Introduction
Gastric cancer is the fifth most frequently
diagnosed cancer type and the third leading cause of
cancer-associated mortality (1). To
treat gastric cancer, surgery is effective but often restrictive.
Therefore, chemotherapy is the main therapy for patients with
advanced gastric cancer. Among them, 5-fluorouracil (5-FU) is one
of the most commonly used chemotherapeutic agents for patients with
gastric cancer. The anticancer mechanism of 5-FU is via inhibition
of thymidylate synthase, which is necessary for the synthesis of
intracellular DNA, and the suppression of cell function by
inhibiting RNA function and production (2). However, numerous patients become
resistant to chemotherapy, limiting the efficacy of commonly used
anticancer drugs. The mechanisms of chemotherapy resistance include
reducing intracellular drug accumulation, increasing drug efflux,
increasing nucleotide repair activity and avoiding apoptosis
(3). In particular, multidrug
resistance protein (MDR) serves an important role in drug efflux,
causing drug resistance (4,5). In gastric cancer, ~21% of patients show
progressive resistance to 5-FU (6).
Therefore, a novel chemotherapeutic agent for gastric cancer is
required to overcome drug resistance.
Chrysin (5,7-dihydroxyflavone) is a biologically
active flavonoid derived from plants and natural products,
including propolis (7). Chrysin
possesses anticancer effects through numerous mechanisms, including
cell cycle arrest through inhibition of histone deacetylase
(8), autophagic cell death (9), suppression of the Jun N-terminal kinase
(JNK) pathway (10) and apoptosis
(11). Furthermore, chrysin inhibits
P-glycoprotein (P-gp), multidrug resistance-related protein (MRP)-2
and breast cancer resistance protein (BCRP). It acts as an MDR
reversing agent in colon carcinoma cells (12) and breast cancer cells (13). These multiple functions of chrysin
suggest that it may be effective in combination chemotherapy.
Therefore, in the present study, the combined effect
of chrysin and 5-FU was investigated in gastric cancer AGS cells.
Furthermore, the effect of chrysin was also investigated in
5-FU-resistant AGS (AGS/FR) cells.
Materials and methods
Cell culture
Human gastric cancer AGS cells were purchased from
the Korean Cell Line Bank (no. 21739). AGS/FR cells were provided
by The Catholic University of Korea. These cells were incubated
with RPMI-1640 medium (GenDEPOT), supplemented with 10% fetal
bovine serum (GenDEPOT) and 1% penicillin/streptomycin (GenDEPOT).
AGS/FR cells were cultured in medium containing 100 µM 5-FU
(Sigma-Aldrich; Merck KGaA) to maintain resistance.
Measurement of cell viability
AGS and AGS/FR cells were seeded onto 96-well plates
at 0.5–1.0×104 cells/well and incubated for 24 h.
Chrysin (Sigma-Aldrich; Merck KGaA) and 5-FU were diluted in 0.5%
dimethyl sulfoxide (DMSO) solution (Sigma-Aldrich; Merck KGaA) and
the cells were treated for 24 or 48 h. For the cell viability
assay, these cells were measured using an MTT assay (14). In brief, MTT (Sigma-Aldrich; Merck
KGaA) was added to the media for 3 h, and then the media were
removed. DMSO was added to the cells for 30 min. Absorbance was
measured using a microplate reader (Infinite M200 PRO; TECAN) at
560 nm.
Calculation of Combination Index
(CI)
To evaluate the combined effect of chrysin and 5-FU,
the Chou-Talalay method was used (15) and the CI value was calculated using
the Calcusyn 2.0 program (Biosoft). The resulting CI value showed
quantitation for the synergistic effect (CI <1), additive effect
(CI = 1) or antagonism (CI >1).
Cell morphology
Cells were treated with 50 µM chrysin, 25 µM 5-FU,
or a combination of chrysin and 5-FU for 24 h. Change of cells
morphology was confirmed through an inverted microscope
(magnification, ×40) (Nikon ECLIPSE TS 100; Nikon Corporation).
Apoptosis staining
Cells were treated with 50 µM chrysin, 25 µM 5-FU,
or a combination of chrysin and 5-FU for 24 h. To measure
apoptosis, cells were washed with cold phosphate-buffered saline
(PBS) and resuspended. The staining solution of Annexin V-FITC (BD
Pharmingen) and propidium iodide (PI) was added to the cells and
gently mixed. The cells were incubated at room temperature for 15
min in the dark. The cells were analyzed using a flow cytometer
ACEA Novocyte 2000 (Agilent Technologies, Inc.). The data was
analyzed using the NovoExpress® software version 1.2.5
(Agilent Technologies, Inc.).
Cell cycle analysis
Cells were treated with 50 µM chrysin, 25 µM 5-FU,
or the combination of chrysin and 5-FU for 24 h. For cell cycle
analysis (16), the collected cells
were washed with cold PBS. After centrifugation at room temperature
(244 × g for 2 min), the cell pellet was fixed with 70% ethanol
overnight at −20°C. The fixed cells were centrifuged at room
temperature (244 × g for 2 min) and supernatant was removed. Cells
were treated with PI solution and incubated for 30 min at room
temperature. Stained cells were analyzed using a flow cytometer
flow cytometer ACEA Novocyte 2000 (Agilent Technologies, Inc.). The
data was analyzed using the NovoExpress® software
version 1.2.5 (Agilent Technologies, Inc.).
Western blotting
Expression levels of cell death-related proteins
were determined by western blot analysis (17). Cells were treated with chrysin (50
µM), 5-FU (25 µM), or the combination of chrysin and 5-FU for 24 h.
Cells were lysed in RIPA buffer (GenDEPOT) with protease inhibitors
(GenDEPOT) and phosphatase inhibitors (Roche, Basel). Protein
concentration was measured using Pierce™ bicinchoninic acid protein
assay kit (cat. no. 23225; Thermo Fisher Scientific, Inc.). The
proteins (15 µg/lane) separated from cell lysates by 10–15%
SDS-polyacrylamide gel electrophoresis were transferred onto
polyvinylidene fluoride membranes (Merck Millipore). Membranes were
blocked with 5% skimmed milk in tris-buffered saline with 0.1%
Tween 20 for 1 h at room temperature, and then incubated with
primary antibodies against p53 (1:1,000; cat. no. 05-224; Merck
KGaA); p21WAF1/Cip1 (1:2,000; cat. no. 05-345; Merck KGaA); Bax
(1:1,000; cat. no. 610982; BD Biosciences); Caspase 9 (1:1,000;
cat. no. 9508; Cell Signaling Technology, Inc.); cleaved Caspase 9
(1:1,000; cat. no. 7237; Cell Signaling Technology, Inc.); Caspase
3 (1:1,000; cat. no. 9665; Cell Signaling Technology, Inc.),
cleaved Caspase 3 (1:1,000; cat. no. 9664; Cell Signaling
Technology, Inc.), phospho-Akt (1:1,000; cat. no. 4060; Cell
Signaling Technology, Inc.); Akt (1:1,000; cat. no. 9272; Cell
Signaling Technology, Inc.); cyclin D1 (1:1,000; cat. no. 2978;
Cell Signaling Technology, Inc.); CDK6 (1:1,000; cat. no. 3136;
Cell Signaling Technology, Inc.), cdc2 (1:1,000; cat. no. 77055;
Cell Signaling Technology, Inc.); cyclin B1 (1:1,000; cat. no.
12231; Cell Signaling Technology, Inc.), MDR1 (1:1,000; cat. no.
sc-55510; Santa Cruz Biotechnology, Inc.) and β-actin (1:5,000;
cat. no. A 5441; Sigma-Aldrich; Merck KGaA) overnight at 4°C. The
next day, membranes were incubated with the following secondary
antibodies for 3 h at room temperature: Goat anti-mouse IgG (H +
L)-horseradish peroxidase (HRP)-conjugated (1:3,000; cat. no.
1706516; BioRad Laboratories, Inc.) and goat anti-rabbit IgG (H +
L)-HRP-conjugated (1:3,000; cat. no. 1706515; BioRad Laboratories,
Inc.). Proteins were detected by Chemi-Doc (FluorChem E system;
ProteinSimple) using enhanced chemiluminescent (ECL) solution [to
use, mix solution A comprised of 250 mM luminol (Sigma-Aldrich;
Merck KGaA), 90 mM p-Coumaric acid (Sigma-Aldrich; Merck KGaA) and
1M Tris-HCl (pH 8.5) (Duchefa Biochemie B.V) and B comprised of 1M
Tris-HCl (pH 8.5) (Duchefa Biochemie B.V) and
H2O2 (DSP Inc.) in a 1:1 ratio] and analyzed
using AlphaView software for FluoChem E system (version 3.4.0,
Proteinsimple Inc.).
Statistical analysis
The data were analyzed using one-way and two-way
analysis of variance, followed by Tukey's post hoc test to
statistically analyze differences among multiple groups. The
statistical analyses were performed using GraphPad Prism 7
(GraphPad Software Inc.). All data are shown as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Synergistic effect of chrysin and 5-FU
in AGS and AGS/FR cells
The viability of AGS cells in response to chrysin,
5-FU, or the combination of chrysin and 5-FU were evaluated with
increasing doses (Fig. 1A).
Conditions 1, 2, 3 and 4 referred to chrysin doses of 40, 50, 60
and 80 µM, and 5-FU doses of 20, 25, 30 and 40 µM, respectively.
The combination of chrysin and 5-FU significantly inhibited cell
viability more than 5-FU alone (Fig.
1A). In particular, conditions 1 and 2 for 5-FU + chrysin
potentiated the inhibition of cell viability more than 5-FU or
chrysin alone. To calculate the combined effect, combination index
(CI) values for each condition were compared and exhibited an
antagonistic (>1), addictive (=1) or synergistic (<1) effect.
As shown in Fig. 1B, CI values of
conditions 1, 2, 3 and 4 were 0.371, 0.548, 1.030 and 1.614,
respectively. These results suggested that the combined treatment
serves a role in the inhibition of cell viability. Furthermore,
addictive or synergistic effects of 24 h treatment similarly showed
synergistic effects in the same conditions as 48 h treatment
(Fig. S1).
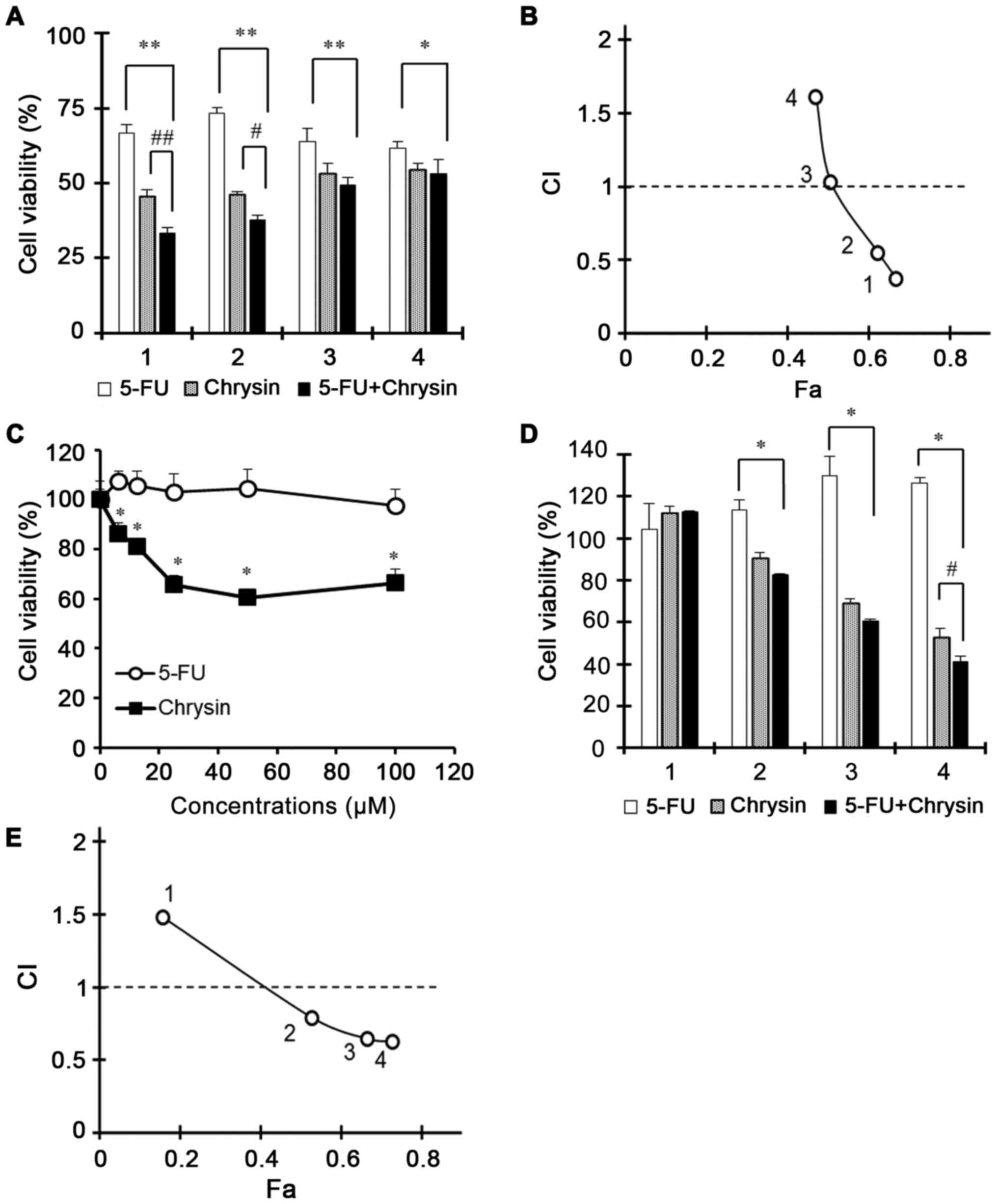 | Figure 1.Synergistic effect of chrysin and 5-FU
in AGS and AGS/FR cells. AGS cells were treated with chrysin, 5-FU
or the combination of chrysin and 5-FU for 24 h. AGS/FR cells were
treated with chrysin, 5-FU or the combination of chrysin and 5-FU
for 24 and 48 h. Treated conditions 1, 2, 3 and 4 were as follows:
(A and B) 40, 50, 60 and 80 µM for chrysin, and 20, 25, 30 and 40
µM for 5-FU, respectively, in AGS cells; and (D and E) 20, 40, 50
and 60 µM for chrysin, and 6.25, 12.5, 25 and 50 µM for 5-FU,
respectively in AGS/FR cells. (A, C, D) Cell viability was measured
by MTT assay. (A) Data are presented as the mean ± SD; *P<0.01;
**P<0.001; #P<0.01; ##P<0.001
(two-way ANOVA). (C) Data are presented as the mean ± SD;
*P<0.001 (one-way ANOVA). (D) Data are presented as the mean ±
SD; *P<0.001; #P<0.05 (two-way ANOVA). 5-FU,
5-fluorouracil; CI, combination index; Fa, fractional effect; SD,
standard deviation; ANOVA, analysis of variance. |
Furthermore, the effect of chrysin or the
combination of chrysin and 5-FU was investigated in 5-FU-resistant
gastric cancer AGS/FR cells. Chrysin significantly inhibited cell
viability in AGS/FR cells (Fig. 1C).
In particular, the combination of chrysin and 5-FU for 48 h showed
a synergistic effect in the inhibition of cell viability (Fig. 1D). For these experiments, conditions
1, 2, 3 and 4 referred to chrysin doses at 20, 40, 50 and 60 µM,
and 5-FU doses at 6.25, 12.5, 25 and 50 µM, respectively. Although
AGS/FR cells remained viable following 5-FU 100 µM treatment, the
viability of the cells was significantly suppressed with the
combination of chrysin and 5-FU more than chrysin alone (Fig. 1D). Furthermore, CI values in
conditions 2, 3, and 4 were 0.79, 0.64 and 0.62, indicating a
synergistic effect at each of these doses (Fig. 1E). These results suggested that
chrysin improved the cytotoxicity of 5-FU in AGS and AGS/FR
cells.
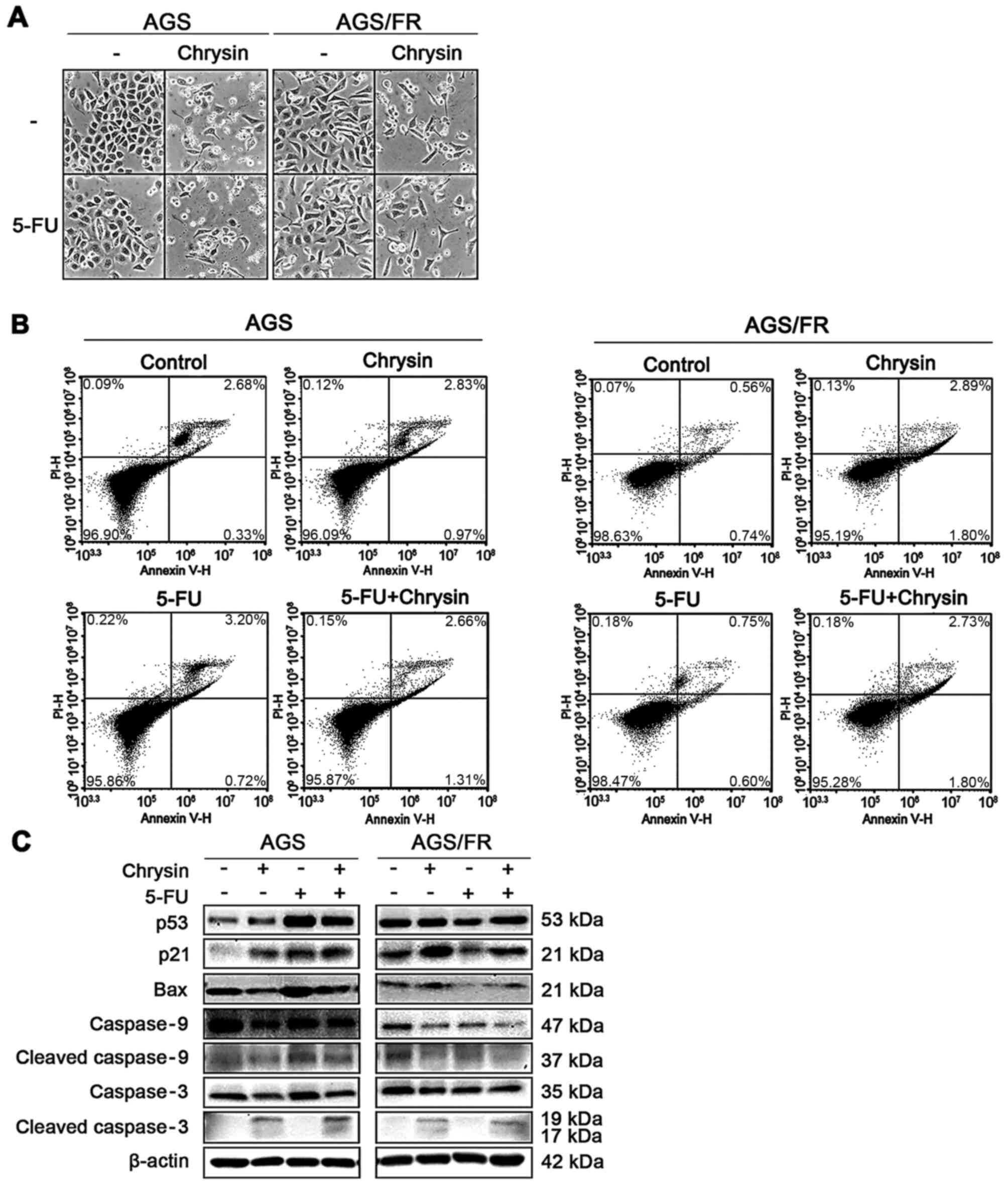
Apoptosis of AGS and AGS/FR cells
following treatment with chrysin and 5-FU
Common condition (condition 2 in AGS cells;
condition 3 in AGS/FR cells) doses were used for further
chrysin/5-FU combination studies. Under these conditions, each
cellular morphology is shown in Fig.
2A.
To elucidate the mechanism of the synergistic
anticancer effect shown in Fig. 1B and
E, the cells were stained using annexin V-PI solution and
analyzed by flow cytometry. Chrysin and the combination of chrysin
and 5-FU caused more apoptosis than the control in AGS and AGS/FR
cells; however, 5-FU caused more apoptosis only in AGS cells
(Fig. 2B). Some cells in Fig. 2B were shown to be slightly spread,
including control. This result is speculated to be caused by the
solvent DMSO. Therefore, the expression levels of apoptosis-related
proteins were further investigated by western blotting.
Semi-quantitative analysis of these protein levels are shown in
Fig. S2 (three repeats). In AGS
cells, 5-FU, chrysin and the combination of chrysin and 5-FU
increased p53 and p21; however, 5-FU caused no change of these
protein expression levels in AGS/FR cells (Fig. 2C). These results suggested that
chrysin inhibited cell viability via a different pathway than that
of 5-FU. Therefore, the complementary mechanism of the combined
treatment was investigated.
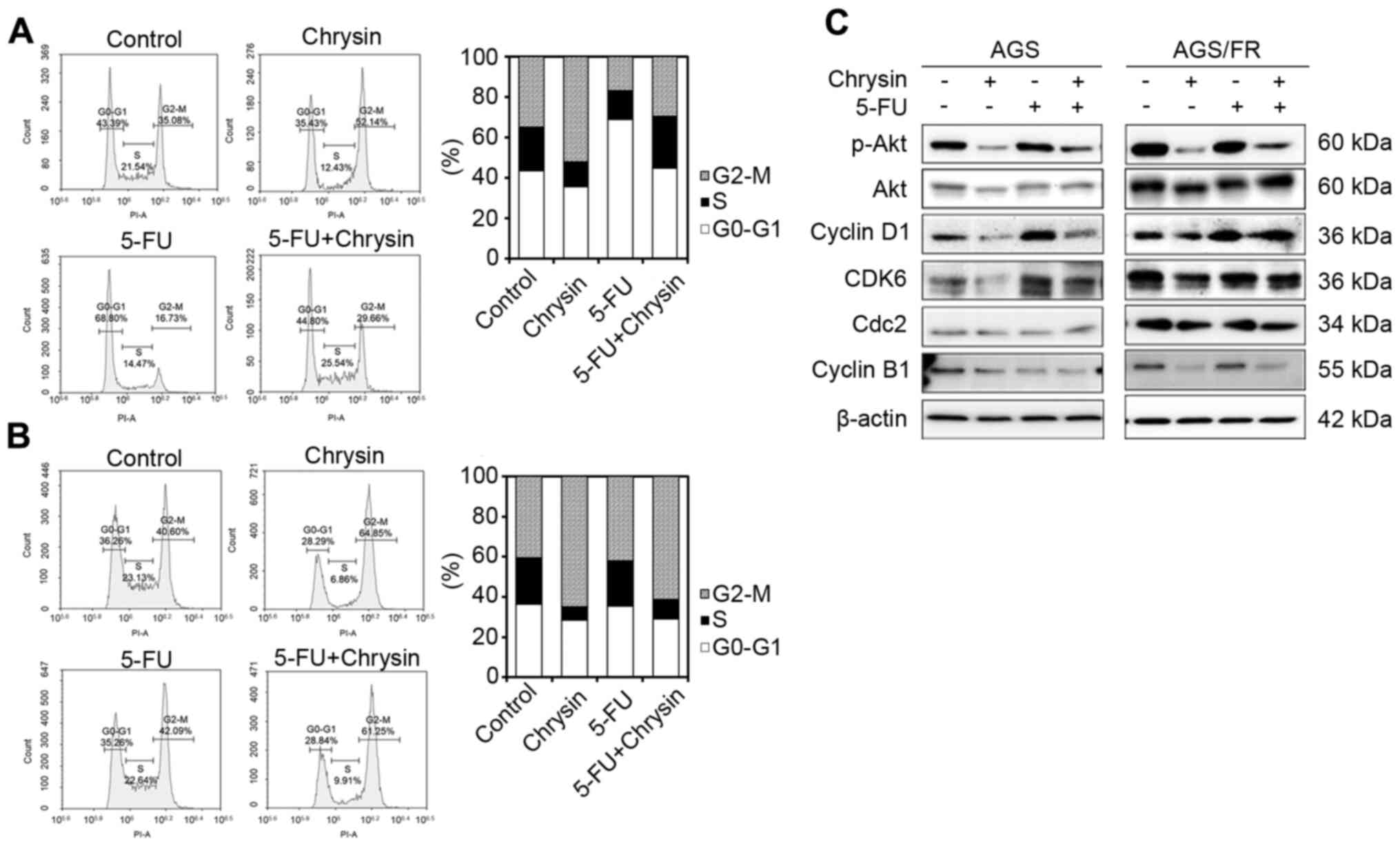
Potentiated anticancer effect of
chrysin occurs via G2/M phase arrest
As an increase in the p21 level induced by chrysin
was observed in the western blot analysis, the cell cycle was
further evaluated in AGS and AGS/FR cells.
Chrysin induced cell arrest in the G2/M phase, while
5-FU induced arrest in the G0/G1 phase in AGS cells. The
combination of chrysin and 5-FU showed cell cycle arrest in the S
phase (Fig. 3A). However, chrysin
(64.85%) and the combination of chrysin and 5-FU (61.25%)
accumulated cells in the G2/M phase more than control treatment
(40.6%) in AGS/FR cells (Fig. 3B).
Cell cycle-related protein levels were measured by western blotting
(Fig. 3C). The combination of
chrysin and 5-FU also decreased cyclin B1, cell cycle division
cycle protein 2 (cdc2) and suppressed phosphorylated Akt (p-Akt)
expression in AGS cells. The combination of chrysin and 5-FU showed
no change in cdc2 expression, but CDK6 levels increased with 5-FU
treatment in AGS cells. On the other hand, chrysin and the
combination of chrysin and 5-FU decreased cdc2 and cyclin B1 though
downregulated p-Akt expression and upregulated p21 expression in
AGS/FR cells. These results indicated that chrysin potentiated the
anticancer effect of 5-FU via G2/M phase arrest (Fig. 3C).
Discussion
The results of the present study have indicated that
the combination of chrysin and 5-FU had synergistic anticancer
effects and overcame 5-FU resistance in vitro. These results
confirm the findings of previous studies (18), which suggest that chrysin may be used
as an anticancer agent for combined therapy. In one study, chrysin
was co-administered with cisplatin in HepG2 liver cancer cells
(19), with docetaxel in A549
non-small cell lung cancer cells (18), and with metformin in breast cancer
cells (20), showing synergistic
effects in all cases. The present study found that chrysin showed
synergy with 5-FU, according to CI analysis. The combination of
chrysin (50 µM) and 5-FU (25 µM) showed significant inhibition of
cell viability, compared with that induced by chrysin or 5-FU
alone, in AGS/FR cells and AGS cells (Fig. 1). As was the case in a previous study
(12,13), chrysin-treated cells showed a
decrease in the MDR1 level (Fig.
S3). However, AGS/FR cells exhibited a higher MDR1 level than
AGS cells, but this was not significant. The combined effect of
chrysin and 5-FU was difficult to understand as a mechanism by
which chrysin inhibits the expression of MDR1 and then accumulates
5-FU in AGS or AGS/FR cells. Therefore, the results of the present
study suggest a mechanism in which the combination of chrysin and
5-FU arrests the cell cycle. In a future study, it would be
beneficial to evaluate the therapeutic efficacy of chrysin in an
AGS/FR-derived xenograft model as the results of the present study
were obtained at the in vitro level. Nevertheless, the
anticancer effects of chrysin observed in this study were
consistent with those in other in vivo studies. Therefore,
the application of chrysin with 5-FU may be clinically implemented
to treat patients with 5-FU-resistant gastric cancer.
The combination of chrysin and 5-FU upregulated p21
expression in AGS cells and AGS/FR cells (Fig. 2C). The cyclin-dependent kinase
inhibitor, p21 serves a key role in the cell cycle and is regulated
by various stimuli, including p53 (21), and the PI3K/Akt pathway (22). Several studies have reported that
chrysin also exerted anticancer effects through upregulating
p21-induced G1 phase arrest in A375 melanoma cells (8) and G2 phase arrest in esophageal
squamous carcinoma (23). In the
present study, chrysin induced G2/M phase arrest and 5-FU induced
G0/G1 phase arrest in AGS cells. The combination of chrysin and
5-FU caused cell accumulation in the S phase, suggesting a
complementary effect of cell arrest by chrysin and 5-FU (Fig. 3A). The S phase of the cell cycle
inhibits cell growth though inhibition of DNA synthesis when
stress-induced DNA damage occurs (24). These results correlated with S phase
arrest through upregulated p53 and p21 expression (Fig. 2C).
AGS/FR cells exhibited downregulated p-Akt
expression (Fig. 3C). Akt regulates
cyclin-dependent kinase inhibitor p21 and the cell cycle.
Phosphorylated Akt promotes cell growth and angiogenesis. Kim et
al (25) reported that Akt
signaling was overactivated in chemo-resistant colon cancer cells,
and that inhibition of Akt signaling may be a good pharmacological
target (25). In the present study,
the combination of chrysin and 5-FU decreased p-Akt expression and
increased p21 expression in AGS/FR cells. Increased p21 inhibits
the cyclin B1/cdc2 complex protein expression, with this complex
regulating G2/M phase. Therefore, the results of the present study
indicated that the combination of chrysin and 5-FU induced G2/M
phase arrest via inhibition of cdc2 and cyclin B1 by p21
upregulation in AGS/FR cells.
As shown in Fig. 1C,
AGS/FR cells were resistant to high doses of 5-FU (100 µM). The
present study found that co-treatment of chrysin with 5-FU caused
similar effects to chrysin treatment alone. Nevertheless, the CI
value of chrysin and 5-FU was <1 at 48 h, suggesting synergy
(Fig. 1E). It is possible that the
combined effect of chrysin and 5-FU occurs though chrysin-induced
cell arrest as previously described (8).
In conclusion, the combination of chrysin and 5-FU
in AGS cells enhanced inhibition of cell viability through S phase
arrest. Furthermore, the results of the present study suggested
that chrysin improved 5-FU resistance via G2/M phase
arrest in AGS/FR cells. These results indicated that chrysin
potentiates the anticancer effect of 5-FU and may be utilized for
the treatment of 5-FU resistant gastric cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Bio &
Medical Technology Development Program of the NRF funded by the
Korean Government (grant no. 2015M3A9B6074045) and the NRF grant
funded by the Korea government, MSIT (grant no.
2017R1A2B4008254).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SYL and JJ were responsible for study conception and
drafting this article. SKL established the AGS/FR cells. JJ
reviewed the article and provided necessary suggestions. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu B and Xie J: Identifying therapeutic
targets in gastric cancer: The current status and future direction.
Acta Biochim Biophys Sin (Shanghai). 48:90–96. 2016.PubMed/NCBI
|
|
4
|
Kimchi-Sarfaty C, Ben-Nun-Shaul O, Rund D,
Oppenheim A and Gottesman MM: In vitro-packaged SV40 pseudovirions
as highly efficient vectors for gene transfer. Hum Gene Ther.
13:299–310. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baguley BC: Multiple drug resistance
mechanisms in cancer. Mol Biotechnol. 46:308–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomas DM and Zalcberg JR: 5-fluorouracil:
A pharmacological paradigm in the use of cytotoxics. Clin Exp
Pharmacol Physiol. 25:887–895. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jung J: Emerging utilization of chrysin
using nanoscale modification. J Nanometer. 2016:72016.
|
|
8
|
Pal-Bhadra M, Ramaiah MJ, Reddy TL,
Krishnan A, Pushpavalli SN, Babu KS, Tiwari AK, Rao JM, Yadav JS
and Bhadra U: Plant HDAC inhibitor chrysin arrest cell growth and
induce p21WAF1 by altering chromatin of STAT response element in
A375 cells. BMC Cancer. 12:1802012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin YM, Chen CI, Hsiang YP, Hsu YC, Cheng
KC, Chien PH, Pan HL, Lu CC and Chen YJ: Chrysin attenuates cell
viability of human colorectal cancer cells through autophagy
induction unlike 5-fluorouracil/oxaliplatin. Int J Mol Sci.
19:17632018. View Article : Google Scholar
|
|
10
|
Xia Y, Lian S, Khoi PN, Yoon HJ, Joo YE,
Chay KO, Kim KK and Do Jung Y: Chrysin inhibits tumor
promoter-induced MMP-9 expression by blocking AP-1 via suppression
of ERK and JNK pathways in gastric cancer cells. PLoS One.
10:e01240072015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sawicka D, Car H, Borawska MH and
Niklinski J: The anticancer activity of propolis. Folia Histochem
Cytobiol. 50:25–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schumacher M, Hautzinger A, Rossmann A,
Holzhauser S, Popovic D, Hertrampf A, Kuntz S, Boll M and Wenzel U:
Chrysin blocks topotecan-induced apoptosis in Caco-2 cells in spite
of inhibition of ABC-transporters. Biochem Pharmacol. 80:471–479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gyémánt N, Tanaka M, Antus S, Hohmann J,
Csuka O, Mándoky L and Molnár J: In vitro search for synergy
between flavonoids and epirubicin on multidrug-resistant cancer
cells. In vivo. 19:367–374. 2005.PubMed/NCBI
|
|
14
|
Hasan MM, Islam MS, Hoque KMF, Haque A and
Reza MA: Effect of Citrus macroptera fruit pulp juice on alteration
of caspase pathway rendering anti-proliferative activity against
Ehrlich's ascites carcinoma in mice. Toxicol Res. 35:271–277. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ittiudomrak T, Puthong S, Roytrakul S and
Chanchao C: α-Mangostin and apigenin induced cell cycle arrest and
programmed cell death in SKOV-3 ovarian cancer cells. Toxicol Res.
35:167–179. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung J, Song DY, Hwang JJ, Park HJ, Lee
JS, Song SY, Jeong SY and Choi EK: Induction of p53-mediated
senescence is essential for the eventual anticancer therapeutic
effect of RH1. Arch Pharm Res. 42:815–823. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim HK, Kim KM, Jeong SY, Choi EK and Jung
J: Chrysin increases the therapeutic efficacy of docetaxel and
mitigates docetaxel-induced edema. Integr Cancer Ther. 16:496–504.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Huang JM, Wang JN, Xiong XK, Yang XF
and Zou F: Combination of chrysin and cisplatin promotes the
apoptosis of Hep G2 cells by up-regulating p53. Chem Biol Interact.
232:12–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rasouli S and Zarghami N: Synergistic
growth inhibitory effects of chrysin and metformin combination on
breast cancer cells through hTERT and cyclin D1 suppression. Asian
Pac J Cancer Prev. 19:977–982. 2018.PubMed/NCBI
|
|
21
|
Tao J, Zhi X, Tian Y, Li Z, Zhu Y, Wang W,
Xie K, Tang J, Zhang X, Wang L and Xu Z: CEP55 contributes to human
gastric carcinoma by regulating cell proliferation. Tumour Biol.
35:4389–4399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuoka T and Yashiro M: The role of
PI3K/Akt/mTOR signaling in gastric carcinoma. Cancers (Basel).
6:1441–1463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khoo BY, Chua SL and Balaram P: Apoptotic
effects of chrysin in human cancer cell lines. Int J Mol Sci.
11:2188–2199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agarwal ML, Agarwal A, Taylor WR, Chernova
O, Sharma Y and Stark GR: A p53-dependent S-phase checkpoint helps
to protect cells from DNA damage in response to starvation for
pyrimidine nucleotides. Proc Natl Acad Sci USA. 95:14775–14780.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim EJ, Kang GJ, Kang JI, Boo HJ, Hyun JW,
Koh YS, Chang WY, Kim YR, Kwon JM, Maeng YH, et al: Over-activation
of AKT signaling leading to 5-Fluorouracil resistance in
SNU-C5/5-FU cells. Oncotarget. 9:19911–19928. 2018. View Article : Google Scholar : PubMed/NCBI
|