Introduction
Breast cancer mainly occurs in glandular epithelial
tissue of breast and is one of the most common malignant tumors in
women (1). Breast cancer has a great
impact on physical and mental health of women, and even endangers
their lives in serious cases. According to statistics, the
incidence rate of breast cancer in the world is increasing year by
year (2,3). At present, the clinical treatment for
breast cancer is mainly surgical operation, radiotherapy,
chemotherapy, endocrine therapy and auxiliary treatment of
traditional Chinese medicine. Surgical resection, the main
treatment for patients with advanced breast cancer, is divided into
breast-conserving therapy and total mastectomy. Total mastectomy is
performed for breast cancer patients who are not suitable for
breast conserving surgery and has a great impact on the physical
and psychological well-being of the patients (4,5). Since
most surgical patients are already in advanced stage of breast
cancer at the time of treatment, breast cancer is likely to
metastasize even if the cancerous tissue is removed (6). Statistics show that the recurrence rate
of breast cancer metastatic patients is high and the 5-year
survival rate is low (7).
Clinically, the etiology of breast cancer is not completely clear,
so early diagnosis of breast cancer patients can be carried out to
improve the survival rate (8).
With the continuous expansion of research on
molecular biology related to breast cancer in recent years,
molecular targeted therapy is one of the most active research
fields at present (9). Molecular
targeting plays an important role in the diagnosis, staging and
comprehensive treatment of breast cancer. Transient receptor
potential channel 1 (TRPC1) and small breast epithelial mucin
(SBEM) were shown to be up-regulated in breast cancer tissues and
cells, and were related to breast cancer metastasis. It has been
reported that SBEM protein in breast cancer patients can be
detected to monitor the condition of breast cancer, and
down-regulation of SBEM gene expression can inhibit the
proliferation of breast cancer cells (10). A recent research report suggests that
a protein called Transient receptor potential channel 1 (TRPC1) is
closely related to the occurrence and development of tumors. TRPCI
belongs to non-selective cation channel families. TRPCI produces
channels for calcium and sodium ions to enter. These channels are
closely related to cell adhesion and drug resistance of cancer
cells. It is particularly important to determine the tools of TRPCI
physiology and the pathophysiological functions of TRPC channels
(11). Among them, TRPCI protein has
been proved to be abnormally up-regulated in renal carcinoma, liver
cancer, breast non-invasive ductal carcinoma and other solid tumors
(12). However, there is lack of
research on the expression and predictive value of TRPC1 and SBEM
in breast cancer. Therefore, our study aimed to provide a new
theoretical basis for the diagnosis and treatment of breast cancer
in molecular biology and to find suitable molecular markers
specific to breast cancer. Predictive value was explored of TRPCI
and SBEM protein in breast cancer metastasis, and whether they play
an important role in the diagnosis, staging and comprehensive
treatment of breast cancer.
Patients and methods
Data collection and patients
From April 2017 to November 2018, 50 female patients
with breast cancer diagnosed by surgery or biopsy were selected.
The patients were aged 29–59 years, with the average age of
50.43±1.02 years. Inclusion criteria: Breast cancer patients
admitted to the hospital; the tissue samples were all examined by
general surgery and pathology department and the patients were
confirmed as breast cancer (13); no
radiotherapy, chemotherapy or other treatment was performed.
Exclusion criteria: Patients with other preoperative complications;
patients with conscious, cognitive and other mental disorders;
pregnant women and patients with other contraindications. The study
was approved by the Ethics Committee of Weifang People's Hospital
(Weifang, China). Patients who participated in this research had
complete clinical data and signed informed consents were obtained
from the patients and/or guardians.
Main reagents, instruments and detection
methods
Main reagents and instruments
Trizol Reagent (Applied; Invitrogen), qRT-PCR kit
and minScript Reverse Transcription kit (Takara), HBS-1096A Enzyme
Labeling Analyzer (Nanjing Detie Experimental Equipment Co., Ltd.),
and Real-time Quantitative PCR instrument (BioRad) were applied.
Primer sequence of TRPC1, SBEM, and internal reference
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and miRNA negative
control were designed by Shanghai Gene Pharma. The sample was not
treated before administration (Table
I).
 | Table I.Primer sequences of TRPC1, SBEM and
internal reference GAPDH. |
Table I.
Primer sequences of TRPC1, SBEM and
internal reference GAPDH.
| Group | Upstream primer | Downstream
primer |
|---|
| TRPC1 |
5′-GCCAGTTTTGTCACTTTGTTATTT-3′ |
5′-CCCATTGTGTTTTTCTTATCCTCA-3′ |
| SBEM |
5′-TAGAGCTAGCGAATTATGAAGTTCTTAGCAGTCC-3′ |
5′-AGATCCTTCGCGGCCTCAGGGACACACTCTACCA-3′ |
| GAPDH |
5′-CGCTGAGTACGTCGTGGAGTC-3′ |
5′-GCTGATGATCTTGAGGCTGTTGTC-3′ |
Detection of mRNAs encoding TRPC1 and
SBEM
qRT-PCR was used to detect the expression of TRPC1
and SBEM in tissues. Total RNA of tissues was extracted according
to Trizol reagent operation instructions and dissolved in 20 µl of
DEPC water. The total RNA was then reverse transcribed using a
reverse transcription kit. The reaction system was as follows: 1 µl
of M-MLV, 1 µl of Olig (d T), 0.5 µl of RNA enzyme inhibitor, 1 µl
of d NTPs, RNase free water was supplemented to 15 µl. The sample
was incubated at 38°C for 60 min. cDNA (1 µl) was taken at 85°C for
5 sec and the synthesized cDNA was used as a template for qRT-PCR
amplification. The PCR reaction system was as follows: 2.5 µl of
10X PCR buffer, 1 µl of dNTPs, 1 µl of upstream and downstream
primers, 0.25 µl of Taq DNA polymerase, dd H2O was
supplemented to 25 µl. Reaction conditions were as follows:
Pre-denaturation at 95°C for 15 min, denaturation at 95°C for 15
sec, annealing at 60°C for 30 sec, for a total of 35 cycles.
Finally, the sample was extended for 15 min at 72°C. Each sample
was provided with three multiple wells for three repeated
experiments, with GAPDH as the internal reference for TRPC1 and
SBEM. After the reaction was completed, the amplification curve and
melting curve of Real-Time PCR were confirmed, and the relative
amount of the target gene was calculated according to the result
parameters. The relative quantification of target gene was
calculated by 2−ΔΔCT (Table
I).
Detection of the expression of TRPC1,
SBEM and GAPDH protein by western blot
Breast cancer tissues of 50 patients with breast
cancer and normal breast tissues adjacent to the cancer were put
into a precooled mortar, and the tissue was ground into powder in
liquid nitrogen. After the protein lysate was added, the total
protein in the tissue was separated and placed into a homogenizer
(Shanghai Active Motif Biotechnology Co., Ltd., item no.
40401/40415), 300 µl of lysate was added and the tissue mass
gradually disappeared through grinding until the lysate was free of
impurities and precipitates, then the sample was cracked on ice for
30 min and centrifuged at 20,000 × g for 20 min at 4°C, and finally
the supernatant was taken as the total cell protein. BCA protein
quantification was carried out by 6–12% of sodium dodecyl sulfate
polyacrylamide gel electrophoresis and transferred to
polyvinylidene fluoride membrane. After selecting the corresponding
band according to the target protein, the sample was sealed with
defatted milk powder with concentration of 5% for 2 h. After
rinsing the membrane, 2 ml of western primary antibody dilution
buffer (Beyotime Biotechnology) with dilution ratio of 1:1,000 was
added and placed at 4°C over night. After the first antibody was
re-warmed for 30 min before the experiment on the second day,
western second antibody (Beyotime Biotechnology) was incubated for
1 h with the same procedure, and developer was added in the dark
for exposure. PVDF film was imaged by Tocan 240 automatic gel
imaging system (Shanghai Tocan Bio-technology Co., Ltd.) and the
results were analyzed by Image LabTM software.
Statistical analysis
SPSS v17.0 software system was used for statistical
analysis. Counting data was expressed by the number of
cases/percentage n(%), and χ2 test was used for
comparison between the two groups. The measurement data were
expressed by mean number (X±sd), and the comparison between groups
was conducted by t-test or F-test. Spearman test was used for
correlation analysis. Logistic univariate and multivariate analysis
were performed on the risk factors related to breast cancer
metastasis in breast cancer patients. P<0.05 was considered to
indicate a statistically significant difference.
Results
General clinical data of patients
The age, tumor size, TNM stage, clinical stage and
lymph node metastasis of breast cancer tissue and normal breast
tissue were compared (P>0.05; Table
II).
 | Table II.General clinical data of patients. |
Table II.
General clinical data of patients.
| Group | n (%) |
|---|
| Age, years |
|
| ≤50 | 25 (50.00) |
|
>50 | 25 (50.00) |
| BMI
(kg/m2) | 19.20±1.04 |
| Residence |
|
|
Urban | 38 (76.00) |
|
Rural | 12 (24.00) |
| Educational
level |
|
| Junior
high school | 6 (12.00) |
| High
school | 14 (28.00) |
| Bachelor
degree or above | 30 (60.00) |
| Histological
classification |
|
| Level
1+level 2 | 26 (52.00) |
| Level
3 | 24 (48.00) |
| Tumor size, cm |
|
| ≤2 | 23 (46.00) |
|
>2 | 27 (54.00) |
| Clinical staging |
|
| Phase
I | 10 (20.00) |
| Phase
II | 17 (34.00) |
| Phase
III | 14 (28.00) |
| Phase
IV | 9
(18.00) |
| TNM staging |
|
| T1 | 10 (20.00) |
| T2 | 18 (36.00) |
| T3 | 13 (26.00) |
| T4 | 9
(18.00) |
| Lymph node
metastasis |
|
| No | 40 (80.00) |
| Yes | 10 (20.00) |
Expression of TRPC1, SBEM protein and
mRNA in breast cancer tissue and normal breast tissue
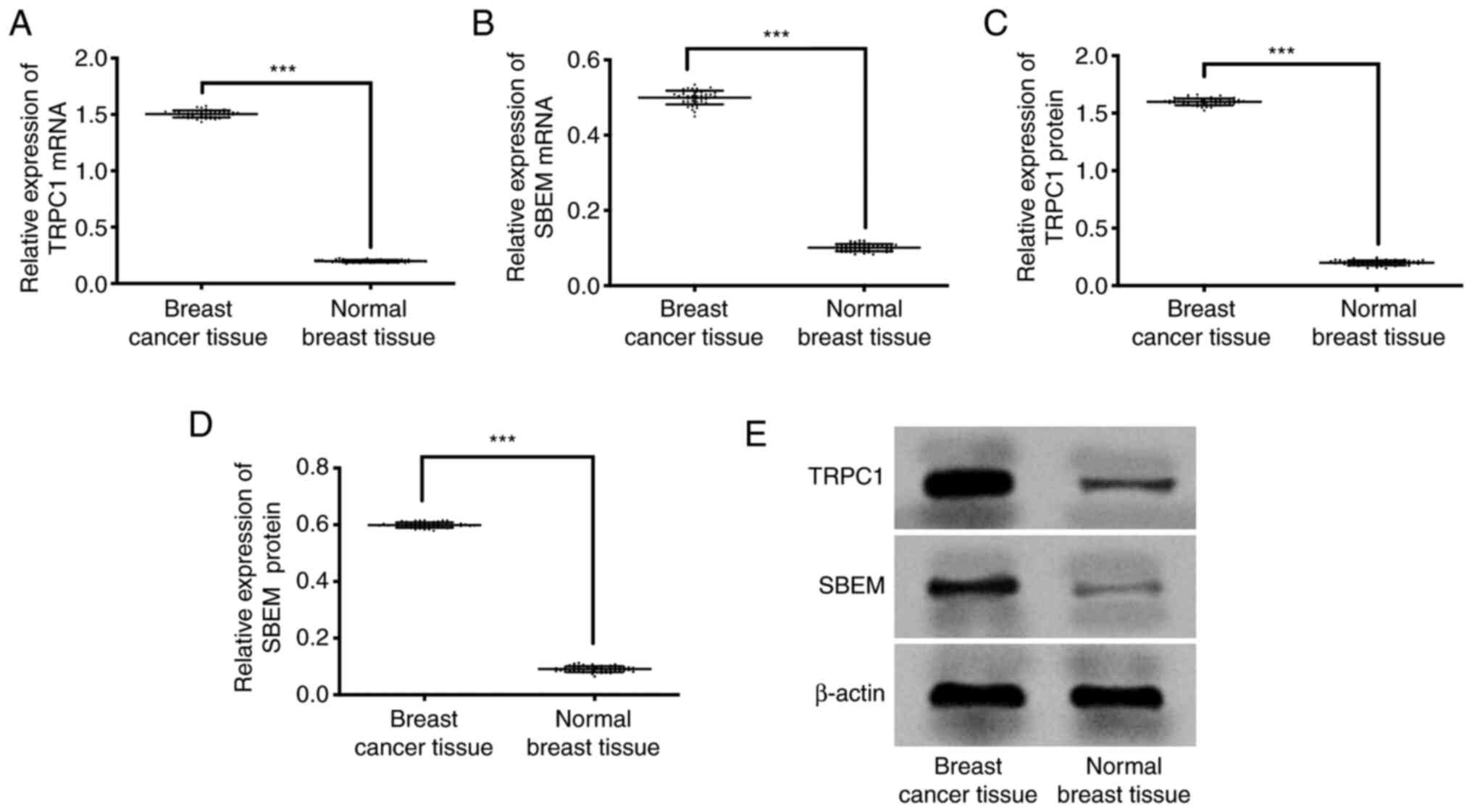
qRT-PCR results showed that the expression of TRPC1
in breast cancer tissue and normal breast tissue were (1.50±0.03),
(0.20±0.01) respectively. Expression of SBEM in breast cancer
tissue and normal breast tissue were (0.50±0.02), (0.10±0.01)
respectively. Western blot results showed that the expression of
TRPC1 protein in breast cancer tissues and normal breast tissues
were (1.60±0.03), (0.20±0.02) respectively. Expression of SBEM
protein in breast cancer tissue and normal breast tissue was
(0.60±0.01), (0.09±0.01) respectively. Compared with the two
groups, the expression of TRPC1 protein and mRNA in breast cancer
tissues was significantly higher than that in normal breast tissues
(P<0.001). However, the expression of SBEM protein and mRNA in
breast cancer tissues was significantly higher than that in normal
breast tissues (P<0.001; Fig.
1).
Relationship between TRPC1 expression
level and clinicopathological features of breast cancer
The expression of TRPC1 mRNA was not related to the
age, tumor size or histological grade of breast cancer patients,
but was related to TNM stage, clinical stage and lymph node
metastasis (Table III).
 | Table III.Relationship between mRNA expression
of TRPC1 and clinicopathological features of breast cancer. |
Table III.
Relationship between mRNA expression
of TRPC1 and clinicopathological features of breast cancer.
| Group | n | TRPC1 | t/F | P-value |
|---|
| Age, years |
|
| 2.000 | 0.051 |
| ≤50 | 25 | 1.49±0.04 |
|
|
|
>50 | 25 | 1.51±0.03 |
|
|
| Histological
classification |
|
| 1.397 | 0.169 |
| Level
1+level 2 | 26 | 1.50±0.02 |
|
|
| Level
3 | 24 | 1.51±0.03 |
|
|
| Tumor size, cm |
|
| 2.017 | 0.05 |
| ≤2 | 23 | 1.49±0.04 |
|
|
|
>2 | 27 | 1.51±0.03 |
|
|
| Clinical
staging |
|
| 5,704.000 | <0.001 |
| Phase
I | 10 | 0.80±0.03 |
|
|
| Phase
II | 17 | 1.07±0.03 |
|
|
| Phase
III | 14 | 1.77±0.03 |
|
|
| Phase
IV | 9 | 2.35±0.03 |
|
|
| TNM staging |
|
| 6,337.000 | <0.001 |
| T1 | 10 | 0.87±0.02 |
|
|
| T2 | 18 | 1.00±0.03 |
|
|
| T3 | 13 | 1.82±0.03 |
|
|
| T4 | 9 | 2.30±0.03 |
|
|
| Lymph node
metastasis |
|
| 140.000 | <0.001 |
| No | 40 | 1.00±0.02 |
|
|
|
Yes | 10 | 2.10±0.03 |
|
|
The expression of TRPC1 protein was not related to
the age, tumor size or histological grade of breast cancer
patients, but was related to TNM stage, clinical stage and lymph
node metastasis (Table IV).
 | Table IV.Relationship between TRPC1 protein
expression and clinicopathological features of breast cancer. |
Table IV.
Relationship between TRPC1 protein
expression and clinicopathological features of breast cancer.
| Group | n | TRPC1 | t/F | P-value |
|---|
| Age, years |
|
| 1.387 | 0.172 |
|
≤50 | 25 | 1.60±0.03 |
|
|
|
>50 | 25 | 1.61±0.02 |
|
|
| Histological
classification |
|
| 1.178 | 0.245 |
| Level
1+level 2 | 26 | 1.60±0.03 |
|
|
| Level
3 | 24 | 1.61±0.03 |
|
|
| Tumor size, cm |
|
| 1.762 | 0.084 |
| ≤2 | 23 | 1.58±0.02 |
|
|
|
>2 | 27 | 1.59±0.02 |
|
|
| Clinical
staging |
|
| 1,837.000 | <0.001 |
| Phase
I | 10 | 1.20±0.02 |
|
|
| Phase
II | 17 | 1.50±0.02 |
|
|
| Phase
III | 14 | 1.69±0.03 |
|
|
| Phase
IV | 9 | 2.02±0.03 |
|
|
| TNM staging |
|
| 1,909.000 | <0.001 |
| T1 | 10 | 1.18±0.02 |
|
|
| T2 | 18 | 1.52±0.02 |
|
|
| T3 | 13 | 1.68±0.03 |
|
|
| T4 | 9 | 2.03±0.03 |
|
|
| Lymph node
metastasis |
|
| 75.420 | <0.001 |
| No | 40 | 1.20±0.03 |
|
|
|
Yes | 10 | 2.00±0.03 |
|
|
Relationship between expression level
of SBEM and clinicopathological characteristics of breast
cancer
Expression of SBEM mRNA was not related to age,
tumor size or histological grade of breast cancer patients, but was
related to TNM staging and metastasis (Table V).
 | Table V.Relationship between mRNA expression
of SBEM and clinicopathological characteristics of breast
cancer. |
Table V.
Relationship between mRNA expression
of SBEM and clinicopathological characteristics of breast
cancer.
| Group | n | SBEM | t/F | P-value |
|---|
| Age, years |
|
| 1.768 | 0.0834 |
|
≤50 | 25 | 0.50±0.02 |
|
|
|
>50 | 25 | 0.51±0.02 |
|
|
| Histological
classification |
|
| 1.767 | 0.837 |
| Level
1+level 2 | 26 | 0.49±0.03 |
|
|
| Level
3 | 24 | 0.50±0.02 |
|
|
| Tumor size, cm |
|
| 1.631 | 0.109 |
| ≤2 | 23 | 0.50±0.03 |
|
|
|
>2 | 27 | 0.51±0.01 |
|
|
| Clinical
staging |
|
| 2,871.000 | <0.001 |
| Phase
I | 10 | 0.25±0.01 |
|
|
| Phase
II | 17 | 0.38±0.02 |
|
|
| Phase
III | 14 | 0.40±0.02 |
|
|
| Phase
IV | 9 | 0.97±0.02 |
|
|
| TNM staging |
|
| 2,687 | <0.001 |
| T1 | 10 | 0.27±0.01 |
|
|
| T2 | 18 | 0.35±0.02 |
|
|
| T3 | 13 | 0.43±0.02 |
|
|
| T4 | 9 | 0.95±0.02 |
|
|
| Lymph node
metastasis |
|
| 135.800 | <0.001 |
| No | 40 | 0.30±0.01 |
|
|
|
Yes | 10 | 0.90±0.02 |
|
|
Expression of SBEM protein was not related to age,
tumor size or histological grade of breast cancer patients, but was
related to TNM staging and metastasis (Table VI).
 | Table VI.Relationship between expression of
SBEM protein and clinicopathological characteristics of breast
cancer. |
Table VI.
Relationship between expression of
SBEM protein and clinicopathological characteristics of breast
cancer.
| Group | n | SBEM | t/F | P-value |
|---|
| Age, years |
|
| 0.000 | 0.999 |
|
≤50 | 25 | 0.59±0.02 |
|
|
|
>50 | 25 | 0.59±0.01 |
|
|
| Histological
classification |
|
|
|
|
| Level
1+level 2 | 26 | 0.59±0.02 | 1.767 | 0.084 |
| Level
3 | 24 | 0.60±0.02 |
|
|
| Tumor size, cm |
|
| 1.631 | 0.109 |
| ≤2 | 23 | 0.59±0.03 |
|
|
|
>2 | 27 | 0.60±0.01 |
|
|
| Clinical
staging |
|
| 1,916.000 | <0.001 |
| Phase
I | 10 | 0.30±0.01 |
|
|
| Phase
II | 17 | 0.35±0.01 |
|
|
| Phase
III | 14 | 0.50±0.01 |
|
|
| Phase
IV | 9 | 1.25±0.01 |
|
|
| TNM staging |
|
| 1,779.000 | <0.001 |
| T1 | 10 | 0.29±0.01 |
|
|
| T2 | 18 | 0.33±0.01 |
|
|
| T3 | 13 | 0.55±0.01 |
|
|
| T4 | 9 | 1.20±0.01 |
|
|
| Lymph node
metastasis |
|
| 169.700 | <0.001 |
| No | 40 | 0.30±0.01 |
|
|
|
Yes | 10 | 0.90±0.01 |
|
|
Correlation between TRPC1 and SBEM
proteins in breast cancer of different clinical stages
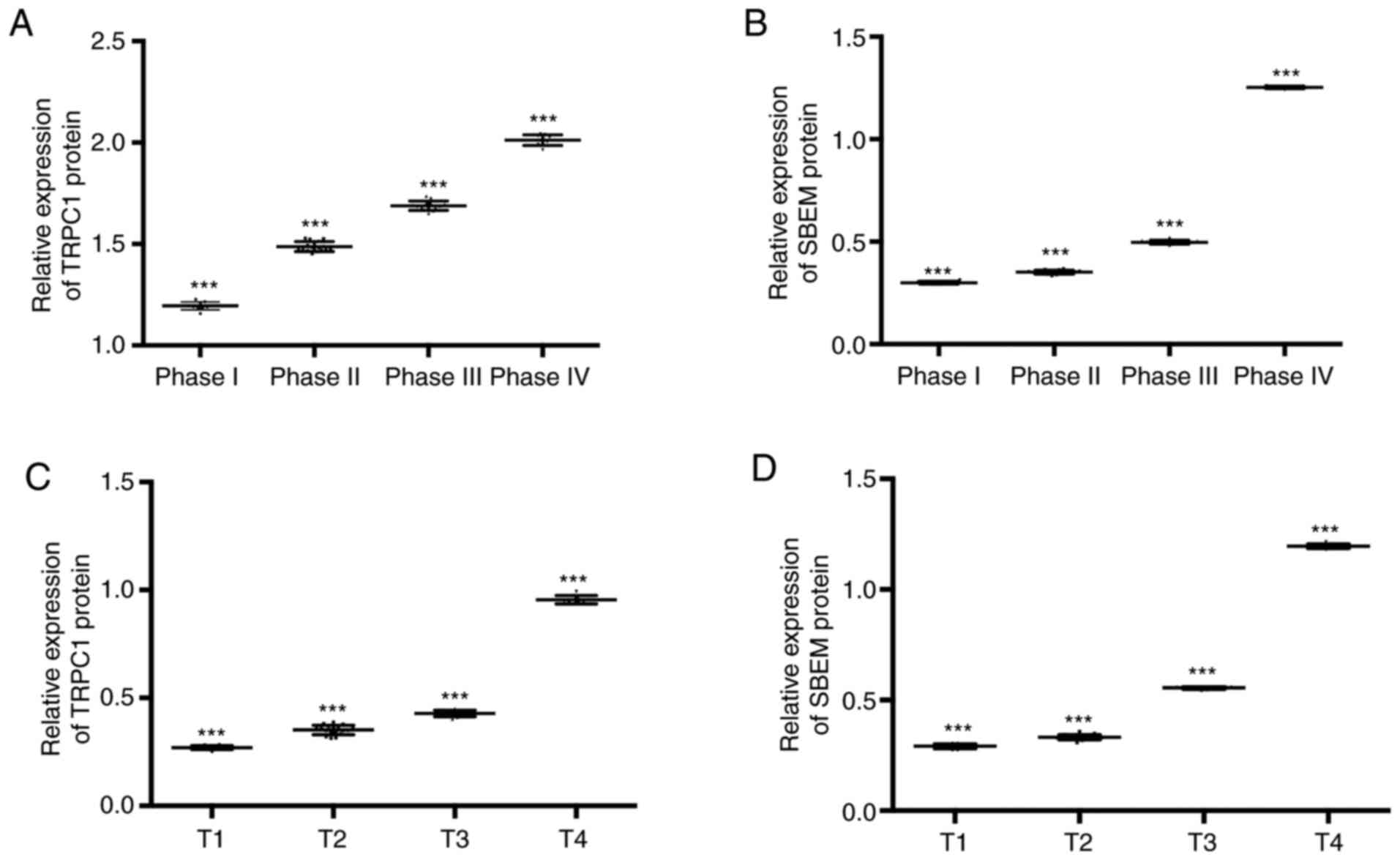
The expression of TRPC1 in the tissues of patients
at T1, T2, T3, and T4 of breast cancer were (1.20±0.02),
(1.50±0.02), (1.69±0.03), and (2.02±0.03) respectively. Expression
levels of SBEM were (0.30±0.01), (0.35±0.01), (0.50±0.01), and
(1.25±0.01). Compared with patients at T1, the relative expression
of TRPC1 and SBEM in tissues of patients at T2 and T3 decreased
significantly (P<0.05). With the increase of clinical stage, the
relative expression of TRPC1 and SBEM in tissues increased
continuously. The expression levels of TRPC1 in tissues at T1, T2,
T3 and T4 were (0.27±0.01), (0.35±0.02), (0.43±0.02), and
(0.95±0.02). Expression levels of SBEM in different TNM stages of
breast cancer were (0.29±0.01), (0.33±0.01), (0.55±0.01), and
(1.20±0.01). Compared with T1 patients, the relative expression of
TRPC1 and SBEM in tissues at the other three stages decreased
significantly (P<0.05). With the increase of clinical stage, the
relative expression of TRPC1 and SBEM in tissues increased
continuously. Stage I, II, III and IV of the TNM stage of the
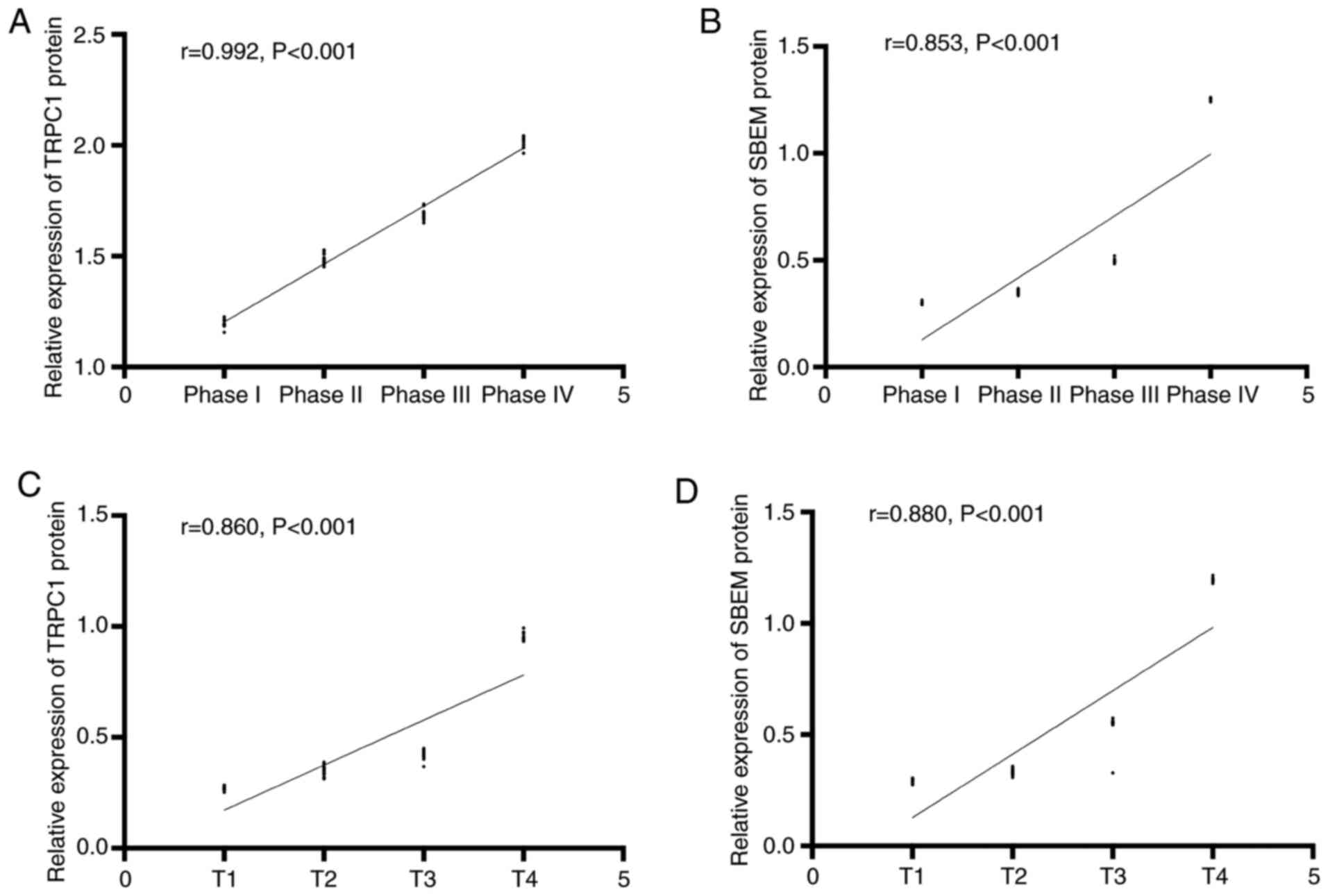
patient were set as 1, 2, 3 and 4 respectively. Spearman
correlation analysis of tissue TRPC1 and SBEM relative expression
with different clinical stages of breast cancer showed that TRPC1
relative expression was positively correlated with clinical stages
of breast cancer (r=0.992, P<0.001). The relative expression of
tissue SBEM was positively correlated with the clinical stage of
breast cancer (r=0.853, P<0.001). The relative expression of
tissue TRPC1 was positively correlated with TNM staging of breast
cancer (r=0.860, P<0.001). The relative expression of SBEM was
positively correlated with TNM staging of breast cancer (r=0.880,
P<0.001). That is, with the worsening of breast cancer, the
expression of TRPC1 and SBEM increased (Figs. 2 and 3).
Predictive value of TRPC1 and SBEM
protein in breast cancer metastasis
Single factor analysis of breast
cancer metastasis and related factors
Logistic single factor analysis of risk factors
related to breast cancer metastasis in breast cancer patients
showed that there were significant differences in age, clinical
stage, TNM stage, TRPC1, and SBEM between breast cancer metastasis
and breast cancer non-metastasis (P<0.05). The patient's age,
clinical stage, TNM stage, TRPC1, SBEM were related to breast
cancer metastasis and were risk factors for breast cancer
metastasis, indicating that the risk of breast cancer increased
with advanced age, worsening condition and high expression of TRPC1
and SBEM, as shown in Tables VII
and VIII.
 | Table VII.Assignments of factors related to
breast cancer metastasis. |
Table VII.
Assignments of factors related to
breast cancer metastasis.
| Correlative
factor | Assignment
description |
|---|
| Age (years) | <50=0;
≥50=1 |
| Histological
classification | Level 1+Level 2=0;
Level 3=1 |
| Tumor size
(cm) | ≤2=0; >2=1 |
| Clinical
staging | Phase I–II=0; Phase
III–IV=1 |
| TNM staging | T0-T1=0;
T2-T3=1 |
| TRPC1 | <0.90=0;
>0.90=1 |
| SBEM | <0.90=0;
>0.90=1 |
 | Table VIII.Single factor analysis of breast
cancer metastasis and related factors. |
Table VIII.
Single factor analysis of breast
cancer metastasis and related factors.
| Factor | Metastasis of
breast cancer (n=10) | Non-metastatic
breast cancer (n=40) |
t/χ2 | P-value |
|---|
| Age, years |
|
| 2.000 | 0.157 |
|
≤50 | 3 (30.00) | 22 (55.00) |
|
|
|
>50 | 7 (70.00) | 18 (45.00) |
|
|
| Histological
classification |
|
| 0.017 | 0.897 |
| Level
1+level 2 | 5 (50.00) | 21 (52.50) |
|
|
| Level
3 | 5 (50.00) | 19 (47.50) |
|
|
| Tumor size, cm |
|
| 0.181 | 0.670 |
| ≤2 | 4 (40.00) | 19 (47.50) |
|
|
|
>2 | 6 (60.00) | 21 (52.50) |
|
|
| TNM staging |
|
| 25.850 | <0.001 |
| T1 | 0 (0.00) | 10 (25.00) |
|
|
| T2 | 0 (0.00) | 18 (45.00) |
|
|
| T3 | 3 (30.00) | 10 (25.00) |
|
|
| T4 | 7 (70.00) | 2 (5.00) |
|
|
| Clinical
staging |
|
| 16.020 | 0.001 |
| Phase
I | 0 (0.00) | 10 (25.00) |
|
|
| Phase
II | 0 (0.00) | 17 (42.50) |
|
|
| Phase
III | 5 (50.00) | 9 (22.50) |
|
|
| Phase
IV | 5 (50.00) | 4 (10.00) |
|
|
|
TRPC1 | 0.90±0.02 | 0.30±0.01 | 135.800 | <0.001 |
|
SBEM | 0.90±0.01 | 0.30±0.01 | 169.700 | <0.001 |
Multivariate analysis of breast cancer
metastasis and related factors
Risk factors related to malignant breast cancer
metastasis were analyzed by multivariate conditional Logistic
regression. The results showed that TNM staging, TRPC1 and SBEM
were independent risk factors for malignant breast cancer
metastasis. Breast cancer is more prone to metastasis with
increasing TNM stage, and breast cancer metastasis is closely
related to TRPC1 and SBEM levels (Table
IX).
 | Table IX.Multivariate analysis of breast
cancer metastasis and related factors. |
Table IX.
Multivariate analysis of breast
cancer metastasis and related factors.
| Factor | β | SE | Wald | P-value | Exp (β) | 95% CI |
|---|
| TNM staging | 1.133 | 1.667 | 0.492 | 0.041 | 1.568 | 1.244–22.018 |
| Clinical
staging | 0.974 | 1.802 | 1.028 | 0.009 | 1.209 | 0.081–4.680 |
| TRPC1 | 1.362 | 1.714 | 0.515 | 0.015 | 2.493 | 0.154–19.200 |
| SBEM | 1.025 | 1.519 | 0.438 | 0.027 | 0.520 | 0.012–4.205 |
Discussion
At present, the overall treatment effect for breast
cancer patients is not ideal. In addition to the untimely diagnosis
and treatment of early breast cancer patients, surgical resection
at this stage has poor treatment effect for breast cancer patients
with lymph node metastasis, and the recurrence rate of patients is
extremely high (14). Traditional
pathological section examination and imaging examination can
accurately diagnose the condition of breast cancer patients, but it
is difficult to monitor the development and changes of specific
conditions of breast cancer. In particular, it is not sensitive to
whether cancer is transferred from breast tissue to outside the
breast in the early stage (15). The
key to reduce the recurrence rate and the mortality rate of breast
cancer is to accurately diagnose the condition of the breast cancer
and to establish the corresponding treatment plan (16). With the deepening research on
oncogenes, tumor suppressor genes and related regulatory proteins,
it is of great significance to analyze the development mechanism of
breast cancer based on the molecular biology of tumors to explore
new therapeutic methods (17).
In this study, the mRNA and protein differences in
TRPC1 and SBEM of breast cancer patients and normal breast cancer
tissues were detected by qRT-PCR and Western blot techniques. The
results showed that the expression of TRPC1 and SBEM in breast
cancer tissues was significantly higher than that in normal breast
tissues, and the difference was statistically significant. TRPC1
can affect the biological function of tumor cells by regulating
gene expression and relevant tumor signal pathways, thus affecting
the development of tumors (18). As
a tumor-related antigen, SBEM is usually highly expressed in tumor
cells. At present, SBEM has been confirmed to be up-regulated in
normal breast tumor cells and can be used as a new marker for
detecting micrometastasis and early diagnosis of breast cancer
(19,20). TRPC1, as a potential target molecule
for diagnosis and treatment of early breast cancer, is abnormally
elevated in a variety of tumor tissues including breast cancer.
Some research indicates that it can play a role in inducing
apoptosis of breast cancer cells by inhibiting expression of
apoptosis protein-related proteins (21,22). By
constructing specific TRPC1 siRNA expression vector and lentivirus
vector mediated TRPC1 siRNA interference system, transfecting cells
and establishing stable passage cell lines, it was found that after
down-regulation of TRPC1 gene, breast cancer cell proliferation,
invasion and migration ability were down-regulated, and apoptosis
rate of cancer cells increased (23,24).
Therefore, we consider that both TRPC1 and SBEM are up-regulated in
breast cancer tissues. Then the clinical data of breast cancer
patients was explored and it was found that the expression of TRPC1
and SBEM was related to TNM stage, clinical stage and lymph node
metastasis of breast cancer. At present, although there is no
specific study on TNM staging, metastasis, TRPC1 and SBEM of breast
cancer, there are reports on the relationship between target
protein regulation and breast cancer. It was suggested that the
expression of TRPC1 detected by qRT-PCR technology showed a
decreasing trend in breast cancer tissues, and miRNA expression in
patients with different TNM staging, lymph node metastasis and
non-metastasis was further detected by experiments. It was found
that the high TNM stage was closely related to the high expression
of TRPC1 in lymph node metastasis cancer tissue, indicating that
the expression change of TRPC1 was related to the breast cancer
metastasis and clinical pathological stage, which is similar to the
results of this study and is an excellent auxiliary evidence for
the results of this study (25).
Then, we analyzed the correlation between TRPC1 and SBEM in breast
cancer of different clinical stages and TNM stages, and found that
the relative expression of TRPC1 and SBEM in tissues increased with
the increase of clinical and TNM stages, and the relative
expression of TRPC1 and SBEM were positively correlated with
clinical stage and TNM stage of breast cancer. miRNAs and related
proteins have been proved to be closely related to tumor staging.
The expression level of miRNAs increased or decreased significantly
with tumor staging by inhibiting or promoting tumor in different
tumors (26). Finally, we analyzed
the risk factors related to breast cancer metastasis in breast
cancer patients by Logistic single factor analysis, and found that
clinical stage, TNM stage and lymph node metastasis were important
prognostic factors of patients (27). However, there has been no previous
study on the diagnostic efficacy and predictive value of TRPC1 and
SBEM expression changes in breast cancer metastatic tissues. In
this study, TRPC1 and SBEM showed certain predictive value for the
diagnosis and prognosis of breast cancer metastasis.
This study confirmed the expression of TRPC1 and
SBEM in breast cancer patients and their predictive value, but
there are still some deficiencies in the study. There is no
specific analysis of the regulatory effects of TRPC1 and SBEM
expression changes on breast cancer cells, or further explanation
of their biological functions. TRPC1 and SBEM, as clinical routine
tumor markers, were analyzed, which have certain influence on the
accuracy of research results. Therefore, further study is still
necessary.
Collectively, the expression of TRPC1 and SBEM in
breast cancer tissues is up-regulated. TRPC1 and SBEM may be
involved in the process of breast cancer occurrence, development
and metastasis, and can be used as potential tissue biomarkers for
diagnosis of breast cancer metastasis and for disease
assessment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ wrote the manuscript, analyzed and interpreted
the patient data. XL performed the PCR and the western blot
analysis. WG assistedƒ with statistical analysis. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Weifang People's Hospital (Weifang, China). Patients who
participated in this research had complete clinical data and signed
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arun G and Spector DL: MALAT1 long
non-coding RNA and breast cancer. RNA Biol. 16:860–863. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kennedy SP, Han JZR, Portman N, Nobis M,
Hastings JF, Murphy KJ, Latham SL, Cadell AL, Miladinovic D,
Marriott GR, et al: Targeting promiscuous heterodimerization
overcomes innate resistance to ERBB2 dimerization inhibitors in
breast cancer. Breast Cancer Res. 21:432019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishay-Ronen D, Diepenbruck M, Kalathur
RKR, Sugiyama N, Tiede S, Ivanek R, Bantug G, Morini MF, Wang J,
Hess C and Christofori G: Gain fat-lose metastasis: Converting
invasive breast cancer cells into adipocytes inhibits cancer
metastasis. Cancer Cell. 35:17–32. e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benjamin MA, Sinnott C, Bawa S, Kaufman
DI, Guarino K and Addona T: Re-excision rate after partial
mastectomy in oncoplastic breast-conserving surgery: A
single-institutional experience and review of the literature. Ann
Plast Surg. 82 (4S Suppl 3):S170–S172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaczmarski K, Wang P, Gilmore R, Overton
HN, Euhus DM, Jacobs LK, Habibi M, Camp M, Weiss MJ and Makary MA:
Surgeon re-excision rates after breast-conserving surgery: A
measure of low-value care. J Am Coll Surg. 228:504–512.e2. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen IX, Chauhan VP, Posada J, Ng MR, Wu
MW, Adstamongkonkul P, Huang P, Lindeman N, Langer R and Jain RK:
Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte
infiltration, and improves immunotherapy in metastatic breast
cancer. Proc Natl Acad Sci USA. 116:4558–4566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baker E, Whiteoak N, Hall L, France J,
Wilson D and Bhaskar P: Mammaglobin-A, VEGFR3, and Ki67 in human
breast cancer pathology and five year survival. Breast Cancer
(Auckl). 13:11782234198589572019.PubMed/NCBI
|
|
8
|
Malmgren J, Hurlbert M, Atwood M and
Kaplan HG: Examination of a paradox: Recurrent metastatic breast
cancer incidence decline without improved distant disease survival:
1990–2011. Breast Cancer Res Treat. 174:505–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Esteva FJ, Hubbard-Lucey VM, Tang J and
Pusztai L: Immunotherapy and targeted therapy combinations in
metastatic breast cancer. Lancet Oncol. 20:e175–e186. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maeda SS, Borba VZ, Camargo MB, Silva DM,
Borges JL, Bandeira F and Lazaretti-Castro M; Brazilian Society of
Endocrinology Metabology (SBEM), : Recommendations of the Brazilian
society of endocrinology and metabology (SBEM) for the diagnosis
and treatment of hypovitaminosis D. Arq Bras Endocrinol Metabol.
58:411–433. 2014.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sambale M, Intemann J, Pap T and Sherwood
J: A homeostatic role for transient receptor potential cation
channel (TRPC1) in articular cartilage. Osteoarthriti Cartilage.
27:S1752019. View Article : Google Scholar
|
|
12
|
Selli C, Pearce DA, Sims AH and Tosun M:
Differential expression of store-operated calcium- and
proliferation-related genes in hepatocellular carcinoma cells
following TRPC1 ion channel silencing. Mol Cell Biochem.
420:129–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Finn RS, Martin M, Rugo HS, Jones S, Im
SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al:
Palbociclib and letrozole in advanced breast cancer. N Engl J Med.
375:1925–1936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Swain SM, Baselga J, Kim SB, Ro J,
Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A,
Heeson S, et al: Pertuzumab, trastuzumab, and docetaxel in
HER2-positive metastatic breast cancer. N Engl J Med. 372:724–734.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang D, Khosla A, Gargeya R, Irshad H and
Beck A: Deep learning for identifying metastatic breast cancer.
arXiv preprint arXiv: 1606.05718. 2016.
|
|
16
|
Murtaza M, Dawson SJ, Pogrebniak K, Rueda
OM, Provenzano E, Grant J, Chin SF, Tsui DWY, Marass F, Gale D, et
al: Multifocal clonal evolution characterized using circulating
tumour DNA in a case of metastatic breast cancer. Nat Commun.
6:87602015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pavan S, Meyer-Schaller N, Diepenbruck M,
Kalathur RKR, Saxena M and Christofori G: A kinome-wide
high-content siRNA screen identifies MEK5-ERK5 signaling as
critical for breast cancer cell EMT and metastasis. Oncogene.
37:4197–4213. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grant CV, Carver CM, Hastings SD,
Ramachandran K, Muniswamy M, Risinger AL, Beutler JA and Mooberry
SL: Triple-negative breast cancer cell line sensitivity to englerin
A identifies a new, targetable subtype. Breast Cancer Res Treat.
177:345–355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue D, Xia T, Wang J, Chong M, Wang S and
Zhang C: Role of regulatory T cells and CD8+ T
lymphocytes in the dissemination of circulating tumor cells in
primary invasive breast cancer. Oncol Lett. 16:3045–3053.
2018.PubMed/NCBI
|
|
20
|
Johnston APR, Rae J, Ariotti N, Bailey B,
Lilja A, Webb R, Ferguson C, Maher S, Davis TP, Webb RI, et al:
Journey to the centre of the cell: Virtual reality immersion into
scientific data. Traffic. 19:105–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Grady S and Morgan MP:
Microcalcifications in breast cancer: From pathophysiology to
diagnosis and prognosis. Biochim Biophys Acta Rev Cancer.
1869:310–320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaemmerer E, Turner D, Peters AA,
Roberts-Thomson SJ and Monteith GR: An automated epifluorescence
microscopy imaging assay for the identification of phospho-AKT
level modulators in breast cancer cells. J Pharmacol Toxicol
Methods. 92:13–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Abdoul-Azize S, Buquet C, Li H, Picquenot
JM and Vannier JP: Integration of Ca2+ signaling
regulates the breast tumor cell response to simvastatin and
doxorubicin. Oncogene. 37:4979–4993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pu Q, Zhao Y, Sun Y, Huang T, Lin P, Zhou
C, Qin S, Singh BB and Wu M: TRPC1 intensifies house dust
mite-induced airway remodeling by facilitating
epithelial-to-mesenchymal transition and STAT3/NF-κB signaling.
FASEB J. 33:1074–1085. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu Z, Shao Z, Wang M, Thorndike EH, Song Y
and Shang ZJ: Expression of transient receptor potential canonical
1 (TRPC1) in tongue squamous cell carcinoma and correlations with
clinicopathological features and outcomes. Int J Clin Exp Pathol.
10:1477–1487. 2017.
|
|
26
|
Pu Q, Huang Y, Lu Y, Peng Y, Zhang J, Feng
G, Wang C, Liu L and Dai Y: Tissue-specific and plasma microRNA
profiles could be promising biomarkers of histological
classification and TNM stage in non-small cell lung cancer. Thorac
Cancer. 7:348–354. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (Eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|