Introduction
Gliomas are the most common central nervous system
tumors in adults, accounting for 44.69% of intracranial tumors and
70% of them are malignant gliomas (1). The incidence of glioma is ~6.04 cases
per 100,000 individuals in 2013, and although this incidence is not
high, the mortality rate is very high (2), and therefore, glioma may be considered
dangerous. Due to the particularity of the intracranial structure,
gliomas exhibit high invasiveness, a high recurrence rate and high
resistance to traditional radiochemotherapy, and under the most
ideal treatment conditions, the prognosis of patients with glioma
is still not ideal (3). The survival
time of patients with GBM is only 12–15 months, the vast majority
of patients die within 2 years and the 5-year survival rate is
<5% (2,4). Due to the characteristics of invasive
growth and progressive malignancy of gliomas, it is difficult to
completely remove the tumor with surgical treatment. Adjuvant
therapies, such as radiation therapy and chemotherapy can only
eliminate tumor cells or delay recurrence to a certain extent and
can also cause certain damage to the patient's body (5,6).
Although numerous advances have been made in the standardized as
well as individualized comprehensive treatment of malignant
gliomas, the clinical efficacy remains limited (7).
In the clinic, certain Traditional Chinese Medicine
prescriptions have been used to treat serious diseases such as
tumors (8). Huang-Lian-Tang (HLT) is
a classic Chinese herbal formula. The major ingredients are
Coptis chinensis, Hebeninos commutaverunt and Gingiberi
exaruit. In China and other Asian countries, HLT has a certain
or a subtle effect in numerous diseases (such as tumor, diabetes,
arthritis, ischemic stroke and liver disease) (9). Previous studies have indicated that
activation of immune cells and metabolic reprogramming the
inflammatory response are involved in the mechanism of action
(9–11). Studies have also suggested that one
of the major components of HLT, Huanglian, has a valuable
effect in the treatment of Alzheimer's disease and brain injury
(12,13). Furthermore, Huanglian has also
been effective in treating certain types of cancer (14–17).
To improve the treatment of gliomas, the present
study turned to Chinese medicine to identify whether it is able to
inhibit key molecular targets and signaling pathways associated
with the occurrence aggressive growth behavior of gliomas to
provide novel approaches for their clinical treatment. The
anti-proliferative effect of HLT on cancer cell growth has been
demonstrated in human myeloma cells (8–10).
However, it has remained elusive whether HLT has any effect on
gliomas and their therapeutic targets. In the present study,
network pharmacological systems analysis technology was used to
explore and analyze the multi-component, multi-target and
multi-pathway interaction laws and regulatory networks involved in
the inhibition of glioma cell growth, cell-cycle arrest and
apoptosis induced by HLT. The present study lays a foundation for
future in-depth study of the mechanism of action of HLT in the
treatment of gliomas and the development of novel valuable drugs
and may provide novel approaches for the clinical treatment of
GBM.
Materials and methods
Data preparation
Drug data were obtained from the Traditional Chinese
Medicines Systems Pharmacology database (TCMSP; http://tcmspw.com/tcmsp.php) (18). TCMSP is a unique systems pharmacology
platform of Chinese herbal medicines that captures the
relationships between drugs, targets and diseases. The database
includes chemicals, targets and drug-target networks, and
associated drug-target-disease networks, as well as pharmacokinetic
properties of natural compounds, including oral bioavailability,
drug-likeness, intestinal epithelial and blood-brain-barrier
permeability, and aqueous solubility.
Enrichment analysis was performed using the Database
for the Annotation, Visualization and Integrated Discovery database
(DAVID; http://david.ncifcrf.gov/) using
tumor gene data from the National Center for Biotechnology
Information (NCBI) Gene Expression Omnibus (GEO) database
(https://www.ncbi.nlm.nih.gov/geo).
Traditional Chinese Medicine
ingredient screening and target detection
In order to obtain the molecular drug composition of
HLT, the TCMIP database was searched to retrieve the name of each
active component included in HLT and their chemical structural
similarity to commercially available drugs was compared using the
US Food and Drug Administration (https://www.fda.gov/). The standard of oral
bioavailability ≥30% and drug-likeness ≥0.18 was used to screen
Chinese medicine ingredients and target analysis was performed.
Using the aforementioned criteria, pharmaceutical ingredients that
serve a therapeutic role were screened.
Gene screening
The GEO datasets (GSE108474, GSE109857, GSE143263,
GSE145940 and GSE150956) included for glioblastoma cells or tissues
were compared with those for normal brain tissue in order to
identify differentially expressed genes in glioma. In addition,
only datasets containing more than 20 samples were selected. The
titles and summaries of 350 potentially relevant datasets were
screened to identify eligible datasets for further evaluation.
Finally, only GSE108474 (72 samples) were selected for further
analysis. The GSE108474 dataset based on the GPL570 platform was
downloaded from the NCBI GEO database, containing the data of 20
normal brain tissues and 52 tumor samples. The Limma version 3.11
software package (https://bioconductor.org/packages/limma/) was used to
screen genes that were differentially expressed between the
high-risk group and the low-risk group (adjusted P-value <0.01,
fold change at least ×2).
Drug-associated active ingredient
inspection and target screenings
The names of the major ingredients of HLT, namely
Coptis chinensis, ume and dried ginger, were inputted
in the TCMSP database and the chemical composition of each drug was
retrieved. The screening conditions were based on oral
bioavailability (OB; ≥30%) and drug-likeness (DL ≥0.18), so as to
obtain the drug composition and targets. Numerous recent studies
have shown that the screening criteria can screen out effective
pharmaceutical ingredients (19–22).
Drug target-GBM network
construction
The drug- disease network was constructed using the
Bisogenet (23) plug-in of Cytoscape
software (version 3.7.2) for drug targets that were active through
TCMSP and the differentially expressed genes in glioma identified
from the GEO dataset. It contains six PPI databases, including
their Interaction Protein Database, the Biological Universal
Repository of Interaction Data Sets, Human Protein Reference
Database, the IntAct Molecular Interaction Database, the
Biomolecular Interaction Network Database and the Molecular
Interaction Action database. Two PPI interactive networks were
built and illustrated using Cytoscape software, including the
screened HLT target and the known GBM target. After combining these
two networks into candidate networks, topology analysis was used to
gradually screen out the central network.
Network topology analysis
The analysis was performed by using the CytoNCA
(24) plug-in in Cytoscape software
by determining these 6 metrics: Degree centrality (DC), topology
intermediateness (BC), closeness centrality (CC), feature vector
centrality (EC), local average connectivity (LAC), and network
centrality (NC). The six metrics provide an in-depth analysis of
the attributes of all nodes in the interactive network. The
definition and calculation equations of all parameters reflect the
significance of the nodes. A higher quantitative value indicates a
greater significance in the network. Through the above methods, the
desired central network was gradually selected.
Screening the collection of core
Protein-Protein Interaction (PPI) networks
The tightly connected area of molecular complexes in
the huge PPI network obtained was defined as topological modules
with pure network characteristics (25,26). The
aggregation of similar nodes and related nodes of drugs in the same
group are defined as the pharmacology modules. Networks that
destroy cell functions or lead to the GBM phenotype are defined as
pathogenic modules. In the topology analysis, pharmacology modules
and pathogenic modules are defined in the same network. Thus, the
pathogenic module is considered to play a role in interference and
destruction. The final core PPI network cluster was obtained by
analyzing the corresponding network using the MCODE plug-in in
Cytoscape software (26).
Gene ontology (GO) and pathway
enrichment analysis
Functional enrichment analyses of the screened genes
were performed through GO analysis (27) in three categories, namely biological
processes (BP), cellular component (CC) and molecular function
(MF), and DAVID was used to perform gene and genomic encyclopedia
of the Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling
pathway analysis. Functional terms and pathways with P≤0.05 were
considered important. The ggplot2 plugin package for R software
(vision 3.6.2) was used to visualize the results.
Results
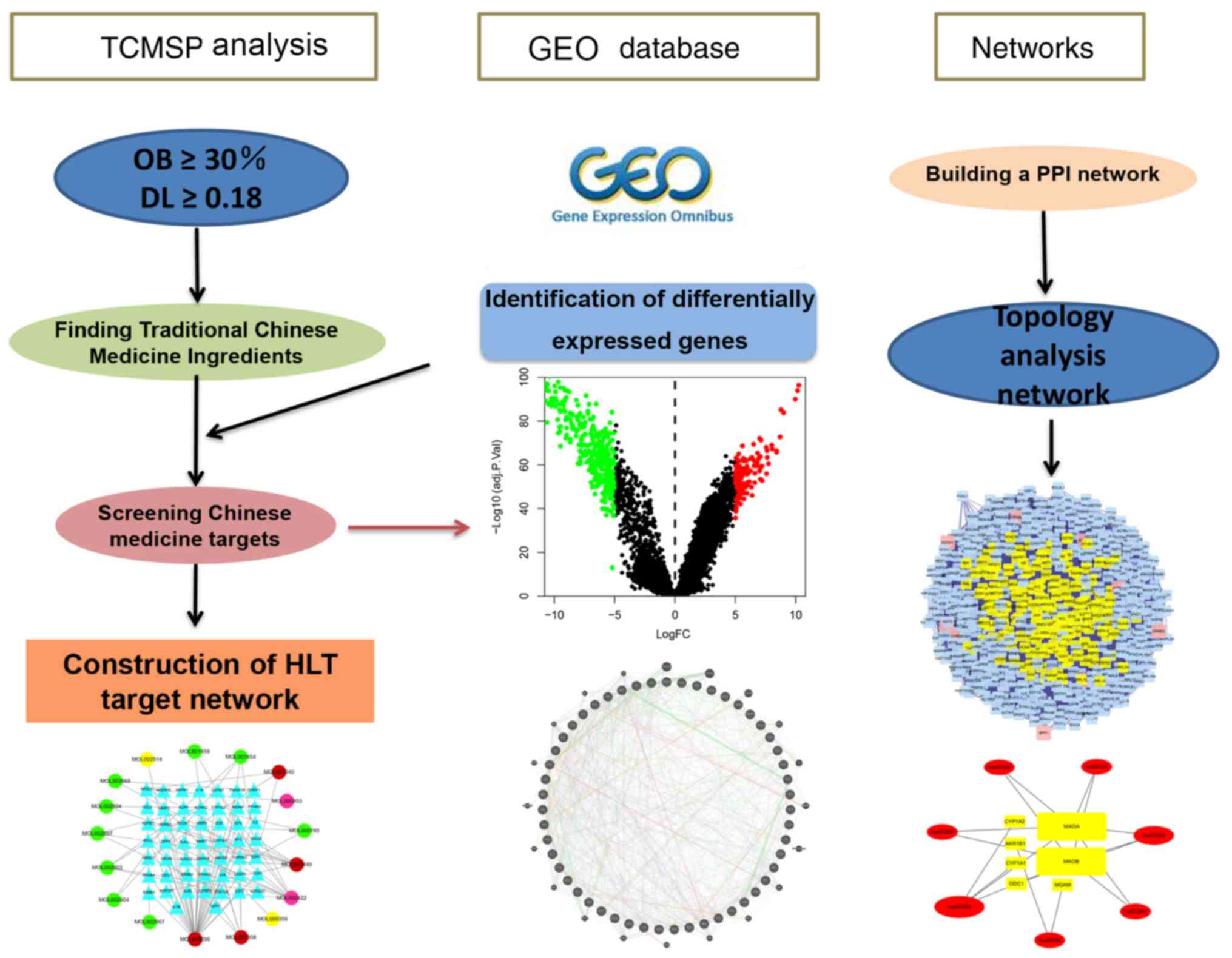
Research process
The present study was divided into three phases in
sequence, and Fig. 1 presents all of
the processes in the systematic analysis. First, the drug
components of HLT were downloaded from TCMIP and the components
were analyzed to build a drug network. Furthermore, the data of
patients with GBM were downloaded from GEO and the differentially
expressed genes were screened to construct a network. Next, the two
networks were merged together and a topology analysis was applied
to filter out the desired core network. The network contained a
total of 171 nodes and 6,309 edges. Finally, GO and KEGG analyses
were performed on these targets to determine the mechanism of
action of HLT against GBM.
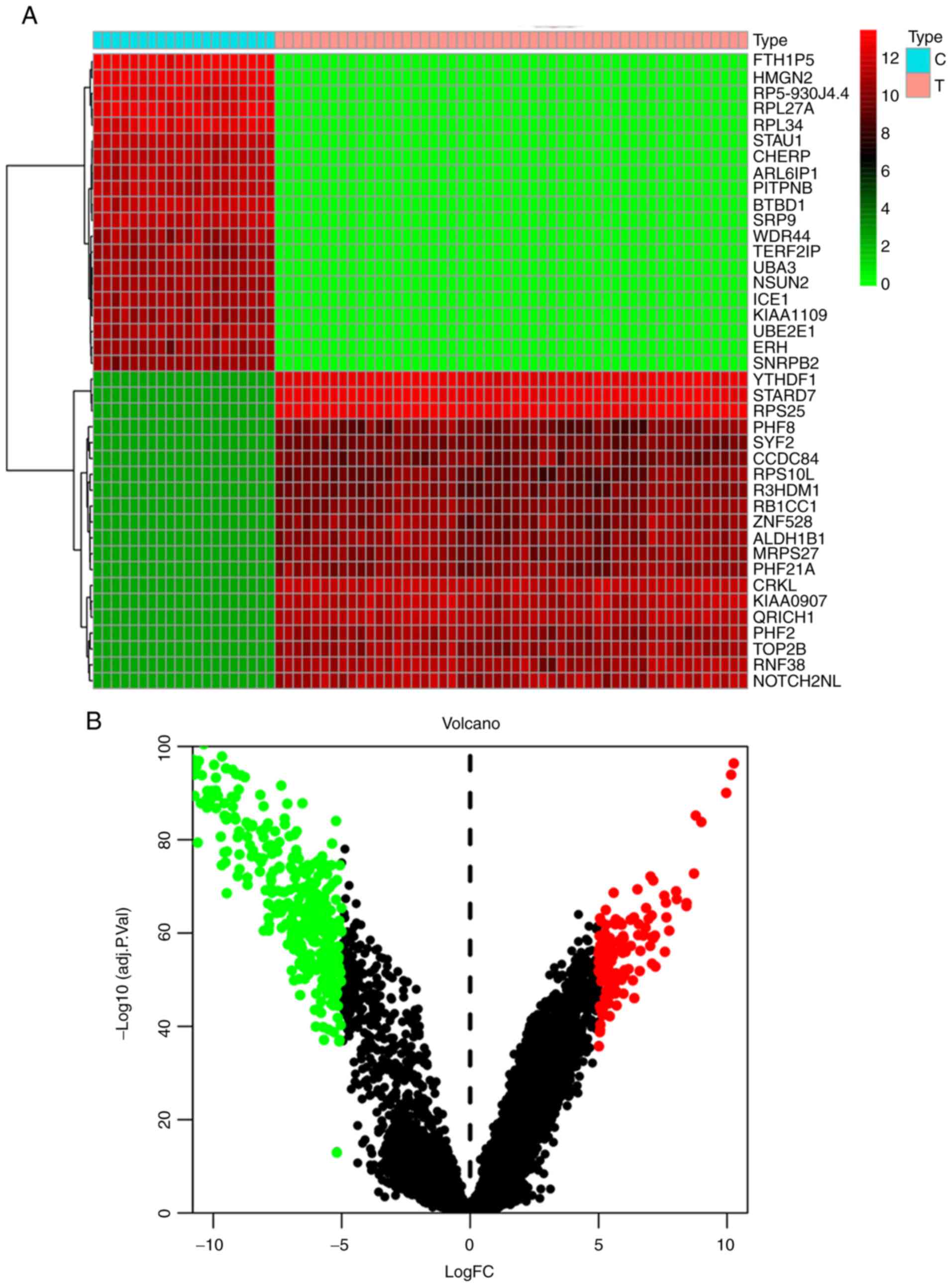
Identification of differentially
expressed genes in GBM
The dataset GSE108474 was selected and downloaded
from the GEO database as a sample, which contained the data of 20
normal tissues and 52 tumor tissues. In order to screen for
differentially expressed genes, a differential expression analysis
on the data was performed obtained using the limma software
package. By performing this analysis, it was determined that
compared with normal brain tissue samples, a total of 5,491 genes
were significantly differentially expressed in GBM, 2,751 of which
were upregulated genes and 2,740 were downregulated genes. Tables I and II list the top 10 most upregulated and
downregulated genes, respectively. According to the fold change,
Ribosomal Protein S25 (RPS25), StAR Related Lipid Transfer Domain
Containing 7 (STARD7) and YTH N6-Methyladenosine RNA Binding
Protein 1 (YTHDF1) are the three genes with the highest degree of
upregulation, while High Mobility Group Nucleosomal Binding Domain
2 (HMGN2), Staufen Double-Stranded RNA Binding Protein 1 (STAU1)
and Ferritin Heavy Chain 1 Pseudogene 5 (FTH1P5) were the three
genes with the highest degree of downregulation. A heat map
(Fig. 2A) and a volcano plot
(Fig. 2B) were drawn to visualize
the differentially expressed genes.
 | Table I.Top 10 most upregulated genes. |
Table I.
Top 10 most upregulated genes.
| Gene name | logFC | Average
expression | t | P-value | Adjusted
P-value | B |
|---|
| RPS25 (Ribosomal
Protein S25) | 10.26617 | 10.03903 | 186.0101 |
5.71×10−100 |
3.86×10−97 | 200.7867 |
| STARD7 (StAR
Related Lipid Transfer Domain Containing 7) | 10.16257 | 9.964207 | 171.7686 |
1.96×10−97 |
9.94×10−95 | 197.2763 |
| YTHDF1 (YTH
N6-Methyladenosine RNA Binding Protein 1) | 9.965356 | 9.821776 | 151.0904 |
2.36×10−93 |
8.60×10−91 | 191.1616 |
| QRICH1 (Glutamine
Rich 1) | 8.783873 | 8.968483 | 128.8207 |
2.76×10−88 |
6.65×10−86 | 182.8256 |
| CRKL (CRK Like
Proto-Oncogene, Adaptor Protein) | 9.000407 | 9.124869 | 123.2493 |
7.00×10−87 |
1.51×10−84 | 180.383 |
| KHDC4 (KH Domain
Containing 4, Pre-MRNA Splicing Facto) | 8.712287 | 8.916781 | 86.2513 |
1.42×10−75 |
1.58×10−73 | 158.9653 |
| MRPL33
(Mitochondrial Ribosomal Protein L33) | 7.022553 | 7.696419 | 84.3207 |
7.39×10−75 |
7.83×10−73 | 157.5233 |
| SYF2 (SYF2 Pre-MRNA
Splicing Factor) | 7.127974 | 7.772555 | 82.1461 |
4.94×10−74 |
5.05×10−72 | 155.8492 |
| PYGO2 (Pygopus
Family PHD Finger 2) | 6.51068 | 7.326732 | 77.2753 |
4.18×10−72 |
3.91×10−70 | 151.8939 |
| PHF2 (PHD Finger
Protein 2) | 8.01312 | 8.411828 | 76.1175 |
1.25×10−71 |
1.12×10−69 | 150.9093 |
 | Table II.Top 10 most downregulated genes. |
Table II.
Top 10 most downregulated genes.
| Id | logFC | Average
expression | t | P-value | Adjusted
P-value | B |
|---|
| HMGN2 (High
Mobility Group Nucleosomal Binding Domain 2) | −12.813 | 3.559164 | −296.316 |
8.37×10−115 |
1.19×10−110 | 216.6827 |
| STAU1 (Staufen
Double-Stranded RNA Binding Protein 1) | −11.6436 | 3.234342 | −290.265 |
3.80×10−114 |
2.70×10−110 | 216.1427 |
| FTH1P5 (Ferritin
Heavy Chain 1 Pseudogene 5) | −12.9861 | 3.607250 | −272.269 |
4.17×10−112 |
1.98×10−108 | 214.3746 |
| CHERP (Calcium
Homeostasis Endoplasmic Reticulum Protein) | −11.5667 | 3.212962 | −260.047 |
1.21×10−110 |
3.86×10−107 | 213.0173 |
| RP5-930J4.4 (RP5
Pre-MRNA Splicing Factor) | −12.3119 | 3.419980 | −259.647 |
1.36×10−110 |
3.86×10−107 | 212.9705 |
| RPL34 (Ribosomal
Protein L34) | −12.4815 | 3.467075 | −251.274 |
1.51×10−109 |
3.56×10−106 | 211.9538 |
| UBA3 (Ubiquitin
Like Modifier Activating Enzyme 3) | −11.0044 | 3.056778 | −243.291 |
1.61×10−108 |
2.96×10−105 | 210.9144 |
| RPL27A (Ribosomal
Protein L27a) | −12.5865 | 3.496262 | −243.174 |
1.67×10−108 |
2.96×10−105 | 210.8986 |
| BTBD1 (BTB Domain
Containing 1) | −11.792 | 3.275543 | −241.309 |
2.93×10−108 |
4.63×10−105 | 210.645 |
| NSUN2 (NOP2/Sun RNA
Methyltransferase 2) | −10.8641 | 3.017812 | −240.118 |
4.21×10−108 |
5.99×10−105 | 210.481 |
Chemical composition of HLT and
traditional Chinese medicine target prediction
A total of 468 chemical ingredients of 7 Chinese
herbs in HLT were collected through the TCMIP database of Chinese
medicinal ingredients, and similarity scores with drugs in the
DrugBank database were obtained. Similar drugs with a score ≥0.8
were considered to be highly similar to the chemical ingredients
contained in HLT. A total of 3,939 ‘component-target’ pairs of HLT
were retrieved. Among them, there were 14 components of Coptis
chinensis with 87 predicted targets, 38 components of dried
ginger with 26 predicted targets, 12 components of cinnamon
sticks with 57 predicted targets, 41 components of Pinellia
ternata with 237 predicted targets, 72 components of
jujube with 1,172 predicted targets, 158 components of
ginseng with 2,000 predicted targets and 133 components of licorice
with 360 predicted targets. The components and targets in the
obtained associations were assigned to the single drugs contained
in HLT to obtain the target information corresponding to each drug.
Among them, Coptis chinensis had 67 component targets and
Guizhi had 46 component targets. Dried ginger, Pinellia
ternata, ginseng, jujube and licorice each contained 7,
196, 876, 429, 115 component targets. The common targets between
the two constituent drugs were analyzed (Table III). The results suggested that
there were different numbers of common targets among the 7
components, of which 57 were common targets of Coptis
chinensis and Ginseng, accounting for 85.1% of the total number
of targets of Coptis chinensis. There were 152 targets for
Pinellia ternata and ginseng.
 | Table III.Common pharmacology-targets between
any two components of Huang-Lian-Tang. |
Table III.
Common pharmacology-targets between
any two components of Huang-Lian-Tang.
| Component (target
number) | Dried ginger
(7) | Ginseng
(876) | Guizhi
(46) | Jujube
(429) | Coptis
chinensis (67) | Pinellia
(196) | Licorice
(115) |
|---|
| Dried ginger
(7) | / | 7 | 0 | 1 | 0 | 1 | 0 |
| Ginseng
(876) | 7 | / | 22 | 265 | 57 | 152 | 110 |
| Guizhi
(46) | 0 | 22 | / | 20 | 7 | 26 | 24 |
| Jujube
(429) | 1 | 265 | 20 | / | 57 | 28 | 105 |
| Coptis
chinensis (67) | 0 | 57 | 7 | 57 | / | 7 | 54 |
| Pinellia
(196) | 1 | 152 | 26 | 28 | 7 | / | 24 |
| Licorice
(115) | 0 | 110 | 24 | 105 | 54 | 24 | / |
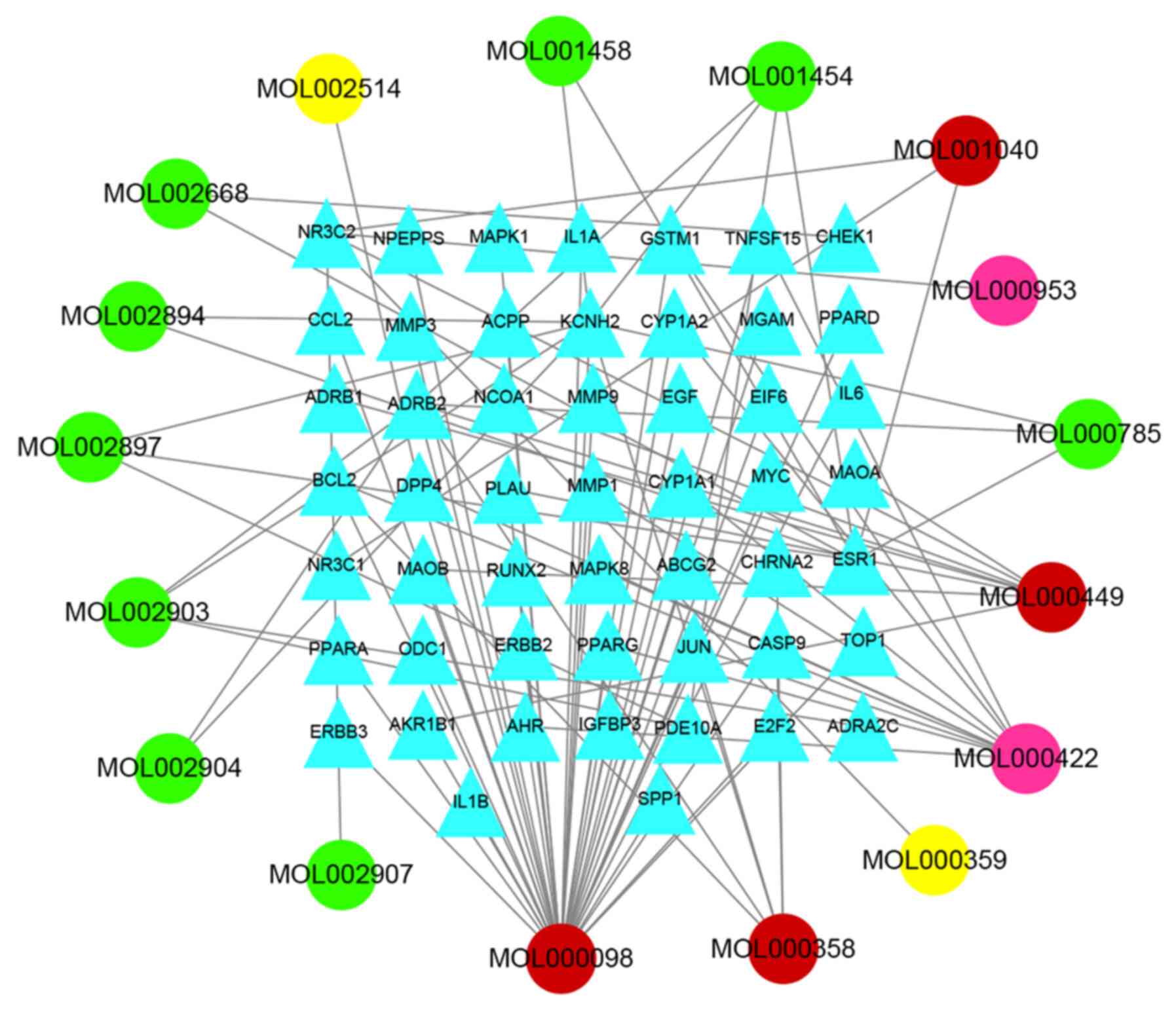
Compound regulation network of
HLT
A compound regulation network of the active
components and molecular relationships of HLT was built and 766
related events and 68 nodes were obtained. There are 17 drug nodes
(round nodes; dried ginger is yellow, Coptis is
green, ume is pink, and various drug combinations are red)
and 51 molecular nodes (triangular nodes). The core target network
was mapped through Cytoscape (Fig.
3).
Construction and analysis of PPI
network
With the rise of network pharmacology, its role in
systems biology research is increasing. In the present study, the
HLT drug target network and the PPI network for GBM were fused
(Fig. 4A and B). In order to reveal
the pharmacological mechanism of HLT against GBM, Cytoscape
software was used to combine two huge networks to build a new and
complex network (2,954 nodes and 72,642 edges), which resembled a
merge of the HLT and GBM networks (Fig.
4B). Subsequently, the CytoNCA plugin in Cytoscape was used to
confirm the core goals. The selection criteria were set as follows:
BC value >450, CC >0.6, DC >70, EC >0.02, NC >20 and
LAC >18 (Fig. 4C and D). Finally,
a core network comprising 171 nodes and 6,309 edges was screened
out.
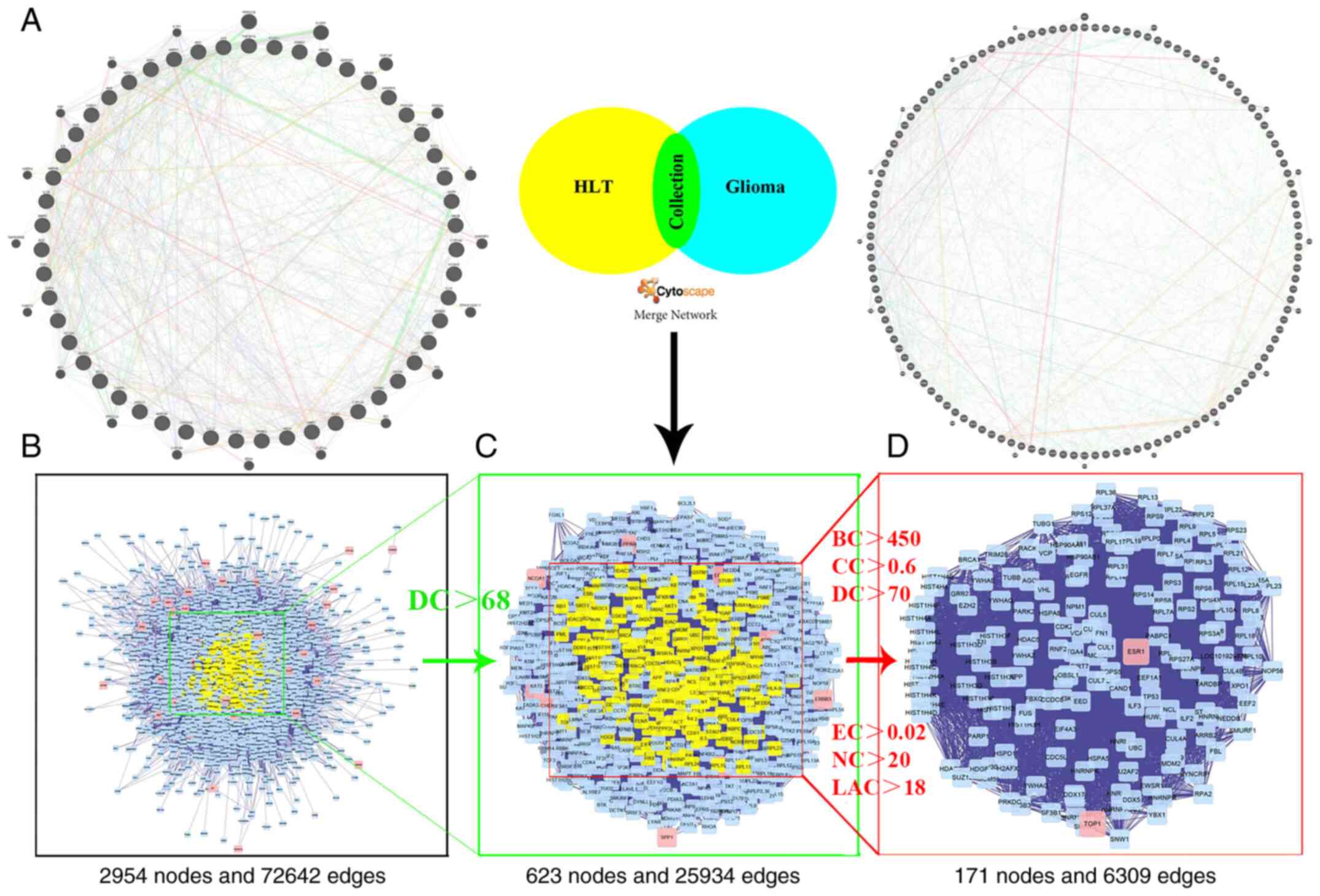 | Figure 4.Identification of a core PPI network
for the mechanism/drug-target interactions. (A) Construction of two
PPI networks, one for HLT targets and the other for glioblastoma
multiforme. (B) The interactive PPI network of HLT and glioblastoma
multiforme targets comprising 2,954 nodes and 72,642 edges. (C) PPI
network of significant proteins extracted from B; this network
comprises 623 nodes and 25,934 edges. (D) PPI network of
significant proteins extracted from C; this network is made up of
171 nodes and 6,309 edges. BC, betweenness centrality; CC,
closeness centrality; DC, degree centrality; EC, eigenvector
centrality; NC, network centrality; LAC, local average
connectivity; PPI, protein-protein interaction; HLT,
Huang-Lian-Tang. |
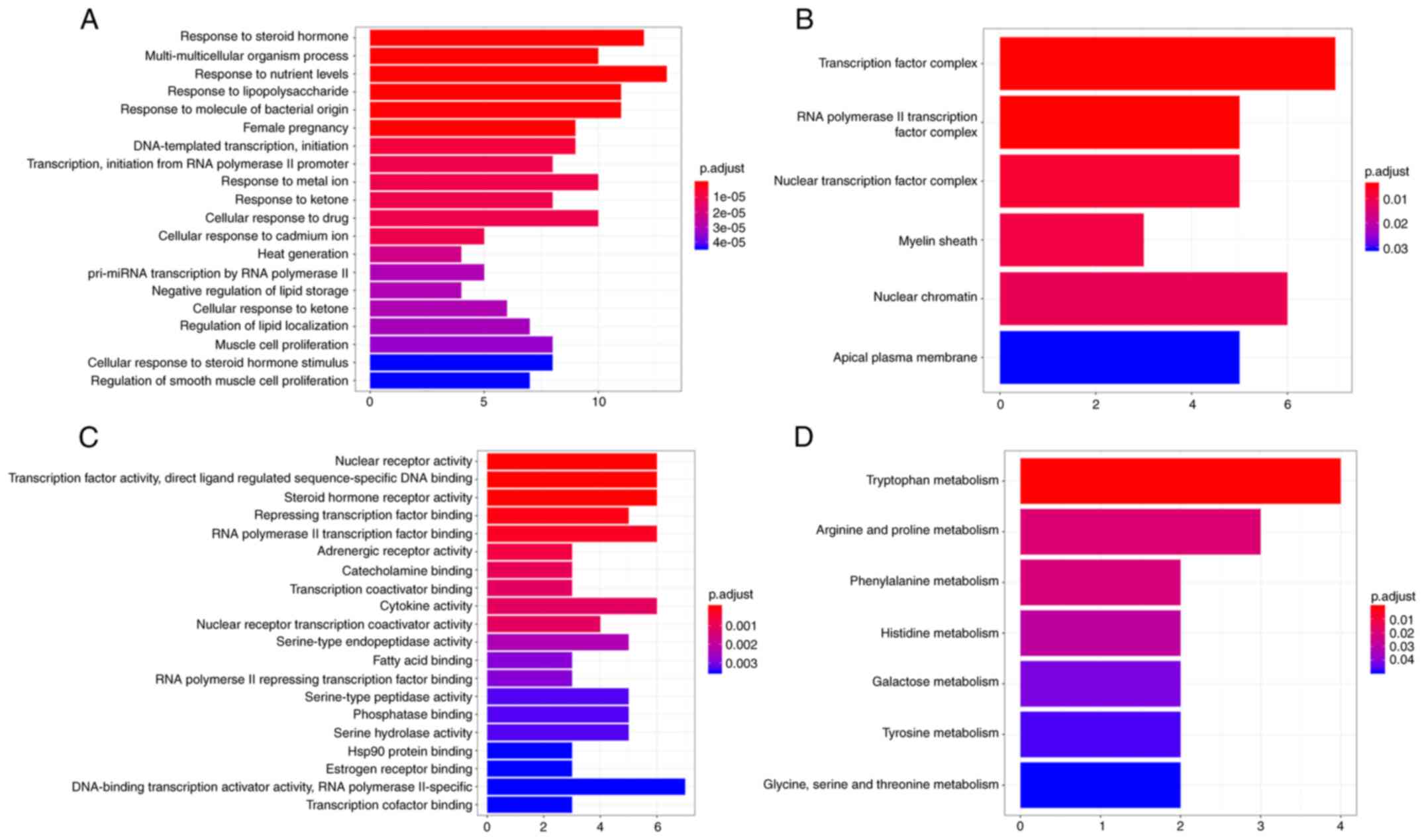
Enrichment analysis and KEGG signaling
pathways of candidate targets for HLT against glioma
The 386 candidate targets for HLT in treating GBM
were subjected to a GO analysis in the DAVID database to explore
the links between the functional units, their potential
significance in the biological systems network. The mechanism of
action is clarified through this functional enrichment analysis The
GO terms were determined in the following categories: Biological
processes (Fig. 5A), cellular
component (Fig. 5B) and molecular
function (Fig. 5C). In the category
biological process, the candidate genes were associated with
response to nutrient levels, response to steroid hormone, response
to lipopolysaccharide and response to molecule of organism process.
In the category cellular component, enriched terms were
transcription factor complex, RNA polymerase II transcription
factor complex, nuclear transcription factor complex, myelin
sheath, nuclear chromatin and apical plasma membrane. Finally, in
the category molecular function, genes were associated with
repression of transcription factor binding and RNA polymerase II
transcription factor binding. A KEGG enrichment analysis was also
performed on these genes, revealing that they are mainly related to
amino acid metabolism (Fig. 5D).
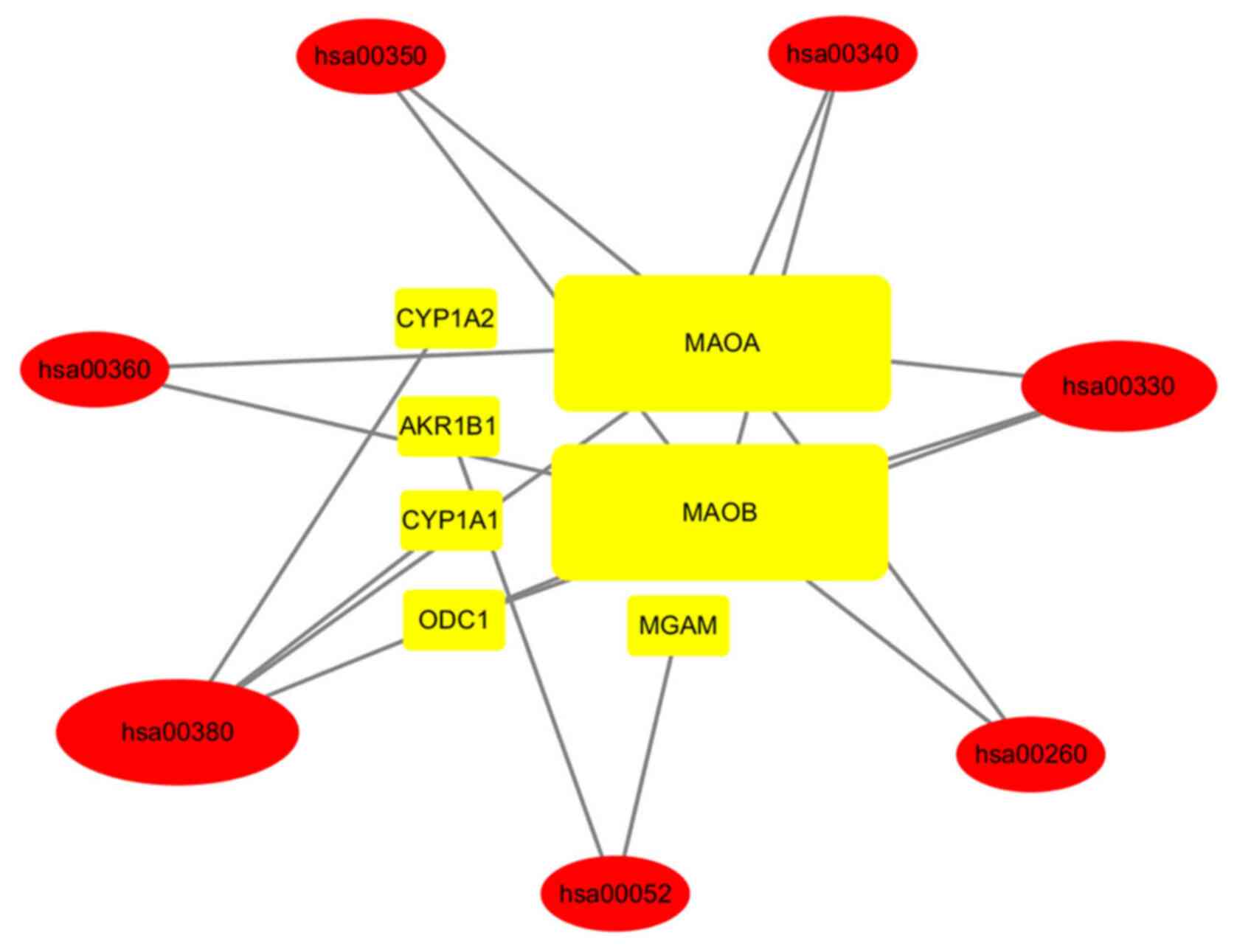
KEGG relationship network
construction
To clarify the overall mechanisms of HLT in the
treatment of gliomas, the above-mentioned steps provided a complete
approach based on the current knowledge of the pathogenesis of
gliomas (Fig. 6). Through
layer-by-layer screening, 7 genes (oval) and 7 KEGG signaling
pathways (rectangular) associated with them were obtained; the size
of the symbols is associated with the number of adjacent nodes. The
7 genes were MAOA (Monoamine Oxidase A), MAOB (Monoamine Oxidase
B), MGAM (Maltase-Glucoamylase),CYP1A2 (Cytochrome P450 Family 1
Subfamily A Member 2), AKR1B1(Aldo-Keto Reductase Family 1 Member
B), CYP1A1(Cytochrome P450 Family 1 Subfamily A Member 1) and
ODC1(Ornithine Decarboxylase 1). The 7 KEGG signaling pathways were
hsa00380, hsa0052, hsa00260, hsa00330, hsa00340, hsa00350 and
hsa00360.
Discussion
In the present study, target genes associated with
GBM were first screened. It was analyzed which genes influence the
occurrence, development and prognosis of GBM. The active components
of HLT were also identified through an online pharmacology
database. Combining the interaction networks of the two provided
the potential mechanisms of action of HLT in the treatment of GBM.
The present study provided a foundation for further research on
Traditional Chinese Medicine treatments for GBM to improve the
prognosis of affected patients. The target genes of HLT identified
in the present study hold promise for the development of novel
targeted therapies for GBM.
Glioma is characterized by rapid growth and
resistance to treatment, and patients usually have a poor prognosis
(28). Diffuse glioma is the most
common intracranial malignancy, accounting for >60% of cases
(29). GBM is the most common and
malignant type of primary brain tumor, accounting for ~80% of
malignant astrocytomas (30). GBM
cells vary in shape and size, which is known as glioblastoma
polymorphisms (30). GBM may develop
rapidly from undiagnosed, less malignant precursor lesions or may
progress slowly from preexisting low-grade glioma (31). In spite of the progress in the
treatment of GBM, including chemotherapy, the prognosis remains
poor (32,33). Therefore, it is necessary to identify
potential pathways for the development of this cancer type and to
prevent the occurrence of GBM.
In the previous decades, scientists have studied the
potential activity of drugs and conducted basic research or
clinical trials for tumor treatment (34). The introduction of the concept of big
data and the continuous development of pharmacology has provided
the opportunity to analyze the relationship between drugs and
molecular targets of diseases in silico (35). Network pharmacology is helpful for
exploring multi-channel signaling pathway regulation, improve drug
efficacy and the success rate of clinical trials and reduce drug
development costs. Chinese herbal medicines and plant ingredients
have positive prospects in the treatment of a variety of complex
diseases (36,37). Network pharmacology has been
extensively applied to study the biological mechanisms of certain
Traditional Chinese Medicine prescriptions and ingredients
(38,39). Therefore, the network pharmacology
method was used to understand the biological mechanisms of action
HLT against GBM at the molecular level.
In southeast Asia, HLT has a significant role in the
treatment of brain diseases. Studies have indicated that HLT is
able to nourish the nerve cell, restore brain function and improve
blood circulation in the brain (40). Studies have also suggested that HLT
is an anti-apoptotic, anti-oxidant and anti-inflammatory agent
(41–43). HLT has also been indicated to promote
neurotrophic factor expression (44)
and regulate neurogenesis of neural precursor cells (45), which is conducive to the recovery of
neural function. In the present study, not only network
pharmacology, but also bioinformatics were used, and a topology
analysis was performed. The central network with 171 nodes and
6,309 edges was screened out. GO analysis was performed and HLT was
indicated to be involved in numerous biological processes
associated with tumorigenesis and development, including RNA
polymerase transcription factor complex and response to nutrient
levels. The KEGG pathway analysis of the core PPI network was used
to identify pathways by which HLT exerts its effects in treating
GBM by regulating tumorigenesis and progression. According to the
P-value of each enriched pathway and its role in GBM, there were
seven related signaling pathways that were significant, and these
pathways were all closely associated with the amino acid metabolism
of tumorigenesis and progression. It is well known that cancer
cells promote the ‘Warburg effect’ (46), which is enhanced glycolysis or
aerobic glycolysis, even when the surrounding oxygen supply is
sufficient. In addition, it has been determined that dysfunctional
anabolism/catabolism of fatty acids and amino acids, particularly
glutamine, serine and glycine, have a role as metabolic regulators
in supporting cancer cell growth (47,48).
Furthermore, extensive crosstalk was reported between dysfunctional
metabolic networks and cancer cell signaling (49,50).
The occurrence and development of tumors are related
to a large number of cellular processes, which ultimately lead to
the formation or recurrence of tumors, including the induction of
cell cycle processes, evasion of apoptotic programs and activation
of cell survival pathways (51). In
the present study, it was observed that HLT disrupts the process by
which these cancer cells are being established, thereby limiting
their growth in vitro and in vivo. It remains elusive
how HLT specifically reduces tumor growth under normal cellular
conditions, so that it does not cause any systemic imbalances or
serious side effects for patients. However, it may be speculated
that individual active ingredients in the prescription have a
mutual synergy, and that the tumor cells are affected by drugs at a
concentration below the lethal dose for normal cells.
There are still certain deficiencies in the present
study that require to be addressed. First, the present study was
only performed in silico. In the next step, a pharmacology
model will be built to further confirm the therapeutic effect of
HLT on GBM through basic experiments. Furthermore, the optimal
dosage of HLT for treating GBM patients and contraindications
require to be established. In the future, in-depth research on the
most important targets or pathways of the final drug candidates
will be performed.
In conclusion, the present network pharmacology
analysis on the effect of HLT in GBM indicated that it may be a
promising chemotherapeutic drug whether it is able to slow/inhibit
tumor growth and recurrence and whether it is used as an adjuvant
treatment for patients subjected to other standard treatments
remains to be elucidated. HLT have few side effects (52), so they may be used in GBM-associated
clinical trials.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed during the
current study are available in the [GEO and TCM] repositories,
[https://www.ncbi.nlm.nih.gov/geo/;https://tcmspw.com/tcmsp.php].
Authors' contributions
Conceptualization: WRL, WHX and CYL. Data collection
and interpretation: WRL, WHX, JFX and RYH. Formal analysis: HNH,
XJL, WRL, WHX, JFX and KH. Funding acquisition: HNH, XJL, WRL and
WHX. Methodology: WRL, WHX, CYL, JFX and RYH. Project
administration: HNH, XJL, KH, HNH, XJL, JFX and RYH. Resources:
HNH, XJL, JFX, RYH and KH. Supervision: JFX, KH and RYH. Writing:
WRL, WHX, CYL, JFX, RYH and KH. Review and editing: HNH and XJL.
Responsible for all aspects of the work to ensure that issues
related to the accuracy or completeness of any part of the work are
properly investigated and resolved: HNH and XJL. All authors were
involved in writing the manuscript and read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ostrom QT, Gittleman H, Farah P, Ondracek
A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 15
(Suppl 2):ii1–ii56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu BL, Cheng JX, Zhang X and Zhang W:
Controversies concerning the application of brachytherapy in
central nervous system tumors. J Cancer Res Clin Oncol.
136:173–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zong G, Wang H, Li J, Xie Y, Bian E and
Zhao B: Inhibition of GPR137 expression reduces the proliferation
and colony formation of malignant glioma cells. Neurol Sci.
35:1707–1714. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baskar R, Lee KA, Yeo R and Yeoh KW:
Cancer and radiation therapy: Current advances and future
directions. Int J Med Sci. 9:193–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rivera Vargas T and Apetoh L: Danger
signals: Chemotherapy enhancers? Immunol Rev. 280:175–193. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
PDQ Pediatric Treatment Editorial Board, .
Childhood brain stem glioma treatment (PDQ(R)): Patient Version.
PDQ Cancer Information Summaries [Internet]. National Cancer
Institute (US); Bethesda, MD: 2002
|
|
8
|
Tao W, Luo X, Cui B, Liang D, Wang C, Duan
Y, Li X, Zhou S, Zhao M, Li Y, et al: Practice of traditional
Chinese medicine for psycho-behavioral intervention improves
quality of life in cancer patients: A systematic review and
meta-analysis. Oncotarget. 6:39725–39739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang WH, Chen CH, Gau RJ, Lin CC, Tsai
CL, Tsai K and Lu FJ: Effect of baicalein on apoptosis of the human
Hep G2 cell line was induced by mitochondrial dysfunction. Planta
Med. 68:302–306. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma Z, Otsuyama K, Liu S, Abroun S,
Ishikawa H, Tsuyama N, Obata M, Li FJ, Zheng X, Maki Y, et al:
Baicalein, a component of Scutellaria radix from
Huang-lian-jie-du-tang (HLJDT), leads to suppression of
proliferation and induction of apoptosis in human myeloma cells.
Blood. 105:3312–3318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee WR, Shen SC, Lin HY, Hou WC, Yang LL
and Chen YC: Wogonin and fisetin induce apoptosis in human
promyeloleukemic cells, accompanied by a decrease of reactive
oxygen species, and activation of caspase 3 and Ca(2+)-dependent
endonuclease. Biochem Pharmacol. 63:225–236. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jia KK, Ding H, Yu HW, Dong TJ, Pan Y and
Kong LD: Huanglian-wendan decoction inhibits NF-κB/NLRP3
inflammasome activation in liver and brain of rats exposed to
chronic unpredictable mild stress. Mediators Inflamm.
2018:30935162018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu XR, Fang SY, Ren W, Wang HJ, Yang J, Si
N, Bian BL and Zhao HY: Pharmacodynamics of Huanglian Jiedu
decoction in Alzheimer's disease (AD) model rats and effect on
improvement of inflammation microenvironment in brain. Zhongguo
Zhong Yao Za Zhi. 43:3006–3011. 2018.(In Chinese). PubMed/NCBI
|
|
14
|
Hu S, Chen CW, Chen ST, Tsui KH, Tang TK,
Cheng HT, Hwang GS, Yu JW, Li YC, Wang PS and Wang SW: Inhibitory
effect of berberine on interleukin-2 secretion from PHA-treated
lymphocytic Jurkat cells. Int Immunopharmacol. 66:267–273. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang JX, Liu J, Wang J, He C and Li FP:
The extract of Huanglian, a medicinal herb, induces cell growth
arrest and apoptosis by upregulation of interferon-beta and
TNF-alpha in human breast cancer cells. Carcinogenesis.
26:1934–1939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gong B, Kao Y, Zhang C, Zhao H, Sun F and
Gong Z: Exploring the pharmacological mechanism of the herb pair
‘HuangLian-GanJiang’ against colorectal cancer based on network
pharmacology. Evid Based Complement Alternat Med. 2019:27350502019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai Y, Sun L and Qiang W: A new strategy
to uncover the anticancer mechanism of Chinese compound formula by
integrating systems pharmacology and bioinformatics. Evid Based
Complement Alternat Med. 2018:67078502018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ru J, Li P, Wang J, Zhou W, Li B, Huang C,
Li P, Guo Z, Tao W, Yang Y, et al: TCMSP: A database of systems
pharmacology for drug discovery from herbal medicines. J
Cheminform. 6:132014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsu YL, Kuo PL, Tzeng TF, Sung SC, Yen MH,
Lin LT and Lin CC: Huang-lian-jie-du-tang, a traditional Chinese
medicine prescription, induces cell-cycle arrest and apoptosis in
human liver cancer cells in vitro and in vivo. J Gastroenterol
Hepatol. 23:e290–e299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang N, Feng Y, Tan HY, Cheung F, Hong M,
Lao L and Nagamatsu T: Inhibition of eukaryotic elongation factor-2
confers to tumor suppression by a herbal formulation
Huanglian-Jiedu decoction in human hepatocellular carcinoma. J
Ethnopharmacol. 164:309–318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang H, Wang Y, Yang T, Ga Y, Zhang Y, Liu
R, Zhang W and Zhao J: Bioinformatics analysis for the
antirheumatic effects of Huang-lian-jie-du-tang from a network
perspective. Evid Based Complement Alternat Med. 2013:2453572013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu T, Ma C, Fan S, Deng N, Lian Y, Tan L,
Du W, Zhang S, Liu S, Ren B, et al: Systematic understanding of the
mechanism of baicalin against ischemic stroke through a network
pharmacology approach. Evid Based Complement Alternat Med.
2018:25828432018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martin A, Ochagavia ME, Rabasa LC, Miranda
J, Fernandez-de-Cossio J and Bringas R: BisoGenet: A new tool for
gene network building, visualization and analysis. BMC
Bioinformatics. 11:912010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang Y, Li M, Wang J, Pan Y and Wu FX:
CytoNCA: A cytoscape plugin for centrality analysis and evaluation
of protein interaction networks. Biosystems. 127:67–72. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barabasi AL, Gulbahce N and Loscalzo J:
Network medicine: A network-based approach to human disease. Nat
Rev Genet. 12:56–68. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
GLASS Consortium: Glioma through the
looking GLASS: Molecular evolution of diffuse gliomas and the
glioma longitudinal analysis consortium. Neuro Oncol. 20:873–884.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Szopa W, Burley TA, Kramer-Marek G and
Kaspera W: Diagnostic and therapeutic biomarkers in glioblastoma:
Current status and future perspectives. Biomed Res Int.
2017:80135752017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sugimoto K, Ideguchi M, Kimura T, Kajiwara
K, Imoto H, Sadahiro H, Ishii A, Kawano H, Ikeda E and Suzuki M:
Epithelioid/rhabdoid glioblastoma: A highly aggressive subtype of
glioblastoma. Brain Tumor Pathol. 33:137–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Farias-Eisner G, Bank AM, Hwang BY,
Appelboom G, Piazza MA, Bruce SS and Sander Connolly E:
Glioblastoma biomarkers from bench to bedside: Advances and
challenges. Br J Neurosurg. 26:189–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Y, Ren Z, Peng Y, Li K, Wang X, Huang
G, Qi S and Liu Y: Classification of glioma based on prognostic
alternative splicing. BMC Med Genomics. 12:1652019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Terstappen GC, Schlupen C, Raggiaschi R
and Gaviraghi G: Target deconvolution strategies in drug discovery.
Nat Rev Drug Discov. 6:891–903. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang GB, Li QY, Chen QL and Su SB:
Network pharmacology: A new approach for chinese herbal medicine
research. Evid Based Complement Alternat Med.
2013:6214232013.PubMed/NCBI
|
|
36
|
Sang Z, Wang K, Shi J, Liu W, Cheng X, Zhu
G, Wang Y, Zhao Y, Qiao Z, Wu A and Tan Z: The development of
advanced structural framework as multi-target-directed ligands for
the treatment of Alzheimer's disease. Eur J Med Chem.
192:1121802020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Wu W, Zhang J, Gao L, Zhang L,
Long H, Hou J, Wu W and Guo D: An integrated strategy for holistic
quality identification of Chinese patent medicine: Liuwei dihuang
pills as a case study. Phytochem Anal. Mar 4–2020.(Epub ahead of
print). doi: 10.1002/pca.2927.
|
|
38
|
Zhu N and Hou J: Exploring the mechanism
of action xianlingubao prescription in the treatment of
osteoporosis by network pharmacology. Comput Biol Chem.
85:1072402020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou X, Hong Y and Zhan Y: Karacoline,
identified by network pharmacology, reduces degradation of the
extracellular matrix in intervertebral disc degeneration via the
NF-κB signaling pathway. J Pharm Anal. 10:13–22. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gu Y, Chen J and Shen J: Herbal medicines
for ischemic stroke: Combating inflammation as therapeutic targets.
J Neuroimmune Pharmacol. 9:313–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen SJ and Cui MC: Systematic
understanding of the mechanism of salvianolic acid a via
computational target fishing. Molecules. 22:6442017. View Article : Google Scholar
|
|
42
|
Wang S, Wang H and Lu Y: Tianfoshen oral
liquid: A CFDA approved clinical traditional Chinese medicine,
normalizes major cellular pathways disordered during colorectal
carcinogenesis. Oncotarget. 8:14549–14569. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang X, Yu S, Jia Q, Chen L, Zhong J, Pan
Y, Shen P, Shen Y, Wang S, Wei Z, et al: NiaoDuQing granules
relieve chronic kidney disease symptoms by decreasing renal
fibrosis and anemia. Oncotarget. 8:55920–55937. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu M, Chen X, Gu Y, Peng T, Yang D, Chang
RC, So KF, Liu K and Shen J: Baicalin can scavenge peroxynitrite
and ameliorate endogenous peroxynitrite-mediated neurotoxicity in
cerebral ischemia-reperfusion injury. J Ethnopharmacol.
150:116–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cao Y, Mao X, Sun C, Zheng P, Gao J, Wang
X, Min D, Sun H, Xie N and Cai J: Baicalin attenuates global
cerebral ischemia/reperfusion injury in gerbils via anti-oxidative
and anti-apoptotic pathways. Brain Res Bull. 85:396–402. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schwartz L, Supuran CT and Alfarouk KO:
The warburg effect and the hallmarks of cancer. Anticancer Agents
Med Chem. 17:164–170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Colomer C, Margalef P, Villanueva A, Vert
A, Pecharroman I, Solé L, González-Farré M, Alonso J, Montagut C,
Martinez-Iniesta M, et al: IKKα kinase regulates the DNA damage
response and drives chemo-resistance in cancer. Mol Cell.
75:669–682.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang Y, Huang Y, Zhang L, Chang A, Zhao
P, Chai X and Wang J: Identification of crucial genes and
prediction of small molecules for multidrug resistance of Hodgkin's
lymphomas. Cancer Biomark. 23:495–503. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hensley CT, Wasti AT and DeBerardinis RJ:
Glutamine and cancer: Cell biology, physiology, and clinical
opportunities. J Clin Invest. 123:3678–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Srinivasan S, Guha M, Kashina A and
Avadhani NG: Mitochondrial dysfunction and mitochondrial
dynamics-The cancer connection. Biochim Biophys Acta Bioenerg.
1858:602–614. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Korf BR: Neurofibromatosis. Handb Clin
Neurol. 111:333–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Singh A and Zhao K: Treatment of insomnia
with traditional Chinese herbal medicine. Int Rev Neurobiol.
135:97–115. 2017. View Article : Google Scholar : PubMed/NCBI
|