe Hospital (grant no. LHYH2019-JC06).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request. The raw datasets used during the current study are
available in the corresponding repositories, including the TCGA
(https://tcga-data.nci.nih.gov/tcga/),
GEO (ncbi.nlm.nih.gov/geo/), UCSC Xena (https://xenabrowser.net/) and the ImmPort database
(https://immport.niaid.nih.gov).
Authors' contributions
XD and DZ conceived and designed the study. XD, XC,
YoZ, XZ and JZ analyzed the data. DZ, TC, and YuZ analyzed the data
and generated the figures. XD and HZ made contributions to analysis
and interpretation of data and wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Grimwade D, Walker H, Oliver F, Wheatley
K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A and
Goldstone A: The importance of diagnostic cytogenetics on outcome
in AML: Analysis of 1,612 patients entered into the MRC AML 10
trial. The medical research council adult and children's leukaemia
working parties. Blood. 92:2322–2333. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kihara R, Nagata Y, Kiyoi H, Kato T,
Yamamoto E, Suzuki K, Chen F, Asou N, Ohtake S, Miyawaki S, et al:
Comprehensive analysis of genetic alterations and their prognostic
impacts in adult acute myeloid leukemia patients. Leukemia.
28:1586–1595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song Y, Zhang W, He X, Liu X, Yang P, Wang
J, Hu K, Liu W, Zhang X, Jing H and Yuan X: High NCALD expression
predicts poor prognosis of cytogenetic normal acute myeloid
leukemia. J Transl Med. 17:1662019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fu L, Shi J, Hu K, Wang J, Wang W and Ke
X: Mitogen-activated protein kinase binding protein 1 (MAPKBP1) is
an unfavorable prognostic biomarker in cytogenetically normal acute
myeloid leukemia. Oncotarget. 6:8144–8154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fu L, Fu H, Qiao J, Pang Y, Xu K, Zhou L,
Wu Q, Li Z, Ke X, Xu K and Shi J: High expression of CPNE3 predicts
adverse prognosis in acute myeloid leukemia. Cancer Sci.
108:1850–1857. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu L, Fu H, Tian L, Xu K, Hu K, Wang J,
Wang J, Jing H, Shi J and Ke X: High expression of RUNX1 is
associated with poorer outcomes in cytogenetically normal acute
myeloid leukemia. Oncotarget. 7:15828–15839. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahn JS, Kim HJ, Kim YK, Lee SS, Ahn SY,
Jung SH, Yang DH, Lee JJ, Park HJ, Lee JY, et al: Assessment of a
new genomic classification system in acute myeloid leukemia with a
normal karyotype. Oncotarget. 9:4961–4968. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slovak ML, Kopecky KJ, Cassileth PA,
Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR,
Rowe JM, et al: Karyotypic analysis predicts outcome of
preremission and postremission therapy in adult acute myeloid
leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology
Group study. Blood. 96:4075–4083. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bullinger L, Döhner K, Bair E, Fröhling S,
Schlenk RF, Tibshirani R, Döhner H and Pollack JR: Use of
gene-expression profiling to identify prognostic subclasses in
adult acute myeloid leukemia. N Engl J Med. 350:1605–1616. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Teague RM and Kline J: Immune evasion in
acute myeloid leukemia: Current concepts and future directions. J
Immunother Cancer. 1:12013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Austin R, Smyth MJ and Lane SW: Harnessing
the immune system in acute myeloid leukaemia. Crit Rev Oncol
Hematol. 103:62–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nishioka C, Ikezoe T, Pan B, Xu K and
Yokoyama A: MicroRNA-9 plays a role in interleukin-10-mediated
expression of E-cadherin in acute myelogenous leukemia cells.
Cancer Sci. 108:685–695. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanchez-Correa B, Bergua JM, Pera A,
Campos C, Arcos MJ, Bañas H, Duran E, Solana R and Tarazona R:
In vitro culture with interleukin-15 leads to expression of
activating receptors and recovery of natural killer cell function
in acute myeloid leukemia patients. Front Immunol. 8:9312017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Galen P, Hovestadt V, Wadsworth Ii MH,
Hughes TK, Griffin GK, Battaglia S, Verga JA, Stephansky J, Pastika
TJ, Story JL, et al: Single-cell RNA-Seq reveals AML hierarchies
relevant to disease progression and immunity. Cell. 176:1265–1281.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakase K, Kita K, Kyo T, Tsuji K and
Katayama N: High serum levels of soluble interleukin-2 receptor in
acute myeloid leukemia: Correlation with poor prognosis and CD4
expression on blasT cells. Cancer Epidemiol. 36:e306–e309. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Q, Munger ME, Highfill SL, Tolar J,
Weigel BJ, Riddle M, Sharpe AH, Vallera DA, Azuma M, Levine BL, et
al: Program death-1 signaling and regulatory T cells collaborate to
resist the function of adoptively transferred cytotoxic T
lymphocytes in advanced acute myeloid leukemia. Blood.
116:2484–2493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bachli EB, Schaer DJ, Walter RB, Fehr J
and Schoedon G: Functional expression of the CD163 scavenger
receptor on acute myeloid leukemia cells of monocytic lineage. J
Leukoc Biol. 79:312–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cancer Genome Atlas Research Network, ;
Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A,
Hoadley K, Triche TJ Jr, Laird PW, et al: Genomic and epigenomic
landscapes of adult de novo acute myeloid leukemia. N Engl J Med.
368:2059–2074. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chuang MK, Chiu YC, Chou WC, Hou HA, Tseng
MH, Kuo YY, Chen Y, Chuang EY and Tien HF: An mRNA expression
signature for prognostication in de novo acute myeloid leukemia
patients with normal karyotype. Oncotarget. 6:39098–39110. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhattacharya S, Andorf S, Gomes L, Dunn P,
Schaefer H, Pontius J, Berger P, Desborough V, Smith T, Campbell J,
et al: ImmPort: Disseminating data to the public for the future of
immunology. Immunol Res. 58:234–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Newman AM, Liu CL, Green MR, Gentles AJ,
Feng W, Xu Y, Hoang CD, Diehn M and Alizadeh AA: Robust enumeration
of cell subsets from tissue expression profiles. Nat Methods.
12:453–457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramzi M, Khalafi-Nezhad A, IravaniSaadi M
and Jowkar Z: Association between TLR2 and TLR4 expression and
response to induction therapy in acute myeloid leukemia patients.
Int J Hematol Oncol Stem Cell Res. 12:303–312. 2018.PubMed/NCBI
|
|
23
|
Yan H, Qu J, Cao W, Liu Y, Zheng G, Zhang
E and Cai Z: Identification of prognostic genes in the acute
myeloid leukemia immune microenvironment based on TCGA data
analysis. Cancer Immunol Immunother. 68:1971–1978. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao G, Guo X and Goff SP: Inhibition of
retroviral RNA production by ZAP, a CCCH-type zinc finger protein.
Science. 297:1703–1706. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu B, Shi N, Sun L and Liu L: Clinical
value of high expression level of CD71 in acute myeloid leukemia.
Neoplasma. 63:809–815. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kollia P, Samara M, Stamatopoulos K,
Belessi C, Stavroyianni N, Tsompanakou A, Athanasiadou A,
Vamvakopoulos N, Laoutaris N, Anagnostopoulos A and Fassas A:
Molecular evidence for transferrin receptor 2 expression in all FAB
subtypes of acute myeloid leukemia. Leuk Res. 27:1101–1103. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kollia P, Stavroyianni N, Stamatopoulos K,
Zoi K, Viniou N, Mantzourani M, Noguchi CT, Paterakis G, Abazis D,
Pangalos C, et al: Molecular analysis of transferrin receptor mRNA
expression in acute myeloid leukaemia. Br J Haematol. 115:19–24.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hou HA, Lu JW, Lin TY, Tsai CH, Chou WC,
Lin CC, Kuo YY, Liu CY, Tseng MH, Chiang YC, et al:
Clinico-biological significance of suppressor of cytokine signaling
1 expression in acute myeloid leukemia. Blood Cancer J. 7:e5882017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Annabi B, Currie JC, Moghrabi A and
Béliveau R: Inhibition of HuR and MMP-9 expression in
macrophage-differentiated HL-60 myeloid leukemia cells by green tea
polyphenol EGCg. Leuk Res. 31:1277–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han S, Cao C, Tang T, Lu C, Xu J, Wang S,
Xue L, Zhang X and Li M: ROBO3 promotes growth and metastasis of
pancreatic carcinoma. Cancer Lett. 366:61–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Narayan G, Goparaju C, Arias-Pulido H,
Kaufmann AM, Schneider A, Dürst M, Mansukhani M, Pothuri B and
Murty VV: Promoter hypermethylation-mediated inactivation of
multiple Slit-Robo pathway genes in cervical cancer progression.
Mol Cancer. 5:162006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koehn J, Huesken D, Jaritz M, Rot A,
Zurini M, Dwertmann A, Beutler B and Korthäuer U: Assessing the
function of human UNC-93B in toll-like receptor signaling and major
histocompatibility complex II response. Hum Immunol. 68:871–878.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goto Y, Arigami T, Kitago M, Nguyen SL,
Narita N, Ferrone S, Morton DL, Irie RF and Hoon DS: Activation of
toll-like receptors 2, 3, and 4 on human melanoma cells induces
inflammatory factors. Mol Cancer Ther. 7:3642–3653. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beghini A, Ripamonti CB, Peterlongo P,
Roversi G, Cairoli R, Morra E and Larizza L: RNA hyperediting and
alternative splicing of hematopoietic cell phosphatase (PTPN6) gene
in acute myeloid leukemia. Hum Mol Genet. 9:2297–2304. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Terwijn M, Feller N, van Rhenen A, Kelder
A, Westra G, Zweegman S, Ossenkoppele G and Schuurhuis GJ:
Interleukin-2 receptor alpha-chain (CD25) expression on leukaemic
blasts is predictive for outcome and level of residual disease in
AML. Eur J Cancer. 45:1692–1699. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fujiwara SI, Muroi K, Yamamoto C, Hatano
K, Okazuka K, Sato K, Oh I, Ohmine K, Suzuki T and Ozawa K: CD25 as
an adverse prognostic factor in elderly patients with acute myeloid
leukemia. Hematology. 22:347–353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Testa U, Riccioni R, Diverio D, Rossini A,
Coco FL and Peschle C: Interleukin-3 receptor in acute leukemia.
Leukemia. 18:219–226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fey MF and Buske C; ESMO Guidelines
Working Group, : Acute myeloblastic leukaemias in adult patients:
ESMO clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 24 (Suppl 6):vi138–vi143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Szczepanski MJ, Szajnik M, Czystowska M,
Mandapathil M, Strauss L, Welsh A, Foon KA, Whiteside TL and
Boyiadzis M: Increased frequency and suppression by regulatory T
cells in patients with acute myelogenous leukemia. Clin Cancer Res.
15:3325–3332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Williams P, Basu S, Garcia-Manero G,
Hourigan CS, Oetjen KA, Cortes JE, Ravandi F, Jabbour EJ, Al-Hamal
Z, Konopleva M, et al: The distribution of T-cell subsets and the
expression of immune checkpoint receptors and ligands in patients
with newly diagnosed and relapsed acute myeloid leukemia. Cancer.
125:1470–1481. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Radpour R, Riether C, Simillion C, Höpner
S, Bruggmann R and Ochsenbein AF: CD8+ T cells expand
stem and progenitor cells in favorable but not adverse risk acute
myeloid leukemia. Leukemia. 33:2379–2392. 2019. View Article : Google Scholar : PubMed/NCBI
|