Introduction
Lung cancer resulted in >1.7 million mortalities
globally in 2018 (1). Non-small cell
lung cancer (NSCLC) is the most prevalent lung cancer subtype that
accounts for >80% of lung cancer cases (2). Chemotherapy is a standard therapeutic
approach for patients with NSCLC that has been demonstrated to
increase the overall survival time of patients (3,4).
5-Fluorouracil (5-FU) is one of most commonly used chemotherapeutic
agents for patients with NSCLC (5).
Mechanistically, it disrupts uracil metabolism in cancer cells and
initially inhibits cancer progression (6); however, resistance to 5-FU frequently
develops and results in poor therapeutic outcomes and patient
mortality (7). Therefore, numerous
studies have attempted to investigate the complex mechanisms
underpinning the development chemotherapeutic resistance in order
to improve the efficacy of chemotherapy treatment regimens and,
ultimately, patient outcomes (8,9).
MicroRNAs (miRNAs) are a group of non-coding,
single-stranded RNA molecules ubiquitously expressed in human cells
(10). In cells, miRNAs serve as
negative regulators of gene expression by binding to the
3′-untranslated region (UTR) of target gene mRNAs and inducing mRNA
degradation or inhibiting translation (11). Expression of miRNAs is essential for
multiple biological processes, such as cell proliferation and
differentiation (12,13). However, the aberrant expression of
certain miRNAs and the subsequent dysregulation of target gene
expression have been associated with the genesis and progression of
multiple types of cancer (14,15).
Downregulation of miRNA (miR)-124-5p is associated with
lymphangiogenesis in human gastric cancer (16); additionally, low expression levels of
miR-124-5p have been detected in glioma and colorectal cancer cells
(17,18).
Astrocyte elevated gene-1 (AEG-1) was originally
identified in primary human fetal astrocytes (19) and it was subsequently revealed that
AEG-1 influenced the progression of several types of cancer. In
lung cancer, the upregulation of AEG-1 was associated with the
epithelial-mesenchymal transition (EMT) and mediated metastasis
(20); furthermore, increased
expression of AEG-1 was associated with chemotherapeutic resistance
in cancer cells (21,22). However, the role of AEG-1 in the
development of resistance to chemotherapy in lung cancer is yet to
be elucidated.
The results of the present study revealed that the
downregulation of miR-124-5p conferred chemotherapeutic resistance
and may, therefore, represent a novel treatment strategy for
patients with NSCLC.
Materials and methods
Patients and tissue samples
A total of 40 pairs of tumors and adjacent
noncancerous tissues (>5 cm from tumors) were surgery removed
from patients with NSCLC at The Third People's Hospital of Linyi
City (Shandong, China) between June 2015 and October 2017. Tissues
were stored at −80°C until further use. The present study was
approved by the Ethics Committee of The Third People's Hospital of
Linyi City, and all participants provided written informed
consent.
Cell culture
A549, H1299 and 293 cell lines were purchased from
the American Type Culture Collection and cultured in Dulbecco's
Modified Eagle's Medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo
Fisher Scientific, Inc.) in a humidified incubator with 5%
CO2 at 37°C. 5-FU was purchased from Sigma-Aldrich;
Merck KGaA, dissolved in dimethyl sulfoxide (DMSO) and diluted in
DMEM prior to use.
Generation of 5-FU resistant A549
(A549/5-FU) cells
A549/5-FU cells were generated from A549 parental
cells. Briefly, A549 cells were treated with gradually increasing
concentrations of 5-FU (0.1–10 µM) in DMEM for >9 months
(23). The proliferation of A549
cells was initially inhibited by 5-FU. However, following culture
with 5-FU for 8 months, the cell viability assay revealed that the
cells were relatively insensitive towards 5-FU compared with cells
treated with DMSO in the control group.
Elevation/inhibition of
miR-124-5p
miR-124-5p mimic (5′-CGUGUUCACAGCGGACCUUGAU-3′);
miR-negative control (NC) (5′-AUUGGAACGAUACAGAGAAGAUU-3′);
miR-124-5p inhibitor (5′-AUCAAGGUCCGCUGUGAACACG-3′); and miR-NC
inhibitor (5′-CAGUACUUUUGUGUAGUACAA-3′) were purchased from Applied
Biological Materials, Inc. The miR-124-5p mimic or inhibitor (50
nM) was transfected into the A549, A549/5-FU and H1299 cell lines
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol. In brief,
miR-124-5p mimic or inhibitor (50 nM) was mixed with
Lipofectamine® 2000 in DMEM at 37°C for 30 min before
being added to the cultured cells. The transfection efficacy was
assessed 72 h after transfection using RT-qPCR.
Flow cytometry analysis
The percentage of apoptotic A549/5-FU cells was
measured using the Annexin V-FITC Apoptosis Detection Kit I (BD
Biosciences) according to the manufacturer's protocol. Briefly, 48
h following treatment with 1 µM 5-FU or DMSO, and transfection with
miR-NC mimic or miR-124-5p mimic, 5×105 A549/5-FU cells
were collected and stained with PI and FITC-Annexin V at room
temperature for 30 min. The samples were analyzed using a
FACSCalibur flow cytometer (BD Biosciences). The data were analyzed
using FlowJo software V. 1.6.0 (FlowJo software LLC).
PI+/Annexin V+ and PI−/Annexin
V+ cells were considered to indicate apoptotic
cells.
Western blotting
Total protein lysates were extracted from A549,
A549/5-FU and H1299 cells using radioimmunoprecipitation assay
lysis buffer (Sigma-Aldrich; Merck KGaA). A total of 25 µg/lane of
the lysates were separated by SDS-PAGE on an 8% gel. The
concentration of lysates was determined using a Bicinchoninic acid
Protein Assay kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The separated proteins were subsequently
transferred onto a polyvinylidene difluoride membrane and blocked
with 5% non-fat milk for 1 h at room temperature. The membrane was
incubated with primary antibodies against AEG-1 (cat. no. 14065;
1:1,000; Cell Signaling Technology, Inc.) and β-actin (cat. no.
A1978; 1:10,000; Sigma Aldrich, Merck KGaA) overnight at 4°C.
Following incubation, the membrane was washed with TBST (0.2%
Tween-20) and incubated with HRP-conjugated secondary antibodies
against rabbit (cat. no. ABL3012-2; 1:100,000; AbSci) and mouse
(cat. no. ABL3032-2; 1:100,000; AbSci) for 1 h at 4°C. The protein
bands were visualized using Pierce™ Enhanced chemiluminescence
Western Blot Substrate (Pierce; Thermo Fisher Scientific, Inc.) and
Image Quant LAS 500 (GE Healthcare Life Sciences).
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted from lung cancer and
adjacent lung tissues, A549, A549/5-FU and H1299 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). For analysis of miR-124-5p expression, RT was performed
using a stem-loop primer and the RevertAid First Strand cDNA kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. For mRNA expression analysis, RNA was reverse transcribed
into cDNA using PrimeScript RT Master mix (Takara Bio, Inc.)
according to the manufacturer's protocol. qPCR was subsequently
performed using a SYBR® Premix Ex Taq kit (Takara Bio,
Inc.). miRNA and mRNA expression levels were quantified using the
2−ΔΔCq method (24) and
normalized to U6 and β-actin levels, respectively. The
thermocycling condition were as follow: 95°C for 30 sec; 35 cycles
of 95°C for 15 sec and 60° for 20 sec. The following primer
sequences were used: Stem loop,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGATCAAG-G-3′; miR-124-5p
forward, 5′-TCGGCAGGCGTGTTCACAGCGG-3′ and reverse,
5′-CTCAACTGGTGTCGTGGA-3′; U6 forward, 5′-CTCAACTGGTGTC-GTGGA-3′ and
reverse, 5′-CTCAACTGGTGTCGTGGA-3′; AEG-1 forward,
5′-AAATGGGCGGACTGTTGAAGT-3′ and reverse,
5′-CTGTTTTGCACTGC-TTTAGCAT-3′; β-actin forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′.
Cytotoxicity assay
The Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) was used to investigate the cytotoxicity of
5-FU in A549 and H1299 cell lines. Briefly, 1,000 cells/well were
seeded in a 96-well plate and incubated for 24 h at 37°C. The cells
were treated with DMSO or increasing concentrations of 5-FU (0, 2,
5, 10, 20 and 40 µM) for 72 h; subsequently 10 µl CCK-8 solution
was added to each well and incubated for an additional 2 h. DMEM
containing the CCK-8 solution was transferred into wells of another
96-well plate, and the absorbance of each well was measured at 450
nm using a microplate reader. The ratio of absorbance of 5-FU- to
DMSO-treated wells was used to determine the cytotoxicity. The half
maximal inhibitory concentration (IC50) was calculated
using the online tool IC50 Calculator (https://www.aatbio.com/tools/ic50-calculator).
Dual-luciferase reporter assay
TargetScan (http://www.targetscan.org/vert_72/) was used to
predict the potential target genes of miR-124-5p. The AEG-1 3′-UTR
was amplified from the cDNA of the 293 cell line and was
subsequently cloned into the pmirGLO plasmid (Promega Corporation)
in order to synthesize pmirGLO-AEG-1 3′UTR-wild-type (WT). A
pmirGLO-AEG-1 3′UTR-mutant (mut) containing two mutations in the
predicted miR-124-5p binding site was generated by introducing
site-specific mutations in the AEG-1 3′UTR-WT using the Quick
Site-Directed Mutagenesis kit (Agilent Technologies, Inc.). A549
cells were transfected with either pmirGLO-AEG-1 3′UTR-WT or
3′UTR-mut, as well as the miR-124-5p mimic or miR-NC mimic, using
Lipofectamine® 2000. Following incubation for 48 h at
37°C, relative luciferase activity was detected using a Dual-Glo
Luciferase Assay system (Promega Corporation) according to the
manufacturer's protocol. For normalization, the firefly luciferase
was normalized to Renilla luciferase.
Knockdown and overexpression of
AEG-1
Control small interfering (si)RNA
(5′-TTCTCCGAACGTGTCACGT-3′) and AEG-1 siRNA
(5′-AACAGAAGAAGAAGAACCGGA-3′) were purchased from Shanghai
GenePharma Co., Ltd. Transient silencing was performed on AEG-1
cells; 50 nM AEG-1 siRNA was mixed with Lipofectamine®
RNAiMax (Invitrogen; Thermo Fisher Scientific, Inc.) in serum-free
DMEM for 5 min at room temperature and added to the A549 and
A549/5-FU cells. The cells were used for further experimentation 72
h post-transfection.
Full length AEG-1 cDNA was amplified from A549 cDNA
and cloned into a pcDNA3.1 vector (Addgene, Inc.) with
PrimeSTAR® GXL DNA Polymerase (Takara Bio, Inc.). The
thermocycling conditions were 30 cycles at 98°C for 10 sec followed
by 68°C for 120 sec. To initiate overexpression of AEG-1, 2 µg
pcDNA3.1-AEG-1 was incubated with Lipofectamine® 2000 in
serum-free DMEM for 15 min at room temperature and subsequently
added to the A549 and A549/5-FU cells. These cells were used for
further experimentation 24 h after transfection.
Statistical analysis
The data were analyzed using GraphPad Prism software
6.0 (GraphPad Software, Inc.) and are expressed as the mean ± SD.
Two-tailed paired Student's t-test was used to evaluate statistical
differences between two groups. One-way ANOVA followed by the
Newman Keul's post-hoc test was used for the analysis of three
groups. Pearson's correlation analysis was used to determine the
correlation between the expression levels of miR-124-5p and AEG-1
in patient tissues. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-124-5p inhibitor decreases A549
and H1299 cell sensitivity to 5-FU
miR-124-5p has previously been identified as a
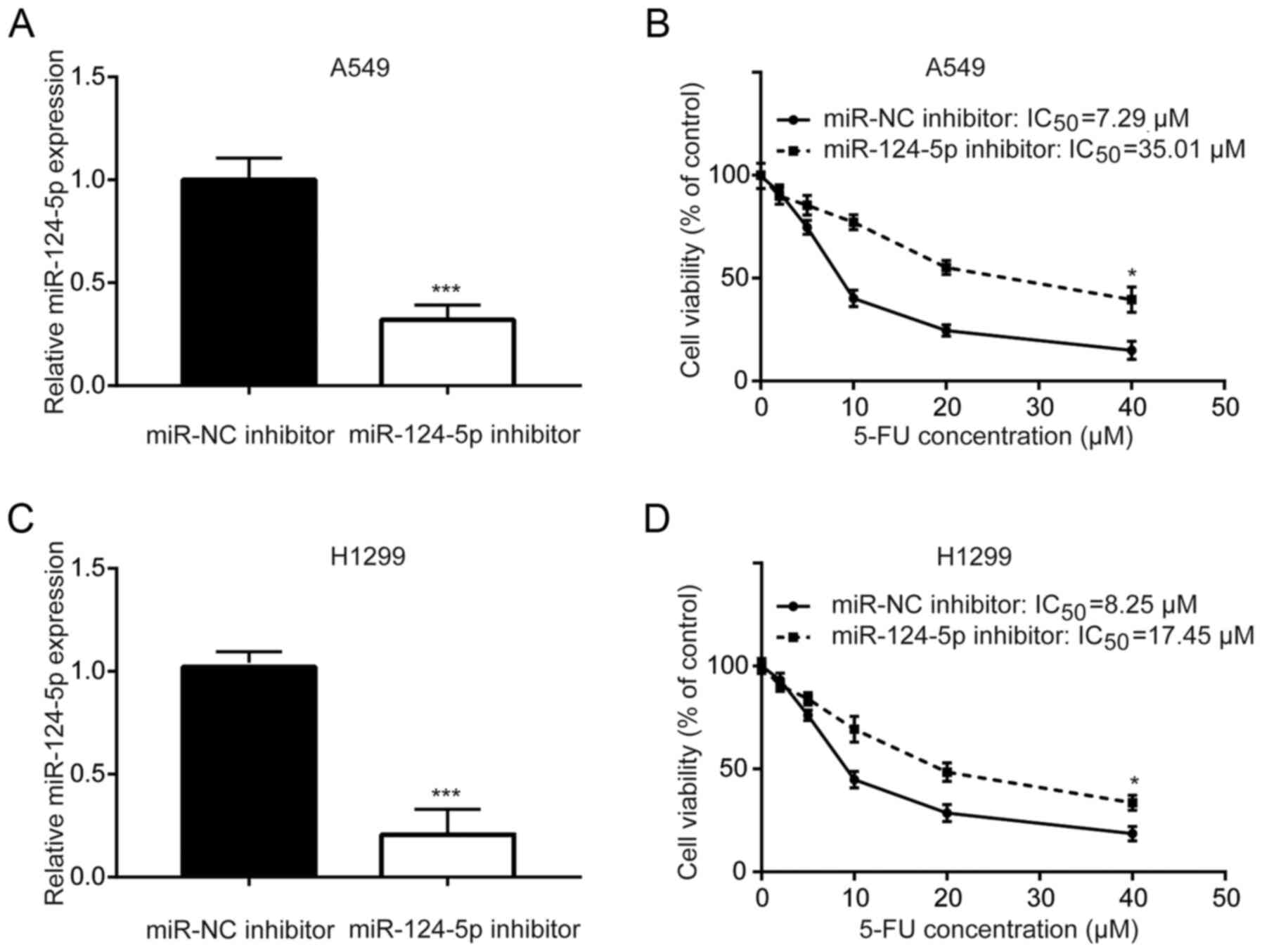
prognostic predictor for patients with NSCLC (25). As demonstrated in Fig. 1A, transfection with the miR-124-5p
inhibitor decreased miR-124-5p expression in A549 cells. Inhibition
of miR-124-5p significantly increased the 5-FU IC50
value (7.29 vs. 35.01 µM) of A549 cells compared with the NC,
suggesting decreased sensitivity of A549 cells to 5-FU (Fig. 1B). Similarly, in another NSCLC cell
line H1299, downregulation of miR-124-5p significantly increased
the 5-FU IC50 value (8.25 vs. 17.45 µM) of H1299 cells
compared with the NC (Fig. 1C and
D). These results indicated that miR-124-5p may mediate 5-FU
sensitivity in A549 and H1299 cells.
miR-124-5p negatively regulates AEG-1
expression in NSCLC cells
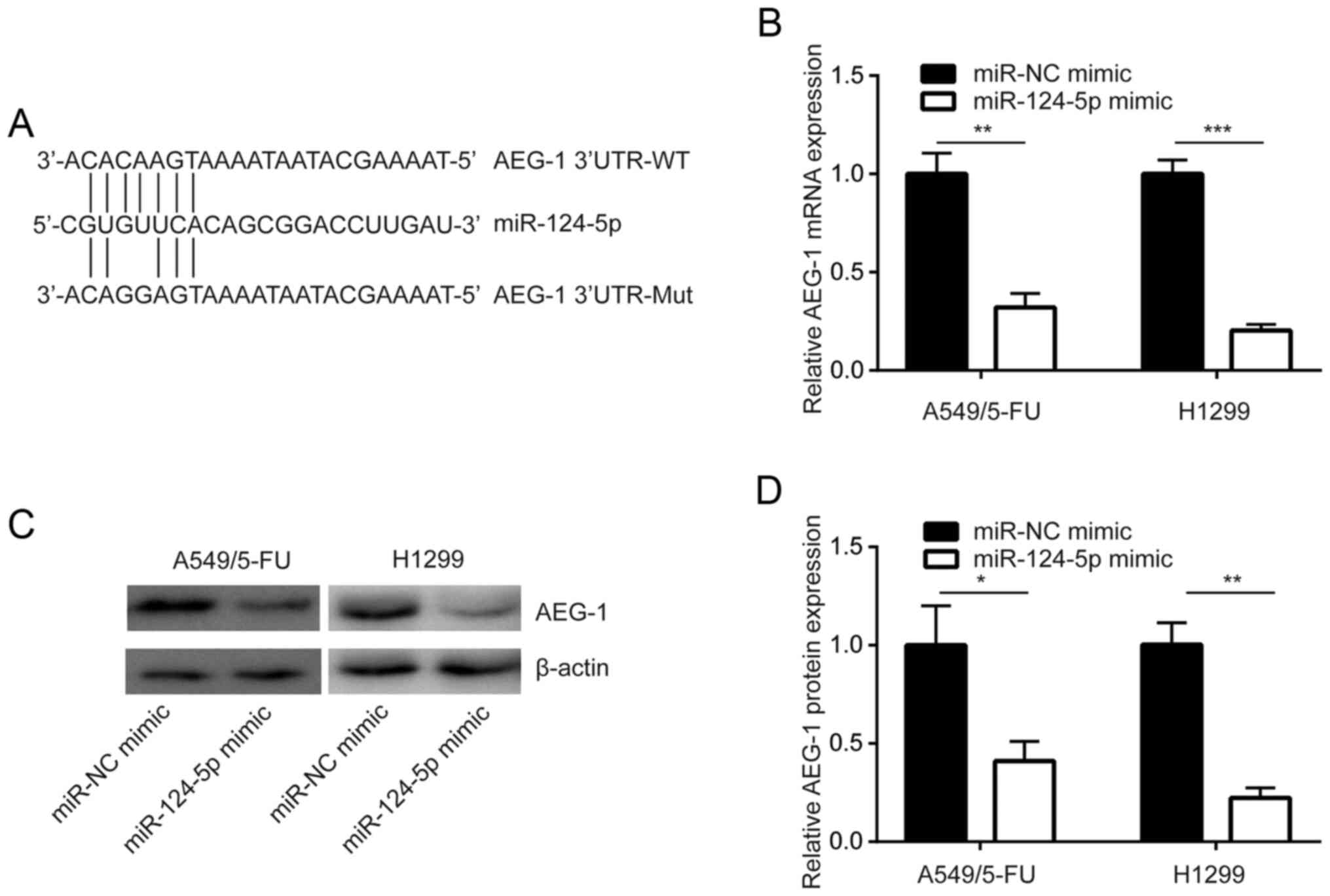
TargetScan was used to predict the potential target
genes of miR-124-5p, which was determined to be complementary to
the 3′-UTR of AEG-1 mRNA, a known sensitizer of chemotherapy
(21). This indicated that
miR-124-5p may regulate AEG-1 expression (Fig. 2A). In addition, in A549/5-FU cells,
overexpression of miR-124-5p reduced AEG-1 mRNA expression
(Fig. 2B). Western blot analysis
revealed that AEG-1 protein expression was decreased following
miR-124-5p overexpression in A549/5-FU cells, which was also
demonstrated in H1299 cells (Fig. 2C and
D). These results revealed that miR-124-5p negatively regulated
the expression of AEG-1 in NSCLC cells.
AEG-1 is a target gene of miR-124-5p
in NSCLC cells
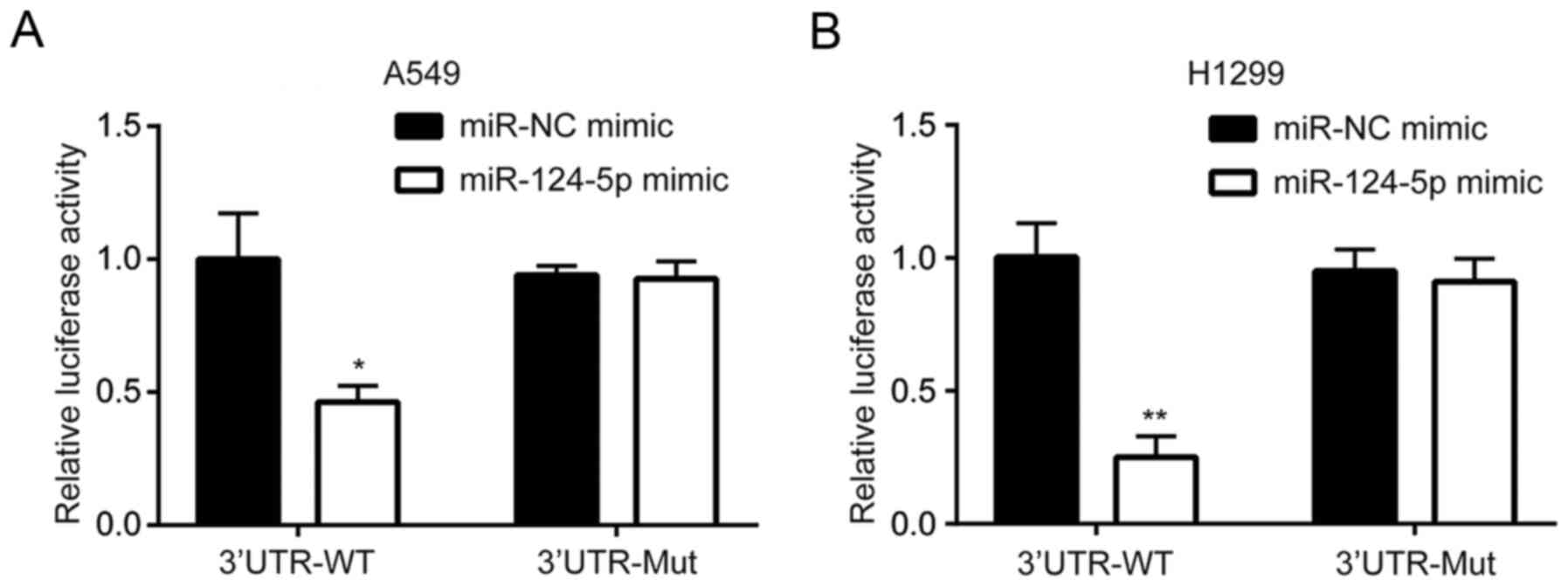
The dual-luciferase reporter assay revealed that the
miR-124-5p mimic reduced luciferase activity in A549/5-FU cells
transfected with AEG-1 3′UTR-WT compared with cells transfected
with AEG-1 3′UTR-mut (Fig. 3A). This
suggested that AEG-1 was a target gene of miR-124-5p. Similar
results were observed in H1299 cells (Fig. 3B).
Downregulation of miR-124-5p and
overexpression of AEG-1 in 5-FU-resistant A549 cells
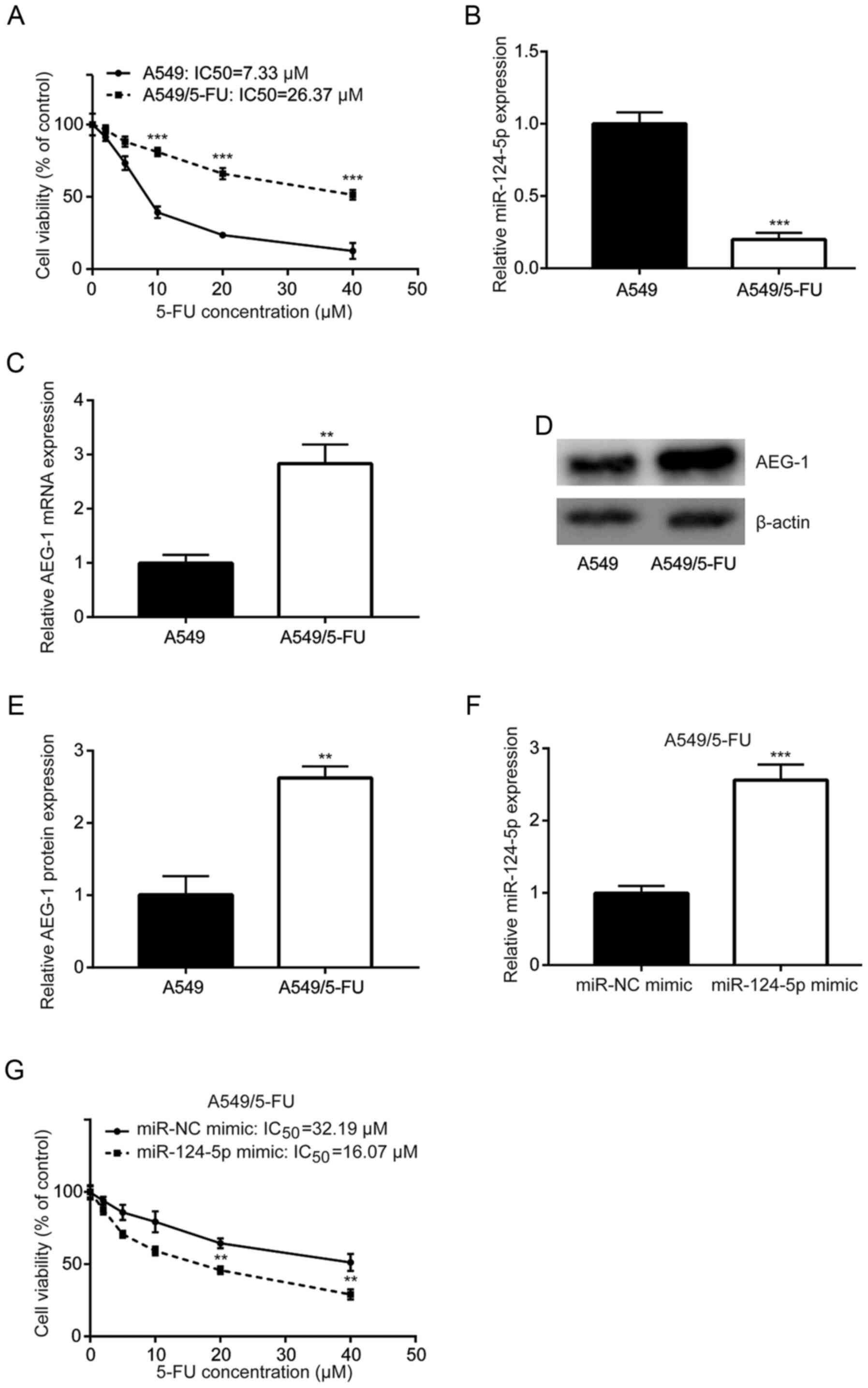
To investigate the mechanism of 5-FU-resistance
development in NSCLC, A549/5-FU cells were established by exposing
parental A549 cells to gradually increasing concentrations of 5-FU.
Cytotoxicity assays revealed that treatment with 5-FU significantly
reduced the cell viability of A549, but not A549/5-FU cells
(Fig. 4A). In addition, RT-qPCR
revealed that miR-124-5p expression was decreased in A549/5-FU
cells compared with the parental cells (Fig. 4B), and AEG-1 mRNA expression was
increased in A549/5-FU cells compared with A549 cells (Fig. 4C). Western blot analysis revealed
similar results in terms of protein expression (Fig. 4D and E). Of note, transfection with
the miR-124-5p mimic sensitized A549/5-FU cells to 5-FU treatment
(Fig. 4F and G).
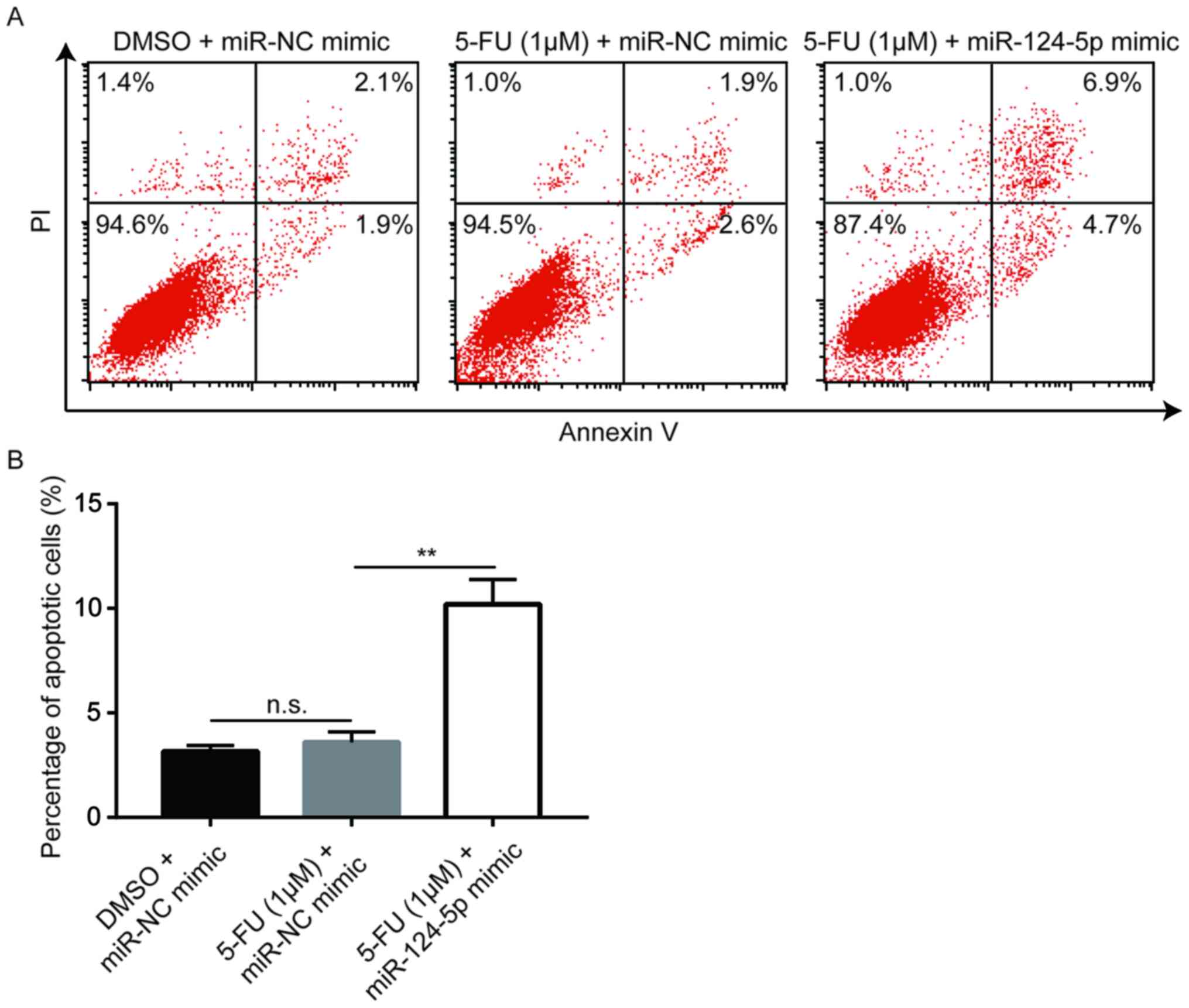
miR-124-5p mimic enhances 5-FU-induced
cell apoptosis in A549/5-FU cells
5-FU blocks thymidylate synthase in cancer cells,
preventing DNA synthesis and leading to apoptosis (8). Flow cytometric analysis revealed that a
low concentration of 5-FU (1 µM) did not induce apoptosis in
A549/5-FU cells; however, following miR-124-5p mimic transfection,
apoptosis was significantly increased in A549/5-FU cells treated
with 1 µM 5-FU (Fig. 5), suggesting
that miR-124-5p reversed 5-FU resistance in NSCLC cells via the
induction of apoptosis.
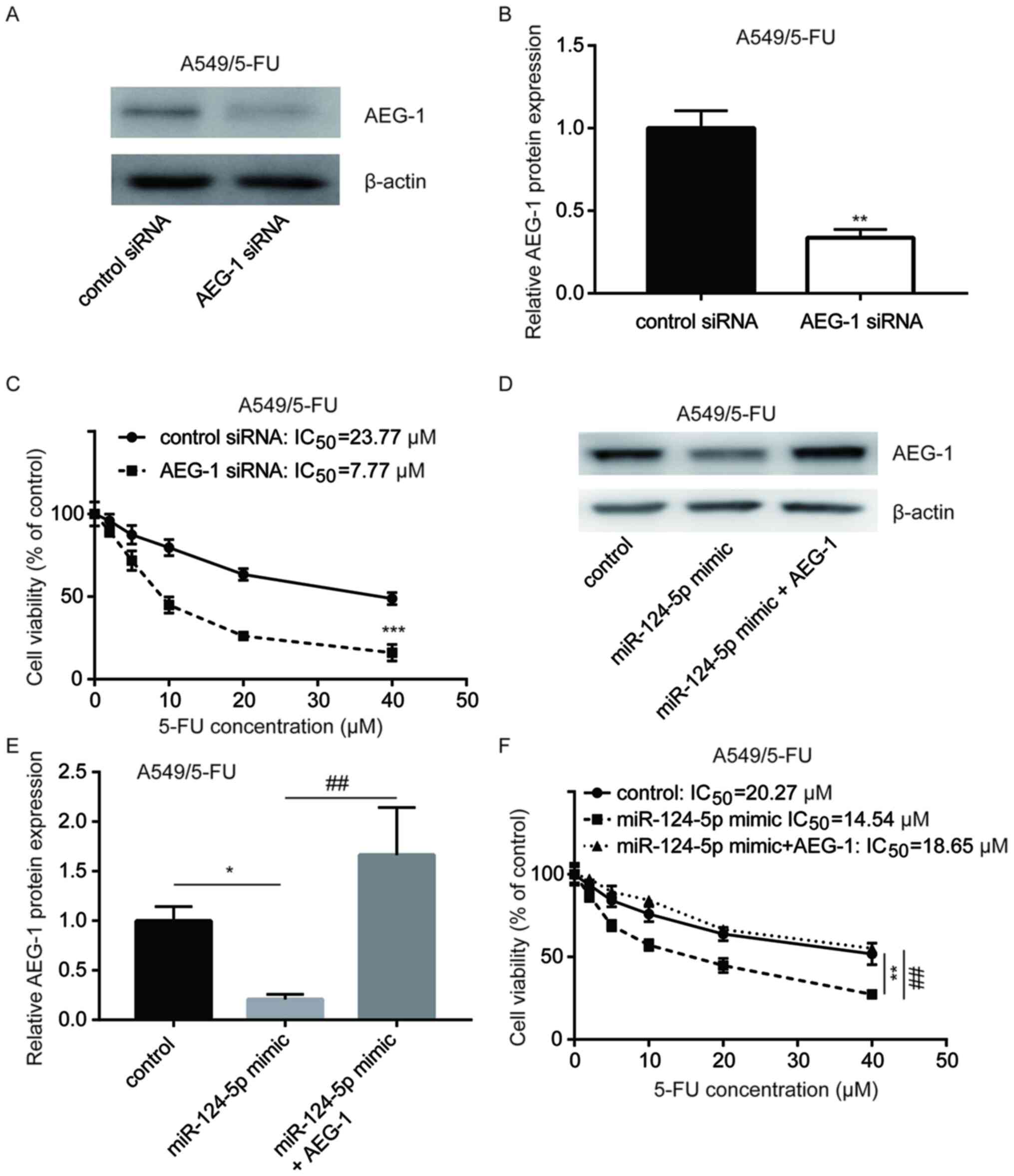
miR-124-5p inhibition mediates 5-FU
resistance of A549/5-FU cells by upregulating AEG-1
AEG-1 protein expression in A549/5-FU cells was
significantly decreased following transfection with AEG-1 siRNA
(Fig. 6A and B). The CCK-8
cytotoxicity assay revealed that silencing of AEG-1 sensitized
A549/5-FU cells to 5-FU treatment (IC50, 23.77 vs. 7.77
µM) (Fig. 6C), suggesting that AEG-1
contributed to A549 cell sensitivity to 5-FU. AEG-1 overexpression
abrogated the decreased AEG-1 protein expression in A549/5-FU cells
transfected with the miR-124-5p mimic (Fig. 6D and E). Compared with the control
group (IC50, 20.27 µM), the miR-124-5p mimic sensitized
A549/5-FU cells to 5-FU (IC50, 14.54 µM), whereas
transfection with the recombinant AEG-1 (IC50, 18.65 µM)
decreased 5-FU sensitivity (Fig.
6F). These results suggested that miR-124-5p affected the
sensitivity of NSCLC cells to 5-FU by decreasing the expression
level of AEG-1.
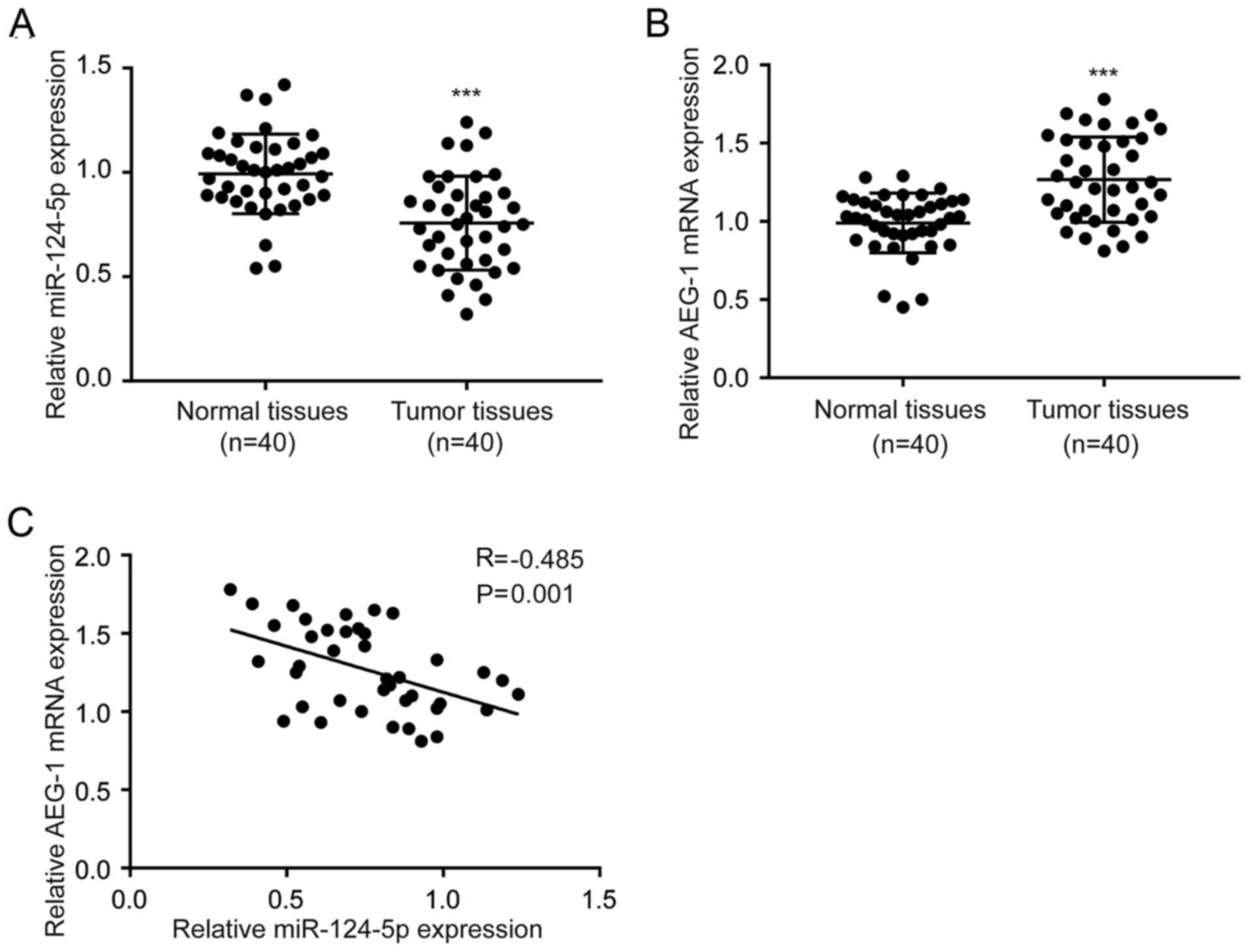
Expression of miR-124-5p is inversely
correlated with AEG-1 expression in NSCLC tumor tissues
RT-qPCR was performed to detect the expression
levels of miR-124-5p and AEG-1 in 40 pairs of tumor and adjacent
noncancerous tissues collected from patients with NSCLC. The
expression of miR-124-5p was significantly decreased in tumor
tissues compared with normal tissues (Fig. 7A). In addition, AEG-1 mRNA levels
were increased in tumor tissues compared with normal tissues
(Fig. 7B). Pearson's correlation
analysis revealed a negative correlation between miR-124-5p and
AEG-1 mRNA expression levels in tumor tissues (r=−0.485; P=0.001;
Fig. 7C).
Discussion
The development of chemoresistance represents a
major challenge for the treatment of patients with NSCLC (26). Previous studies have revealed that
dysregulation of miRNA expression is associated with the
development of chemoresistance (27,28). In
addition, several miRNAs have been identified as predictors of the
magnitude of the response to chemotherapy in patients with NSCLC
(29–31). The present study demonstrated that
the downregulation of miR-124-5p reduced sensitivity to 5-FU
treatment and promoted the development of chemoresistance in A549
and H1299 cells via the downregulation of AEG-1.
Previous studies have demonstrated that miR-124-5p
serves as a tumor suppressor in several types of cancer. For
example, in glioma, miR-124-5p inhibited tumor growth in
vitro and in vivo by downregulating laminin subunit β-1
(17). In colorectal cancer, high
expression of miR-124-5p was associated with an increase in overall
survival (18). A recent study
demonstrated that miR-124-5p was downregulated in NSCLC tissue
samples compared with matched paracancerous tissues, and was also
associated with radiation sensitivity in NSCLC cells (32). Furthermore, miR-124-5p suppressed
NSCLC cell proliferation by directly targeting signal transducer
and activator of transcription 3 and protein kinase B (33,34). The
results of the present study revealed that miR-124-5p was
downregulated in A549/5-FU cells compared with parental A549 cells.
Additionally, the CCK-8 assay demonstrated that the miR-124-5p
mimic increased A549/5-FU cell sensitivity to 5-FU, whereas
inhibition of miR-124-5p reduced A549 cell sensitivity to 5-FU
treatment. The miR-124-5p mimic induced apoptosis in A549/5-FU
cells treated with 1 µM 5-FU. These results indicated that
miR-124-5p may sensitize NSCLC cells to chemotherapeutic treatment
with 5-FU.
AEG-1 regulates several key pathways associated with
carcinogenesis in a number of organs and tissues (35) and has also been demonstrated to
mediate cisplatin resistance in serous ovarian cancer cells
(36). Furthermore, AEG-1 is
upregulated in NSCLC tissues compared with matched normal tissues,
and promotes EMT through the activation of the Wnt signaling
pathway (20). Patients with NSCLC
with a low-expression level of AEG-1 also exhibited an improved
response to postoperative chemotherapy and radiotherapy compared
with patients exhibiting high AEG-1 expression (37). Additionally, miR-136 sensitized
glioma cells to chemotherapy via the regulation of AEG-1 expression
(38). The results of the present
study revealed that AEG-1 was upregulated in A549/5-FU cells
compared with the parental cells, and that the miR-124-5p mimic
decreased AEG-1 mRNA and protein expression levels in A549/5-FU
cells. Subsequently, a bioinformatics tool was used to predict that
AEG-1 was a target gene of miR-124-5p, which was subsequently
validated using a dual-luciferase reporter assay. Finally, RT-qPCR
analysis suggested that the expression of miR-124-5p was inversely
associated with AEG-1 mRNA expression levels in tumor tissues
obtained from patients with NSCLC.
There are limitations of the current study. The role
of miR-124-5p was solely investigated in vitro. In addition,
a single miRNA may target several target genes in cells. In the
future, further investigation of the target genes of miR-124-5p
should be conducted and the role of miR-124-5p should be examined
in vivo.
In conclusion, the present study demonstrated that
miR-124-5p directly decreased AEG-1 expression and sensitized NSCLC
cells to treatment with 5-FU. Therefore, miR-124-5p may serve as a
novel target for the treatment of NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XT, CZ, WG, BS, BJ and PS designed and performed the
experiments. CZ and WG collection the samples and clinical data. XT
supervised the study and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All protocols involving human participants were
approved by the Ethics Committee of The Third People's Hospital of
Linyi City (Linyi, China). Written informed consent was provided by
all participants prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang H, Yang T and Wu X: 5-Fluorouracil
preferentially sensitizes mutant KRAS non-small cell lung carcinoma
cells to TRAIL-induced apoptosis. Mol Oncol. 9:1815–1824. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He X, Li C, Wu X and Yang G: Docetaxel
inhibits the proliferation of non-small-cell lung cancer cells via
upregulation of microRNA-7 expression. Int J Clin Exp Pathol.
8:9072–9080. 2015.PubMed/NCBI
|
|
4
|
Heigener DF, Kerr KM, Laing GM, Mok TSK,
Moiseyenko FV and Reck M: Redefining treatment paradigms in
first-line advanced Non-small-cell lung cancer. Clin Cancer Res.
25:4881–4887. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noro R, Miyanaga A, Minegishi Y, Okano T,
Seike M, Soeno C, Kataoka K, Matsuda K, Yoshimura A and Gemma A:
Histone deacetylase inhibitor enhances sensitivity of
non-small-cell lung cancer cells to 5-FU/S-1 via down-regulation of
thymidylate synthase expression and up-regulation of p21(waf1/cip1)
expression. Cancer Sci. 101:1424–1430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Hou N, Faried A, Tsutsumi S,
Takeuchi T and Kuwano H: Inhibition of autophagy by 3-MA enhances
the effect of 5-FU-induced apoptosis in colon cancer cells. Ann
Surg Oncol. 16:761–771. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yi H, Cho HJ, Cho SM, Jo K, Park JA, Lee
SH, Chang BJ, Kim JS and Shin HC: Effect of 5-FU and MTX on the
expression of drug-resistance related cancer stem cell markers in
non-small cell lung cancer cells. Korean J Physiol Pharmacol.
16:11–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan X, Zhang X, Sun H, Zhang J, Yan M and
Zhang H: Autophagy inhibition promotes 5-fluorouraci-induced
apoptosis by stimulating ROS formation in human non-small cell lung
cancer A549 cells. PLoS One. 8:e566792013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oguri T, Bessho Y, Achiwa H, Ozasa H,
Maeno K, Maeda H, Sato S and Ueda R: MRP8/ABCC11 directly confers
resistance to 5-fluorouracil. Mol Cancer Ther. 6:122–127. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berezikov E, Chung WJ, Willis J, Cuppen E
and Lai EC: Mammalian mirtron genes. Mol Cell. 28:328–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hara ES, Ono M, Eguchi T, Kubota S, Pham
HT, Sonoyama W, Tajima S, Takigawa M, Calderwood SK and Kuboki T:
miRNA-720 controls stem cell phenotype, proliferation and
differentiation of human dental pulp cells. PLoS One. 8:e835452013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen L, Holmstrom K, Qiu W, Ditzel N, Shi
K, Hokland L and Kassem M: MicroRNA-34a inhibits osteoblast
differentiation and in vivo bone formation of human stromal stem
cells. Stem Cells. 32:902–912. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Jiang M and Xia S: miR-339-5p
increases radiosensitivity of lung cancer cells by targeting
phosphatases of regenerating liver-1 (PRL-1). Med Sci Monit.
24:8408–8416. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang B, Jing C, Wang J, Guo X, Chen Y, Xu
R, Peng L, Liu J and Li L: Identification of microRNAs associated
with lymphangiogenesis in human gastric cancer. Clin Transl Oncol.
16:374–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Q, Lu G, Cai Y, Li Y, Xu R, Ke Y and
Zhang S: MiR-124-5p inhibits the growth of high-grade gliomas
through posttranscriptional regulation of LAMB1. Neuro Oncol.
16:637–651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jinushi T, Shibayama Y, Kinoshita I,
Oizumi S, Jinushi M, Aota T, Takahashi T, Horita S, Dosaka-Akita H
and Iseki K: Low expression levels of microRNA-124-5p correlated
with poor prognosis in colorectal cancer via targeting of SMC4.
Cancer Med. 3:1544–1552. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su ZZ, Kang DC, Chen Y, Pekarskaya O, Chao
W, Volsky DJ and Fisher PB: Identification and cloning of human
astrocyte genes displaying elevated expression after infection with
HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid
subtraction hybridization, RaSH. Oncogene. 21:3592–3602. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He W, He S, Wang Z, Shen H, Fang W, Zhang
Y, Qian W, Lin M, Yuan J, Wang J, et al: Astrocyte elevated gene-1
(AEG-1) induces epithelial-mesenchymal transition in lung cancer
through activating Wnt/β catenin signaling. BMC Cancer. 15:1072015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoo BK, Gredler R, Vozhilla N, Su ZZ, Chen
D, Forcier T, Shah K, Saxena U, Hansen U, Fisher PB and Sarkar D:
Identification of genes conferring resistance to 5-fluorouracil.
Proc Natl Acad Sci USA. 106:12938–12943. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang P, Yin B, Shan L, Zhang H, Cui J,
Zhang M and Song Y: RNA interference-mediated knockdown of
astrocyte elevated gene-1 inhibits growth, induces apoptosis, and
increases the chemosensitivity to 5-fluorouracil in renal cancer
Caki-1 cells. Mol Cells. 37:857–864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao JG, Ren KM and Tang J: Overcoming
5-Fu resistance in human non-small cell lung cancer cells by the
combination of 5-Fu and cisplatin through the inhibition of glucose
metabolism. Tumor Biol. 35:12305–12315. 2014. View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, Li H, Han J and Zhang Y:
Down-regulation of microRNA-124 is correlated with tumor metastasis
and poor prognosis in patients with lung cancer. Int J Clin Exp
Pathol. 8:1967–1972. 2015.PubMed/NCBI
|
|
26
|
Chang A: Chemotherapy, chemoresistance and
the changing treatment landscape for NSCLC. Lung Cancer. 71:3–10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lei L, Huang Y and Gong W: miR-205
promotes the growth, metastasis and chemoresistance of NSCLC cells
by targeting PTEN. Oncol Rep. 30:2897–2902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai J, Fang L, Huang Y, Li R, Xu X, Hu Z,
Zhang L, Yang Y, Zhu X, Zhang H, et al: Simultaneous overactivation
of Wnt β-catenin and TGF β signalling by miR-128-3p confers
chemoresistance-associated metastasis in NSCLC. Nat Commun.
8:158702017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou LK, Ma YS, Han Y, Lu GX, Luo P, Chang
ZY, Xie RT, Yang HQ, Chai L, Cai MX, et al: Association of
microRNA-33a molecular signature with non-small cell lung cancer
diagnosis and prognosis after chemotherapy. PLoS One.
12:e01704312017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo P, Yang Q, Cong LL, Wang XF, Li YS,
Zhong XM, Xie RT, Jia CY, Yang HQ, Li WP, et al: Identification of
miR124a as a novel diagnostic and prognostic biomarker in nonsmall
cell lung cancer for chemotherapy. Mol Med Rep. 16:238–246. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu J, Zhan Y, Feng J, Luo J and Fan S:
MicroRNAs associated with therapy of non-small cell lung cancer.
Int J Biol Sci. 14:390–397. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang M, Meng B and Liu Y, Yu J, Chen Q and
Liu Y: MiR-124 inhibits growth and enhances radiation-induced
apoptosis in non-small cell lung cancer by inhibiting STAT3. Cell
Physiol Biochem. 44:2017–2028. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Yu Z, Li Y, Liu S, Gao C, Hou X, Yao
R and Cui L: The tumor suppressor miR-124 inhibits cell
proliferation by targeting STAT3 and functions as a prognostic
marker for postoperative NSCLC patients. Int J Oncol. 46:798–808.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao X, Lu C, Chu W, Zhang B, Zhen Q, Wang
R, Zhang Y, Li Z, Lv B, Li H and Liu J: MicroRNA-124 suppresses
proliferation and glycolysis in non-small cell lung cancer cells by
targeting AKT-GLUT1/HKII. Tumour Biol. 39:10104283177062152017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi X and Wang X: The role of MTDH/AEG-1
in the progression of cancer. Int J Clin Exp Med. 8:4795–4807.
2015.PubMed/NCBI
|
|
36
|
Meng X, Thiel KW and Leslie KK: Drug
resistance mediated by AEG-1/MTDH/LYRIC. Adv Cancer Res.
120:135–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lu S, Xu J, Xu X, Hu S, Li B and Li W: The
expression of astrocyte elevated gene-1 in human non-small-cell
lung cancer and its relationship with postoperative chemotherapy
and radiotherapy. Histopathology. 67:817–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Y, Wu J, Guan H, Cai J, Fang L, Li J
and Li M: MiR-136 promotes apoptosis of glioma cells by targeting
AEG-1 and Bcl-2. FEBS Lett. 586:3608–3612. 2012. View Article : Google Scholar : PubMed/NCBI
|