Introduction
Gastric cancer (GC) is the third most common
malignant tumor and a leading cause of cancer-related mortality
worldwide according to statistics from 2008 (1). Certain risk factors have been
identified to be responsible for the initiation of GC, including
excessive salt intake and Helicobacter pylori infection
(2). Although the therapeutic
strategies of GC are advanced, including surgical resection,
radiation and chemotherapy, the overall survival rate and the
prognosis of patients with GC remain poor due to a significant
proportion of patients presenting with advanced tumors at initial
diagnosis (3). Therefore, it is
crucial to identify specific diagnostic and prognostic biomarkers
to improve GC treatment.
MicroRNAs (miRNAs or miRs) are small non-coding RNAs
approximately 18–25 nucleotides in length with important regulatory
effects on gene expression at the post-transcriptional level
(4,5). MiRNAs bind to the 3′-untranslated
region of target mRNA, leading to the inhibition of target gene
expression (6). Additionally, miRNAs
have been reported to be involved in various cellular processes,
including proliferation, migration and invasion (7). Accumulating studies have reported the
association between miRNAs and human cancers, and the results have
indicated that miRNAs function as tumor suppressors or oncogenes
during tumorigenesis (8,9). There are certain abnormally expressed
miRNAs in GC, such as miR-216b (10)
and miR-519a (11), which are
closely associated with the diagnosis, prognosis and progression of
GC. miR-4636 has been reported to be downregulated in cervical
cancer (12) and its decreased
expression has been reported in GC tissues by Zhang et al
(13). However, the functional role
and clinical significance of miR-4636 in GC remain unknown.
To further improve the treatment of GC, the present
study analyzed the expression levels of miR-4636 in patients with
GC and in GC cell lines to evaluate the diagnostic and prognostic
significance of miR-4636 and its biological function in GC
progression. The results may provide a potential novel biomarker
and therapeutic target for the diagnosis, prognosis and treatment
of GC.
Materials and methods
Patients and tissue collection
A total of 112 patients (65 males and 47 females)
who underwent surgery at Zibo Central Hospital (Zibo, China)
between June 2009 and May 2013 were recruited. The mean age of the
patients was 64.87±11.31 years (range, 45–85 years). The inclusion
criteria were as follows: i) All the patients were pathologically
diagnosed with GC; ii) none of the patients had received any
therapy prior to surgery; and iii) had complete clinicopathological
data. The exclusion criteria were as follows: i) Patients younger
than 18 years or older than 80 years; ii) pregnant or lactating
patients; and iii) patients that had autoimmune diseases or other
malignancies. Furthermore, 60 healthy individuals with an average
age of 65.23±10.96 years (range, 46–84 years) were recruited during
the same time period. The healthy volunteers included 35 males and
25 females, and had no history of malignancy. Venous blood was
obtained from all the participants and serum was isolated by
centrifugation at 1,500 × g for 15 min at 4°C, that was then stored
at −80°C until further use. Additionally, GC tissue samples and
adjacent normal tissue samples, which were located 3 cm from the
edge of the tumors, were collected from the patients and frozen
with liquid nitrogen at −80°C for future use. All the patients
received a 5-year follow-up survey and their survival information
was recorded. Prior to sampling, all the patients and healthy
volunteers provided written informed consent for clinical sample
use and analysis, and the experimental methods were approved by the
Ethics Committee of Zibo Central Hospital (approval no.
ZCHh-090618).
Cell culture and transfection
The GC cell lines AGS, HGC27, HS746T and MKN45 and
the gastric epithelial cell line GES-1 were purchased from The Cell
Bank of Type Culture Collection of the Chinese Academy of Sciences.
The cells were cultured in RPMI-1640 medium (BioTek Corporation)
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at
37°C with 5% CO2.
GC cell lines AGS and MGN45 were subjected to cell
transfection. Sequences for 50 nM miR-4636 mimics
(5′-AACUCGUGUUCAAAGCCUUUAG-3′), 100 nM miR-4636 inhibitors
(5′-CUAAAGGCUUUGAACACGAGUU-3′) or their respective negative
controls (50 nM mimic NC, 5′-UUCUCCGAACGUGUCACGU-3′ and 100 nM
inhibitor NC, 5′-CAGUACUUUUGUGUAGUACAA-3′) were transfected into
AGS and MGN45 cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C according to
the manufacturer's protocol. After 48 h of cell transfection, the
cells were used for subsequent experiments. The cells transfected
with only transfection reagents were used as a mock group.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from GC tissues and cell
lines using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was synthesized from RNA using a
PrimeScript RT reagent kit (Takara Bio, Inc.), according to the
manufacturer's protocol. The expression levels of miR-4636 were
detected by qPCR, which was conducted using a SYBR-Green I Master
Mix kit (Invitrogen; Thermo Fisher Scientific, Inc.) on a 7500
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The thermocycling conditions were as follows: 95°C, 10 min
for initial denaturation, 40 cycles of 95°C, 30 sec for
denaturation, 58°C, 20 sec for annealing, 72°C, 30 sec for
elongation, and 72°C, 10 min for final extension. U6 was used as
the internal control and the final expression values of miR-4636
were quantified using the 2−ΔΔCq method (14). The sequences of primers used were as
follows: miR-4636 forward, 5′-GCCGAGAACTCGTGTTCAA-3′ and reverse,
5′-CTCAACTGGTGTCGTGGA-3′; U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′.
Cell proliferation assay
At 48-h post-transfection, AGS and MKN45 cells
(3×103 cells/well) were seeded into 96-well plates and
the cell proliferation was analyzed using a MTT assay. The cell
plates were retained in an incubator at 37°C for 3 days. A total of
10 µl MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added to each
well every 24 h followed by incubation for a further 4 h. Following
this, 150 µl DMSO was added in each well to dissolve the violet
formazan crystals. Following incubation, the absorbance of the
cultures was determined at a wavelength of 570 nm using a
microplate reader.
Cell migration and invasion assay
The invasion and migration abilities of AGS and
MKN45 cells were analyzed using Transwell chambers with 8 µm pore
membranes (Corning, Inc.) precoated with Matrigel (for invasion
assays) or without Matrigel (for migration assays). GC cells
(3×105 cells/well) were seeded into the upper chamber
with serum-free RPMI-1640 medium. The lower chambers contained
medium supplemented with 10% FBS. Following 24 h of incubation at
37°C, the cells in the lower chambers were stained using 0.1%
crystal violet for 10 min at room temperature, and were calculated
under an inverted light microscope (Olympus Corporation).
Statistical analysis
The statistical analyses were performed using SPSS
(version no. 21.0; IBM Corp.) and GraphPad Prism (version no. 7.0;
GraphPad Software, Inc.) software. Differences between groups were
analyzed using Student's t-test (paired t-test for
tissue miR-4636 expression examination; unpaired t-test for serum
miR-4636 expression between healthy controls and cancer patients)
or one-way ANOVA followed by Tukey's post-hoc test. A χ2
test was used to assess the association between miR-4636 expression
and the clinicopathological features of patients with GC. A
receiver operating characteristics (ROC) curve was performed to
evaluate the diagnostic value of serum miR-4636 expression. The
survival curves for patients with GC were constructed using the
Kaplan-Meier (KM) method and log-rank test. A multivariate Cox
regression analysis was applied to evaluate the prognostic value of
miR-4636. P<0.05 was considered to indicate a statistically
significant difference.
Results
Downregulation of miR-4636 in patients
with GC and GC cell lines
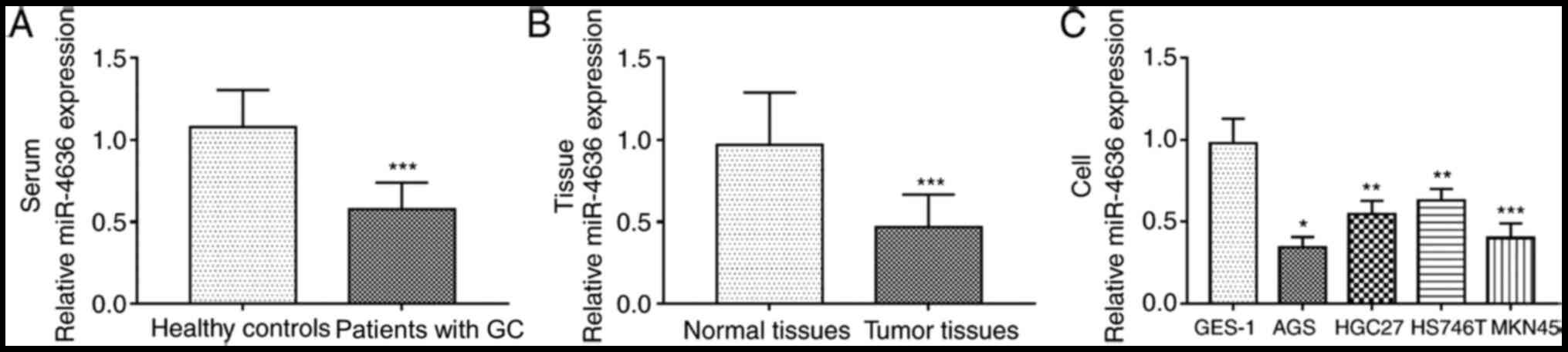
The serum expression levels of miR-4636 in patients
with GC were significantly lower compared with healthy controls
(P<0.001; Fig. 1A). Additionally,
the expression of miR-4636 in GC tissues was significantly
downregulated compared with normal tissues (P<0.001; Fig. 1B). The expression levels of miR-4636
in GC cell lines were significantly lower compared with GES-1 cells
(all, P<0.05; Fig. 1C).
Association of miR-4636 with the
clinicopathological characteristics of patients with GC
As presented in Table
I, GC patients were divided into low and high miR-4636
expression groups based on the mean expression value (serum, 0.576;
tissue, 0.470). The χ2 test results revealed that the
expression of miR-4636 in both serum and tissue samples was
associated with lymph node metastasis and TNM stage (both,
P<0.05). No significant association was found between miR-4636
and age, sex, tumor size or differentiation (all, P>0.05).
 | Table I.Association between miR-4636 and the
clinical characteristics of patients with gastric cancer. |
Table I.
Association between miR-4636 and the
clinical characteristics of patients with gastric cancer.
|
|
| miR-4636 in
serum |
| miR-4636 in
tissues |
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | Total (n=112) | Low (n=60) | High (n=52) | P-value | Low (n=62) | High (n=50) | P-value |
|---|
| Age (years) |
|
|
| 0.821 |
|
| 0.734 |
|
<60 | 40 | 22 | 18 |
| 23 | 17 |
|
| ≥60 | 72 | 38 | 34 |
| 39 | 33 |
|
| Sex |
|
|
| 0.945 |
|
| 0.572 |
|
Female | 47 | 25 | 22 |
| 24 | 22 |
|
| Male | 65 | 35 | 30 |
| 38 | 28 |
|
| Tumor size (cm) |
|
|
| 0.143 |
|
| 0.068 |
|
<5 | 52 | 24 | 28 |
| 24 | 28 |
|
| ≥5 | 60 | 36 | 24 |
| 38 | 22 |
|
| Differentiation |
|
|
| 0.054 |
|
| 0.052 |
|
Well/moderate | 58 | 26 | 32 |
| 27 | 31 |
|
|
Poor | 54 | 34 | 20 |
| 35 | 19 |
|
| Lymph node
metastasis |
|
|
| 0.026 |
|
| 0.010a |
|
Negative | 52 | 22 | 30 |
| 22 | 30 |
|
|
Positive | 60 | 38 | 22 |
| 40 | 20 |
|
| TNM stage |
|
|
| 0.017 |
|
| 0.019a |
|
I–II | 49 | 20 | 29 |
| 21 | 28 |
|
|
III–IV | 63 | 40 | 23 |
| 42 | 22 |
|
Clinical significance of miR-4636 in
the diagnosis and prognosis of GC
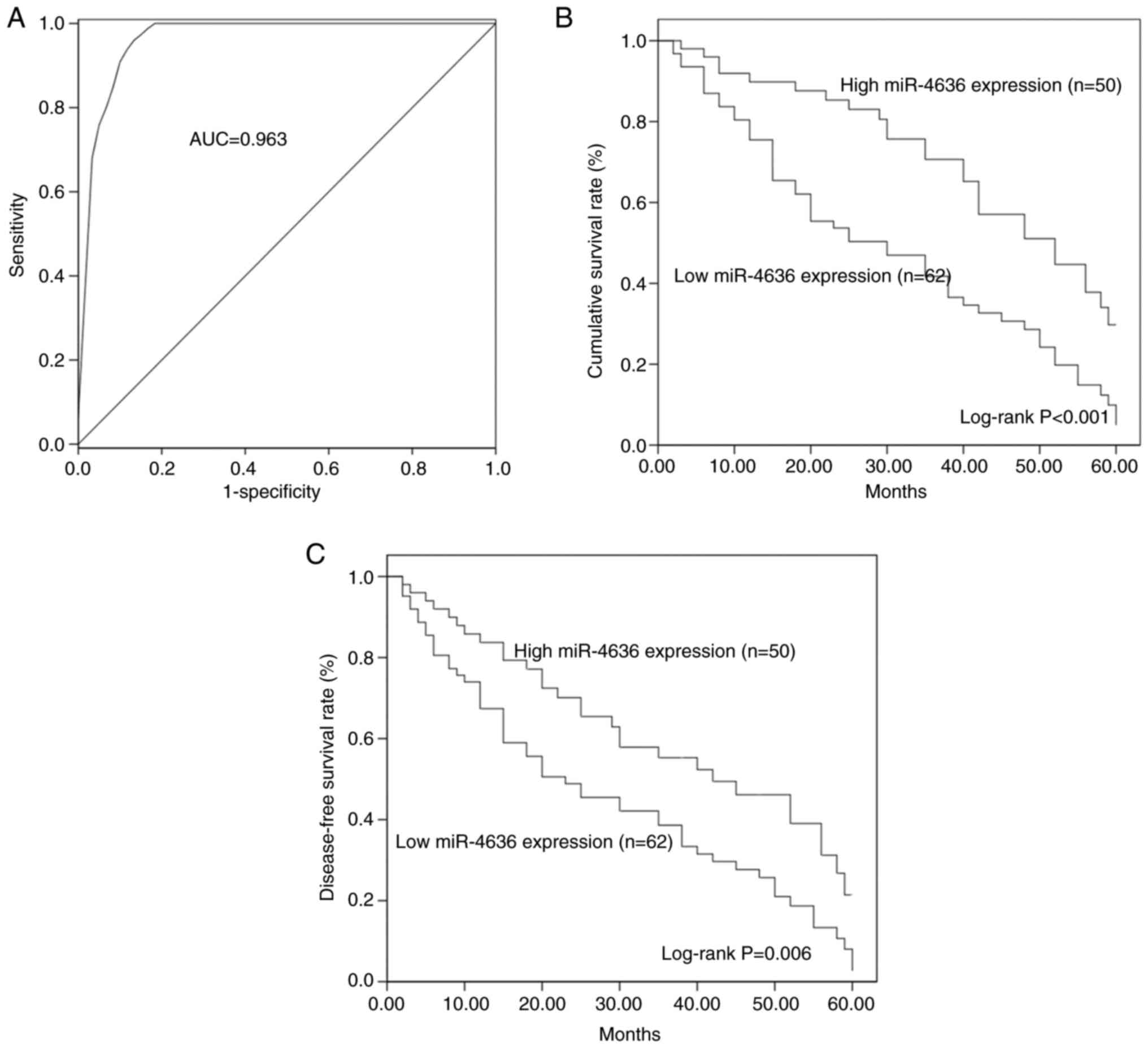
A ROC curve based on serum miR-4636 levels was
constructed, which demonstrated that the area under the curve (AUC)
was 0.963, indicating the diagnostic accuracy of serum miR-4636 in
patients with GC (Fig. 2A). At a
cut-off value of 0.855, the values for diagnosis sensitivity and
specificity were 97.3 and 86.7%, respectively.
By evaluating the 5-year follow-up data, the KM
method was used to construct the overall survival and disease-free
survival curves of patients with GC. Patients with GC and low
miR-4636 expression had shorter survival times compared with
patients with a high miR-4636 expression (log-rank, P<0.001;
Fig. 2B). Furthermore, a low
expression of miR-4636 was associated with poor disease-free
survival in patients with GC (log-rank P=0.006; Fig. 2C). The multivariate Cox regression
analysis revealed that aberrant miR-4636 expression (HR=3.522; 95%
CI=2.016–6.154; P<0.001; Table
II) and TNM stage (HR=1.800; 95% CI=1.093–2.966; P=0.021) were
two independent prognostic factors for the overall survival rate in
patients with GC.
 | Table II.Cox regression analysis in patients
with gastric cancer. |
Table II.
Cox regression analysis in patients
with gastric cancer.
| Parameter | HR value | 95% CI | P-value |
|---|
| miR-4636 | 3.522 | 2.016–6.154 | <0.001 |
| Age | 1.681 | 0.997–2.829 | 0.053 |
| Sex | 1.397 | 0.880–2.217 | 0.156 |
| Tumor size | 1.377 | 0.855–2.217 | 0.189 |
|
Differentiation | 1.149 | 0.717–1.842 | 0.564 |
| Lymph node
metastasis | 1.527 | 0.927–2.516 | 0.097 |
| TNM stage | 1.800 | 1.093–2.966 | 0.021 |
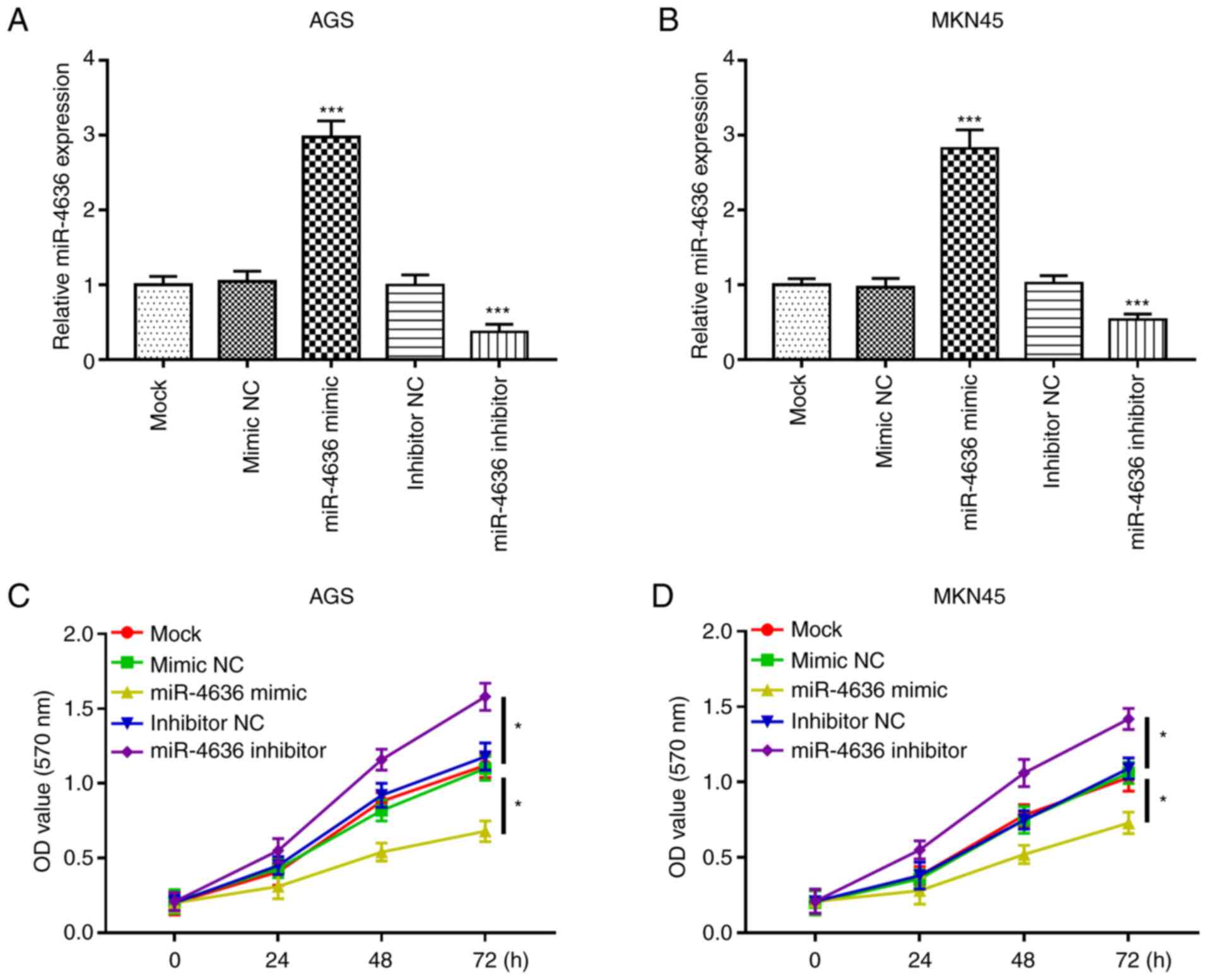
miR-4636 overexpression inhibits GC
cell proliferation
The functional role of miR-4636 in GC progression
was further investigated. GC cell lines AGS and MKN45 were selected
for the cell experiments due to their significantly lower
expression of miR-4636 expression compared with the normal cell
line GES-1. The expression of mi-4636 was upregulated by miR-4636
mimics and downregulated by miR-4636 inhibitors compared with the
mock group in the AGS and MKN45 cell lines (all, P<0.001;
Fig. 3A and B). Furthermore, the MTT
assay results demonstrated that cell proliferation in the AGS and
MKN45 cell lines was significantly inhibited by the overexpression
of miR-4636 and promoted by the knockdown of miR-4636 compared with
the mock group (all, P<0.05; Fig. 3C
and D).
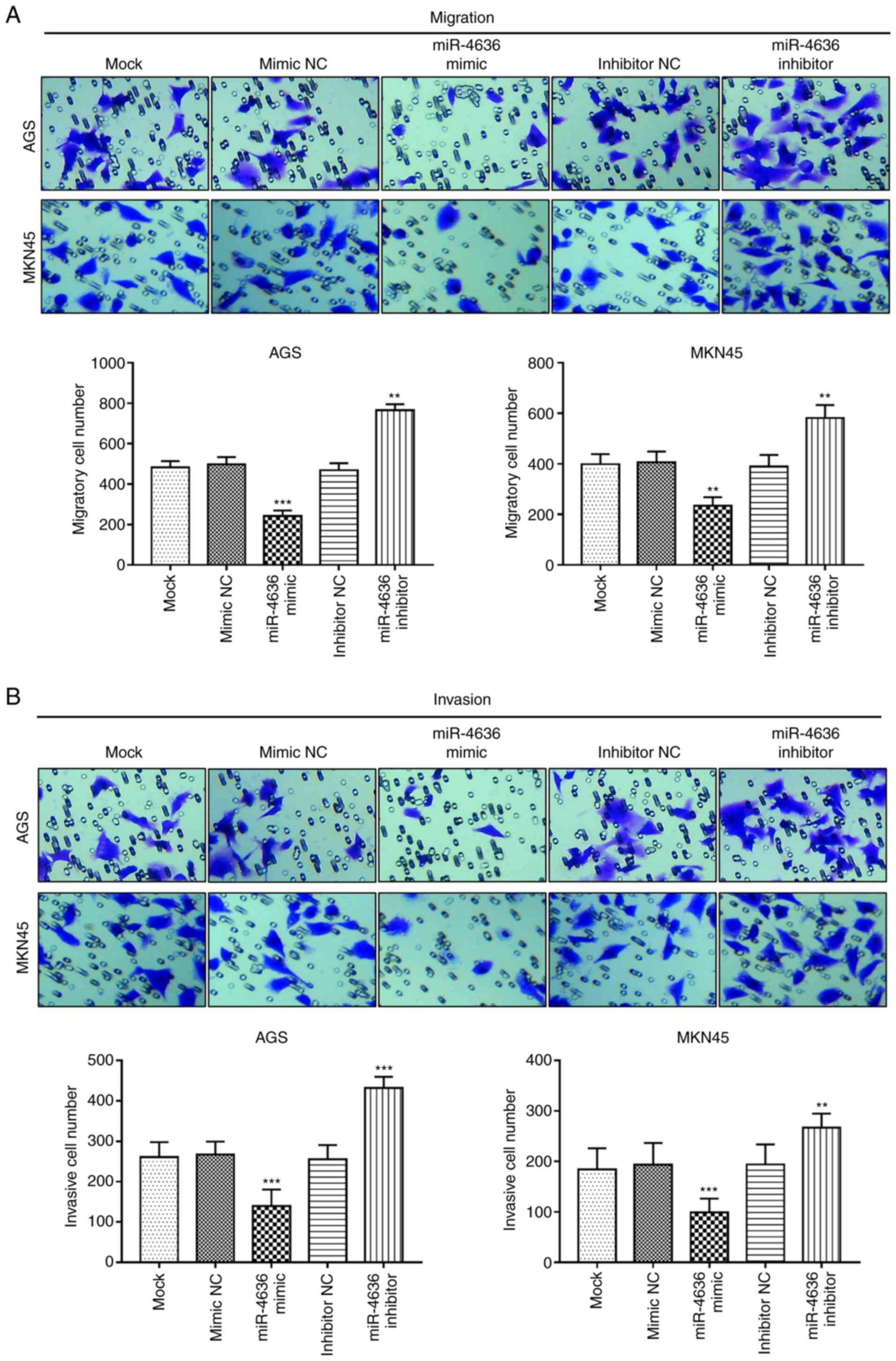
Effects of miR-4636 on GC cell
migration and invasion
The Transwell assay results demonstrated that the
overexpression of miR-4636 inhibited GC cell migration, while the
miR-4636 knockdown promoted cell migration in the AGS and MKN45
cell lines compared with the mock group (P<0.01 or P<0.001;
Fig. 4A). Furthermore, the cell
invasion ability of the AGS and MKN5 cells was suppressed by the
overexpression of miR-4636 and enhanced by the knockdown of
miR-4636 compared with the mock group (P<0.01 or P<0.001;
Fig. 4B).
Discussion
GC is a heterogeneous malignant disease (15). Although the global incidence and
mortality of GC have declined significantly over the past decades,
GC remains a leading cause of cancer-associated death worldwide
based on statistics from 2010 (16).
Despite the development of therapeutic strategies for GC, prognosis
remains poor, with the median survival time ranging from 4–12
months (17,18). Previous studies have reported that
certain aberrantly expressed miRNAs were associated with disease
development and served as biomarkers to improve the diagnosis and
prognosis of various cancers, such as lung cancer and
hepatocellular carcinoma (19–21).
Moreover, aberrant miRNA expression has been documented to be
involved in the regulation of tumor progression. For instance, the
downregulated expression of miR-145 was detected in colorectal
cancer and correlated with tumor size, grade of differentiation,
invasion, metastasis and clinical stage (22). In GC, certain aberrant miRNAs
participate in tumorigenesis and tumor progression. Cai et
al (23) revealed that the
downregulated expression of miR-519a predicted the poor prognosis
of GC and was involved in the regulation of GC progression.
Additionally, Li and Zou (24)
reported that miR-652 was significantly elevated in GC tissues and
cell lines compared with normal tissues and cells and functioned as
an oncogene in GC by promoting tumor progression through targeting
RAR-related orphan receptor α (24).
These previous studies demonstrated that miRNAs serve roles in
tumor progression. Therefore, the present study aimed to
investigate the biological function and clinical value of miR-4636
in GC.
miR-4636 has been reported to be deregulated in
certain human cancers. For instance, Yin et al (12) revealed that miR-4636 expression
levels were correlated with gross tumor volume and the depth of
invasion in cervical cancer. Notably, Zhang et al (13) reported that miR-4636 expression was
reduced in GC tissues. The present study confirmed downregulated
expression of miR-4636 in GC tissues compared with normal tissues.
Additionally, the expression of miR-4636 was decreased in GC serum
samples and cell lines compared with controls. The expression of
miR-4636 was significantly associated with lymph node metastasis
and TNM stage in patients with GC. However, there was no
significant association between miR-4636 expression and tumor size,
which may be due to the limited sample size. Therefore, further
investigation with larger study populations are required. These
findings indicated that miR-4636 acted as a potential tumor
suppressor and may be involved in GC development.
Given the results by Yin et al (12), which indicated that miR-4636 may
serve as a clinical biomarker in cervical cancer, the diagnostic
and prognostic value of miR-4636 in patients with GC was evaluated.
The ROC curve of serum miR-4636 levels indicated a relatively high
diagnostic value of miR-4636 in patients with GC. Furthermore, by
analyzing the recorded survival data from a 5-year follow-up
survey, the association between miR-4636 and the survival rate of
patients with GC was investigated. The KM curves demonstrated that
patients with lower miR-4636 expressions had shorter overall
survivals time and poor disease-free survival compared with
patients with higher miR-4636 expression. The Cox regression
analysis revealed that the expression of miR-4636 was an
independent factor for the prognosis of GC. Therefore, miR-4636 may
serve as a biomarker for the diagnosis and prognosis of GC.
Numerous downregulated miRNAs in human malignancies
have been reported to be involved in the regulation of tumor
progression (25). These functional
miRNAs have been proposed as candidate therapeutic targets for the
treatment of cancer (26). In the
pathogenesis of GC, previous studies have reported that miR-625-3p
inhibited tumor cell migration (27)
and that miR-26a-5p overexpression suppressed GC cell
proliferation, migration and invasion (12). The current study demonstrated that
the overexpression of miR-4636 significantly inhibited GC cell
proliferation, migration and invasion, while downregulated
expression resulted in the opposite effects on these biological
processes. Therefore, we hypothesized that miR-4636 may be a tumor
suppressor in GC progression. However, to the best of our
knowledge, miR-4636-related signaling pathways or target genes have
not been reported until now. Thus, the underlying molecular
mechanisms involved in the functional role of miR-4636 in GC
require further investigation. Furthermore, the current study was a
preliminary examination of the biological function of miR-4636
in vitro. Therefore, the role of miR-4636 in GC
tumorigenesis requires corroboration by further investigations,
including colony formation analysis, wound healing assays, flow
cytometry and in vivo experiments.
In conclusion, the present study demonstrated that
miR-4636 expression was decreased in the serum and tissues of
patients with GC and that miR-4636 dysregulation may serve as a
biomarker for the diagnosis and prognosis of GC. Additionally, the
results revealed a significant inhibitory effect of miR-4636 on GC
cell proliferation, migration and invasion, indicating that may
miR-4636 be a potential therapeutic target for the treatment of GC.
Methods to increase miR-4636 expression may help in the development
of novel therapeutic approaches for GC therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JT and CG conducted the clinical study, analyzed
clinical data and wrote the manuscript. YH and CZ performed the
cell experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Prior to sampling, all the patients and healthy
volunteers provided written informed consent for clinical sample
use and analysis, and the experimental methods were approved by the
Ethics Committee of Zibo Central Hospital, Zibo, China (approval
no. ZCHh-090618).
Patient consent for publication
Written informed consent for publication was
obtained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Piazuelo MB and Correa P: Gastric cancer:
Overview. Colomb Med (Cali). 44:192–201. 2013.PubMed/NCBI
|
|
2
|
Correa P: Human gastric carcinogenesis: A
multistep and multifactorial process-First American Cancer Society
Award Lecture on Cancer Epidemiology and Prevention. Cancer Res.
52:6735–6740. 1992.PubMed/NCBI
|
|
3
|
Stock M and Otto F: Gene deregulation in
gastric cancer. Gene. 360:1–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Y, Li M, Zang W, Ma Y, Wang N, Li P,
Wang T and Zhao G: MiR-429 up-regulation induces apoptosis and
suppresses invasion by targeting Bcl-2 and SP-1 in esophageal
carcinoma. Cell Oncol (Dordr). 36:385–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee YS and Dutta A: MicroRNAs in cancer.
Annu Rev Pathol. 4:199–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Liu F, Fu Y, Chen X and Zhang D:
MiR-520d-5p functions as a tumor-suppressor gene in cervical cancer
through targeting PTK2. Life Sci. 254:1175582020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu HY and Pan SS: MiR-202-5p suppressed
cell proliferation, migration and invasion in ovarian cancer via
regulating HOXB2. Eur Rev Med Pharmacol Sci. 24:2256–2263.
2020.PubMed/NCBI
|
|
10
|
Chen X, Zhang L, Song Q and Chen Z:
MicroRNA-216b regulates cell proliferation, invasion and cycle
progression via interaction with cyclin T2 in gastric cancer.
Anticancer Drugs. 31:623–631. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cai H, Lin H, Cao W, Sun J, Huang Y and
Fang Y: The downregulation of miR-519a predicts poor prognosis and
contributes to tumor progression in gastric cancer. Int J Clin Exp
Pathol. 12:2496–2505. 2019.PubMed/NCBI
|
|
12
|
Yin S, Yang M, Li X, Zhang K, Tian J, Luo
C, Bai R, Lu Y and Wang M: Peripheral blood circulating
microRNA-4636/-143 for the prognosis of cervical cancer. J Cell
Biochem. 121:596–608. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C, Zhang CD, Ma MH and Dai DQ:
Three-microRNA signature identified by bioinformatics analysis
predicts prognosis of gastric cancer patients. World J
Gastroenterol. 24:1206–1215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Strong VE: Progress in gastric cancer.
Updates Surg. 70:157–159. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng XJ, Lin JC and Tu SP: Etiology and
prevention of gastric cancer. Gastrointest Tumors. 3:25–36. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wagner AD, Unverzagt S, Grothe W, Kleber
G, Grothey A, Haerting J and Fleig WE: Chemotherapy for advanced
gastric cancer. Cochrane Database Syst Rev. CD0040642010.PubMed/NCBI
|
|
18
|
Pyrhonen S, Kuitunen T, Nyandoto P and
Kouri M: Randomised comparison of fluorouracil, epidoxorubicin and
methotrexate (FEMTX) plus supportive care with supportive care
alone in patients with non-resectable gastric cancer. Br J Cancer.
71:587–591. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
von Felden J and Villanueva A: Role of
molecular biomarkers in liver transplantation for hepatocellular
carcinoma. Liver Transpl. 26:823–831. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan QZ, Liu Q, Zhou YQ, Zhao JJ, Wang QJ,
Li YQ, Tang Y, Gu JM, He J, Chen SP, et al: CIK cell cytotoxicity
is a predictive biomarker for CIK cell immunotherapy in
postoperative patients with hepatocellular carcinoma. Cancer
Immunol Immunother. 69:825–834. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Backes C, Meese E and Keller A: Specific
miRNA disease biomarkers in blood, serum and plasma: Challenges and
prospects. Mol Diagn Ther. 20:509–518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Q, Yang W, Luo Y, Hu S and Zhu L:
Correlation between miR-21 and miR-145 and the incidence and
prognosis of colorectal cancer. J Buon. 23:29–35. 2018.PubMed/NCBI
|
|
23
|
Cai H, Lin H, Cao W, Sun J, Huang Y and
Fang Y: Downregulation of miR-519a predicts poor prognosis and
contributes to tumor progression in gastric cancer. Oncol Res
Treat. 43:19–26. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J and Zou X: MiR-652 serves as a
prognostic biomarker in gastric cancer and promotes tumor
proliferation, migration, and invasion via targeting RORA. Cancer
Biomark. 26:323–331. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu ML, Xiong SW, Zhu SX, Xue XX and Zhou
XD: MicroRNAs in gastric cancer: From bench to bedside. Neoplasma.
66:176–186. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin W, Han H and Liu D: Downregulation
miR-539 is associated with poor prognosis of gastric cancer
patients and aggressive progression of gastric cancer cells. Cancer
Biomark. 26:183–191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Zhou HC, Zhang Y and Huang H:
MicroRNA-625-3p inhibits gastric cancer metastasis through
modulating EZH2. Eur Rev Med Pharmacol Sci. 24:1177–1185.
2020.PubMed/NCBI
|