Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer worldwide (1). Due
to the continuous development of endoscopic treatments, including
endoscopic mucosal resection and endoscopic submucosal dissection,
which allow radical resection of a CRC tumor without the need for
open surgery (2–4), the 5-year survival rate of patients
with early CRC is >90% (5,6). By
contrast, for patients with advanced CRC with distant metastasis,
5-fluorouracil (5-FU)-based chemotherapy is the first-line option;
however, the development of drug resistance is the primary cause of
treatment failure, and the median survival time is <2 years
(7). Therefore, the development of
alternative approaches to overcome CRC resistance is urgently
required.
Halofuginone (HF), a derivative of a traditional
Chinese medicine and the effective constituent of febrifugine, has
attracted increasing attention for its wide range of biological
activities in malaria, autoimmune diseases, fibrosis and cancer
(8–11). HF suppresses cancer cell
proliferation and decreases tumor metastasis in hepatoma, melanoma
and multiple myeloma in vitro (12–14). HF
has already been trialed for use as an anticarcinogenic drug for
advanced solid tumors in one phase I clinical trial (15). The trial found that the
pharmacokinetics of HF were linear over the dose range studied,
with a large interpatient variability. The dose-limiting toxicities
of HF were nausea, vomiting and fatigue. The recommended dose for
phase II studies of HF was 0.5 mg administered orally, once
daily.
In the present study, the regulatory effects of HF
on the proliferation, migration and invasion of 5-FU-resistant
human CRC HCT-15/FU cells were assessed. Additionally, using
bioinformatics analysis, alterations in the microRNA (miRNA/miR)
expression profiles were determined following treatment with HF.
The results of the present study highlight the potential of
miR-132-3p as a potential therapeutic target, and the potential of
HF as a novel therapeutic agent in 5-FU-resistant CRC.
Materials and methods
Chemicals and reagents
MTT was purchased from Sigma-Aldrich; Merck KGaA.
Antibodies against β-catenin (cat. no. 8480), poly(ADP-ribose)
polymerase (PARP; cat. no. 9532), ERK1/2 (cat. no. 4696), AKT (cat.
no. 4691), Survivin (cat. no. 2808) and integrin β4 (ITGβ4; cat.
no. 14803) were obtained from Cell Signaling Technology, Inc. An
antibody against EGFR (cat. no. ab52894) was purchased from Abcam.
GAPDH (1A6) monoclonal antibody (cat. no. MB001) and goat
anti-mouse IgG (H+L)-HRP (cat. no. BS12478) were purchased from
Bioworld Technology, Inc. Goat anti-rabbit IgG (H+L) HRP (cat. no.
S0001) was purchased from Affinity Biosciences. All tissue culture
reagents and other routine laboratory reagents were obtained from
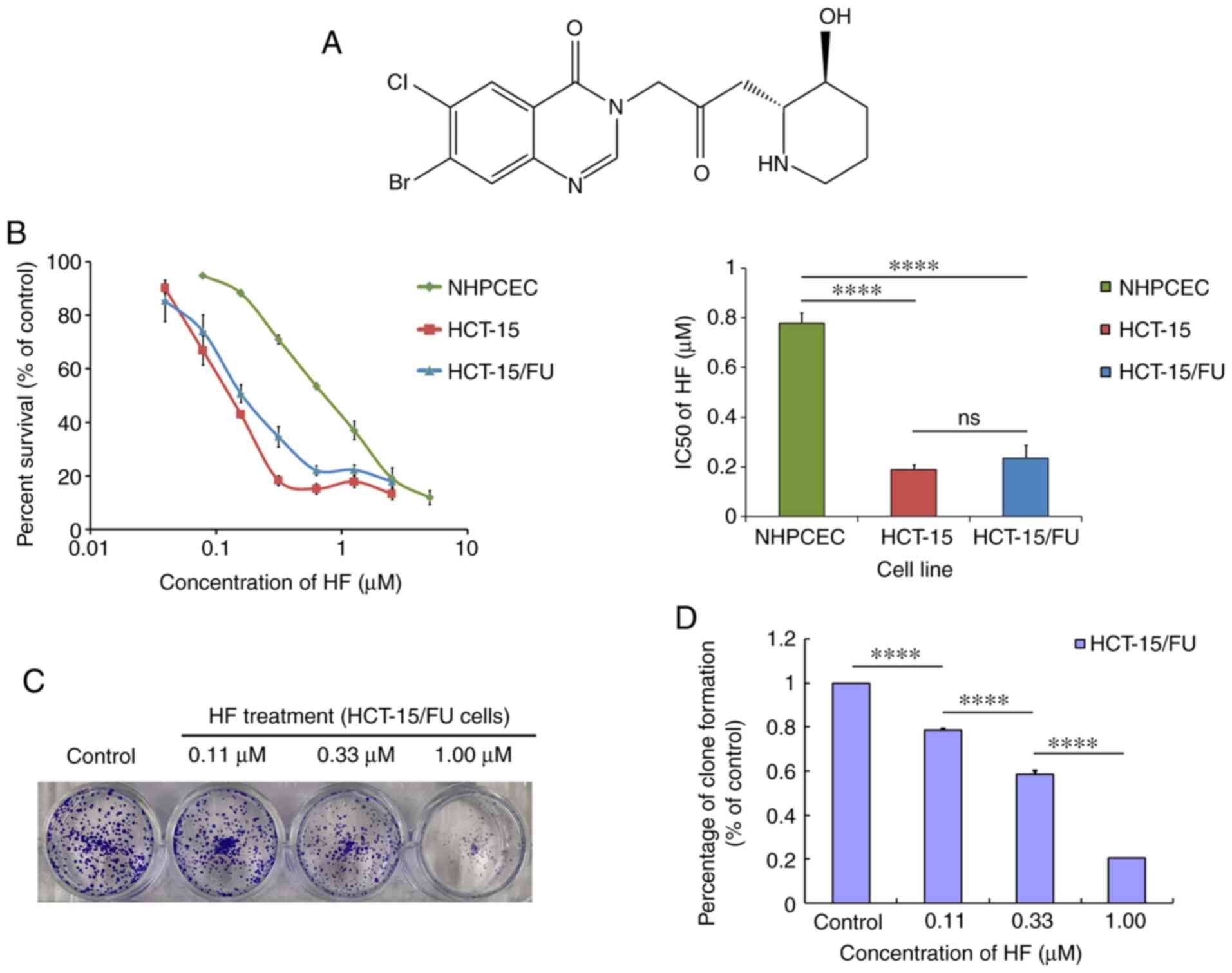
Guangzhou Whiga Technology Co., Ltd. HF (Fig. 1A), extracted from Dichroa
febrifuga Lour of traditional Chinese herbs, with a purity of
99.05% was obtained from Selleck Chemicals (cat. no. S8144).
Cell culture
The normal human primary colonic epithelial cells
(NHPCECs; HUM-iCELL-d010), the 5-FU-resistant human CRC HCT-15/FU
cell line (iCell-h073) and the corresponding 5-FU-sensitive HCT-15
cells (iCell-h072), the identities of which were confirmed using
Short Tandem Repeat profiling, were purchased form iCell
Bioscience, Inc. In detail, by gradually increasing the 5-FU
concentration, HCT-15 cells were successfully induced to
5-FU-resistant cell lines and named HCT-15/FU. Specifically, HCT-15
cells in the logarithmic growth phase were seeded into 6-well
plates. When the cell proliferation was stable and the confluence
was ~80%, the medium was replaced with complete medium containing
7.5 µg/ml 5-FU. The drug concentration could be increased until the
cells maintained normal growth at a concentration of 7.5 µg/ml for
4 days. The increasing drug concentrations were: 7.5, 10, 12.5, 15,
17.5 and 20 µg/ml. By day 208, the cells could be cultured in 20
µg/ml 5-FU-containing medium for 7 days, and the cells grew
normally. At this point, HCT-15/FU cells were successfully
constructed. 5-FU-resistant HCT-15/FU cells were authenticated by
comparing their fold resistance with that of the parental
5-FU-sensitive HCT-15 cells (Fig.
S1). The aforementioned two CRC cell lines were cultured in
RPMI-1640 medium supplemented with 1% penicillin-streptomycin and
10% fetal bovine serum (FBS) (Tianhang Biotech Co., Ltd.). NHPCECs
were cultured in complete medium of the iCell primary epithelial
cell culture system (PriMed-Icell-001; iCell Bioscience, Inc.).
Both cultures were maintained at 37°C in a 5% CO2
incubator.
Cell cytotoxicity and colony formation
assays
Cell cytotoxicity was evaluated using MTT assays as
previously described (16).
Specifically, 20 µl MTT (5 mg/ml) was added to each well for a 4-h
reaction. Next, the supernatant was discarded, and the formed
formazan product was dissolved in 100 µl DMSO/well. The optical
density was detected at an absorbance wavelength of 540 nm using a
Model 550 Microplate reader (Bio-Rad Laboratories, Inc.), with
reference filter of 655 nm. Colony formation assays were performed
to determine the cloning capability of HCT-15/FU cells. HCT-15/FU
cells were treated with HF (0.11, 0.33, 1.00 µM) for 48 h. A total
of 500 cells/well were seeded into 6-well plates and cultured for
an additional 10 days in RPMI-1640 medium supplemented with 10% FBS
at 37°C until most of the single colonies contained >50 cells.
Subsequently, the colonies were fixed with 4% formaldehyde at room
temperature, stained with 0.01% crystal violet for 30 min. Images
were captured using ChemiDoc™ XRS+ (Bio-Rad Laboratories) (17).
Wound healing assay
A wound healing assay was performed to determine
cell migration in vitro. Briefly, cells were plated in
6-well plates and grown overnight to reach 100% confluence.
Subsequently, a 1-ml pipette tip was used to scratch the cell
monolayer, the wounded cell layer was washed with PBS to remove
dead cells, and then FBS-free medium was added. The HCT-15/FU cells
were incubated with HF (0.11, 0.33 and 1.00 µM) for 0, 12, 24 or 48
h. Images were captured using a light microscope (Olympus
Corporation) at ×40 magnification. The surface area was calculated
using ImageJ software (version 1.51j8; National Institutes of
Health). The cell motility rate was calculated as follows: Cell
motility rate (%) = migrated cell surface area/total surface area
×100.
Invasion assay
The bottom of the Transwell apparatus was pre-coated
for 3 h with Matrigel (Corning Inc.), which was diluted 5 times
with basal medium. HCT-15/FU cells were transferred into the upper
chamber of the Transwell apparatus at a density of
2.5×104 cells/well in 200 µl serum-free RPMI-1640
medium, which contained the HF (0, 0.11, 0.33 and 1.00 µM). The
lower chamber contained 600 µl RPMI-1640 medium with 10% FBS, which
was a chemoattractant. After incubation for 48 h, cells that
migrated into the lower chamber were fixed with methanol and
stained with 0.05% crystal violet at room temperature for 30 min.
Cells on the top layer were removed using a cotton swab, and images
of the migrated cells were captured using an inverted microscope
(Leica Microsystems) at ×200 magnification. Six random fields were
imaged for quantification of migrated cells. The cell number that
had invaded to the bottom chamber was calculated for each group by
ImageJ software (version 1.51j8).
Western blotting
Cultured cells from different samples were collected
and washed twice with ice-cold PBS. The pellet was vortexed and
lysed in lysis buffer (Cell Signaling Technology, Inc.),
supplementary with proteinase inhibitor cocktail (Cell Signaling
Technology, Inc.) and phenylmethylsulfonyl fluoride (Cell Signaling
Technology, Inc.). The protein concentration was determined using a
BCA Protein assay kit (Thermo Fisher Scientific, Inc.). Proteins
(25 µg/lane) were separated on 8–12% gels using SDS-PAGE and
subsequently transferred onto PVDF membranes (EMD Millipore). The
membranes were then blocked with TBST buffer [150 mmol/l NaCl, 20
mmol/l Tris-HCl (pH 7.4) and 0.4% (v/v) Tween-20] containing 5%
dried non-fat milk, and subsequently incubated with primary
antibodies overnight at 4°C. After being washed with TBST buffer
three times, the membranes were incubated with horseradish
peroxidase (HRP) conjugated secondary antibodies for 2 h at room
temperature. Blots were visualized using a Western
Lightning® Plus-ELC kit (PerkinElmer, Inc.) and analyzed
by ChemiDoc™ XRS+ (Bio-Rad Laboratories, Inc.). Antibodies used in
this study included: β-catenin (1:1,000 dilution), PARP (1:1,000
dilution), ERK1/2 (1:1,000 dilution), AKT (1:1,000 dilution),
Survivin (1:1,000 dilution), ITGβ4 (1:1,000 dilution), EGFR
(1:1,000 dilution), GAPDH (1:10,000 dilution), goat anti-mouse IgG
(H+L) HRP (1:5,000 dilution) and goat anti-rabbit IgG (H+L) HRP
(1:5,000 dilution).
Construction and sequencing of the
miRNA library and subsequent miRNA trend analysis
HCT-15/FU cells were treated with HF (0.11, 0.33 and
1.00 µM) for 48 h, and total RNA was extracted using
TRIzol® (Thermo Fisher Scientific, Inc.). An miRNA
sequencing library was constructed using the NEBNext Multiplex
Small RNA Library Prep Set for Illumina (New England Biolabs Inc.;
NEB #E7580L), which contains the adaptors, primers, enzymes and
buffers required to convert small RNAs into indexed libraries for
next generation sequencing on the Illumina platform. This
construction was performed by KangChen Bio-tech Co., Ltd.
Specifically, the experiment was performed according to the
following steps: i) 3′-Adaptor ligation; ii) 5′-adaptor ligation;
iii) cDNA synthesis; iv) PCR amplification; v) size selection of
135 to 155-bp PCR-amplified fragments (corresponding to 15 to 35-nt
small RNAs). The libraries were denatured into single-stranded DNA
molecules by 0.1 M NaOH, captured on Illumina flow cells (Illumina,
Inc.), amplified in situ as clusters (TruSeq Rapid SR
Cluster kit (#GD-402-4001; Illumina, Inc.) and finally sequenced
for 51 cycles on an Illumina NextSeq 500 platform. Based on the
miRNA sequencing results, the subsequent trend analysis was
performed using Short Time-series Expression Miner software v1.3.8
(Kangchen BioTech Co., Ltd.). The miRNAs were assigned to the
corresponding profile according to the number of genes assigned to
that profile, the number of genes expected and the enrichment
P-value.
miRNA extraction and reverse
transcription-quantitative (RT-q)PCR
Following treatment of HCT-15/FU cells with 0.33 and
1.00 µM HF for 48 h, total RNA was extracted using
TRIzol® reagent (Thermo Fisher Scientific, Inc.). A
Mir-X™ miRNA RT-qPCR SYBR kit (Takara Bio, Inc.) was used to
reverse transcribe miRNAs into cDNA, and qPCR was performed
according to the manufacturer's protocol. The thermocycling
conditions were: 95°C for 10 sec; followed by 40 cycles of 95°C for
5 sec and 64°C for 35 sec; followed by 95°C for 30 sec and 55°C for
30 sec. U6 was used as the internal control. The primers were
synthesized by Takara Bio, Inc. The sequences of the primers were:
U6 forward, 5′-CGCAAGGATGACACG-3′ and reverse,
5′-GAGCAGGCTGGAGAA-3′; and miR-132-3p forward,
5′-TAACAGTCTACAGCCATGGTC-3′ and reverse, 5′-CAGTGCGTGTCGTGGAGT-3′.
The relative expression levels of the genes were determined using
the 2−ΔΔCq method (18).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 7 software (GraphPad Software, Inc.). Single factor
variance analysis (one-way ANOVA) followed by Tukey's post-hoc test
was used when multiple sets of data met the criteria for normality
and the homogeneity of the variance. All results were presented as
mean ± standard deviation (SD). P<0.05 was considered to
indicate a statistically significant difference.
Results
HF is cytotoxic to HCT-15/FU
cells
MTT assays were used to measure cell viability
following treatment with HF. As shown in Fig. 1B, the IC50 values were
0.19±0.02 and 0.23±0.05 µmol/l for HCT-15 and HCT-15/FU cells,
respectively (P>0.05), whereas for NHPCECs, the IC50
was 0.78±0.04 µmol/l. These data suggest that HF exhibited potent
and similar cytotoxicity against both HCT-15/FU and HCT-15 cells.
Compared with the two tumor cell lines, HF was less cytotoxic to
NHPCECs (both P<0.05). Additionally, HF treatment inhibited the
clone formation of HCT-15/FU cells as demonstrated by the colony
formation assays (Fig. 1C and
D).
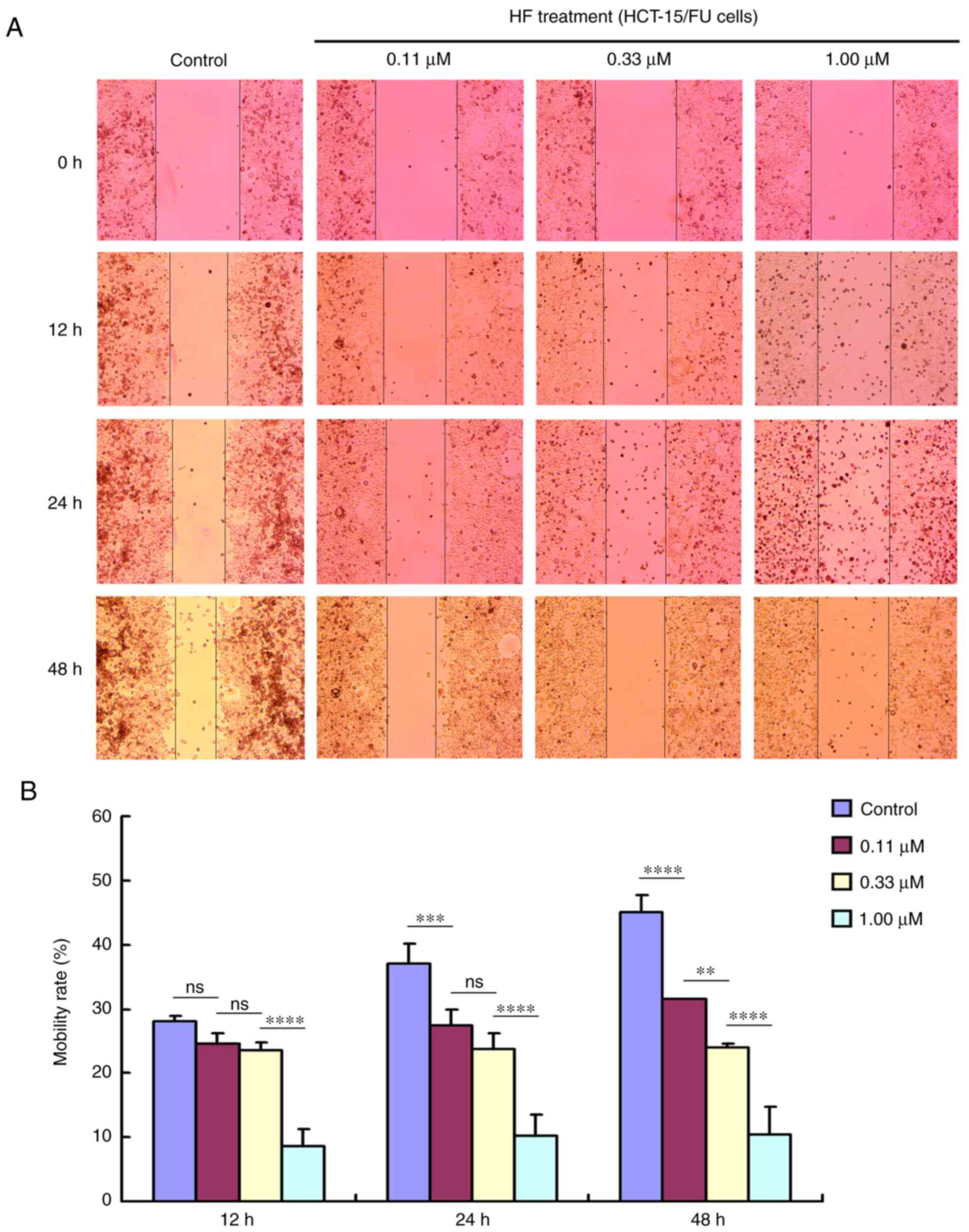
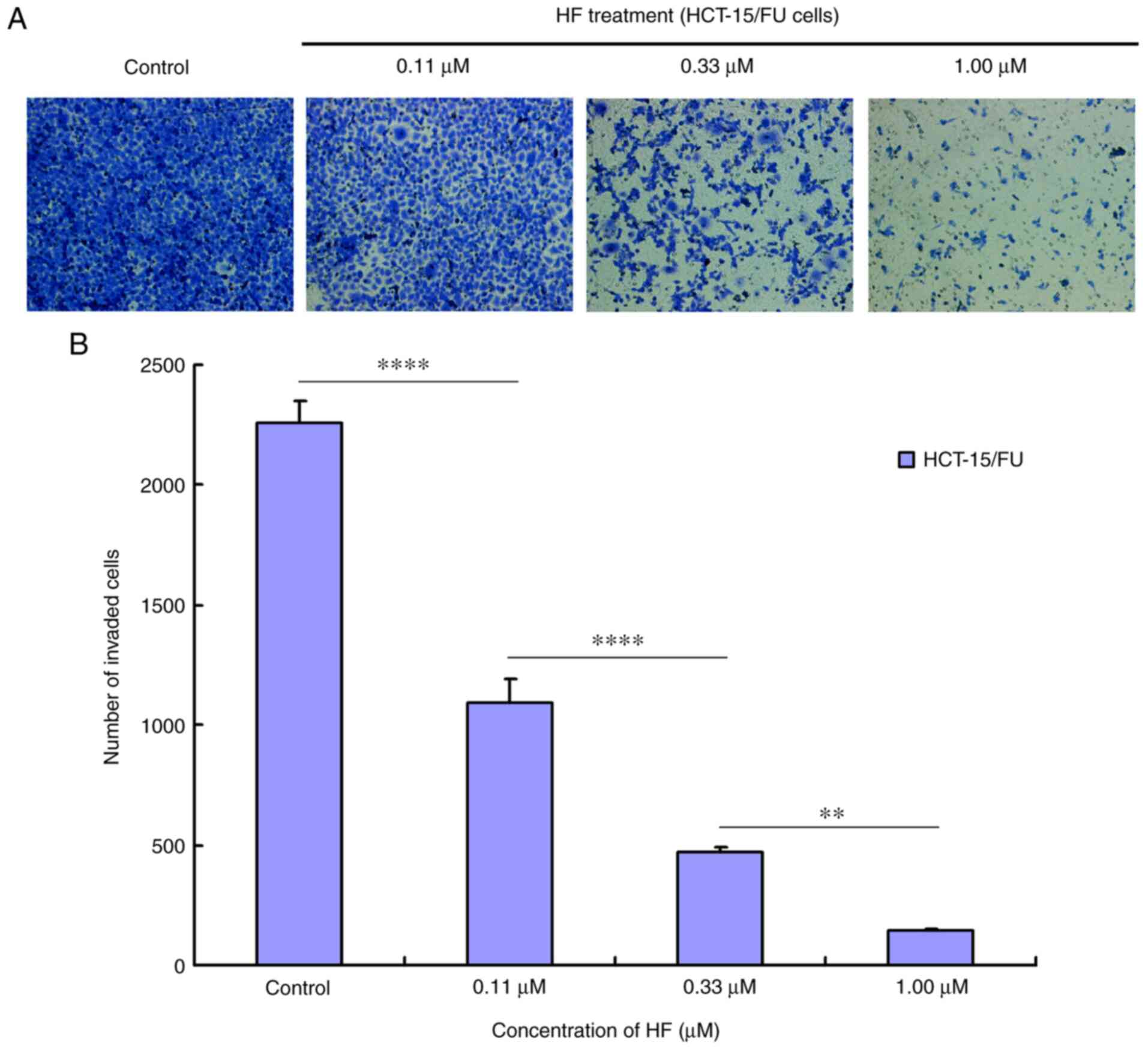
HF inhibits migration and invasion of
HCT-15/FU cells
Tumor cell migration and invasion promote tumor
progression. To determine the effects of HF on migration and
invasion, HCT-15/FU cells were treated with 0.11, 0.33 or 1.00
µmol/l HF for 48 h to assess changes in cell migration and
invasion. The results demonstrated that HF inhibited HCT-15/FU cell
migration (Fig. 2) and invasion
(Fig. 3) in a dose-dependent manner.
Notably, the results presented in Fig.
2 indicated that HF could also induce apoptosis of HCT-15/FU
cells (consistent with the western blotting results for PARP
protein). Cell mobility was quantified from living cells on the
edge of the scratch. At 48 h after HF treatment, numerous apoptotic
cells covered visible gaps and the edges of the scratch were not
visible. Therefore, dead cells were removed to calculate cell
mobility at 48 h.
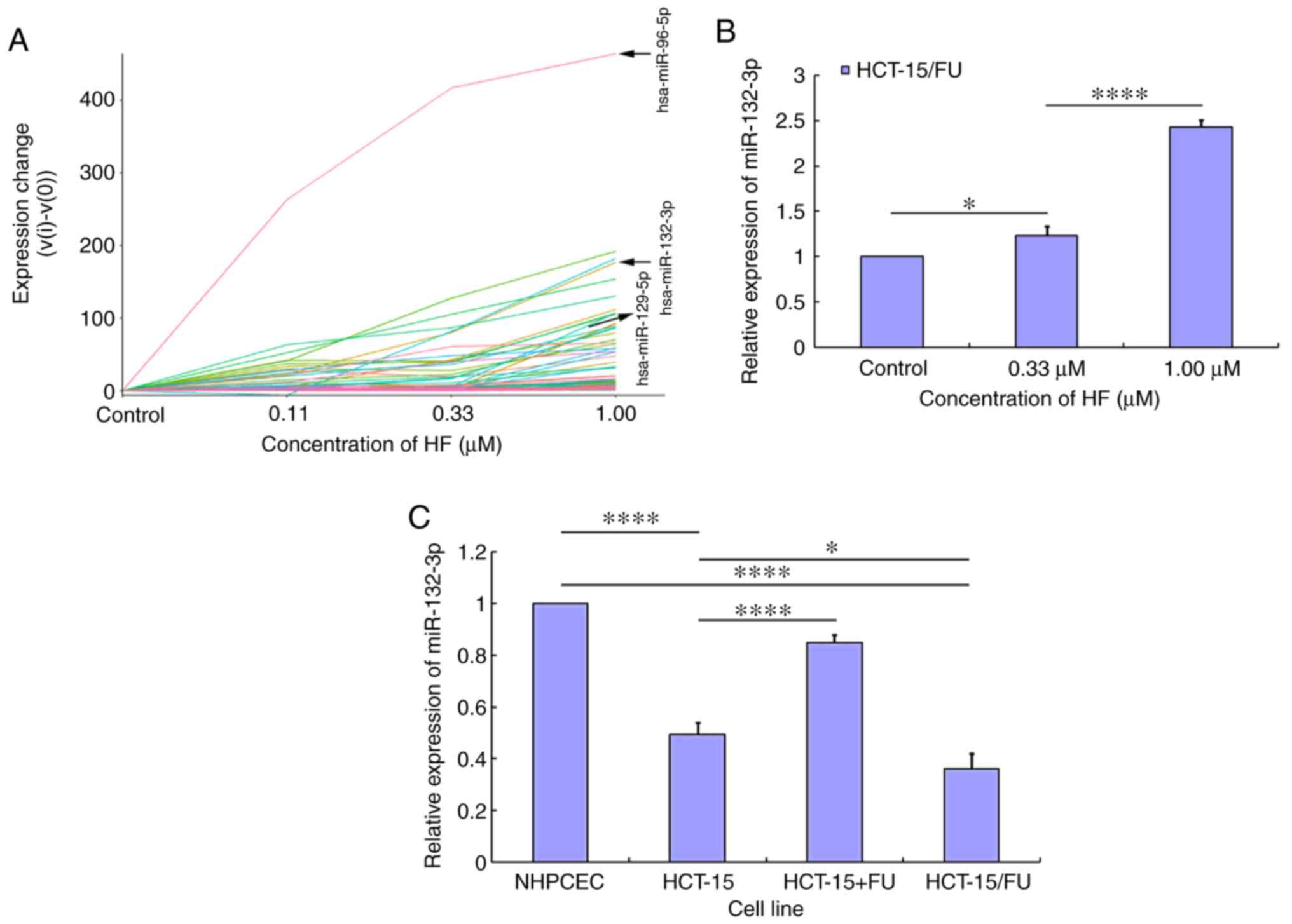
miRNA trend analysis in HCT-15/FU
cells treated with HF
Using miRNA sequencing analysis and subsequent miRNA
trend analysis, the expression profiles of miRNAs in HCT-15/FU
cells treated with HF were determined. The miRNA trend analysis
results demonstrated that the expression levels of certain miRNAs
were increased in a dose-dependent manner (Fig. 4A), and other miRNAs were assigned to
the corresponding profile (Figs. S2
and S3). The labeled lines in
Fig. 4A represent miR-96-5p,
miR-132-3p and miR-129-5p. Subsequently, RT-qPCR was used to
validate the expression levels of the aforementioned specific
miRNAs. Following treatment with HF, only miR-132-3p expression was
demonstrated to be increased in a dose-dependent manner (Fig. 4B), while the expression level of
miR-96-5p and miR-129-5p changed irregularly (data not shown). To
further elucidate the mechanism by which HF inhibited progression
of HCT-15/FU cells, subsequent experiments focused on the role of
miR-132-3p. miR-132-3p expression was lower in CRC cells (HCT-15
and HCT-15/FU cells) compared with in NHPCECs (P<0.01). When
HCT-15 cells were treated with 5-FU, the expression levels of
miR-132-3p were increased. Additionally, the expression levels of
miR-132-3p were lower in HCT-15/FU cells compared with HCT-15 cells
(P<0.05; Fig. 4C). These results
suggest that HF may inhibit HCT-15/FU cell progression via
upregulation of miR-132-3p expression.
HF inhibits progression of HCT-15/FU
cells through miR-132-3p-mediated targeting of proteins involved in
proliferation, invasion and metastasis
Using TargetScan Human 7.2 prediction (http://www.targetscan.org/vert_72/), search
results demonstrated that miR-132-3p might be predicted to target
the 3′-untranslated regions (UTRs) of proteins involved in
proliferation, invasion and metastasis, including MAPK1, MAPK3,
MAPKBP1, AKT3 and integrin subunit α9. In the present study,
western blotting results revealed that the protein expression
levels of EGFR, AKT, ERK1/2, Survivin, β-Catenin, PARP and ITGβ4,
which are associated with cell proliferation, apoptosis, invasion
or metastasis, were decreased in HCT-15/FU cells treated with HF
(Fig. 5). Overall, these results
suggest that HF may inhibit HCT-15/FU cell progression via
miR-132-3p-mediated targeting of proteins involved in
proliferation, invasion and metastasis.
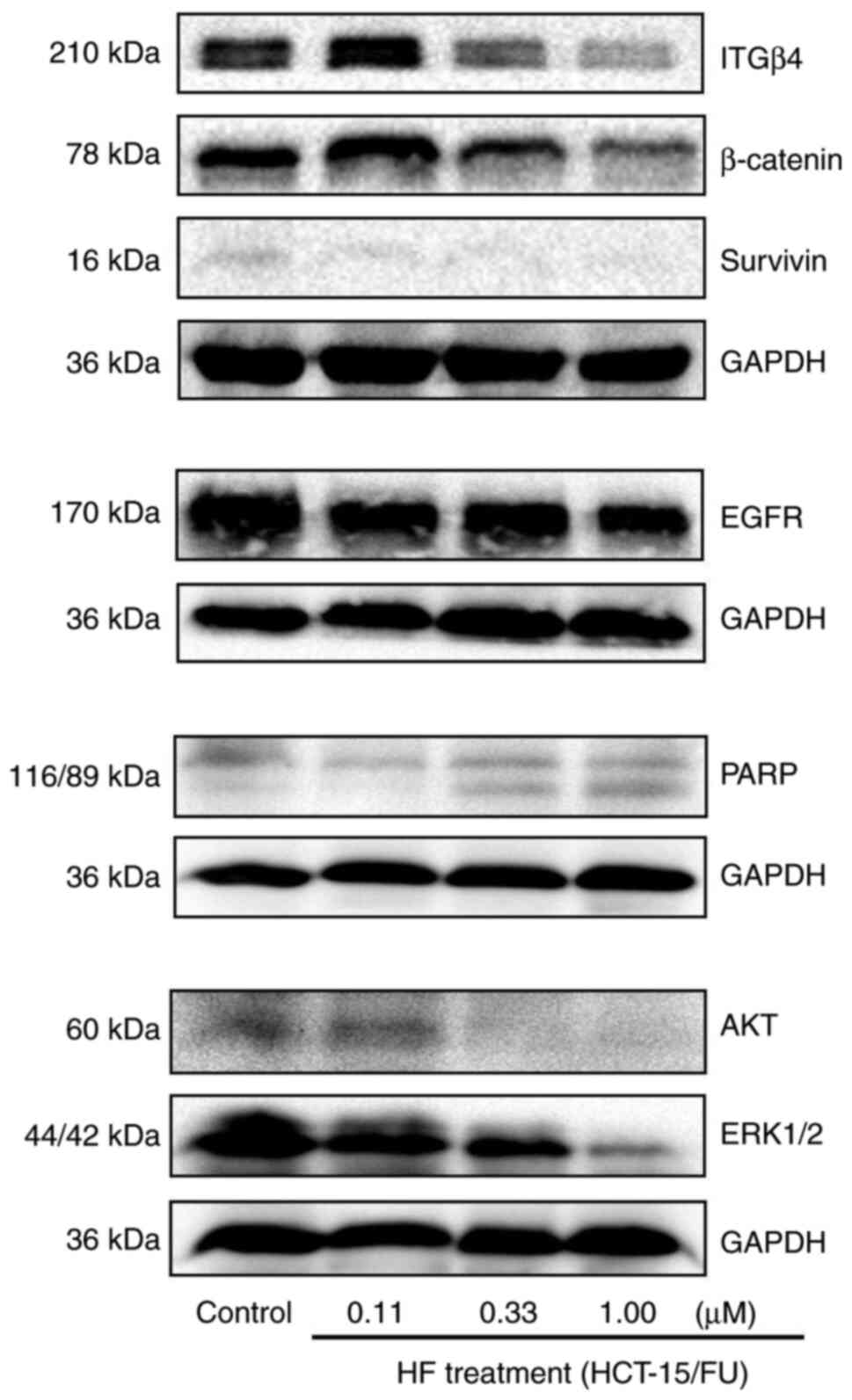 | Figure 5.HF inhibits progression of HCT-15/FU
cells via microRNA-132-3p-mediated targeting of proteins involved
in proliferation, invasion and metastasis. Following treatment of
HCT-15/FU cells with various concentrations of HF, the expression
levels of proteins associated with cell proliferation, invasion and
metastasis were detected. The protein expression levels of EGFR,
AKT, ERK1/2 (MAPK1/2), Survivin, β-Catenin, PARP and ITGβ4 were
downregulated. PARP, poly(ADP-ribose) polymerase; ITGβ4, Integrin
β4; HF, halofuginone. |
Discussion
HF has been demonstrated to exhibit anticancer
activity via several mechanisms, including the induction of
apoptosis, inhibition of proliferation and regulation of multiple
downstream cancer-associated signaling molecules (19,20).
Since not having more 5-FU-resistant CRC cell lines is a
limitation, the present study detected the effects of HF on two
pairs of cancer cell lines: KB and it corresponding resistant
KBv200 cell line (data not shown), and HCT-15 and it corresponding
resistant HCT-15/FU cell line. The results indicated that HF
exhibited potent cytotoxicity against the two pairs of cells. To
further elucidate the molecular mechanisms underlying the antitumor
activity of HF, the effects of HF on the tumorigenic progression of
human 5-FU-resistant HCT-15/FU cells were examined. MTT, colony
formation, wound healing and invasion assays demonstrated that when
HCT-15/FU cells were treated with HF, cell viability, migration and
invasion were reduced, and western blotting indicated that the
protein expression levels of EGFR, AKT, ERK1/2, Survivin,
β-catenin, PARP and ITGβ4 were downregulated. Therefore, the
effects of HF on HCT-15/FU cells may be attributed to two models:
i) Inhibition of protein expression of EGFR, AKT, ERK1/2, Survivin,
β-catenin, PARP, downstream of MAPK and/or PI3K/AKT signaling
pathways, resulting in inhibition of HCT-15/FU cell proliferation
and induction of apoptosis; and ii) inhibiting the protein
expression of ITGβ4, thus inhibiting invasion and metastasis.
Therefore, it was hypothesized that HF may exert beneficial effects
in 5-FU-resistant CRC treatment.
miRNAs are small, endogenous single-stranded 21–23
nucleotide non-coding RNAs that serve as protooncogenes or tumor
suppressor genes, and are abnormally expressed in a variety of
tumors. Increasing bioinformatics evidence and subsequent
functional assays have revealed that miRNAs serve key roles in
numerous biological processes, including tumor occurrence,
development and metastasis, in several types of cancer (21). miRNAs can bind to the 3′-UTR of
target gene mRNAs and induce their post-transcriptional repression
(22–24).
Studies have demonstrated that miRNAs regulate the
expression of various genes associated with human cancer (25,26).
Several other studies have revealed that miRNAs may serve as
biomarkers of CRC, and the majority of these miRNAs have a tumor
suppressor role (27,28). miR-132 has been demonstrated to
regulate cancer via regulation of several cellular behaviors,
including tumorigenesis, proliferation, resistance and progression
(29). Previous studies have
indicated that miR-132 exerts tumor-suppressing functions in
various types of cancer, including prostate cancer, thyroid cancer,
pancreatic cancer, cervical cancer and renal carcinoma (30–35).
Furthermore, miR-132 affects drug resistance. Studies have
demonstrated that overexpression of miR-132 enhances the
chemotherapy or radiotherapy sensitivity of CRC, ovarian cancer,
nasopharyngeal carcinoma and cervical cancer (36–39).
However, the biological functions of miR-132-3p in 5-FU-resistant
CRC remain poorly understood, which largely limits its potential as
a biomarker of resistance. In the present study, miR-132-3p
expression was determined to be downregulated in CRC cells compared
with NHPCECs. Additionally, the levels of miR-132-3p were lower in
HCT-15/FU cells compared with the sensitive HCT-15 cells.
Therefore, it was hypothesized that miR-132-3p may be involved not
only in the tumorigenesis, but also in the process of CRC
resistance to 5-FU. Overall, the present study identified that
miR-132-3p may regulate CRC resistance to 5-FU.
Studies have demonstrated that natural compounds,
including curcumin, tectorigenin and avenanthramide A can regulate
the expression of numerous miRNAs, which increases the sensitivity
of cancer cells to conventional chemotherapeutics and thereby
effectively suppresses tumor cell proliferation (40–42).
Previous studies have demonstrated that anticarcinogenic effects of
HF on various types of cancer (43–45). In
order to determine the mechanisms by which HF affected HCT-15/FU
cells, bioinformatics analysis, miRNA-sequencing data, subsequent
miRNA trend analysis and RT-qPCR verification were used, and it was
demonstrated that miR-132-3p expression was increased in a
dose-dependent manner.
ERK1/2 are critical molecules in the MAPK signaling
pathway, which is an important pathway involved in tumor
proliferation (46). Using
TargetScan prediction software, it was suggested that miR-132-3p
bound to the 3′-UTR of MAPK1 (ERK1). Consistent with this
prediction, the present study demonstrated that HF treatment
increased miR-132-3p expression and reduced the protein expression
levels of ERK1/2. Therefore, it was hypothesized that miR-132-3p
served as a tumor suppressor in HCT-15/FU cells via reduction of
ERK1/2 expression. Additionally, western blotting suggested that
the protein expression levels of EGFR, AKT, Survivin, β-Catenin,
PARP and ITGβ4 were reduced following treatment of HCT-15/FU cells
with HF. In addition, cell cycle-related proteins are closely
associated with tumor growth pathways. In our other work, after
HCT15/FU cells were added to HF, it was demonstrated using
label-free quantitative proteomics that the expression levels of
some cell cycle-related proteins, such as CDK2 and CDK11B, were
altered (data not shown). Overall, these results suggested that HF
inhibited proliferation of HCT-15/FU cells via upregulation of
miR-132-3p-mediated targeting of proteins involved in
proliferation, apoptosis, invasion and metastasis, providing a
novel perspective on anti-5-FU-resistant CRC. Both miR-132-3p and
cancer progression-related proteins may potentially serve as
5-FU-resistant biomarkers and/or targets for developing CRC
therapeutics. These results suggest that HF and its derivatives may
serve as novel therapeutics for treatment of 5-FU-resistant
CRC.
In conclusion, the present study demonstrated that
downregulated expression of miR-132-3p in HCT-15/FU cells promoted
CRC resistance to 5-FU, and thus subsequent progression. miR-132-3p
expression was increased in HCT-15/FU cells treated with HF in a
dose-dependent manner. HF inhibited proliferation of HCT-15/FU
cells via upregulation of miR-132-3p, which in turn targeted genes
associated with cancer progression. To the best of our knowledge,
the present study was the first to report the relationship among HF
treatment, miR-132-3p and cancer progression-related proteins in
HCT-15/FU cells, and HF and its derivatives may be a novel
therapeutic agent for treatment of 5-FU-resistant CRC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Jianye
Zhang, Dr Jiajun Li and Dr Qiaoru Guo (affiliated to both to the
Guangdong Provincial Key Laboratory of Molecular Target and
Clinical Pharmacology, School of Pharmaceutical Sciences and to the
Fifth Affiliated Hospital, Guangzhou Medical University, Guangzhou,
China) for their assistance with the statistical analysis.
Funding
This work was supported by grants from the Fund
project of Inner Mongolia Autonomous Region Applied Technology
Research and Development (2018–3rd batch-1; Wuhai Municipal
People's Hospital), the National Natural Science Foundation of
China (grant no. 81902152), and the Fund of Shanxi Province Higher
Education Technology Innovation Project (grant no. 2019L0753).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YYY designed the study. CW and JBZ wrote the
manuscript and performed the MTT, reverse
transcription-quantitative PCR and western blotting assays. XJG and
XW performed the migration and invasion assays. WZ and XLW
performed miRNA trend analysis. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chiba H, Takahashi A, Inamori M, Goto T,
Ohata K, Matsuhashi N and Nakajima A: Early colon cancer presenting
as intussusception and successfully treated using endoscopic
submucosal dissection. Endoscopy. 46:E326–E327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kiriyama S, Saito Y, Yamamoto S, Soetikno
R, Matsuda T, Nakajima T and Kuwano H: Comparison of endoscopic
submucosal dissection with laparoscopic-assisted colorectal surgery
for early-stage colorectal cancer: A retrospective analysis.
Endoscopy. 44:102410–102430. 2012.
|
|
4
|
Kudo S: Endoscopic mucosal resection of
flat and depressed types of early colorectal cancer. Endoscopy.
25:455–461. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang D, Othman M and Draganov PV:
Endoscopic mucosal resection vs endoscopic submucosal dissection
for barrett's esophagus and colorectal neoplasia. Clin
Gastroenterol Hepatol. 17:1019–1028. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dumoulin FL and Hildenbrand R: Endoscopic
resection techniques for colorectal neoplasia: Current
developments. World J Gastroenterol. 25:300–307. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fakih MG: Metastatic colorectal cancer:
Current state and future directions. J Clin Oncol. 33:1809–1824.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang S, Zeng Q, Gettayacamin M, Tungtaeng
A, Wannaying S, Lim A, Hansukjariya P, Okunji CO, Zhu S and Fang D:
Antimalarial activities and therapeutic properties of febrifugine
analogs. Antimicrob Agents Chemother. 49:1169–1176. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pines M, Snyder D, Yarkoni S and Nagler A:
Halofuginone to treat fibrosis in chronic graft-versus-host disease
and scleroderma. Biol Blood Marrow Transplant. 9:417–425. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pines M and Nagler A: Halofuginone: A
novel antifibrotic therapy. Gen Pharmacol. 30:445–450. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia X, Wang L, Zhang X, Wang S, Lei L,
Cheng L, Xu Y, Sun Y, Hang B, Zhang G, et al: Halofuginone-induced
autophagy suppresses the migration and invasion of MCF-7 cells via
regulation of STMN1 and p53. J Cell Biochem. 119:4009–4020. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagler A, Ohana M, Shibolet O, Shapira MY,
Alper R, Vlodavsky I, Pines M and Ilan Y: Suppression of
hepatocellular carcinoma growth in mice by the alkaloid
coccidiostat halofuginone. Eur J Cancer. 40:1397–1403. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Juárez P, Mohammad KS, Yin JJ, Fournier
PG, McKenna RC, Davis HW, Peng XH, Niewolna M, Javelaud D, Chirgwin
JM, et al: Halofuginone inhibits the establishment and progression
of melanoma bone metastases. Cancer Res. 72:6247–6256. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leiba M, Jakubikova J, Klippel S,
Mitsiades CS, Hideshima T, Tai YT, Leiba A, Pines M, Richardson PG,
Nagler A and Anderson KC: Halofuginone inhibits multiple myeloma
growth in vitro and in vivo and enhances cytotoxicity of
conventional and novel agents. Br J Haematol. 157:718–731. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Jonge MJ, Dumez H, Verweij J, Yarkoni
S, Snyder D, Lacombe D, Marréaud S, Yamaguchi T, Punt CJ and van
Oosterom A; EORTC New Drug Development Group (NDDG), : Phase I and
pharmacokinetic study of halofuginone, an oral quinazolinone
derivative in patients with advanced solid tumours. Eur J Cancer.
42:1768–1774. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan YY, Bi H, Zhang W, Wen Q, Liu H, Li
JX, Zhang HZ, Zhang YX and Li JS: Downregulation and subcellular
distribution of HER2 involved in MDA-MB-453 breast cancer cell
apoptosis induced by lapatinib/celastrol combination. J BUON.
22:644–651. 2017.PubMed/NCBI
|
|
17
|
Wei M, Li J, Qiu J, Yan Y, Wang H, Wu Z,
Liu Y, Shen X, Su C, Guo Q, et al: Costunolide induces apoptosis
and inhibits migration and invasion in H1299 lung cancer cells.
Oncol Rep. 43:1986–1994. 2020.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Assis PA, De Figueiredo-Pontes LL, Lima
AS, Leão V, Cândido L, Pintão CT, Garcia AB, Saggioro FP, Panepucci
RA, Chahud F, et al: halofuginone inhibits phosphorylation of
SMAD-2 reducing angiogenesis and leukemia burden in an acute
promyelocytic leukemia mouse model. J Exp Clin Cancer Res.
34:652015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Figueiredo-Pontes LL, Assis PA,
Santana-Lemos BA, Jácomo RH, Lima AS, Garcia AB, Thomé CH, Araújo
AG, Panepucci RA, Zago MA, et al: Halofuginone has
anti-proliferative effects in acute promyelocytic leukemia by
modulating the transforming growth factor beta signaling pathway.
PLoS One. 6:e267132011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Babaei K, Shams S, Keymoradzadeh A, Vahidi
S, Hamami P, Khaksar R, Norollahi SE and Samadani AA: An insight of
microRNAs performance in carcinogenesis and tumorigenesis; an
overview of cancer therapy. Life Sci. 240:1170772020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
An X, Sarmiento C, Tan T and Zhu H:
Regulation of multidrug resistance by microRNAs in anti-cancer
therapy. Acta Pharm Sin B. 7:38–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Denli AM, Tops BB, Plasterk RH, Ketting RF
and Hannon GJ: Processing of Primary microRNAs by the
microprocessor Complex. Nature. 432:231–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Li D, Zhang Y, Ding Z, Zheng Y,
Chen S and Wan Y: Integrative analysis of mRNA and miRNA expression
profiles reveals seven potential diagnostic biomarkers for nonsmall
cell lung cancer. Oncol Rep. 43:99–112. 2020.PubMed/NCBI
|
|
26
|
Jung G, Hernández-Illán E, Moreira L,
Balaguer F and Goel A: Epigenetics of colorectal cancer: Biomarker
and therapeutic potential. Nat Rev Gastroenterol Hepatol.
17:111–130. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng Y, Zheng Y, Lei W, Xiang L and Chen
M: miR-1307-3p overexpression inhibits cell proliferation and
promotes cell apoptosis by targeting ISM1 in colon cancer. Mol Cell
Probes. 48:1014452019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bandres E, Agirre X, Bitarte N, Ramirez N,
Zarate R, Roman-Gomez J, Prosper F and Garcia-Foncillas J:
Epigenetic regulation of microRNA expression in colorectal cancer.
Int J Cancer. 125:2737–2743. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen L, Zhu Q, Lu L and Liu Y: MiR-132
inhibits migration and invasion and increases chemosensitivity of
cisplatin-resistant oral squamous cell carcinoma cells via
targeting TGF-β1. Bioengineered. 11:91–102. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li SL, Sui Y, Sun J, Jiang TQ and Dong G:
Identification of tumor suppressive role of microRNA-132 and its
target gene in tumorigenesis of prostate cancer. Int J Mol Med.
41:2429–2433. 2018.PubMed/NCBI
|
|
31
|
Chen X, Li M, Zhou H and Zhang L: miR-132
targets FOXA1 and exerts tumor-suppressing functions in thyroid
cancer. Oncol Res. 27:431–437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khan S, Ebeling MC, Chauhan N, Thompson
PA, Gara RK, Ganju A, Yallapu MM, Behrman SW, Zhao H, Zafar N, et
al: Ormeloxifene suppresses desmoplasia and enhances sensitivity of
gemcitabine in pancreatic cancer. Cancer Res. 75:2292–2304. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Zhu H, Wang Y, Song Y, Zhang P,
Wang Z, Gao J, Li Z and Du Y: MicroRNA-132 plays an independent
prognostic role in pancreatic ductal adenocarcinoma and acts as a
tumor suppressor. Technol Cancer Res Treat.
18:15330338188243142019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao JL, Zhang L, Guo X, Wang JH, Zhou W,
Liu M, Li X and Tang H: miR-212/132 downregulates SMAD2 expression
to suppress the G1/S phase transition of the cell cycle and the
epithelial to mesenchymal transition in cervical cancer cells.
IUBMB life. 67:380–394. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu Y, Lu W, Zhou X, Huang H, Shen S and
Guo L: MicroRNA-132 suppresses migration and invasion of renal
carcinoma cells. J Clin Lab Anal. 34:e229692020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Y and Zhang M: miR-132 Regulates
adriamycin resistance in colorectal cancer cells through targeting
extracellular signal-regulated kinase 1. Cancer Biother Radiopharm.
34:398–404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang XL, Sun BL, Tian SX, Li L, Zhao YC
and Shi PP: MicroRNA-132 reverses cisplatin resistance and
metastasis in ovarian cancer by the targeted regulation on Bmi-1.
Eur Rev Med Pharmacol Sci. 23:3635–3644. 2019.PubMed/NCBI
|
|
38
|
Li YL, Zhao YG, Chen B and Li XF:
MicroRNA-132 sensitizes nasopharyngeal carcinoma cells to cisplatin
through regulation of forkhead box A1 protein. Pharmazie.
71:715–718. 2016.PubMed/NCBI
|
|
39
|
Liu GF, Zhang SH, Li XF, Cao LY, Fu ZZ and
Yu SN: Overexpression of microRNA-132 enhances the radiosensitivity
of cervical cancer cells by down-regulating Bmi-1. Oncotarget.
8:80757–80769. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Saini S, Arora S, Majid S, Shahryari V,
Chen Y, Deng G, Yamamura S, Ueno K and Dahiya R: Curcumin modulates
microRNA-203-mediated regulation of the Src-Akt axis in bladder
cancer. Cancer Prev Res (Phila). 4:1698–1709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang H, Liu X, Chen S, Wu J, Ye X, Xu L,
Chen H, Zhang D, Tan R and Wang Y: Tectorigenin inhibits the in
vitro proliferation and enhances miR-338* expression of pulmonary
fibroblasts in rats with idiopathic pulmonary fibrosis. J
Ethnopharmacol. 131:165–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fu R, Yang P, Sajid A and Li Z:
Avenanthramide A induces cellular senescence via
miR-129-3p/Pirh2/p53 signaling pathway to suppress colon cancer
growth. J Agric Food Chem. 67:4808–4816. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xia X, Wang X, Zhang S, Zheng Y, Wang L,
Xu Y, Hang B, Sun Y, Lei L, Bai Y and Hu J: miR-31 shuttled by
halofuginone-induced exosomes suppresses MFC-7 cell proliferation
by modulating the HDAC2/cell cycle signaling axis. J Cell Physiol.
234:18970–18984. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Demiroglu-Zergeroglu A, Turhal G, Topal H,
Ceylan H, Donbaloglu F, Karadeniz Cerit K and Odongo RR:
Anticarcinogenic effects of halofuginone on lung-derived cancer
cells. Cell Biol Int. 44:1934–1944. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang Y, Xie Z and Lu H: Significance of
halofuginone in esophageal squamous carcinoma cell apoptosis
through HIF-1α-FOXO3a pathway. Life Sci. 257:1181042020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Robert Roskoski Jr: Targeting ERK1/2
protein-serine/threonine kinases in human cancers. Pharmacol Res.
142:151–168. 2019. View Article : Google Scholar : PubMed/NCBI
|