Introduction
Pancreatic cancer is the fourth leading cause of
cancer-associated death in Japan according to Japan's National
Cancer Center (1). Most patients
present with locally advanced disease or systemic metastasis at
diagnosis, at which point only 15–20% of tumors are resectable
(2). Furthermore, pancreatic cancer
has a high relapse rate after radical surgery (relapse-free
survival rate, 6.7–13.4 months; five-year survival rate,
10.4–20.7%) and is often resistant to conventional chemotherapy and
radiation therapy (3,4). There is ongoing research regarding
effective adjuvant chemotherapy and new immunotherapies (5), although new therapeutic targets are
still needed. Based on the high rates of local recurrence and
distant metastasis, the lack of response to conventional treatment
may be associated with the presence of cancer stem-like cells
(CSLCs) (6–9); however, further research is needed
regarding the biological properties of CSLCs and how these may be
therapeutically targeted.
The tumorigenic subpopulation of pancreatic cancer
cells is reported to have high expression of CD44, CD24 and
epithelial-specific antigen (10).
Furthermore, pancreatic (P-)CSLCs are characterized by increased
expression of aldehyde dehydrogenase 1 (11), doublecortin-like kinase 1 (12), CD133 (13), c-Met (14) and CD44 mutant isoforms (CD44v)
(15). Nevertheless, few studies
have examined this population of cells, and it would be useful to
identify new biomarkers for P-CSLCs. Our previous study developed a
method for generating a P-CSLC-enriched population of cells with
high CD44 and CD24 expression using pancreatic cancer cell lines
(16), therefore this method maybe
useful for identifying P-CSCL biomarkers.
Cathepsin B (CTSB) is a type of lysosomal cysteine
protease (17,18) that is synthesized as a 339-amino acid
preproenzyme with a 17 amino acid signal peptide on the rough
endoplasmic reticulum and is associated with general protein
turnover in lysosomes (19,20). This protein is subject to multiple
levels of regulation and may be involved in generally cancer
progression (21). The SP1, SP3 and
ETS1 proteins activate CTSB transcription (22), with SP1 and SP3 proteins being highly
expressed generally in cancer cells and tissues (23), and ETS1 is associated with cancer
invasion (24,25). Therefore, the present study evaluated
whether CTSB expression was upregulated in P-CSLCs and whether this
was associated with patients' postoperative outcomes.
Materials and methods
Patients and tissue samples
The present study involved in vitro
experiments using human pancreatic cancer cell lines, as well as a
retrospective review of specimens from Japanese patients who
underwent surgery for invasive ductal carcinoma. The patients were
diagnosed according to the Japan Pancreas Society classification
(26) and underwent radical
resection with D2 or higher lymph node dissection between June 2001
and June 2013 at Yamaguchi University Hospital (Yamaguchi, Japan),
and between March 2008 and October 2012 at Osaka University
Hospital (Osaka, Japan). Written informed consent was obtained from
participant at each institution. Treatment with gemcitabine alone,
gemcitabine plus radiation, gemcitabine/S-1 plus radiation or
gemcitabine plus heavy ion radiation was administered if patients
received preoperative therapy. Treatment with gemcitabine alone,
S-1 alone, gemcitabine plus immune cell therapy or immune cell
therapy alone was administered if patients received postoperative
therapy. Patients were excluded if they died from surgery-related
causes or if they had other cancer types, serous and mucinous
cystic pancreas neoplasms, pancreatic cancer derived from
intraductal papillary-mucinous neoplasms, were pathologically
cancer positive or had indeterminate surgical margins. Resected
specimens without residual cancer were not considered. In total, 77
patients from Yamaguchi University Hospital and 64 patients from
Osaka University Hospital were evaluated, although only 69 patients
were considered eligible. The patients' medical records were
reviewed to obtain their clinicopathological characteristics as
listed in Table I, and the study
protocol was approved by the Institutional Review Boards of
Yamaguchi University Hospital (Yamaguchi, Japan) and Osaka
University Hospital (Osaka, Japan). Tumor-Node-Metastasis (TNM)
staging was performed according to the Union for International
Cancer Control criteria (27).
 | Table I.Association between 30 cases of low
cathepsin B expression and 39 cases of high expression and clinical
features of patients with pancreatic cancer. |
Table I.
Association between 30 cases of low
cathepsin B expression and 39 cases of high expression and clinical
features of patients with pancreatic cancer.
|
| Cathepsin B
expression |
|
|---|
|
|
|
|
|---|
| Clinical
feature | Low | High | P-value |
|---|
| Age, years |
|
| 0.366 |
|
<60 | 8 | 6 |
|
|
≥60 | 22 | 33 |
|
| Sex |
|
|
|
|
Male | 8 | 19 | 0.083 |
|
Female | 22 | 20 |
|
| Tumor location |
|
| 0.008 |
|
Pancreatic head | 15 | 32 |
|
|
Pancreatic body and tail | 15 | 7 |
|
| Tumor size, mm |
|
| 0.045 |
|
<30 | 23 | 20 |
|
|
≥30 | 28 | 35 |
|
|
Differentiation |
|
| 0.690 |
|
Well | 2 | 4 |
|
|
Moderate-poor | 28 | 35 |
|
| Invasion depth |
|
| 0.002 |
|
T1+T2 | 9 | 1 |
|
| T3 | 21 | 38 |
|
| Lymph node
metastasis |
|
| 0.016 |
|
Negative | 19 | 13 |
|
|
Positive | 11 | 26 |
|
| TNM stage |
|
| 0.038 |
| I | 6 | 1 |
|
| II | 24 | 38 |
|
| Perineural
invasion |
|
| 0.488 |
|
Negative | 5 | 4 |
|
|
Positive | 25 | 35 |
|
| Portal
invasion |
|
| 0.803 |
|
Negative | 19 | 26 |
|
|
Positive | 11 | 13 |
|
| Preoperative
therapy† |
|
| 0.058 |
|
None | 18 | 32 |
|
|
Performed | 12 | 7 |
|
| Postoperative
therapy‡ |
|
| 0.532 |
|
None | 4 | 8 |
|
|
Performed | 26 | 31 |
|
Cell lines and culture conditions
The human pancreatic cancer cell line YPK2 has been
established at Yamaguchi University School of Medicine (28). A common pancreatic cancer cell line,
PANC-1, was purchased from the American Type Culture Collection.
The cells were maintained at 37°C and 5% CO2 in DMEM-F12
(Sigma-Aldrich; Merck KGaA) supplemented with 10% heat-inactivated
fetal bovine serum (FBS) (Thermo Fisher Scientific, Inc.).
Generation of P-CSLCs
The P-CSLC-enriched populations were generated from
YPK2 and PANC-1 cells as previously described (16). The cells were initially cultured in
serum-free medium containing leukemia inhibitory factor (Merck
KGaA), neural survival factor-1 (Lonza Group, Ltd.) and
N-acetyl-L-cysteine (Sigma-Aldrich; Merck KGaA) to induce tumor
sphere formation. The spheres were collected and transferred to
laminin-coated dishes with culture medium containing B27 supplement
(Thermo Fisher Scientific, Inc.), human recombinant epidermal
growth factor (Sigma-Aldrich; Merck KGaA) and basic fibroblast
growth factor (Merck KGaA). One-half of the culture medium was
changed every week. The resultant cells were designated YPK2-Lm and
PANC-1-Lm.
Two-dimensional (2D) electrophoresis
and matrix-assisted laser desorption/ionization time of flight mass
spectrometry and tandem mass spectrometry (MALDI TOF/TOF MS)
Dead cells were eliminated from the cultures by
labeling with Dead Cell Removal MicroBeads (Miltenyi Biotech GmbH)
and separated using an LS column with a MidiMACS Separator
(Miltenyi Biotech GmbH). CD44v9-positive cells were selected using
rat anti-CD44v9 IgG (1:50; clone RV3; cat. no. LKG-M003; Cosmo Bio
Co., Ltd.), mouse biotin-conjugated anti-rat IgG (1:2,000; cat. no.
13-4813-85; eBioscience; Thermo Fisher Scientific, Inc.) and
microbeads carrying mouse anti-biotin IgG (ready to use; cat. no.
130-090-485; Miltenyi Biotech GmbH), with isolation performed using
the MidiMACS Separator according to the manufacturer's
instructions.
Each sample was suspended in 0.2% pharmalyte and
homogenized in lysis buffer containing 5 M urea, 2 M thiourea, 2%
(w/v) CHAPS, 2% (w/v) SB3-10 and 1% (w/v) DTT (all reagents from
Sigma-Aldrich; Merck KGaA). Protein concentrations were measured
using a protein assay kit (Bio-Rad Laboratories, Inc.). The samples
were subjected to 2D electrophoresis as previously described
(29,30). Briefly, the samples were applied to
18-cm Immobiline DryStrips (pH 3.0–10.0; GE Healthcare) and then
subjected to isoelectric focusing using CoolPhoreStar IPG-IEF
Type-P (Anatech-Analytical Technology). The DryStrips were then
subjected to 2D gradient electrophoresis (9–18% acrylamide;
FUJIFILM Wako Pure) using an ANDERSON ISO-DALT Multiple
Electrophoresis system (Hoefer Inc.). After staining with SYPRO
Ruby stain (S21900; Thermo Fisher Scientific, Inc.), protein spots
were detected using a Molecular Imager FX (Bio-Rad Laboratories,
Inc.) and analyzed using ImageMaster 2D Platinum software version
5.0 (GE Healthcare). Common protein spots with higher intensities
in the YPK2-Lm cells (vs. the respective parental cells) were
excised and subjected to MALDI TOF/TOF MS analysis.
The excised samples were de-stained, washed and
dehydrated with acetonitrile. The gels were rehydrated in a
digestion solution containing 50 mM NH4HCO3,
5 mM CaCl2 and 0.01 µg/µl trypsin (Promega Corporation),
and the digestion was then terminated using 5% TFA. Peptides were
extracted using 5% TFA in 50% acetonitrile. The samples were
absorbed to ZipTip C18 pipette tips (Merck KGaA) and the peptides
were eluted using 0.1% TFA in 50% acetonitrile. An aliquot of the
eluted sample was mixed with an equal volume of matrix solution
(0.3 g/l alpha-cyano-4-hydroxycinnamic acid, 33% acetone and 66%
ethanol; all from Wako Chemicals GmbH), placed onto a target plate
(MTP Anchorchip 600/384; Bruker Corporation), dehydrated using MTP
Anchorchip and analyzed using a mass spectrometer (Ultraflex
TOF/TOF; Bruker Corporation) in the positive ion reflector mode
(20–4,000 m/z). The MS/MS spectra were searched against the NCBI NR
database within the Mascot database search engine (Matrix Science,
Ltd.).
Western blotting
Cells were lysed in a buffer containing 50 mM
Tris-HCl (pH 8.0), 5 mM EDTA, 5 mM EGTA, 0.2% SDS, 0.5% Nonidet
P-40, 1 mM Na3VO4, 20 mM sodium pyrophosphate
and Roche complete protease inhibitor mixture. The protein amount
was quantified by the Lowry method using DC Protein Assay (Bio-Rad
Laboratories, Inc.). The proteins (10 µg/lane) were loaded
onto a 8% gel, resolved using SDS-PAGE and transferred onto a PVDF
membrane (Bio-Rad Laboratories, Inc.). Membranes were blocked with
3% skimmed milk at room temperature for 1 h and treated with
anti-CTSB (cat. no. ab58802; Abcam) and anti-valosin-containing
protein (VCP; cat. no. GTX113030; GeneTex, Inc.) primary antibodies
at room temperature for 1 h, before the immunoreactive bands were
visualized using an ECL Pro kit (PerkinElmer, Inc.) and Amersham
Imager (GE Healthcare), with quantification using ImageJ software
version 1.5.1 (National Institutes of Health). VCP was used as the
loading control, because the protein levels of VCP is more stable
compared with used loading controls such as GAPDH and actin
(31–33). Moreover, the size of VCP (97 kDa) is
useful as a loading control for most of the proteins analyzed in
this study were <75 kDa.
Flow cytometry
Expression of CTSB on the surface of YPK2 and
YPK2-Lm cells was analyzed using flow cytometry. Dissociated cells
were suspended in PBS with 2% FBS (106 cells/100 µl) and
then incubated with each antibody at 4°C for 30 min. The antibodies
for this assay were rat anti-human CD44v9 (1:50; clone RV3; cat.
no. LKG-M003; Cosmo Bio Co., Ltd.), anti-calreticulin (CALR; 1:50;
cat. no. ab196159; Abcam) and anti-CTSB (1:20; cat. no. ab58802;
Abcam). The mouse FITC-conjugated anti-rat IgG2a secondary antibody
(1:10; cat. no. 11-4811-85; eBioscience; Thermo Fisher Scientific,
Inc.) was used for the anti CD44v9 primary antibody. The anti-CTSB
and cognate isotype control antibodies were conjugated with APC
using an APC conjugation kit (cat. no. ab201807; Abcam) according
to the manufacturer's instructions. Rat IgG2a κ Isotype Control
(cat. no. 14-4321-82; eBioscience; Thermo Fisher Scientific, Inc.),
rabbit IgG Isotype Control Alexa Fluor 647 (cat. no. ab199093;
Abcam) and mouse IgG2a κ monoclonal Isotype Control (cat. no.
ab18415; Abcam) were used as negative controls for anti-CD44v9,
anti-CRT Alexa Fluor 647 and anti-CTSB with corresponding dilution
factors. Flow cytometry analysis was performed using a FACS
ARIA-III (BD Biosciences) and a MACSQuant analyzer version 2.4
(Miltenyi Biotec GmbH).
ELISA
To quantify CTSB secretion, conditioned medium was
collected and analyzed using a human cathepsin B ELISA kit (cat.
no. ab119584; Abcam) according to the manufacturer's instructions.
Signals were detected using an EnVision plate reader (PerkinElmer,
Inc.).
Immunohistochemistry
Immunohistochemical staining for CTSB was performed
using formalin-fixed paraffin-embedded surgical specimens (fixed in
10% formalin at room temperature overnight). Antigens in the
specimens of 4-µm thickness were retrieved by heating the
tissues for 20 min at 95°C in 10 mM Target Retrieval Solution, pH
6.0 (Dako; Agilent Technologies, Inc.) and then boiling for 10 min
at 105°C. Endogenous peroxidase activity was blocked using 3%
H2O2 in methanol for 5 min at room
temperature, and non-specific binding was blocked using Protein
Block serum-free (Dako; Agilent Technologies, Inc.) for 10 min at
room temperature. The slides were incubated with the mouse
monoclonal anti-CTSB antibodies (1:3,000; cat. no. ab58802; Abcam)
for 1 h at room temperature and followed by the rat anti-mouse
antibodies (undiluted solution; cat. no. K4001; Dako; Agilent
Technologies, Inc.) for 30 min at room temperature. Tissue sections
were incubated with 3,3′-diaminobenzidine tetrahydrochloride (Dako;
Agilent Technologies, Inc.), and then counter-stained using
hematoxylin for 2 min at room temperature.
Intensity of CTSB staining in the infiltrating
pancreatic cancer specimen was classified as either negative or
positive following examination of the positive area by a
pathologist (AO) who was blinded to the patients' characteristics.
High CTSB was defined as positive staining that occupied >30% of
>5 fields of view at 100-fold magnification using a
phase-contrast microscope (BZ-X700; KEYENCE). Low CTSB was defined
as both negative and positive staining that occupied <30% of the
fields of view. Moderate staining was defined as a result between
high and low staining based on the criteria using only occupation
of positive cells as aforementioned. The brightness/contrast
adjustment was applied to the whole image.
Statistical analysis
Each experiment was repeated at least three times.
Data are expressed as mean ± standard deviation. For the
statistical analyses, patients with high and low CTSB expression
were compared. The patients' clinicopathological characteristics
and clinical outcomes were compared using the Fisher's exact test.
Survival outcomes were analyzed using the Kaplan-Meier method and
log-rank test. Significant differences between two or three groups
were evaluated by Welch's t-test or ANOVA with Scheffe's test,
respectively. All analyses were performed using R version 3.4.0
software (http://www.r-project.org/). P<0.05
was considered to indicate a statistically significant
difference.
Results
Identification and expression of
CTSB
The protein expressions from YPK2-Lm and YPK5-Lm
cells (generated P-CSLCs) and parental YPK2 and YPK5 cells were
compared using proteome analysis using MALDI TOF/TOF MS data from
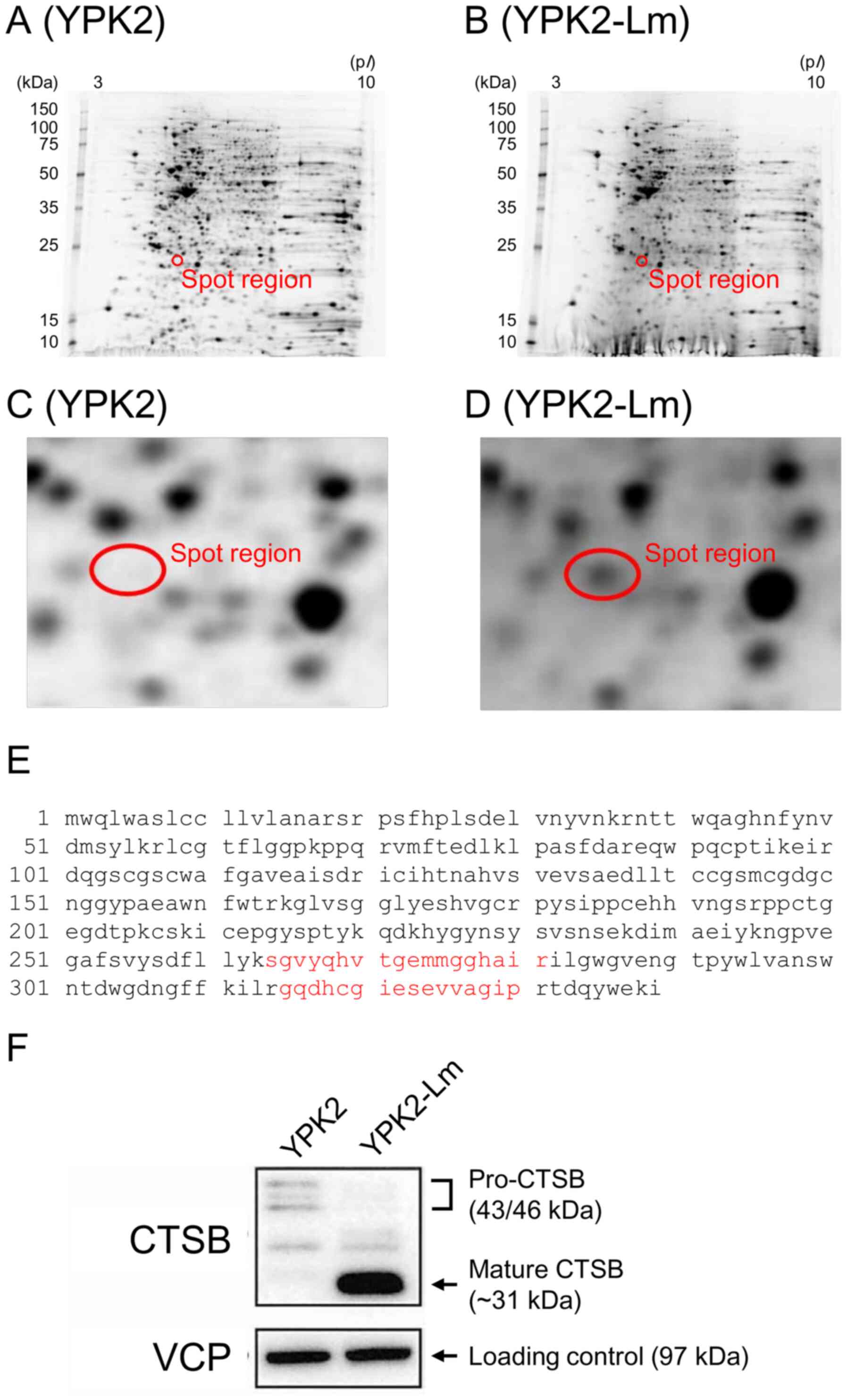
previously performed 2D gel electrophoresis (30). The 2D gel electrophoresis revealed a
10-fold stronger band in YPK2-Lm compared with YPK2 cells (Fig. 1C and D), and the MALDI TOF/TOF MS
assay identified CTSB (Fig. 1E).
Western blotting confirmed that CTSB expression was elevated in
YPK2-Lm cells compared with parental cells (Fig. 1F). Corresponding spots identified as
CTSB were also obtained in the gels from YPK5 derivatives (data not
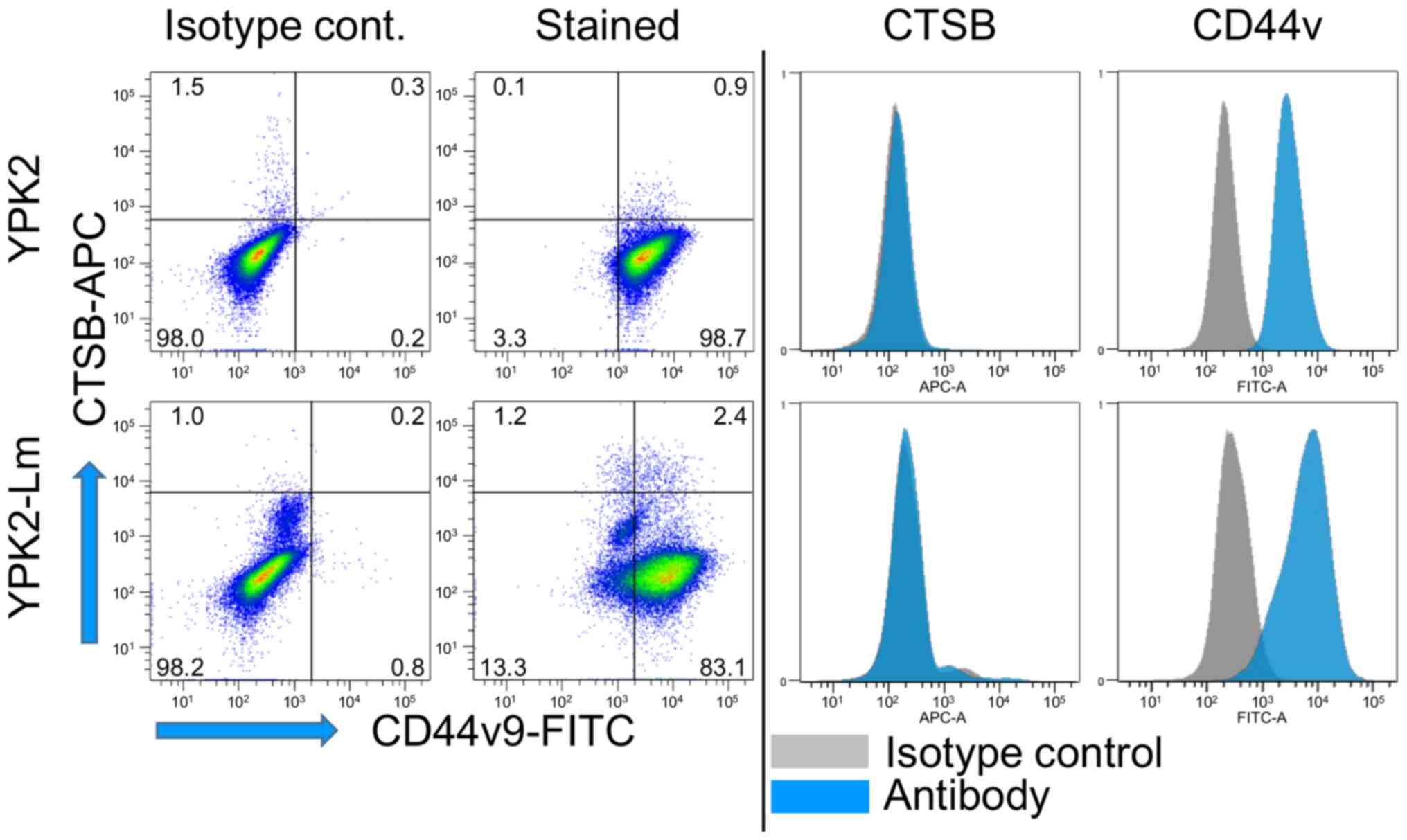
shown). Protein expression on the surface of YPK2 and YPK2-Lm cells
was evaluated using flow cytometry, and the results confirmed a
slight elevated expression of CTSB on the surface of YPK2-Lm cells
(Fig. 2). Slightly elevated
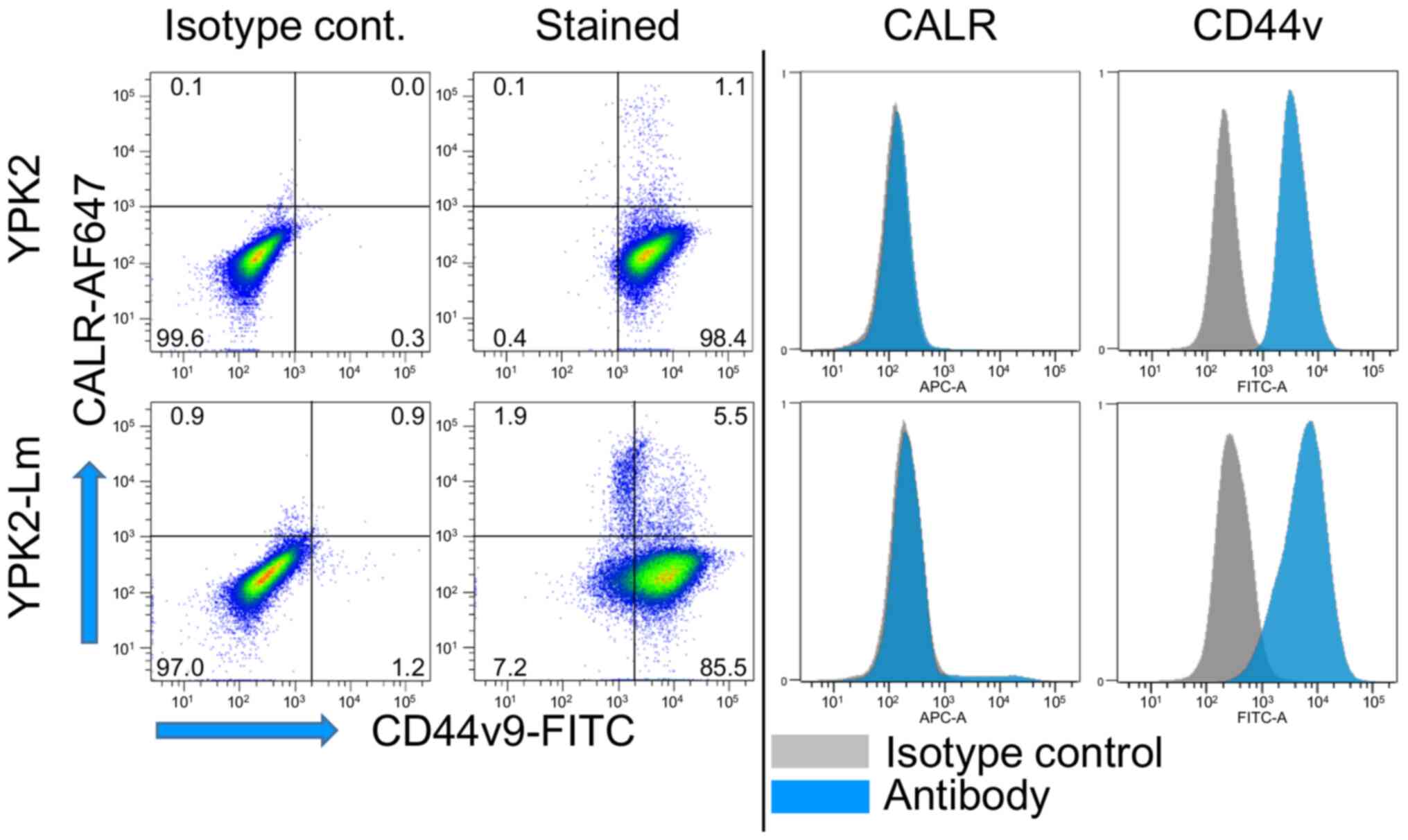
expressions of CD44v9 and CALR were observed on the YPK2-Lm cell
(Fig. 3) confirmed that P-CSLCs had
been generated as described in a previous study (30). Elevated CTSB and CD44v9 expression
was also seen in PANC-1-Lm cells compared with those in parental
PANC-1 cells (Figs. S1 and S2).
Association between CTSB expression
and clinical outcomes
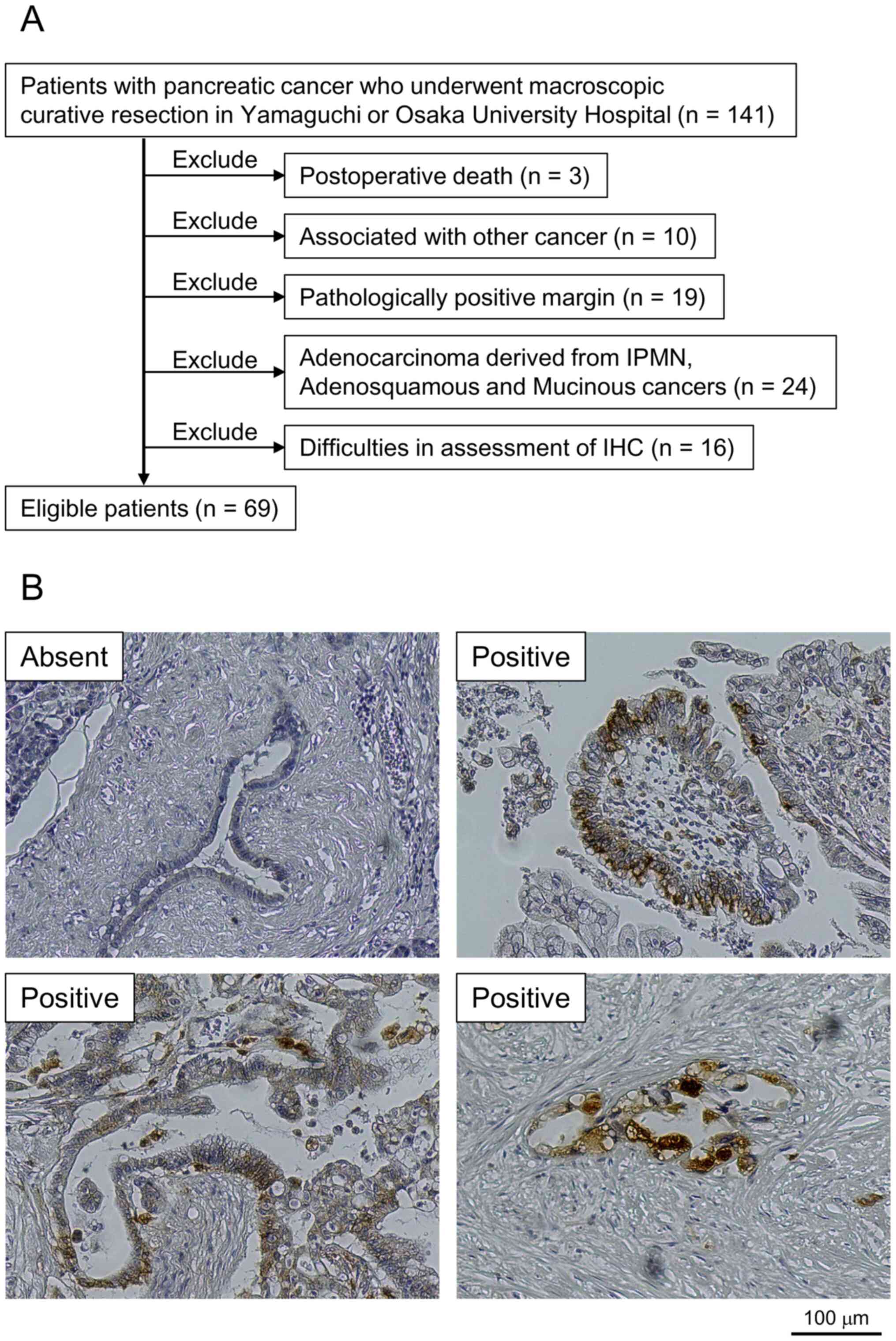
The patient selection process is shown in Fig. 4A, and representative images of CTSB
expression in the tumor specimens are shown in Fig. 4B. While normal tissues exhibited
minimal CTSB staining, CTSB expression was predominantly detected
in the cytoplasm of cancerous tissues, although some cases also
exhibited nucleus or membranous staining (Fig. 4B). In addition to those staining
location, there was broad variation, ranging from diffuse staining
to staining involving granulocytes or even the Golgi area.
Comparison of the clinical outcomes between the groups with low and
high CTSB expression revealed that high expression was
significantly associated with more advanced disease parameters,
including tumor size (P=0.045), invasion depth (P=0.002), lymph
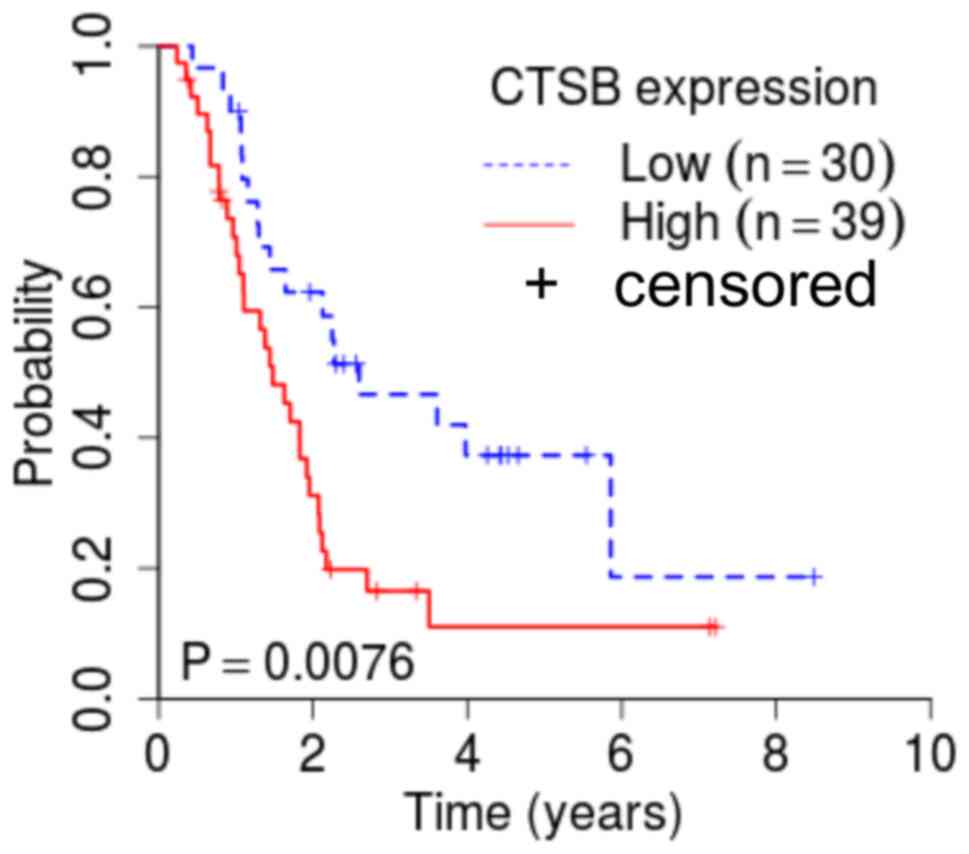
node metastasis (P=0.016) and TNM stage (P=0.038) (Table I). Furthermore, the high expression
group had significantly more cases with pancreatic head tumors, as
well as a lower overall survival rate, compared with those with low
CTSB expression (Fig. 5).
Secretion of CTSB into YPK2 growth
medium
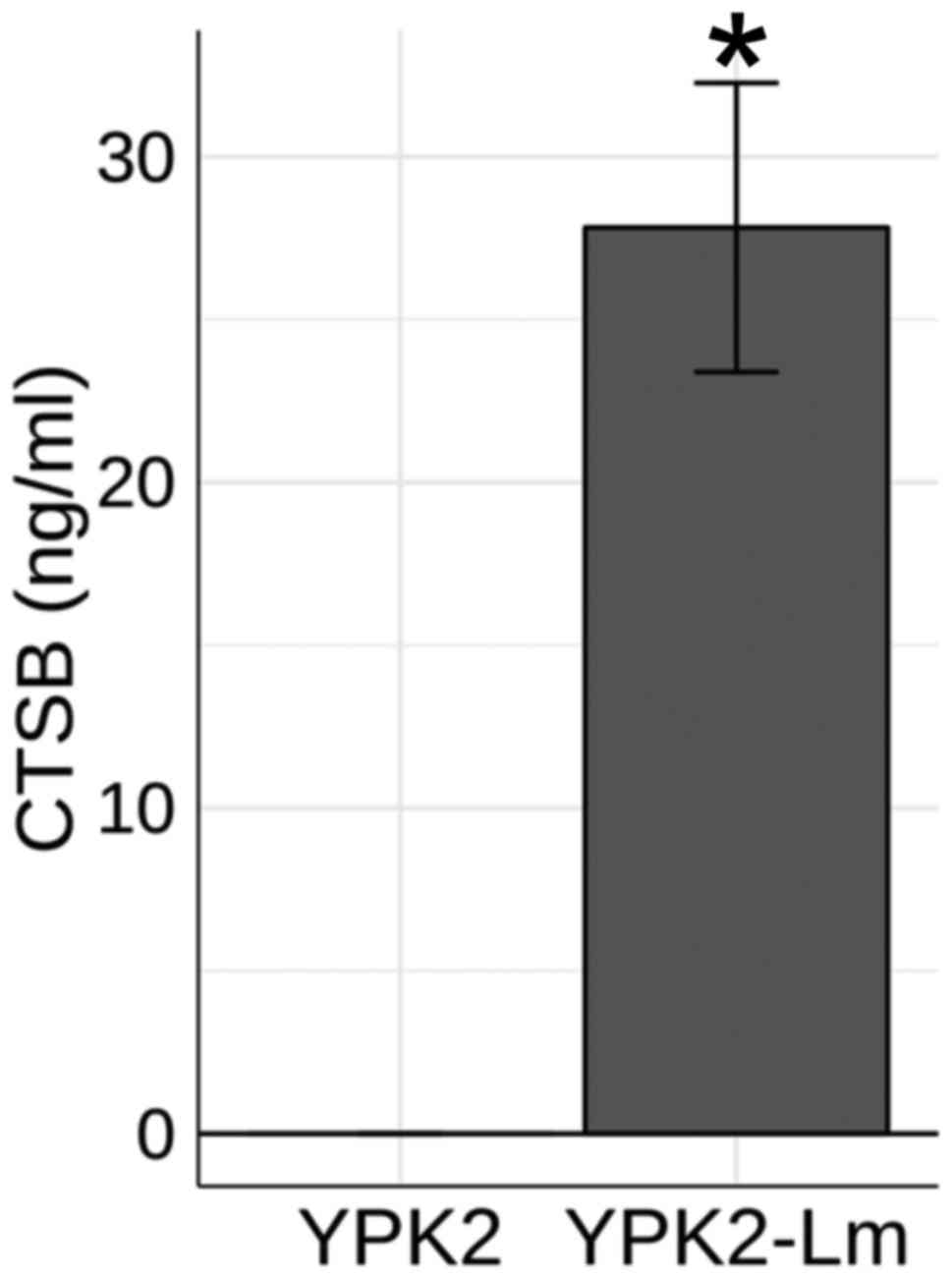
ELISA was used to evaluate the cellular secretions
of CTSB by YPK2 and YPK2-Lm cells. The results revealed
significantly elevated concentrations of CTSB in the medium for
YPK2-Lm cells compared with YPK2 cells (P<0.05; Fig. 6). The same result was obtained with
for PANC-1-Lm compared with PANC-1 cells (P<0.05; Fig. S3).
Discussion
The function of CTSB is dynamic and poorly
understood; however, it is hypothesized that CTSB encoding
lysosomal proteinase plays a role in not only in normal
physiological conditions but also in pathological conditions, such
as inflammation, infection and neurodegenerative disease, and the
malignant progression of several cancer types including gastric,
colorectal, breast and pancreatic cancers (34–38). It
remains unclear whether CTSB is expressed in tumor cells,
tumor-related cells, or both, and whether CTSB expression affects
or is affected by interactions with cellular and/or non-cellular
microenvironments (21). A previous
study have previously described an association between CSC and CTSB
expression (39).
The present study generated P-CSLCs using our
previously reported method (16,30), in
which YPK2-Lm cells showed not only a much higher population of
CD44v compared with parental YPK2 cells, but also much higher
side-population and higher ALDH activity. In addition, the
enhancement of tumorigenicity and epithelial-mesenchymal transition
was confirmed by both mouse subcutaneous injection and quantitative
PCR analyses. Protein analyses revealed that CTSB was more highly
expressed in P-CSLCs compared with in the parent pancreatic cancer
cell lines. Western blot analysis also confirmed that CTSB was
highly expressed in the P-CSLCs, while flow cytometry revealed
elevated CTSB expression on the surface of P-CSLCs. CD24, CD44,
CD44v and CALR markers expressed on the cell surface are useful for
the identification of pancreatic cancer stem cells (30). The present results suggested that
CTSB cell surface expression and total protein levels could be used
as prognostic biomarkers. The current study examined tumor
specimens with high CTSB staining intensity, and observed that most
of these cases exhibited strong cytoplasmic staining, although some
cases also exhibited nucleus or membranous staining. There was
broad variation in the staining location for these combined cases,
ranging from diffuse staining to staining involving granulocytes or
even the Golgi area. Moreover, high CTSB expression in resected
specimens was significantly associated with advanced disease
parameters (larger tumor size, deeper invasion depth, positive for
lymph node metastasis and higher TNM stage) and lower overall
survival rate, which is consistent with previously reported results
(40). These results support the
hypothesis that CTSB is associated with pancreatic cancer
progression. It has been reported that CTSB contributes generally
to tumor growth, migration, invasion and angiogenesis (38). Notably, the observation of both
expressions of CTSB and its inhibitors in tumor tissues and
tumor-infiltrating immune cells had suggested that those may be
involved in pancreatic ductal adenocarcinoma-related inflammation
(41).
Given the diffuse CTSB staining observed in the
pancreatic cancer tissues as aforementioned, the secretion of CTSB
was examined in vitro. Higher CTSB concentrations in the
culture medium for P-CSLCs compared with the medium for the
parental cells was observed, which suggested that CTSB is expressed
on the cell surface of P-CSLCs and is also secreted by these cells.
Thus, CTSB in P-CSLCs may directly or indirectly influence the
extracellular microenvironment and potentially contribute to the
progression of malignancy. These results were further confirmed
using P-CSLCs generated from two pancreatic cancer cell lines
(PANC-1 and YPK2), which suggests that the present findings were
not cell line-specific.
It should be highlighted that the present results do
not clarify the functional role of CTSB in P-CSLCs, although CTSB
in various locations (surface, intracellular and secretions) has
been widely reported to be involved in the progression of various
cancer types (40–49) including glioblastoma, malignant
meningioma, pancreatic, breast, esophageal and prostate cancers.
Notably, the subcellular localization of various cancer markers is
generally limited to a single compartment, although their
localization varies according to malignancy in some high-grade
carcinomas. For example, cytokeratin is a common marker for solid
tumor cells and is often expressed in the cell membrane in
well-differentiated adenocarcinoma, but is expressed in the
membrane and Golgi in high-grade poorly differentiated
adenocarcinoma (50). Thus, multiple
localizations for cancer-associated proteins may indicate a
high-grade tumor. Similar to cytokeratin (50), CTSB is also a cancer-associated
protein with multiple cellular localizations as shown in the
present study, and the association between its localization and
malignancy is not clear, although the present findings suggested
that CTSB in cancer stem cells may be involved in the degree of
malignancy.
The present study revealed that CTSB expression was
elevated in P-CSLCs generated from common pancreatic cancer cell
lines, and that its expression in resected tumor specimens was
associated with poor postoperative outcomes. While it is unclear
whether this protein is a useful therapeutic target, it might be a
useful prognostic marker that is associated with pancreatic cancer
stem cells, especially if it is present in multiple subcellular
locations. Further studies with gene-targeting experiments such as
genome-editing and in vivo murine experiments are needed to
clarify the prognostic implications of CTSB expression in this
setting, as well as whether it is a potential therapeutic
target.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Akiko Sano, Ms.
Kaori Kaneyasu and Ms. Hiroko Takenouchi for their technical
assistance (Department of Gastroenterological, Breast and Endocrine
Surgery, Yamaguchi University Graduate School of Medicine).
Funding
The study was funded by JSPS KAKENHI Grants (grant
nos. 24390317, 17H06903, 18K16365, and 18K08646).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TF, SM, KY and HN conceived and designed the present
study. TF, SM, AO, NF and YF performed the experiments. Statistical
analysis was performed by TF and RT. TF, RT and HN wrote the
manuscript. RT, KY, HM, YS, YT, NS, SK, SH and HE interpreted the
data and edited the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Boards of Yamaguchi University Hospital (protocol number,
H27-007) and Osaka University Hospital (protocol number, 15208).
All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CTSB
|
cathepsin B
|
|
CD44v
|
variant isoforms of CD44
|
|
CALR
|
calreticulin
|
|
CSC
|
cancer stem cell
|
|
CSLC
|
cancer stem-like cell
|
|
P-CSLC
|
pancreatic cancer stem-like cell
|
References
|
1
|
The Editorial Board of the Cancer
Statistics in Japan, . Cancer statistics in Japan 2018. Foundation
for Promotion of Cancer Research; Tokyo: pp. 152019
|
|
2
|
Ryan DP, Hong TS and Bardeesy N:
Pancreatic adenocarcinoma. N Engl J Med. 371:1039–1049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murakami T, Hiroshima Y, Matsuyama R,
Homma Y, Hoffman RM and Endo I: Role of the tumor microenvironment
in pancreatic cancer. Ann Gastroenterol Surg. 3:130–137. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oettle H, Neuhaus P, Hochhaus A, Hartmann
JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J,
Arning MB, et al: Adjuvant chemotherapy with gemcitabine and
long-term outcomes among patients with resected pancreatic cancer:
The CONKO-001 randomized trial. JAMA. 310:1473–1481. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Torphy RJ, Zhu Y and Schulick RD:
Immunotherapy for pancreatic cancer: Barriers and breakthroughs.
Ann Gastroenterol Surg. 2:274–281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clevers H: The cancer stem cell: Premises,
promises, and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Lewis MT, Huang J, Gutierrez C,
Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC,
et al: Intrinsic resistance of tumorigenic breast cancer cells to
chemotherapy. J Natl Cancer Inst. 100:672–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou BB, Zhang H, Damelin M, Geles KG,
Grindley JC and Dirks PB: Tumour-initiating cells: Challenges and
opportunities for anticancer drug discovery. Nat Rev Drug Discov.
8:806–823. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsunedomi R, Yoshimura K, Suzuki N, Hazama
S and Nagano H: Clinical implications of cancer stem cells in
digestive cancers: Acquisition of stemness and prognostic impact.
Surg Today. Feb 5–2020.(Epub ahead of print). doi:
10.1007/s00595-020-01968-x. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsay V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rasheed ZA, Yang J, Wang Q, Kowalski J,
Freed I, Murter C, Hong SM, Koorstra JB, Rajeshkumar NV, He X, et
al: Prognostic significance of tumorigenic cells with mesenchymal
features in pancreatic adenocarcinoma. J Natl Cancer Inst.
102:340–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sureban SM, May R, Qu D, Weygant N,
Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier RG
and Houchen CW: DCLK1 regulates pluripotency and angiogenic factors
via microRNA-dependent mechanisms in pancreatic cancer. PLoS One.
8:e739402013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C, Wu JJ, Hynes M, Dosch J, Sarkar B,
Welling TH, Pasca di Magliano M and Simeone DM: c-Met is a marker
of pancreatic cancer stem cells and therapeutic target.
Gastroenterology. 141:2218–2227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ishimoto T, Nagano O, Yae T, Tamada M,
Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, et al:
CD44 variant regulates redox status in cancer cells by stabilizing
the xCT subunit of system xc (−) and thereby promotes tumor growth.
Cancer Cell. 19:387–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Watanabe Y, Yoshimura K, Yoshikawa K,
Tsunedomi R, Shindo Y, Matsukuma S, Maeda N, Kanekiyo S, Suzuki N,
Kuramasu A, et al: A stem cell medium containing neural stimulating
factor induces a pancreatic cancer stem-like cell-enriched
population. Int J Oncol. 45:1857–1866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rawlings ND, Barrett AJ and Bateman A:
MEROPS: The database of proteolytic enzymes, their substrates and
inhibitors. Nucleic Acids Res. 40:D343–D350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Turk V, Stoka V, Vasiljeva O, Renko M, Sun
T, Turk B and Turk D: Cysteine cathepsins: From structure, function
and regulation to new frontiers. Biochim Biophys Acta. 1824:68–88.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kirschke H, Barrett AJ and Rawlings ND:
Proteinases 1: Lysosomal cysteine proteinases. Protein Profile.
2:1581–1643. 1995.PubMed/NCBI
|
|
20
|
Mort JS and Buttle DJ: Cathepsin B. Int J
Biochem Cell Biol. 29:715–720. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aggarwal N and Sloane BF: Cathepsin B:
Multiple roles in cancer. Proteomics Clin Appl. 8:427–437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan S, Berquin IM, Troen BR and Sloane BF:
Transcription of human cathepsin B is mediated by Sp1 and Ets
family factors in glioma. DNA Cell Biol. 79:79–91. 2000. View Article : Google Scholar
|
|
23
|
Li L and Davie JR: The role of Sp1 and Sp3
in normal and cancer cell biology. Ann Anat. 192:275–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dittmer J: The biology of the Ets1
proto-oncogene. Mol Cancer. 2:292003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buggy Y, Maguire TM, McGreal G, McDermott
E, Hill ADK, O'Higgins N and Duffy MJ: Overexpression of the Ets-1
transcription factor in human breast cancer. Br J Cancer.
91:1308–1315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Japan Pancreas Society: General rules for
the study of pancreatic cancer, The 6th Edition, Revised Version
edn. Tokyo, Japan: Kanehara; pp. 3–14. 2013
|
|
27
|
Union for International Cancer Control
(UICC): TNM Classification of Malignant Tumours, 7th edition. Sobin
LH, Gospodarowicz MK and Wittekind C: Wiley; New York, NY: 2010
|
|
28
|
Yamamoto K, Yahara N, Gondo T, Ishihara T
and Oka M: Establishment and characterization of a new human
pancreatic cancer cell line, YPK-1. Bull Yamaguchi Med Sch.
49:33–42. 2002.
|
|
29
|
Gerashchenko BI, Yamagata A, Oofusa K,
Yoshizato K, de Toledo SM and Howell RW: Proteome analysis of
proliferative response of bystander cells adjacent to cells exposed
to ionizing radiation. Proteomics. 7:2000–2008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsukuma S, Yoshimura K, Ueno T, Oga A,
Inoue M, Watanabe Y, Kuramasu A, Fuse M, Tsunedomi R, Nagaoka S, et
al: Calreticulin is highly expressed in pancreatic cancer stem-like
cells. Cancer Sci. 107:1599–1609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujiwara N, Usui T, Ohama T and Sato K:
Regulation of beclin 1 protein phosphorylation and autophagy by
protein phosphatase 2A (PP2A) and death-associated protein kinase 3
(DAPK3). J Biol Chem. 291:10858–10866. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Enjoji S, Yabe R, Tsuji S, Yoshimura K,
Kawasaki H, Sakurai M, Sakai Y, Takenouchi H, Yoshino S, Hazama S,
et al: Stemness is enhanced in gastric cancer by a SET/PP2A/E2F1
axis. Mol Cancer Res. 16:554–563. 2008. View Article : Google Scholar
|
|
33
|
Yabe R, Tsuji S, Mochida S, Ikehara T,
Usui T, Ohama T and Sato K: A stable association with PME-1 may be
dispensable for PP2A demethylation-implications for the detection
of PP2A methylation and immunoprecipitation. FEBS Open Bio.
8:1486–1496. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ebert MP, Krüger S, Fogeron ML, Lamer S,
Chen J, Pross M, Schulz HU, Lage H, Heim S, Roessner A, et al:
Overexpression of cathepsin B in gastric cancer identified by
proteome analysis. Proteomics. 5:1693–1704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McKerrow JH, Bhargava V, Hansell E, Huling
S, Kuwahara T, Matley M, Coussens L and Warren R: A functional
proteomics screen of proteases in colorectal carcinoma. Mol Med.
6:450–460. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Srisomsap C, Subhasitanont P, Otto A,
Mueller EC, Punyarit P, Wittmann-Liebold B and Svasti J: Detection
of cathepsin B up-regulation in neoplastic thyroid tissues by
proteomic analysis. Proteomics. 2:706–712. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wulfkuhle JD, Sgroi DC, Krutzsch H, McLean
K, McGarvey K, Knowlton M, Chen S, Shu H, Sahin A, Kurek R, et al:
Proteomics of human breast ductal carcinoma in situ. Cancer Res.
62:6740–6749. 2002.PubMed/NCBI
|
|
38
|
Mijanović O, Branković A, Panin AN,
Savchuk S, Timashev P, Ulasov I and Lesniak MS: Cathepsin B: A
sellsword of cancer progression. Cancer Lett. 449:207–214. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang KD, Liu J, Jovanovic L, An J, Hill
MM, Vela I, Lee TK, Ma S, Nelson C, Russell PJ, et al: Adipocytes
promote prostate cancer stem cell self-renewal through
amplification of the cholecystokinin autocrine loop. Oncotarget.
7:4939–4948. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Niedergethmann M, Wostbrock B, Sturm JW,
Willeke F, Post S and Hildenbrand R: Prognostic impact of cysteine
proteases cathepsin B and cathepsin L in pancreatic adenocarcinoma.
Pancreas. 29:204–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Komura T, Takabatake H, Harada K, Yamato
M, Miyazawa M, Yoshida K, Honda M, Wada T, Kitagawa H, Ohta T, et
al: Clinical features of cystatin A expression in patients with
pancreatic ductal adenocarcinoma. Cancer Sci. 108:2122–2129. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Krueger S, Haeckel C, Buehling F and
Roessner A: Inhibitory effects of antisense cathepsin B cDNA
transfection on invasion and motility in a human osteosarcoma cell
line. Cancer Res. 59:6010–6014. 1999.PubMed/NCBI
|
|
43
|
Shimizu A, Nakayama H, Wang P, König C,
Akino T, Sandlund J, Coma S, Italiano JE Jr, Mammoto A, Bielenberg
DR and Klagsbrun M: Netrin-1 promotes glioblastoma cell
invasiveness and angiogenesis by multiple pathways including
activation of RhoA, cathepsin B, and cAMP-response element-binding
protein. J Biol Chem. 288:2210–2222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Withana NP, Blum G, Sameni M, Slaney C,
Anbalagan A, Olive MB, Bidwell BN, Edgington L, Wang L, Moin K, et
al: Cathepsin B inhibition limits bone metastasis in breast cancer.
Cancer Res. 72:1199–1209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tummalapalli P, Spomar D, Gondi CS,
Olivero WC, Gujrati M, Dinh DH and Rao JS: RNAi-mediated abrogation
of cathepsin B and MMP-9 gene expression in a malignant meningioma
cell line leads to decreased tumor growth, invasion and
angiogenesis. Int J Oncol. 31:1039–1050. 2007.PubMed/NCBI
|
|
46
|
Gocheva V, Zeng W, Ke D, Klimstra D,
Reinheckel T, Peters C, Hanahan D and Joyce JA: Distinct roles for
cysteine cathepsin genes in multistage tumorigenesis. Genes Dev.
20:543–556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Andl CD, McCowan KM, Allison GL and Rustgi
AK: Cathepsin B is the driving force of esophageal cell invasion in
a fibroblast-dependent manner. Neoplasia. 12:485–498. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guo M, Mathieu PA, Linebaugh B, Sloane BF
and Reiners JJ Jr: Phorbol ester activation of a proteolytic
cascade capable of activating latent transforming growth
factor-betaL a process initiated by the exocytosis of cathepsin B.
J Biol Chem. 277:14829–14837. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Steffan JJ, Snider JL, Skalli O, Welbourne
T and Cardelli JA: Na+/H+ exchangers and RhoA
regulate acidic extracellular pH-induced lysosome trafficking in
prostate cancer cells. Traffic. 10:737–753. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bayrak R, Yenidünya S and Haltas H:
Cytokeratin 7 and cytokeratin 20 expression in colorectal
adenocarcinoma. Pathol Res Pract. 207:156–60. 2011. View Article : Google Scholar : PubMed/NCBI
|