Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide, and the incidence of this
disease is increasing incrementally (1). There are nearly one million newly
diagnosed cases annually, and the incidence is increasing at a rate
of 26.96% each year worldwide (2).
At present, despite the development of chemotherapy, radiotherapy
and molecular targeted therapy (3–5), the
5-year survival rate for patients with lung cancer remains
unsatisfactory (6,7). The resistance of cancer cells to
anticancer drugs is a principal reason for the reduced curative
effect of chemotherapy (8).
Therefore, it is imperative to understand the potential molecular
mechanisms governing lung cancer metastasis and recurrence, and to
identify effective molecular markers.
At present, the standard therapy for lung cancer is
surgical intervention followed by combination chemotherapy,
consisting of Taxol and platinum-based drugs (9). Cisplatin is one of the most effective
non-specific drugs administered for the treatment of lung cancer
(10,11), and Taxol is a primary
chemotherapeutic agent. Combination therapy with cisplatin serves a
pivotal role in the treatment of lung cancer (12). The development of drug resistance has
become a principal cause of poor survival rates in patients with
lung cancer (8). Chemoresistance is
a prevalent clinical challenge resulting in treatment failure and
recurrence (13), and, consequently,
there is an urgent need to develop targeted solutions to overcome
drug resistance, based on the distinct molecular background of lung
cancer and the mechanisms of platinum-based drug resistance.
Cytokines, including interleukins (ILs),
interferons, cancer necrosis factors, colony stimulating factors,
chemokines and growth factors, serve an important role in immune
regulation (14,15). Cytokines also participate in the
occurrence and development of a variety of diseases, including
acute or chronic inflammatory disease, autoimmune disease and
cancers (16). Changes in the
expression levels of cytokines can regulate the immune response
(17). ILs are soluble proteins
secreted by a group of white blood cells, which can adjust the
function of white blood cells and tissues (18). ILs are primarily involved in the
activation and regulation of immune cells, T-cell and B-cell
proliferation, differentiation and inflammation (19). Numerous ILs, such as IL-6 and IL-33,
are closely associated with gastric cancer (20,21).
IL-27, a member of the cytokine family, exhibits antitumor cell
proliferation and antiangiogenetic properties (22–25).
However, the roles of IL-27 in lung cancer cells, chemotherapeutic
sensitivity and drug resistance mechanisms have not been completely
elucidated.
The aim of the present study was to investigate the
effect of IL-27 on chemotherapy resistance in the A549 lung cancer
cell line and analyze its potential molecular mechanism in lung
cancer tissues. This may provide a theoretical basis for the
development of more effective cancer therapeutics.
Materials and methods
Tissue samples
A total of 60 patients with lung cancer were
included in the present study, and 30 cancer and 30 normal tissues
(≥5 cm from tumor) were obtained. Patients (32–73 years; mean age,
51±20.9 years) were recruited at Suizhou Central Hospital (Suizhou,
China) between August 2015 and December 2016, and all provided
written informed consent prior to resection. The patients enrolled
in this study had not received prior chemotherapy and radiotherapy.
The present study was approved by the Ethics Committee of Suizhou
Central Hospital (Suizhou, China). All patients provided the
informed consent. Tissue samples were stored in liquid nitrogen at
−80°C until subsequent experimentation. The clinical information of
the patients is presented in Table
I.
 | Table I.Clinical information of the
patients. |
Table I.
Clinical information of the
patients.
| Characteristic | Total, n | Relative IL-27
expression | P-value |
|---|
| Age, years |
|
| 0.319 |
|
<60 | 14 | 0.513±0.015 |
|
| ≥60 | 16 | 0.508±0.012 |
|
| Sex |
|
| 0.304 |
| Male | 18 | 0.511±0.008 |
|
|
Female | 12 | 0.515±0.013 |
|
| Tumor
differentiation |
|
| 0.006 |
|
Well/moderate | 17 | 0.521±0.021 |
|
| Poor | 13 | 0.503±0.007 |
|
| Metastasis |
|
|
|
|
Absent | 19 | 0.519±0.015 | <0.001 |
|
Present | 11 | 0.497±0.009 |
|
Cell culture and treatment
The lung adenocarcinoma cell line A549 was obtained
from the American Type Culture Collection (Manassas, VA, USA).
Cells were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 100 U/ml
penicillin and 100 µg/ml streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.) in an incubator with 5% CO2 at 37°C.
Cells were passaged every other day. Cells in the logarithmic phase
were selected for experimentation and divided into three groups: i)
Cisplatin, ii) cisplatin + IL-27, and iii) the control group. The
A549 cells were treated with or without 50 ng/ml of IL-27 at 37°C.
Following treatment with IL-27 for 72 h, the cisplatin (40 µM,
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) + IL-27 group and
the cisplatin-only group were treated with 40 µM cisplatin at 37°C
for 48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA of cells and tissues were extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The concentration and purity of RNA were determined by
spectrophotometry. Following RNA extraction, a PrimeScript™ one
step RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China) was
used for cDNA synthesis at 72°C for 10 min. RT-qPCR was performed
to amplify the cDNA template using a SYBR® premix dimmer
eraser kit (Takara Biotechnology Co., Ltd.), performed according to
the manufacturer's protocol. The thermocycling conditions were as
follows: Denaturing for 5 min at 95°C, 40 cycles of 10 sec at 95°C
and an extension for 1 min at 60°C. GAPDH was used as an internal
control, and mRNA values were normalized to GAPDH. The expression
levels were quantified using the 2−∆∆Cq method (26). In addition, the expression of Bax,
Bcl-2, caspase-3 and Akt were compared among the experimental
groups. The sequences of the primers were: IL-27 forward,
5′-AGCCTTCGCATCATCAGC-3′ and reverse, 5′-TTATTGGGCACCCAGCAT-3′; Bax
forward, 5′-TCCACCAAGAAGCTGAGCGAG-3′ and reverse,
5′-GTCCAGCCCATGATGGTTCT-3′; Bcl-2 forward,
5′-TTCTTTGAGTTCGGTGGGGTC-3′ and reverse,
5′-TGCATATTTGTTTGGGGCAGG-3′; caspase-3 forward,
5′-AGGACTCAAACTGTTGCCACC-3′ and reverse,
5′-TGGAACAAATGACCTGTTGACC-3′; Akt forward,
5′-GCAGCACGTGTACGAGAAGA-3′ and reverse, 5′-GTGTCAGTCTCCGACGTG-3′;
and GAPDH forward, 5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse,
5′-AGGGGCCATCCACAGTCTTC-3′.
Western blot analysis
The cells and tissue samples were collected
following treatment with cisplatin and IL-27, and lysed with RIPA
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
on ice. The supernatant was retained to detect the protein
concentration following high-speed by centrifugation at 12,000 × g
for 20 min at 4°C. The protein concentration was determined using a
BCA kit (Pierce; Thermo Fisher Scientific, Inc.). Equivalent
protein samples (50 µg/lane) were loaded onto 10% SDS-PAGE gels,
and then transferred to polyvinylidene fluoride membranes. The
membranes were blocked with 5% skimmed milk for 2 h at 37°C. The
primary antibodies were as follows: Anti-Bax (1:1,000; cat. no.
B3428), anti-Bcl-2 (1:1,000; cat. no. PRS3335), anti-caspase-3
(1:1,000, cat. no. C8487), anti-Akt (1:1,000; cat. no. SAB4500796),
anti-GAPDH (1:100; cat. no. G9545; all from Sigma-Aldrich; Merck
KGaA). The membrane was incubated with the primary antibodies
overnight at 4°C and were then treated with the HRP-conjugated
secondary antibodies (1:5,000; cat. no. R2655, Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) at room temperature for 1 h. Blots were
evaluated using an ECL Plus immunoblotting detection system (Thermo
Fisher Scientific, Inc.) and quantified by densitometry using
Gel-Pro analyzer software version 6.3 (Media Cybernetics).
Cell Counting Kit-8 (CCK-8)
proliferation assay
Following 48-h treatment, Cell proliferation in
vitro was evaluated using a CCK-8 assay (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan). Cells were divided into 3
groups: Control, cisplatin and cisplatin + IL-27. Lung cancer cells
were seeded separately into 96-well plates at a density of
5.0×103 cells/well and incubated with serum-free DMEM
(HyClone; GE Healthcare Life Sciences Logan, UT, USA) at 37°C
(final volume of 100 µl) for 48 h. A total of 10 µl CCK-8 was added
into each well and incubated for an additional 2 h. The absorbance
of each sample was measured at 450 nm using a microplate
reader.
Flow cytometry
Following 48-h treatment, A549 cells were divided
into 3 groups: Control, cisplatin and cisplatin + IL-27. Cells were
collected and re-suspended in binding solution at a concentration
of 5.0×105 cells/ ml. Cells were transferred to a flow
tube (100 µl/tube) and then incubated for 5 min with 5 µl
Annexin V/FITC (Nanjing KeyGen Biotech, Co., Ltd., Nanjing,
China) for 15 min at room temperature. At the end of incubation,
cells were washed three times with ice-cold PBS and the
fluorescence intensity of cells was measured using a FACScalibur
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and
analyzed by FlowJo 7.6.1 software (Tree Star, Inc.).
Statistical analysis
SPSS v19.0 software (IBM Corp., Armonk, NY, USA) was
used to analyze the data obtained by the present study. Data are
presented as the mean ± standard deviation of at least three
independent experiments. A two-tailed Student's t-test was applied
to analyze the difference between two groups. One-way analysis of
variance followed by a Newman-Keuls post-hoc test was employed to
analyze differences among three groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
The expression of IL-27 and clinical
characteristics
The results of patient clinical information analysis
demonstrated that the expression levels of IL-27 were significantly
associated with tumor differentiation and lymphatic metastasis,
whereas no significant differences were observed between IL-27
expression and age or sex (Table
I).
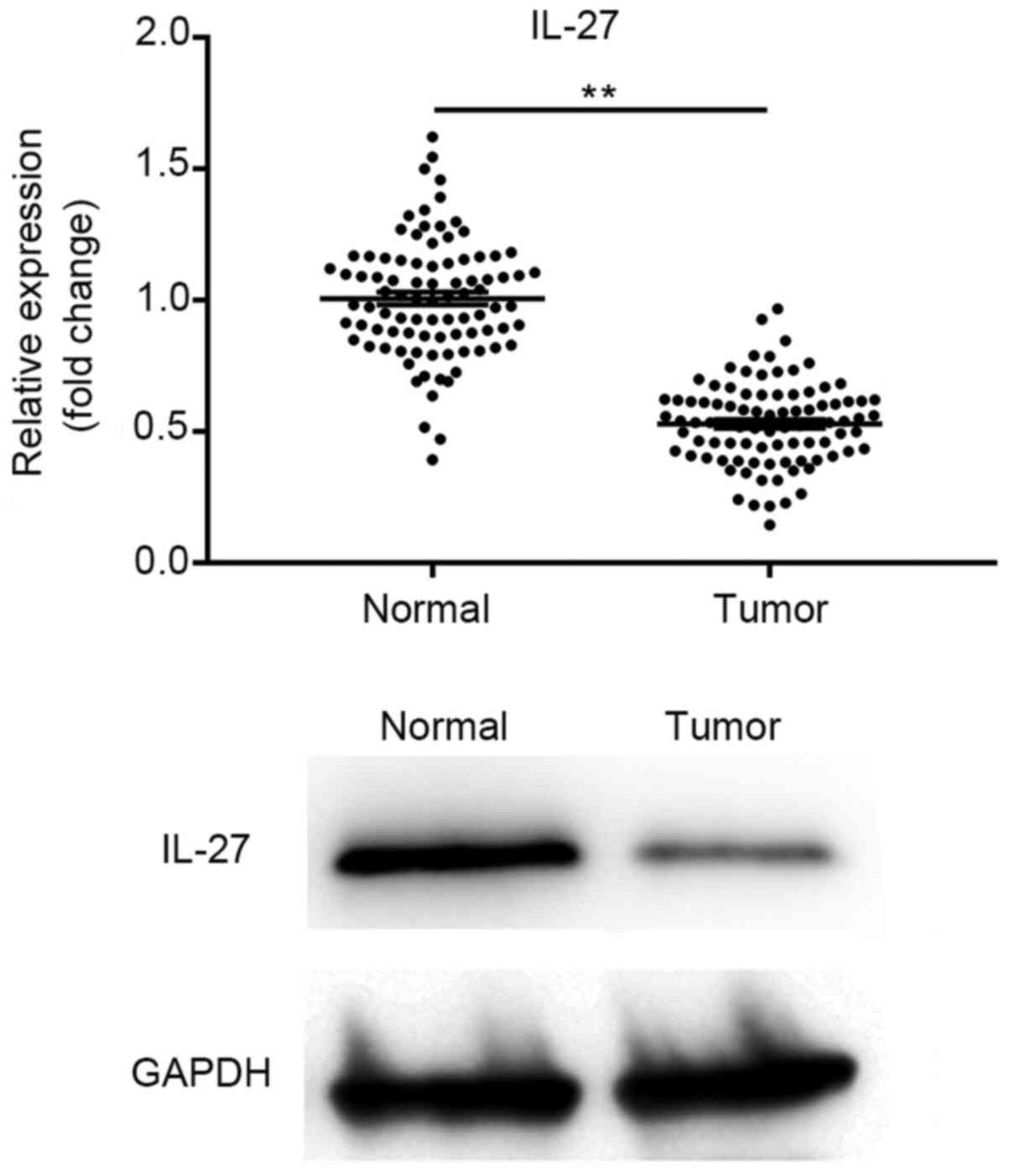
IL-27 expression is suppressed in lung
cancer tissues
The expression of IL-27 in lung cancer and adjacent
tissues was evaluated using RT-qPCR and western blot analysis. The
results demonstrated that the expression of IL-27 was significantly
suppressed in cancer tissues when compared with that in the
paracarcinoma tissues (P<0.01). The results of the western blot
analysis were consistent with those obtained by RT-qPCR, with IL-27
being expressed at a low level in cancer tissues (Fig. 1). These results suggest that IL-27
exhibits low expression levels in lung cancer tissue.
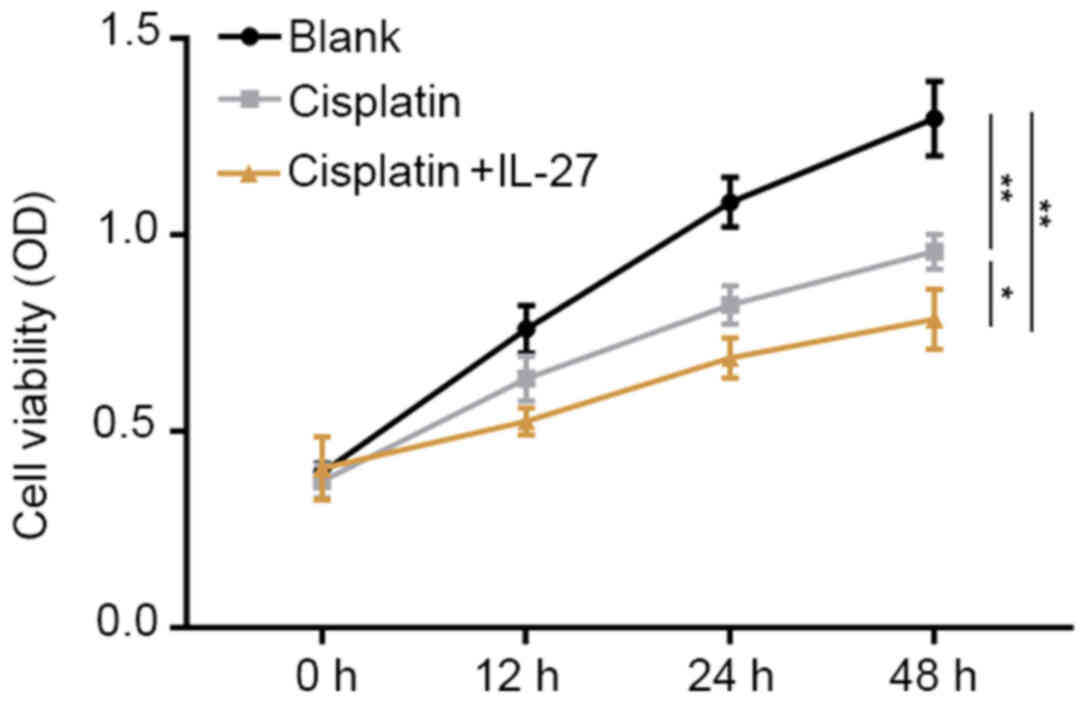
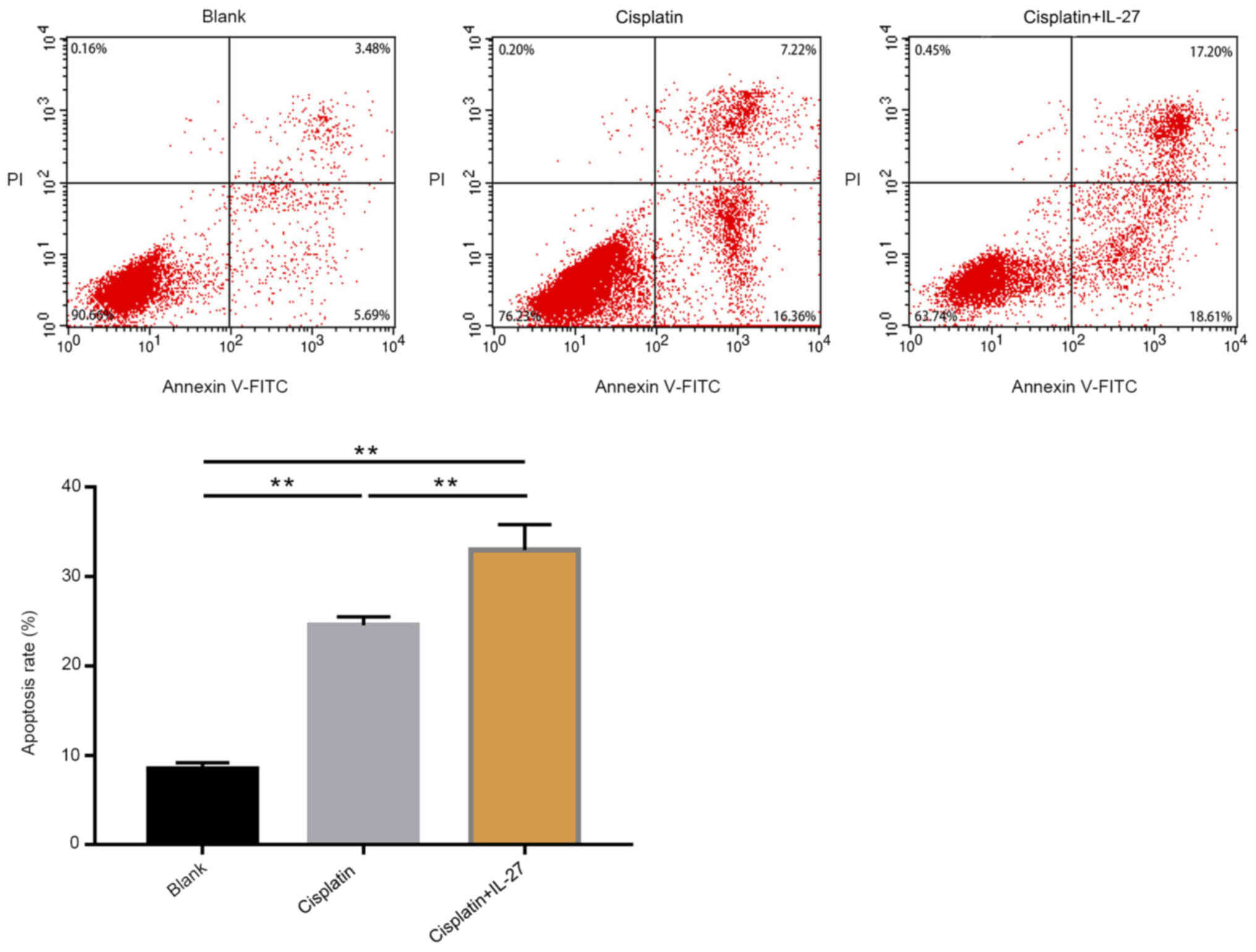
Proliferation ability and cell
apoptosis of A549 lung cancer cells
A CCK-8 assay was conducted to evaluate cell
proliferation ability (Fig. 2), and
an apoptosis assay was performed to evaluate cell apoptosis
(Fig. 3). The results of the CCK-8
assay demonstrated that, compared with the control group, treatment
with cisplatin significantly promoted apoptosis and inhibited
proliferation in lung cancer cells (P<0.01). The A549 cells
treated with cisplatin + IL-27 exhibited a significant increased
rate of apoptosis, and the ability to suppress proliferation was
also increased compared with the cisplatin group (P<0.05). In
summary, these data suggest that IL-27 is capable of inhibiting the
proliferation ability of A549 lung cancer cells, enhancing
apoptosis and promoting sensitivity to cisplatin.
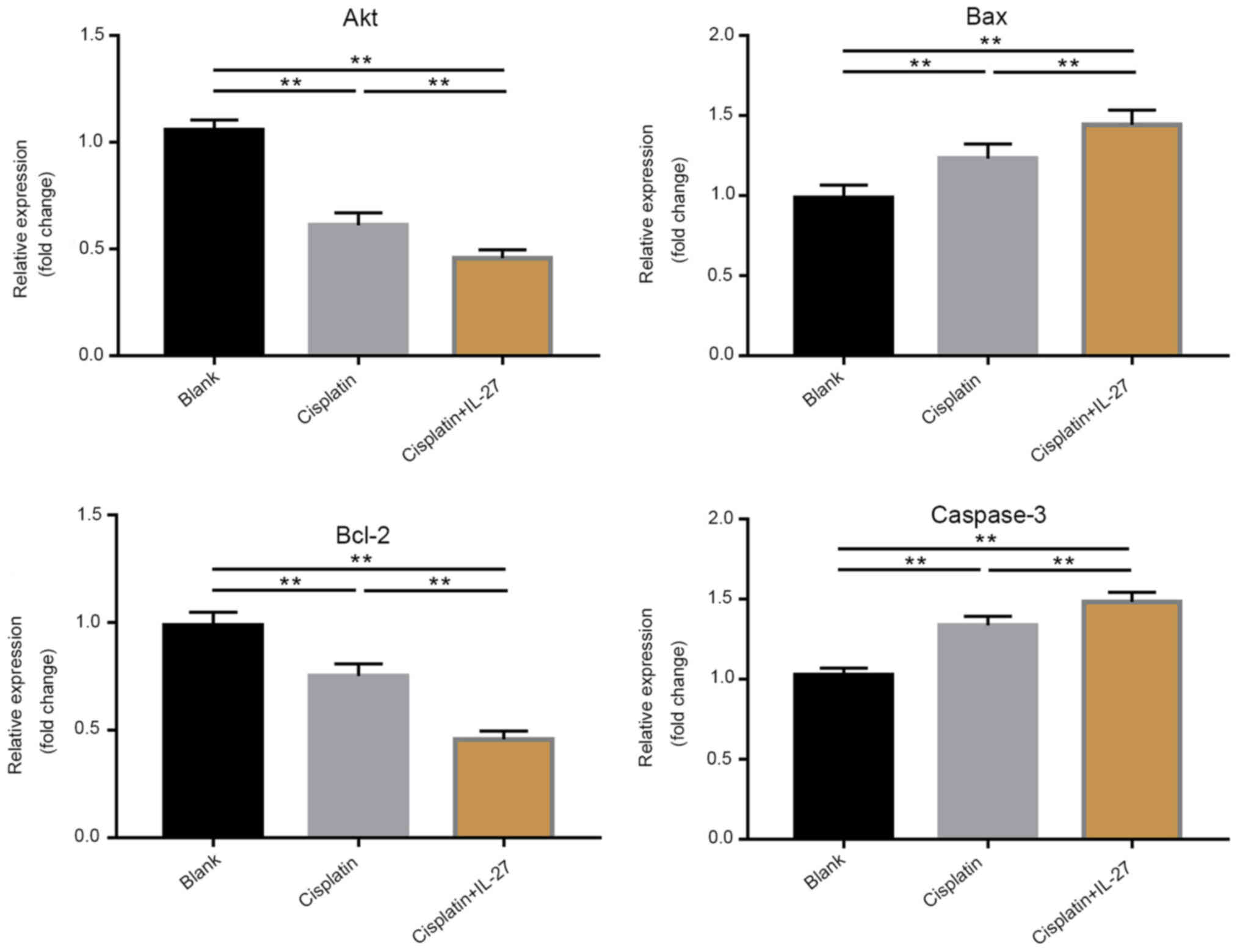
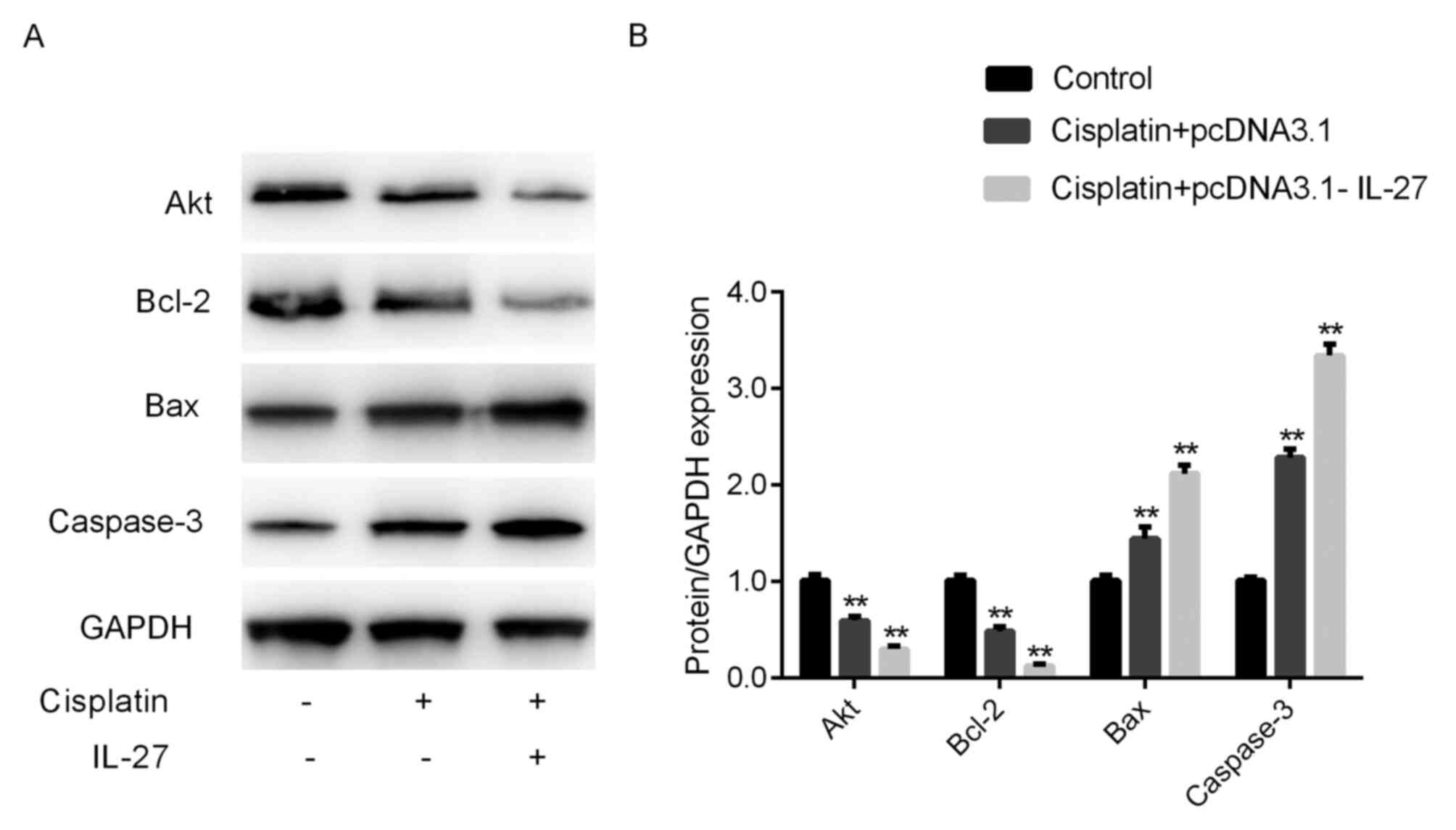
IL-27 regulated Akt, Bcl-2, Bax and
caspase-3 expression in A549 lung cancer cells
The RT-qPCR and western blot analysis of Akt, Bcl-2,
Bax and caspase-3 corroborated the apoptosis assay results. The
results of the RT-qPCR assay demonstrated that the expression
levels of pro-apoptotic genes, including Bax and caspase-3, were
significantly upregulated in the cisplatin group compared with that
in the control group, whereas the expression of the anti-apoptotic
gene Bcl-2 was significantly downregulated in the cisplatin group
compared with that in the control group (Fig. 4, P<0.01). Furthermore, Bax and
caspase-3 expression levels in the cisplatin + IL-27 treatment
group were significantly downregulated compared with that in the
cisplatin alone group (P<0.01; Fig.
4), while the expression of Bcl-2 was significantly
downregulated in the cisplatin + IL-27 treatment group compared
with that in the cisplatin alone group. The results of the western
blot analysis were consistent with those obtained by RT-qPCR
(Fig. 5). The expression levels of
Bax and caspase-3 in the cisplatin group were significantly
increased, and the expression of Bcl-2 was significantly decreased
when compared with the control (P<0.05); these effects were
further enhanced in the cisplatin + IL-27 group. The relative
expression levels of genes associated with apoptosis indicate that
IL-27 can significantly increase the sensitivity of A549 lung
cancer to cisplatin.
Discussion
Numerous studies have verified that IL-27 exhibits
an antitumor function in various types of human cancer. It has
previously been reported that IL-27 directly inhibits cell
proliferation and angiogenesis in prostate cancer (27). Furthermore, transfection of human
pancreatic carcinoma cells with IL-27 has previously been reported
to inhibit proliferation, induce cell cycle arrest and promote
apoptosis (28). Increasing the
expression levels of IL-27, combined with treatment with rapamycin
and cisplatin, has been demonstrated to inhibit the proliferation
and metastasis of endometrial cancer (29). The findings of the present study
suggest that IL-27 is downregulated in lung cancer tissues in
comparison with that in normal tissues, and verified that IL-27 is
an anti-oncogene, which is consistent with the findings of the
aforementioned previous studies.
The potential mechanisms by which IL-27 promotes
cisplatin chemosensitivity in lung cancer cells were investigated.
The present study demonstrated that IL-27 induced chemosensitivity
in human lung cancer cells by inhibiting proliferation and
invasion, and promoting apoptosis. Cell viability was significantly
inhibited in the cisplatin group, and these effects were even
greater in the cisplatin + IL-27 group, concordant with the
apoptosis assay results.
Subsequently, the underlying mechanisms by which
IL-27 enhances lung cancer cell sensitivity to cisplatin were
investigated. A previous study demonstrated that IL-27 could
inhibit the proliferation of various cancer cells by suppressing
the Akt signaling pathway (30). In
a previous study, IL-27 and sorafenib each suppressed bladder
cancer cell proliferation, migration and invasion, and increased
apoptosis through the AKT/mTOR/mitogen-activated protein kinase
pathway (31). It is hypothesized
that IL-27 may strengthen the sensitivity of lung cancer cells to
drugs by inhibiting the Akt pathway. The present study revealed
that treating lung cancer cells with cisplatin + IL-27
significantly decreased expression levels of the anti-apoptotic
factors Akt and Bcl-2, and augmented expression of the apoptotic
factors Bax and caspase-3. This was consistent with our hypothesis
and the results obtained in previous studies (31,32).
To the best of our knowledge, the present study is
the first to investigate the mechanisms of IL-27 in enhancing the
chemosensitivity of lung cancer cells to cisplatin. However, the
present study was limited by a small patient cohort and, at
present, lacks an efficient system to record patient outcomes.
In conclusion, the current data suggest that IL-27
may be a potential target for antitumor therapy. IL-27 can enhance
the sensitivity of A549 cells to chemotherapeutic drugs by
inhibiting the Akt pathway. These findings provide evidence that
supports IL-27 as a potential biomarker and treatment strategy for
patients with lung cancer, and lays a foundation for the
application of IL-27 in a clinical setting.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BJ drafted the manuscript and cooperated with WS and
PL to collect the data, and with YW and YL to analyze and interpret
the data. CB was involved in the concept and design of the
study.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Suizhou Central Hospital (Suizhou, China) and all
patients provided written informed consent prior to surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gu Y, Liu S, Zhang X, Chen G, Liang H, Yu
M, Liao Z, Zhou Y, Zhang CY, Wang T, et al: Oncogenic miR-19a and
miR-19b co-regulate tumor suppressor MTUS1 to promote cell
proliferation and migration in lung cancer. Protein Cell.
8:455–466. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deng L, Tang Y, Lin J, Lu XJ, Xue JX, Wang
LS, Zhou L, Zou Y, Ying BW, Li GD and Lu Y: Detection of epidermal
growth factor receptor gene mutation in non-small cell lung cancer
by allele-specific oligonucleotide-PCR and bi-loop probe specific
primer quantitative PCR. Zhonghua Bing Li Xue Za Zhi. 41:20–22.
2012.(In Chinese). PubMed/NCBI
|
|
3
|
Lo Russo G, Proto C and Garassino MC:
Afatinib in the treatment of squamous non-small cell lung cancer: A
new frontier or an old mistake? Transl Lung Cancer Res. 5:110–114.
2016.PubMed/NCBI
|
|
4
|
Kazandjian D, Suzman DL, Blumenthal G,
Mushti S, He K, Libeg M, Keegan P and Pazdur R: FDA approval
summary: Nivolumab for the treatment of metastatic non-small cell
lung cancer with progression on or after platinum-based
chemotherapy. Oncologist. 21:634–642. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antonia S, Goldberg SB, Balmanoukian A,
Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel
JJ and Rizvi NA: Safety and antitumour activity of durvalumab plus
tremelimumab in non-small cell lung cancer: A multicentre, phase 1b
study. Lancet Oncol. 17:299–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou YY, Hu ZG, Zeng FJ and Han J:
Clinical profile of cyclooxygenase-2 inhibitors in treating
non-small cell lung cancer: A meta-analysis of nine randomized
clinical trials. PLoS One. 11:e0151932016.
|
|
7
|
Fenchel K, Sellmann L and Dempke WC:
Overall survival in non-small cell lung cancer-what is clinically
meaningful? Transl Lung Cancer Res. 5:115–119. 2016.PubMed/NCBI
|
|
8
|
Wang W, Zhang L, Wang Y, Ding Y, Chen T,
Wang Y, Wang H, Li Y, Duan K, Chen S, et al: Involvement of miR-451
in resistance to paclitaxel by regulating YWHAZ in breast cancer.
Cell Death Dis. 8:e30712017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu D, Lu P, Mi X and Miao J:
Downregulation of mir-503 contributes to the development of drug
resistance in ovarian cancer by targeting pi3k p85. Arch Gynecol
Obstet. 297:699–707. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He L, Luo L, Zhu H, Yang H, Zhang Y, Wu H,
Sun H, Jiang F, Kathera CS, Liu L, et al: FEN1 promotes tumor
progression and confers cisplatin resistance in non-small-cell lung
cancer. Mol Oncol. 11:640–654. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang PF, Sheng LL, Wang G, Tian M, Zhu
LY, Zhang R, Zhang J and Zhu JS: miR-363 promotes proliferation and
chemo-resistance of human gastric cancer via targeting of FBW7
ubiquitin ligase expression. Oncotarget. 7:35284–35292. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krevvata M, Shan X, Zhou C, Dos Santos C,
Habineza Ndikuyeze G, Secreto A, Glover J, Trotman W, Brake-Silla
G, Nunez-Cruz S, et al: Cytokines increase engraftment of human
acute myeloid leukemia cells in immunocompromised mice but not
engraftment of human myelodysplastic syndrome cells. Haematologica.
103:959–971. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nasiri M, Karimi MH, Azarpira N and Saadat
I: Gene expression profile of toll-like receptor/adaptor/interferon
regulatory factor/cytokine axis during liver regeneration after
partial ischemia-reperfusion injury. Exp Clin Transplant.
2018.(Epub ahead of prin). PubMed/NCBI
|
|
16
|
Jiang YX, Yang SW, Li PA, Luo X, Li ZY,
Hao YX and Yu PW: The promotion of the transformation of quiescent
gastric cancer stem cells by IL-17 and the underlying mechanisms.
Oncogene. 36:1256–1264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mantovani A, Barajon I and Garlanda C:
IL-1 and IL-1 regulatory pathways in cancer progression and
therapy. Immunol Rev. 281:57–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anuradha R, Munisankar S, Bhootra Y, Dolla
C, Kumaran P, Nutman TB and Babu S: Modulation of CD4+ and CD8+ T
Cell Function and Cytokine Responses in Strongyloides stercoralis
Infection by Interleukin-27 (IL-27) and IL-37. Infect Immun.
85(pii): e00500–17. 2017.PubMed/NCBI
|
|
19
|
Xu L, Zhu LL, Ye LL, Meng LJ, Liu WQ and
Wang J: Percentages of peripheral blood gammadelta t cells and
regulatory t cells and expression of associated cytokines in
infants with human cytomegalovirus infection. Zhongguo Dang Dai Er
Ke Za Zhi. 20:204–208. 2018.(In Chinese). PubMed/NCBI
|
|
20
|
Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X,
Li J, Li C, Yan M, Zhu Z, et al: IL-6 secreted by cancer-associated
fibroblasts promotes epithelial-mesenchymal transition and
metastasis of gastric cancer via JAK2/STAT3 signaling pathway.
Oncotarget. 8:20741–20750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu XX, Hu Z, Shen X, Dong LY, Zhou WZ and
Hu WH: IL-33 promotes gastric cancer cell invasion and migration
via ST2-ERK1/2 pathway. Dig Dis Sci. 60:1265–1272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kopinski P, Wandtke T, Wedrowska E and
Chorostowska-Wynimko J: Interleukin 27 in bronchoalveolar lavage
fluid in patients with non- small cell lung cancer. Authors' reply.
Pol Arch Intern Med. 128:266–268. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Q, Yu YX, Wang XJ and Wang Z and Wang
Z: Diagnostic accuracy of interleukin-27 between tuberculous
pleural effusion and malignant pleural effusion: A meta-analysis.
Respiration. 95:469–477. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patel MV, Shen Z, Rossoll RM and Wira CR:
IL-27 expression and responsiveness in human uterine epithelial
cells and fibroblasts in vitro and the role of estradiol. J
Interferon Cytokine Res. 38:101–110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu J, Liu JQ, Shi M, Cheng X, Ding M,
Zhang JC, Davis JP, Varikuti S, Satoskar AR, Lu L, et al: IL-27
gene therapy induces depletion of tregs and enhances the efficacy
of cancer immunotherapy. JCI Insight. 3(pii): 987452018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Di Carlo E, Sorrentino C, Zorzoli A, Di
Meo S, Tupone MG, Ognio E, Mincione G and Airoldi I: The antitumor
potential of Interleukin-27 in prostate cancer. Oncotarget.
5:10332–10341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu L, Meng J, Zhang C, Duan Y, Zhao L,
Wang S and Shan B: Effects on apoptosis and cell cycle arrest
contribute to the antitumor responses of interleukin-27 mediated by
retrovirus in human pancreatic carcinoma cells. Oncol Rep.
27:1497–1503. 2012.PubMed/NCBI
|
|
29
|
Zhou WJ, Chang KK, Wu K, Yang HL, Mei J,
Xie F, Li DJ and Li MQ: Rapamycin synergizes with cisplatin in
antiendometrial cancer activation by improving IL-27-stimulated
cytotoxicity of NK cells. Neoplasia. 20:69–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Z, Zhou B, Zhang K, Song Y, Zhang L
and Xi M: IL-27 suppresses SKOV3 cells proliferation by enhancing
STAT3 and inhibiting the Akt signal pathway. Mol Immunol.
78:155–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao JY, Yin HS, Li HS, Yu XQ and Han X:
Interleukin-27 augments the inhibitory effects of sorafenib on
bladder cancer cells. Braz J Med Biol Res. 50:e62072017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Z, Liu JQ, Talebian F, Wu LC, Li S and
Bai XF: IL-27 enhances the survival of tumor antigen-specific CD8+
T cells and programs them into IL-10-producing, memory
precursor-like effector cells. Eur J Immunol. 43:468–479. 2013.
View Article : Google Scholar : PubMed/NCBI
|