Introduction
Leukemia is a hematological malignancy characterized
by an increase of abnormal leukocytes in the blood and bone marrow
(1). In 2018, a total of 437,000 new
cases and 309,000 deaths of leukemia were estimated worldwide
(2). Chemotherapy is the main
treatment for leukemia for decreasing the excessive number of
leukocytes, but the efficacy is limited by its side effects, such
as nausea, hair loss and changes in appetite (3). Therefore, complementary and alternative
medicine using natural compounds has been used to enhance the
efficacy of leukemia treatment (4).
Sesamin is the major oil-soluble furofuran lignan abundantly found
in sesame seeds and related products (5). Several studies have reported the
medicinal properties of sesamin, including antioxidant (6), anti-inflammatory (7), anti-hypertensive (8) and anti-hypercholesterolemic activities
(9). The anticancer activity of
sesamin has been revealed in a number of types of cancer, such as
lung cancer, breast cancer and leukemia (10). Sesamin is able to inhibit the
proliferation and induce apoptosis of lung cancer cells via
cyclooxygenase (COX)-2 (11).
Additionally, it advocates cell cycle arrest by increasing the
levels of p53 and checkpoint kinase 1 phosphorylation, and inducing
apoptosis via Bax and caspase 3 in MCF-7 cells (12). Moreover, sesamin has been reported to
potentiate autophagy; it triggers autophagy through the regulation
of EPH receptor (Eph)A1 and EphB2 in colon cancer cells (13). Additionally, it activates autophagic
cell death in the HeLa cervical cancer cell line (14). However, evidence of the anti-leukemic
effects of sesamin is limited.
Sesamin possesses anti-leukemic activities by
decreasing the proliferation and inducing apoptosis of HL-60 cells
via the mitochondrial and endoplasmic reticulum stress pathways
(15). It promotes TNF-induced
apoptosis by suppressing the expression of survival genes (such as
Bcl-2 and survivin), cell proliferative genes (such as cyclin D1
and COX-2) and genes involved in invasion and angiogenesis,
including intracellular adhesion molecule 1 and matrix
metalloproteinase 9, in KBM-5 cells (16).
Thus, the present study investigated the effects of
sesamin on MOLT-4 and NB4 leukemic cells in vitro and
examined the underlying mechanisms associated with apoptosis and
autophagy.
Materials and methods
Leukemic cell culture and peripheral
blood mononuclear cell (PBMC) isolation
The human T-lymphocytic leukemic MOLT-4 cell line
and human acute promyelocytic leukemic NB4 cell line were purchased
from CLS Cell Lines Service GmbH. The cells were cultured in
RPMI-1640 medium supplemented with 10% FBS and 1%
penicillin-streptomycin (all from Gibco; Thermo Fisher Scientific,
Inc.) in a humidified incubator at 37°C with 5% CO2. The
isolation of PBMCs from healthy adult volunteers (1 female and 2
males; mean age, 27 years; age range, 26–27 years) was performed
using Lymphoprep™ (Axis-Shield PoC AS) according to the
manufacturer's protocol. Briefly, 10 ml of blood was collected from
volunteers in August 2019 and diluted with an equal volume of PBS.
The diluted blood was gently layered onto the Lymphoprep solution.
The separation was done by centrifugation at 800 × g for 30 min at
25°C. The PBMC layer consisting of lymphocytes and monocytes was
collected and washed twice with medium before being used for the
cell viability assay. All healthy adult volunteers provided written
informed consent for donating peripheral blood to the present
study, which was in accordance with the guidelines of the Committee
for Research of the Faculty of Medicine Ramathibodi Hospital,
Mahidol University (Bangkok, Thailand), based on the Declaration of
Helsinki.
Determination of cell viability by MTT
assay
The effects of sesamin on cell viability were
determined using an MTT assay. Sesamin (Sigma-Aldrich; Merck KGaA)
was dissolved in DMSO at a stock concentration of 50 mg/ml and
small aliquots were stored at −20°C. Immediately before use,
individual aliquots were thawed and diluted in cell culture medium
to the required concentrations. The leukemic cells and PBMCs were
treated with various concentrations of sesamin (0, 100, 300 and 500
µg/ml). Following 24 and 48 h of incubation at 37°C, 5 mg/ml MTT
(Thermo Fisher Scientific, Inc.) was added, followed by incubation
for 4 h at 37°C. The formazan crystals were dissolved with
solubilizing solution (10% SDS in 0.01 N HCl) and the optical
density was measured at a wavelength of 570 nm using a
spectrophotometer. The IC50 was calculated from linear
regression for further experiments.
Analysis of apoptotic cells using flow
cytometry
The leukemic cells were treated with sesamin at the
IC50 concentration (400 µg/ml for MOLT-4 and 600 µg/ml
for NB4). Following 48 h of incubation at 37°C, the cells were
washed twice with cold PBS and resuspended in 1X binding buffer.
Apoptosis was examined using the FITC Annexin V Apoptosis Detection
kit (BD Biosciences) by staining the cells with 5 µl Annexin V and
5 µl PI for 15 min at room temperature in the dark. The apoptotic
cells were detected using a FACS Canto II flow cytometer (BD
Biosciences) within 1 h and analyzed using the BD FACSDiva software
version 6.1.3 (BD Biosciences).
Analysis of autophagy induction using
flow cytometry
The leukemic cells were treated with sesamin at the
IC50 concentration (400 µg/ml for MOLT-4 and 600 µg/ml
for NB4) at 37°C for 48 h. The LC3-II level was determined using
the FlowCellect™ Autophagy LC3 Antibody-based Assay kit (Merck
KGaA) following the manufacturer's protocol. At 30 min before the
end of the time point, 10 µl diluted reagent A were added, followed
by incubation at 37°C for 30 min. The medium was aspirated and 100
µl reagent B were added, followed by immediate spinning. The cells
were resuspended in 1X assay buffer and stained with anti-LC3/FITC
antibody for 30 min at room temperature in the dark, followed by
flow cytometric detection using a FACS Canto II flow cytometer (BD
Biosciences) within 1 h. The level of LC3-II was calculated from
the mean fluorescence intensity analyzed using the BD FACSDiva
software version 6.1.3 (BD Biosciences).
Reverse transcription-quantitative PCR
(RT-qPCR)
Leukemic cells were treated with sesamin at the
IC50 concentration (400 µg/ml for MOLT-4 and 600 µg/ml
for NB4) at 37°C for 48 h. Total RNA was extracted using GENEzol™
reagent (Geneaid Biotech Ltd.) and converted to 2 µg of cDNA using
the RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. qPCR
was performed using the Bio-Rad CFX96 touch™ real-time PCR
detection system (Bio-Rad Laboratories, Inc.) with 2 µl template,
0.5 µl of each primer, 10 µl Luna® Universal qPCR Master
Mix (New England Biolabs, Inc.) and nuclease-free water to reach a
final volume of 20 µl. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 15 sec, followed by 40
cycles of denaturation at 95°C for 15 sec and extension at 60°C for
30 sec. The following primers were used for detection: Caspase 3
(CASP3) forward, 5′-TTCAGAGGGGATCGTTGTAGAAGTC-3′ and reverse,
5′-CAAGCTTGTCGGCATACTGTTTCAG-3′; mTOR forward,
5′-CGCTGTCATCCCTTTATCG-3′ and reverse, 5′-ATGCTCAAACACCTCCACC-3′;
unc-51 like autophagy activating kinase 1 (ULK1) forward,
5-′GGCAAGTTCGAGTTCTCCCG-3′ and reverse,
5′-CGACCTCCAAATCGTGCTTCT−3′; and GAPDH forward,
5′-GCACCGTCAAGGCTGAGAA-3′ and reverse, 5′-AGGTCCACCACTGACACGTTG−3′.
GAPDH was used as an endogenous control for normalization. The mRNA
expression was calculated from the mean Cq value using
the 2−∆∆Cq method (17).
Prediction of protein-chemical
interactions using bioinformatics tools
The interaction network between sesamin and possible
targets was predicted using the Search Tool for Interactions of
Chemicals (STITCH) version 5.0, which is a search tool used for
predicting the interactions of chemicals and proteins that
integrates information and databases, including metabolic pathways,
crystal structures, binding experiments and drug-target
associations to predict biological events (18). The parameters were determined as
follows: List of names, sesamin, caspase3, mTOR and ULK1; species,
Homo sapiens; confidence score, medium (0.400).
Statistical analysis
The experiments were performed in triplicate. All
data are presented as the mean ± SEM. For comparisons between 2
groups, unpaired Student's t-test was used. For comparisons between
>2 groups, data were analyzed using a one-way ANOVA followed by
Dunnett's multiple comparisons test using GraphPad Prism 6
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Sesamin selectively exhibits
cytotoxicity to leukemic cells
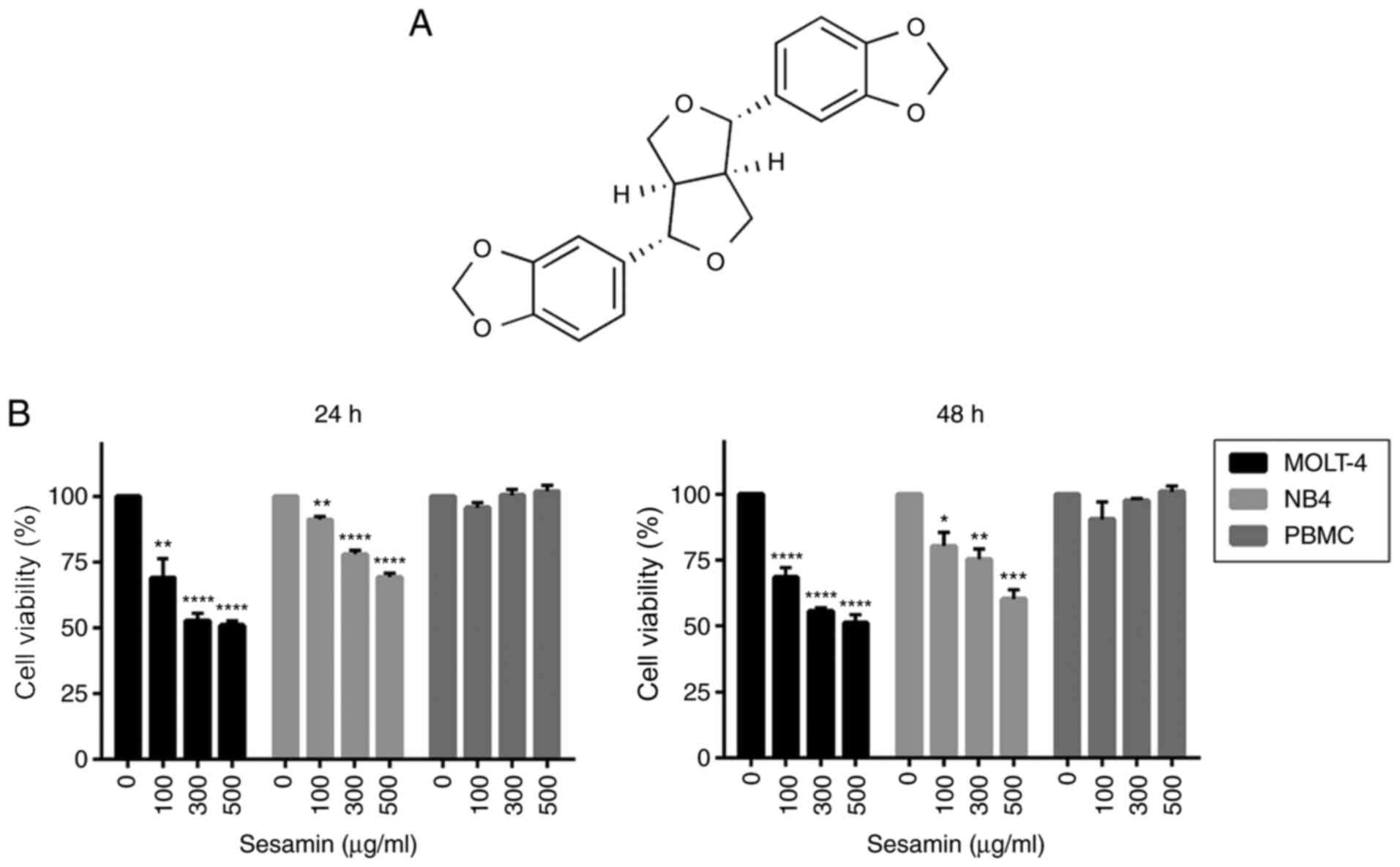
The chemical structure of sesamin is shown in
Fig. 1A. To determine the
anti-leukemic potential of sesamin, MOLT-4 and NB4 cells were
treated with various concentrations of sesamin (0, 100, 300 and 500
µg/ml). Following 24 and 48 h of incubation, cell viability was
examined using an MTT assay. Sesamin significantly decreased the
viability of MOLT-4 and NB4 cells in a dose-dependent manner
(Fig. 1B). The IC50 value
of sesamin at 48 h of incubation was 400 and 600 µg/ml in the
MOLT-4 and NB4 cells, respectively. Notably, sesamin did not affect
the viability of healthy PBMCs (Fig.
1B).
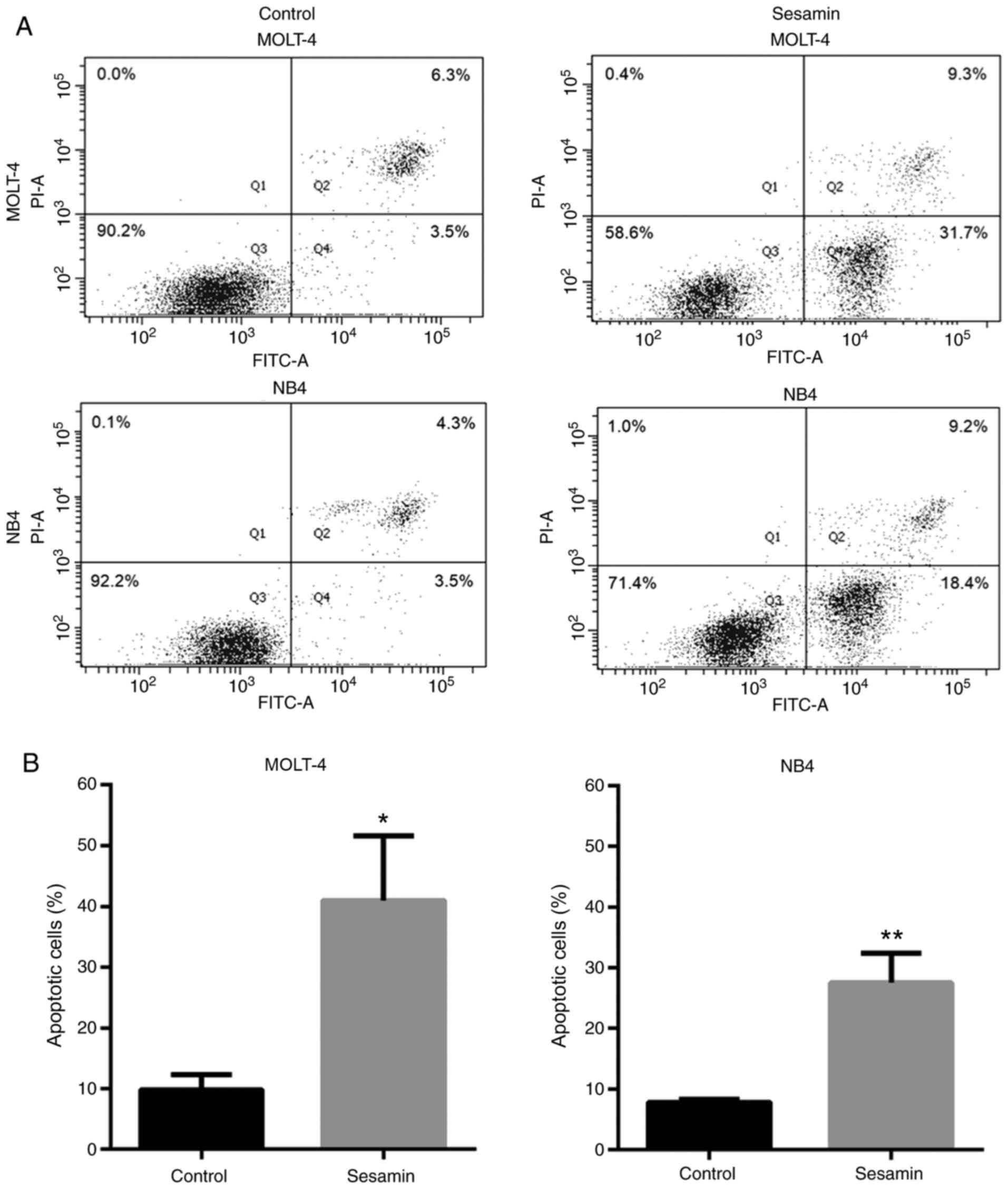
Sesamin induces the apoptosis of
MOLT-4 and NB4 cells
To investigate the apoptosis induced by sesamin,
MOLT-4 and NB4 leukemic cells were treated with sesamin at their
IC50 concentration for 48 h. The assessment of apoptosis
was performed via Annexin V-FITC/PI staining and flow cytometry.
The Annexin V-FITC+ cells in Q2 (late apoptotic cells)
and Q4 (early apoptotic cells) were defined as total apoptotic
cells. The results revealed that sesamin significantly induced
apoptosis of MOLT-4 and NB4 cells (Fig.
2A and B).
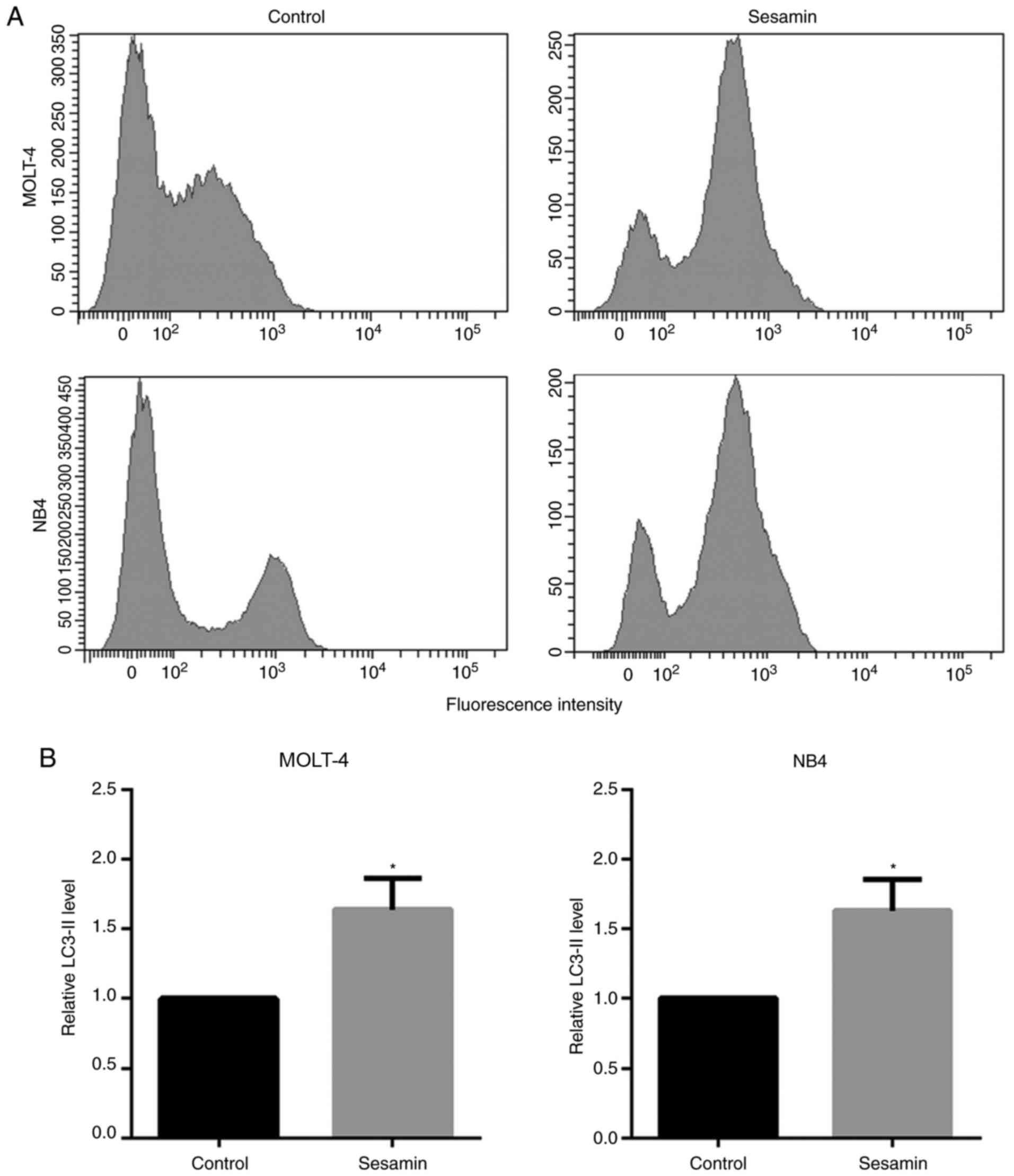
Sesamin induces autophagy by
increasing the LC3-II level
To evaluate the induction of autophagy, leukemic
cells were treated with sesamin at their IC50
concentration for 48 h, and autophagy assay was performed using
flow cytometry. The detection was based on the level of LC3-II in
the cells that accordingly indicated the level of autophagy.
Compared with the control, sesamin significantly increased the
LC3-II level by ~1.6-fold in both cell lines (Fig. 3A and B).
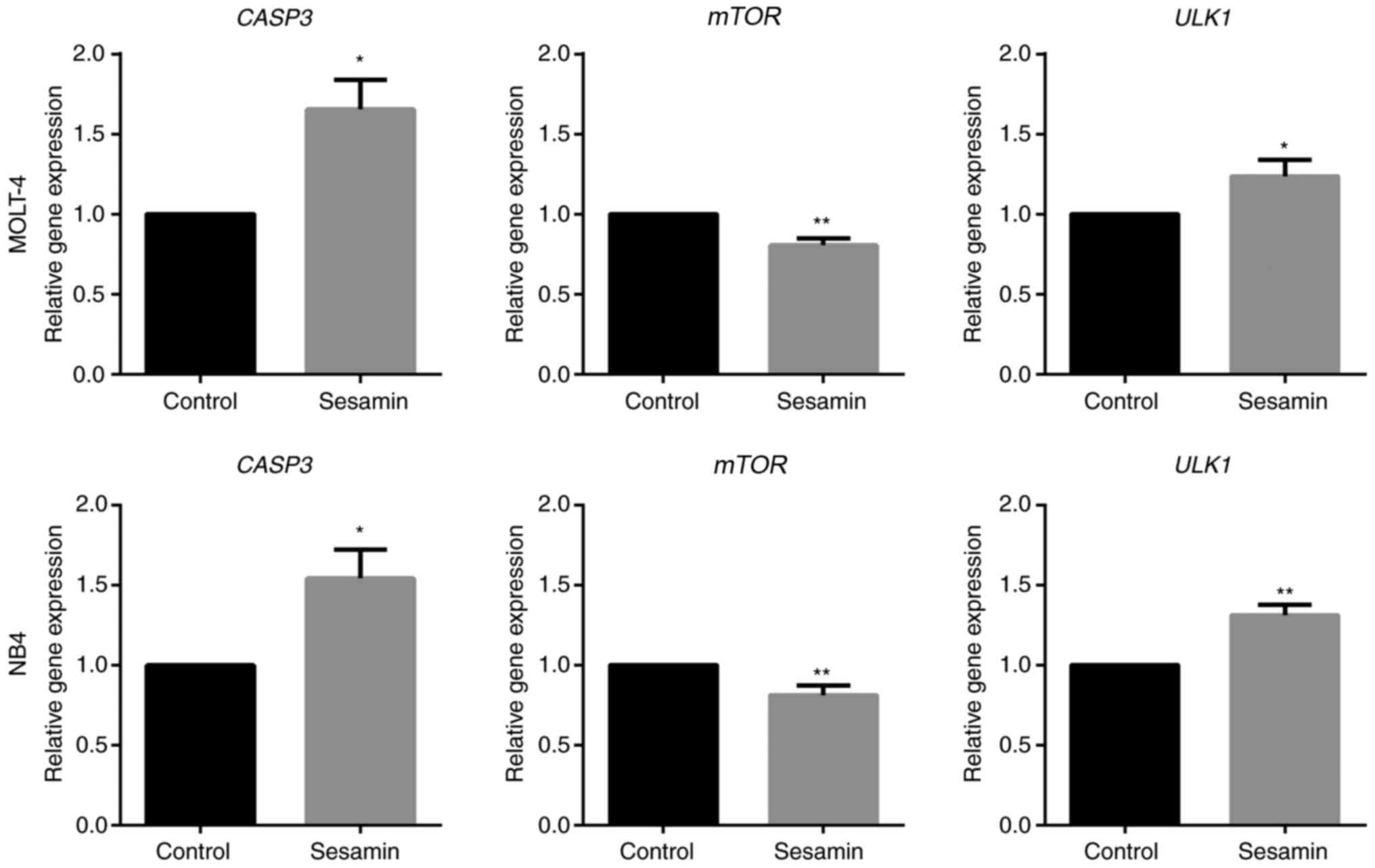
Sesamin contributes to the alteration of mRNA
expression involved in apoptosis and autophagy. To examine the
alteration of genes resulting from sesamin treatment, the
expression levels of key genes associated with apoptosis, namely
CASP3, and autophagy, namely mTOR and ULK1, were evaluated. The
results of mRNA expression analysis in both cells demonstrated that
sesamin induced apoptosis through the upregulation of CASP3
expression, and induced autophagy through the downregulation of
mTOR and upregulation of ULK1 expression (Fig. 4).
Protein-chemical interaction of
sesamin predicted by STITCH
STITCH was used to predict the association between
sesamin and the proposed proteins (CASP3, mTOR and ULK1). The
results from STITCH software analysis revealed that sesamin
exhibited an association with CASP3 and mTOR via nitric oxide
synthase 3. Moreover, ULK1, which is directly downstream of mTOR,
exhibited high-score interactions with the target of rapamycin
complex subunit LST8 (MLST8), regulatory-associated protein of mTOR
(RPTOR) and proline-rich AKT1 substrate 1 (AKT1S1), as well as
low-score interactions with ras homolog enriched in brain (RHEB),
rapamycin-insensitive companion of mTOR (RICTOR), eukaryotic
translation initiation factor 4E-binding protein 1 (EIF4EBP1) and
eukaryotic translation initiation factor 4E (EIF4E). This
interaction network indicated that CASP3, mTOR and ULK1 served a
role in apoptosis and autophagy in response to sesamin treatment,
similar to rapamycin, AZD8055, Torin1 and Torin2 (Fig. 5).
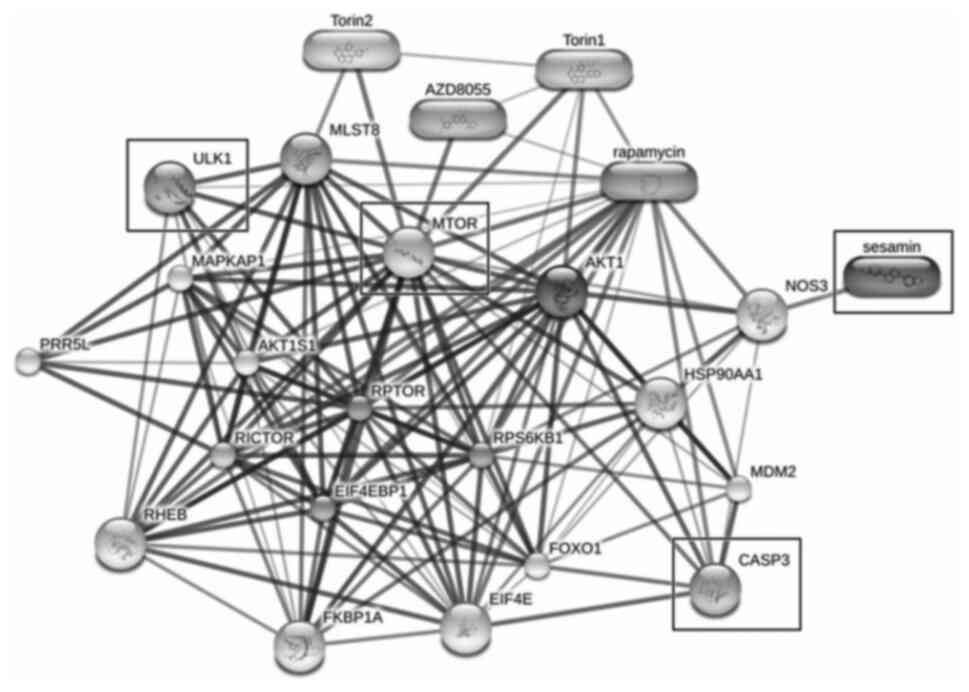 | Figure 5.Prediction of protein-chemical
interaction using the Search Tool for Interactions of Chemicals
with the input of sesamin and associated factors (CASP3, mTOR and
ULK1). Thick lines represent strong associations, while thin lines
represent weak interactions. CASP3, caspase 3; ULK1, unc-51 like
autophagy activating kinase 1; NOS3, nitric oxide synthase 3;
MLST8, target of rapamycin complex subunit LST8; RPTOR,
regulatory-associated protein of mTOR; AKT1S1, proline-rich AKT1
substrate 1; RHEB, ras homolog enriched in brain; RICTOR,
rapamycin-insensitive companion of mTOR; EIF4EBP1, eukaryotic
translation initiation factor 4E-binding protein 1; EIF4E,
eukaryotic translation initiation factor 4E. |
Discussion
In the present study, the most substantial lignan in
sesame seeds, known as sesamin, was investigated for its
anti-leukemic effects on MOLT-4 and NB4 cell lines. It was revealed
that sesamin exerted selective cytotoxic effects by inhibiting the
viability of leukemic cells in a dose-dependent manner; however, it
had a minimal effect on healthy PBMCs. Additionally, it increased
apoptosis of both leukemic cell lines via the regulation of CASP3.
When comparing the two cell lines, the MOLT-4 cells exhibited lower
cell viability and a higher percentage of apoptosis compared with
NB4 cells, suggesting that MOLT-4, an acute lymphoblastic leukemic
cell line, was more sensitive to sesamin compared with NB4, an
acute promyelocytic leukemic cell line. Previous studies have
reported the induction of apoptosis by sesamin in several types of
cancer. For example, the regulation of the apoptotic proteins
Bax/Bcl-2 and endoplasmic reticulum stress proteins in the
inositol-requiring enzyme 1α/JNK signaling pathway have been
reported as key factors in cervical cancer cell apoptosis induced
by sesamin (14). Additionally,
sesamin is able to induce cell cycle arrest and apoptosis through
the inhibition of STAT3 in hepatocellular carcinoma HepG2 cells
(19).
In addition, previous studies have focused on the
induction of autophagic cancer cell death by natural products. For
example, ailanthone, the extract from Ailanthus altissima,
induces autophagic cell death through the modulation of Beclin1,
p62 and LC3 expression in HL-60 cells (20). Allicin and diallyl disulfide, the
bioactive compounds in garlic extract, promote the mTOR-mediated
autophagic death of human liver cancer and leukemic cells (21,22).
mTOR is a protein kinase that serves a role in cell proliferation,
survival, metabolism and autophagy; the activation of mTOR is
frequently observed in cancer (23).
Thereby, the inhibition of mTOR has been considered for cancer
therapy (24). ULK1 is an autophagic
marker, and its downstream signals are triggered by decreased mTOR
levels; it has been reported as a promising target for cancer
therapy (25). Baicalein, a
flavonoid from Scutellaria baicalensis, and LYN-1604, a
candidate ULK1 agonist, exert antitumor effects through ULK1
activation in cancer cells (26,27). The
results of the present study demonstrated that sesamin treatment
stimulated autophagy, represented by the increased levels of LC3-II
in MOLT-4 and NB4 leukemic cells. This effect was accompanied by
the downregulation of mTOR and the upregulation of ULK1 expression.
This suggested that the induction of autophagy through mTOR/ULK1
signaling by sesamin may be an alternative treatment option for
leukemia.
Bioinformatics tools have facilitated the
comprehensive overview of available databases that are helpful to
the understanding of pharmacology and biochemistry. The study of
the interaction between proteins and small molecules is essential
for the development of therapeutic drugs for diseases (28). The association between sesamin and
possible associated molecules (CASP3, mTOR and ULK1) demonstrated
that several targets participated in the response to sesamin.
MLST8, RPTOR and AKT1S1, which exhibited strong interaction
networks, were recognized as subunits of mTOR and their
overexpression has been reported in a number of types of cancer
cells, including colon, prostate, breast and lung cancer cells
(29–31). It has been reported that the
downregulation of MLST8, RPTOR and AKT1S1 expression inhibited
tumor growth and induced cell death in colon cancer, lymphoma and
sarcoma (29,30,32,33).
RHEB and RICTOR, which exhibited an interaction in the current
network, are also involved in the mTOR signaling pathway (24). The deletion of RHEB and RICTOR can
enhance cell cycle arrest and apoptosis of acute leukemic cells
(34,35). The other identified associated
proteins, the translation factors EIF4E and EIF4EBP1, affect
leukemogenesis by promoting growth and impairing the
differentiation of blood cell precursors (36). Additionally, STITCH predicted that
other anticancer agents, including rapamycin, AZD8055, Torin1 and
Torin2, exhibited similar mechanisms to sesamin. These chemicals
are categorized as mTOR inhibitors with anti-leukemic properties.
Rapamycin treatment enhances the anti-neoplastic effects of the
chemotherapeutic drug arabinozide cytarabine in acute myeloid
leukemia (AML) (37). AZD8055 exerts
cytotoxic effects in clinical AML cells through cell cycle
blocking, caspase-dependent apoptosis and autophagic cell death
(38). The mTOR inhibitors Torin1
and 2 have been investigated for their anticancer effects,
revealing that they can suppress cell proliferation and promote the
death of colon cancer and hepatocellular carcinoma cells (39,40) and
have thus progressed to clinical trials (41).
The present study suggested that sesamin may be a
potent anti-leukemic agent, since it exerted anti-proliferative
effects on MOLT-4 and NB4 leukemic cell lines. Further experiments
indicated that these effects were mediated via the modulation of
apoptosis and autophagy. The expression levels of the effective
apoptotic factor CASP3 were increased in sesamin-treated cells.
Autophagy occurred through the regulation of LC3-II triggered via
the mTOR and ULK1 signaling pathway. The protein-chemical
interaction analysis indicated that CASP3, mTOR and ULK1 were
associated with several biomolecules in response to sesamin. The
underlying mechanisms of sesamin were associated with the effects
of the anti-neoplastic drug rapamycin and those of the well-known
agents AZD8055, Torin1 and 2.
The findings of the present study demonstrated the
anti-leukemic effects of sesamin on MOLT-4 and NB4 cell lines
through apoptotic and autophagic signaling pathways. These findings
may be beneficial for the improved understanding of the role of
sesamin in human leukemia. However, the present study only
performed in vitro experiments. Thus, further studies on the
effects of sesamin in vivo are required in the future for
the development of alternative therapeutic strategies for
leukemia.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Royal Golden
Jubilee PhD scholarship (grant no. PHD/0051/2558) from the Thailand
Research Fund.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DT was involved in the conception of the study. KD
and DT were involved in the experimental procedures. KD, CC, SR, UA
and DT were involved in the interpretation and discussion of the
results. KD was involved in the manuscript preparation. SR and DT
were involved in the revision of the manuscript. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The experiments involving human blood samples were
approved by the Committee for Research of the Faculty of Medicine
Ramathibodi Hospital, Mahidol University (Bangkok, Thailand;
approval no. MURA2019/679). All healthy adult volunteers provided
written informed consent for donating peripheral blood.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wong SW, Lenzini S and Shin JW:
Perspective: Biophysical regulation of cancerous and normal blood
cell lineages in hematopoietic malignancies. APL Bioeng.
2:0318022018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramirez LY, Huestis SE, Yap TY, Zyzanski
S, Drotar D and Kodish E: Potential chemotherapy side effects: What
do oncologists tell parents? Pediatr Blood Cancer. 52:497–502.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saedi TA, Md Noor S, Ismail P and Othman
F: The effects of herbs and fruits on leukaemia. Evid Based
Complement Alternat Med. 2014:4941362014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moazzami AA, Haese SL and Kamal-Eldin A:
Lignan contents in sesame seeds and products. Eur J Lipid Sci
Technol. 109:1022–1027. 2007. View Article : Google Scholar
|
|
6
|
Ruankham W, Suwanjang W, Wongchitrat P,
Prachayasittikul V, Prachayasittikul S and Phopin K: Sesamin and
sesamol attenuate H2O2-induced oxidative
stress on human neuronal cells via the SIRT1-SIRT3-FOXO3a signaling
pathway. Nutr Neurosci. 1–12. 2019.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong P, Chen G, Jiang A, Wang Y, Song C,
Zhuang J, Xi C, Wang G, Ji Y and Yan J: Sesamin inhibits
IL-1β-stimulated inflammatory response in human osteoarthritis
chondrocytes by activating Nrf2 signaling pathway. Oncotarget.
7:83720–83726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyawaki T, Aono H, Toyoda-Ono Y, Maeda H,
Kiso Y and Moriyama K: Antihypertensive effects of sesamin in
humans. J Nutr Sci Vitaminol (Tokyo). 55:87–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang YT, Chen J, Jiao R, Peng C, Zuo Y,
Lei L, Liu Y, Wang X, Ma KY, Huang Y and Chen ZY:
Cholesterol-lowering activity of sesamin is associated with
down-regulation on genes of sterol transporters involved in
cholesterol absorption. J Agric Food Chem. 63:2963–2969. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Majdalawieh AF, Massri M and Nasrallah GK:
A comprehensive review on the anti-cancer properties and mechanisms
of action of sesamin, a lignan in sesame seeds (Sesamum
indicum). Eur J Pharmacol. 815:512–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fang Q, Zhu Y, Wang Q, Song M, Gao G and
Zhou Z: Suppression of cyclooxygenase 2 increases chemosensitivity
to sesamin through the Akt-PI3K signaling pathway in lung cancer
cells. Int J Mol Med. 43:507–516. 2019.PubMed/NCBI
|
|
12
|
Siao AC, Hou CW, Kao YH and Jeng KC:
Effect of sesamin on apoptosis and cell cycle arrest in human
breast cancer mcf-7 cells. Asian Pac J Cancer Prev. 16:3779–3783.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanabe H, Kuribayashi K, Tsuji N, Tanaka
M, Kobayashi D and Watanabe N: Sesamin induces autophagy in colon
cancer cells by reducing tyrosine phosphorylation of EphA1 and
EphB2. Int J Oncol. 39:33–40. 2011.PubMed/NCBI
|
|
14
|
Dou H, Yang S, Hu Y, Xu D, Liu L and Li X:
Sesamin induces ER stress-mediated apoptosis and activates
autophagy in cervical cancer cells. Life Sci. 200:87–93. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Banjerdpongchai R, Yingyurn S and
Kongtawelert P: Sesamin induces human leukemic cell apoptosis via
mitochondrial and endoplasmic reticulum stress pathways. World J
Oncol. 1:78–86. 2010.PubMed/NCBI
|
|
16
|
Harikumar KB, Sung B, Tharakan ST, Pandey
MK, Joy B, Guha S, Krishnan S and Aggarwal BB: Sesamin manifests
chemopreventive effects through the suppression of NF-kappa
B-regulated cell survival, proliferation, invasion, and angiogenic
gene products. Mol Cancer Res. 8:751–761. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuhn M, von Mering C, Campillos M, Jensen
LJ and Bork P: STITCH: Interaction networks of chemicals and
proteins. Nucleic Acids Res. 36:D684–D688. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng P, Wang C, Chen L, Wang C, Du Y, Yan
X, Chen M, Yang G and He G: Sesamin induces cell cycle arrest and
apoptosis through the inhibition of signal transducer and activator
of transcription 3 signalling in human hepatocellular carcinoma
cell line HepG2. Biol Pharm Bull. 36:1540–1548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei C, Chen C, Cheng Y, Zhu L, Wang Y, Luo
C, He Y, Yang Z and Ji Z: Ailanthone induces autophagic and
apoptotic cell death in human promyelocytic leukemia HL-60 cells.
Oncol Lett. 16:3569–3576. 2018.PubMed/NCBI
|
|
21
|
Chu YL, Ho CT, Chung JG, Rajasekaran R and
Sheen LY: Allicin induces p53-mediated autophagy in Hep G2 human
liver cancer cells. J Agric Food Chem. 60:8363–8371. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suangtamai T and Tanyong DI: Diallyl
disulfide induces apoptosis and autophagy via mTOR pathway in
myeloid leukemic cell line. Tumour Biol. 37:10993–10999. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim J and Guan KL: mTOR as a central hub
of nutrient signalling and cell growth. Nat Cell Biol. 21:63–71.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hua H, Kong Q, Zhang H, Wang J, Luo T and
Jiang Y: Targeting mTOR for cancer therapy. J Hematol Oncol.
12:712019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Yan L, Liao N, Wu WQ and Shi JL: A
review of ULK1-mediated autophagy in drug resistance of cancer.
Cancers (Basel). 12:3522020. View Article : Google Scholar
|
|
26
|
Aryal P, Kim K, Park PH, Ham S, Cho J and
Song K: Baicalein induces autophagic cell death through AMPK/ULK1
activation and downregulation of mTORC1 complex components in human
cancer cells. FEBS J. 281:4644–4658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Fu L, Zhang S, Zhang J, Zhao Y,
Zheng Y, He G, Yang S, Ouyang L and Liu B: Discovery of a small
molecule targeting ULK1-modulated cell death of triple negative
breast cancer in vitro and in vivo. Chem Sci. 8:2687–2701. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia X: Bioinformatics and drug discovery.
Curr Top Med Chem. 17:1709–1726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kakumoto K, Ikeda J, Okada M, Morii E and
Oneyama C: mLST8 promotes mTOR-mediated tumor progression. PLoS
One. 10:e01190152015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gulhati P, Cai Q, Li J, Liu J, Rychahou
PG, Qiu S, Lee EY, Silva SR, Bowen KA, Gao T and Evers BM: Targeted
inhibition of mammalian target of rapamycin signaling inhibits
tumorigenesis of colorectal cancer. Clin Cancer Res. 15:7207–7216.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Malla R, Ashby CR Jr, Narayanan NK,
Narayanan B, Faridi JS and Tiwari AK: Proline-rich AKT substrate of
40-kDa (PRAS40) in the pathophysiology of cancer. Biochem Biophys
Res Commun. 463:161–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin R, Desponds C, Eren RO, Quadroni M,
Thome M and Fasel N: Caspase-mediated cleavage of raptor
participates in the inactivation of mTORC1 during cell death. Cell
Death Discov. 2:160242016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lv D, Liu J, Guo L, Wu D, Matsumoto K and
Huang L: PRAS40 deregulates apoptosis in Ewing sarcoma family
tumors by enhancing the insulin receptor/Akt and mTOR signaling
pathways. Am J Cancer Res. 6:486–497. 2016.PubMed/NCBI
|
|
34
|
Gao Y, Gao J, Li M, Zheng Y, Wang Y, Zhang
H, Wang W, Chu Y, Wang X, Xu M, et al: Rheb1 promotes tumor
progression through mTORC1 in MLL-AF9-initiated murine acute
myeloid leukemia. J Hematol Oncol. 9:362016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hua C, Guo H, Bu J, Zhou M, Cheng H, He F,
Wang J, Wang X, Zhang Y, Wang Q, et al: Rictor/mammalian target of
rapamycin 2 regulates the development of Notch1 induced murine
T-cell acute lymphoblastic leukemia via forkhead box O3. Exp
Hematol. 42:1031–1040.e1-e4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Topisirovic I, Guzman ML, McConnell MJ,
Licht JD, Culjkovic B, Neering SJ, Jordan CT and Borden KLB:
Aberrant eukaryotic translation initiation factor 4E-dependent mRNA
transport impedes hematopoietic differentiation and contributes to
leukemogenesis. Mol Cell Biol. 23:8992–9002. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Janus A, Linke A, Cebula B, Robak T and
Smolewski P: Rapamycin, the mTOR kinase inhibitor, sensitizes acute
myeloid leukemia cells, HL-60 cells, to the cytotoxic effect of
arabinozide cytarabine. Anticancer Drugs. 20:693–701. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Willems L, Chapuis N, Puissant A, Maciel
TT, Green AS, Jacque N, Vignon C, Park S, Guichard S, Herault O, et
al: The dual mTORC1 and mTORC2 inhibitor AZD8055 has anti-tumor
activity in acute myeloid leukemia. Leukemia. 26:1195–1202. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Francipane MG and Lagasse E: Selective
targeting of human colon cancer stem-like cells by the mTOR
inhibitor Torin-1. Oncotarget. 4:1948–1962. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang C, Wang X, Su Z, Fei H, Liu X and Pan
Q: The novel mTOR inhibitor Torin-2 induces autophagy and
downregulates the expression of UHRF1 to suppress hepatocarcinoma
cell growth. Oncol Rep. 34:1708–1716. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun SY: mTOR kinase inhibitors as
potential cancer therapeutic drugs. Cancer Lett. 340:1–8. 2013.
View Article : Google Scholar : PubMed/NCBI
|