Introduction
E3 ligases of the ubiquitin proteasome system are
involved in the regulation of protein functions, stability and
degradation; some of the components of the E3 ligase complexes are
considered oncogenes or tumor suppressors with regards to the
development of melanoma (1,2). Previous studies have focused on
identifying gene products whose expression is altered as a disease
progresses, as these may be novel molecular targets for the
treatment of melanoma or new prognostic markers to track the course
of the disease (1,3).
F-box and WD repeat domain-containing 7 (FBXW7) is a
well-studied F-box-containing protein (4–6). F-box
proteins are subunits of SCF-type E3 ligases that recognize the
substrate, bind to it and target it for ubiquitination and
degradation (4). It has been
demonstrated that FBXW7 can act as a tumor suppressor by negatively
regulating the expression levels of several protein oncogenes,
including c-Myc, Notch, cyclin E and c-Jun (5–8). In
melanoma, FBXW7 acts as a tumor suppressor. Studies have revealed
that FBXW7 expression is significantly decreased in primary and
metastatic melanoma samples compared with samples of dysplastic
nevi, and that this decreased FBXW7 expression is associated with
melanoma progression (5,9). However, the mechanism underlying the
decrease in FBXW7 expression in tumors remains unclear.
One of the most important regulators of FBXW7 is p53
(10,11). p53 is a major tumor suppressor
protein, with mutations detected in several types of human cancer,
such as breast cancer, bone and soft tissue sarcoma, brain tumor,
adrenocortical carcinoma, leukemia, stomach cancer and colorectal
cancer (12–17). Mao et al (11) revealed that FBXW7 mediates the
critical role of p53 in responding to DNA damage, suggesting that
FBXW7 may be a p53-dependent gene tumor suppressor involved in
tumor development. Further studies have demonstrated that FBXW7
expression may be restored by targeting the p53 signaling pathway
(10,11).
c-Myc is a protein that is found in ~70% of all
types of human cancer, including leukemia (18,19),
sarcoma (20) and hepatocellular
carcinoma (21,22). c-Myc is important for normal cell
growth, and cellular Myc protein levels are tightly controlled
(23,24). At least four different ubiquitin
ligase complexes can target c-Myc for proteasomal degradation,
including FBXW7 (23). Previous
studies have revealed increased c-Myc expression in advanced and
metastatic melanoma (25–29).
The E3 ubiquitin ligase MDM2 is a major negative
regulator of the p53 tumor suppressor protein, and by suppressing
p53, MDM2 promotes tumor development (30). In normal cells, the presence of MDM2
is essential for maintaining p53 protein expression at a basal
level by regulating its ubiquitination and degradation in the 26S
proteasome (31,32). In addition, MDM2 suppresses p53
function by directly binding to the transcriptional binding site of
p53, thereby preventing its interaction with transcription factors
(33–35). p53 and MDM2 interact and form a
negative autoregulation loop in which elevated p53 transcriptional
levels activate MDM2, which in turn decrease p53 levels (15,30,36).
The aim of the present study was to determine the
protein expression levels of FBXW7, c-Myc, MDM2 and p53 in patients
with dysplastic nevi or melanoma using tissue samples.
Additionally, the associations between the expression levels of
these proteins with clinicopathological parameters and prognosis of
the disease were evaluated to determine whether these proteins may
be used as prognostic factors for patients with melanoma,
potentially allowing for improved modeling of effective
personalized treatment of melanoma.
Materials and methods
Study population
The present study was performed on tissue microarray
(TMA) sections obtained from paraffin blocks of postoperative
material from 100 patients with dysplastic nevi or skin melanoma
treated at the National Cancer Institute (Vilnius, Lithuania)
between January 2013 and December 2018. Histological and
immunohistochemical analysis was performed at the National Center
of Pathology affiliated to Vilnius University Hospital
SantarosKlinikos (Vilnius, Lithuania). The present study was
approved by the Vilnius Regional Committee of Biomedical Research
(approval no. 158200-16-878-387; approval date, 2016-12-13).
The present study included patients >18 years old
with surgically removed and histologically confirmed dysplastic
nevi or melanoma. The present study included 16 patients diagnosed
with dysplastic nevi, 16 with in situ melanoma, 17 with
pathological tumor (pT) 1 stage, 17 with pT2 stage, 17 with pT3
stage and 17 with pT4 stage melanoma. The following
clinicopathological parameters of patients were evaluated: Sex,
age, pT stage (37), morphological
tumor type, ulceration and localization. Of the 100 cases under
analysis, 39 were male and 61 were female, with a median age of 61
years (range, 21–92 years).
Immunohistochemical (IHC)
analysis
The TMAs were constructed from 10% buffered
formalin-fixed (at room temperature for ~24 h) paraffin-embedded
tissue blocks; 2-mm diameter cores were punctured from the tumor
block as randomly selected by a pathologist (1 core per patient),
thus producing 2 TMAs constructed using the tissue arraying
instrument (TMA Master; 3DHISTECH, Ltd.). The TMA blocks for
immunohistochemistry were cut into 2-µm-thick sections and mounted
on TOMO adhesion glass slides (Matsunami Glass Ind., Ltd.). The
sections were deparaffinized and rehydrated in a descending alcohol
series, and antigen retrieval for antibodies against p53 and c-Myc
proteins was performed using DAKO PTLink system with EnVision FLEX
Target buffer (pH 8.0) at 95°C for 20 min (both from Dako; Agilent
Technologies, Inc.), while that for antibodies against FBXW7 and
MDM2 proteins was performed using a Ventana Benchmark Ultra system
with Cell Conditioning solution (pH 8.5) at 100°C for 36 min. (both
from Ventana Medical Systems, Inc.; Roche Diagnostics). The
sections were blocked with FLEX Peroxide Block (cat. no. SM801;
Dako; Agilent Technologies, Inc.) at room temperature for 5 min for
p53, 10 min for c-Myc and 8 min for FBXW7 and MDM2.Subsequently,
the sections were incubated with antibodies against p53 (1:200;
cat. no. M7001, clone DO-7; Dako; Agilent Technologies, Inc.) and
c-Myc (1:40; cat. no. ab32072, cloneY69; Abcam) at room temperature
for 30 min, then incubated using a DAKO EnVision FLEX system (Dako;
Agilent Technologies, Inc.) for 20 min at room temperature.
Incubation with antibodies against FBXW7 (1:50; cat. no. MA5-26562,
clone OTI6F5; Thermo Fisher Scientific, Inc.) and MDM2 (1:250; cat.
no. MA1-113, clone IF2; Invitrogen; Thermo Fisher Scientific, Inc.)
was performed at 37°C for 32 min and then using the
VentanaUltraview DAB detection kit (Ventana Medical Systems, Inc.;
Roche Diagnostics) for 8 min at 37°C. Finally, the sections were
developed using DAB, counterstained using Mayer's hematoxylin at
room temperature for 10 min and mounted. Negative controls were
performed by omitting the application of the primary antibody. The
IHC slides were observed using a light microscope at a
magnification of ×20 (0.5 µm resolution) using a bright field
AperioScanScope XT Slide Scanner (Leica Microsystems, Inc.).
Assessment of FBXW7, c-Myc, MDM2 and
p53 expression
The pathologist performed a visual assessment of
staining intensity and overall percentage of cells staining. The
intensity of protein immunostaining was scored as 0–3: 0, Negative
staining; 1, weak staining; 2, moderate staining; and 3, strong
staining. The percentage of nuclear staining was graded in 4
categories: 1, 0–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%. The
combined score obtained by multiplying the staining intensity score
with the staining percentage score was graded as follows: 0–6, Low
expression; and 7–12, high expression.
Statistical analysis
Statistical analysis was performed using STATA v11.2
(StataCorp LP). Fisher's exact test was used to evaluate the
association between protein expression and the patient
clinicopathological parameters. Univariate and multivariate Cox
proportional and hazard regression analyses were performed to
estimate the crude hazard ratios (HRs), adjusted HRs and 95%
confidence intervals (CIs) of HRs. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
Based on the morphological type of melanoma, there
were 48 cases of superficial melanoma, 27 cases of nodular melanoma
and 9 cases of lentigomaligna. Of all the melanoma cases analyzed,
26 had melanoma depths of ≤1 mm and 42 cases had depths of invasion
>1 mm, while 32 cases were non-invasive. Of the 68 melanoma
cases ranging from pathological stages pT1-pT4, 22 were ulcerated.
In 77% of the cases, the tumor was diagnosed in sun-exposed areas
(Table I).
 | Table I.Association between FBXW7 protein
expression and clinicopathological variables. |
Table I.
Association between FBXW7 protein
expression and clinicopathological variables.
|
|
| FBXW7 expression, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
variables | N | High | Low | P-value |
|---|
| All cases | 100 | 53 (53.0) | 47 (47.0) | – |
| Sex |
|
|
| 0.41 |
|
Male | 39 | 23 (54.8) | 16 (45.2) |
|
|
Female | 61 | 30 (49.2) | 31 (50.8) |
|
| Age, years |
|
|
| 0.69 |
|
≤58 | 44 | 25 (56.8) | 19 (43.2) |
|
|
>58 | 56 | 28 (50.0) | 28 (50.0) |
|
| pT stage |
|
|
| 0.001a |
|
Dysplastic nevi | 16 | 15 (93.7) | 1 (6.25) |
|
|
pTis | 16 | 14 (73.7) | 2 (12.5) |
|
|
pT1 | 17 | 15 (88.2) | 2 (11.8) |
|
|
pT2 | 17 | 6 (35.3) | 11(64.7) |
|
|
pT3 | 17 | 1 (5.9) | 16 (94.1) |
|
|
pT4 | 17 | 2 (11.8) | 15 (88.2) |
|
| Depth of invasion,
mm |
|
|
| 0.001a |
| ≤1 | 26 | 20 (76.9) | 6 (23.1) |
|
|
>1 | 42 | 4 (9.5) | 38 (90.5) |
|
|
Non-invasive | 32 | 29 (90.6) | 3 (9.4) |
|
| Morphology |
|
|
| 0.001a |
|
Superficial spreading | 48 | 26 (54.2) | 22 (45.8) |
|
|
Lentigomaligna | 9 | 7 (77.8) | 2 (22.2) |
|
|
Nodular | 27 | 5 (18.5) | 22 (81.5) |
|
|
Dysplastic nevi | 16 | 15 (93.7) | 1 (6.5) |
|
| Site |
|
|
| 0.54 |
|
Sun-protected | 13 | 5 (38.5) | 8 (61.5) |
|
|
Sun-exposed | 77 | 42 (54.5) | 35 (45.5) |
|
|
Unknown | 10 | 6 (60.0) | 4 (4.0) |
|
| Ulceration in
melanoma (pT1-pT4) | 68 |
|
| 0.18 |
|
Present | 22 | 5 (22.7) | 17 (77.3) |
|
|
Absent | 46 | 19 (41.3) | 27 (58.7) |
|
FBXW7 expression is associated with
pathological stage, melanoma invasion depth and tumor morphological
type
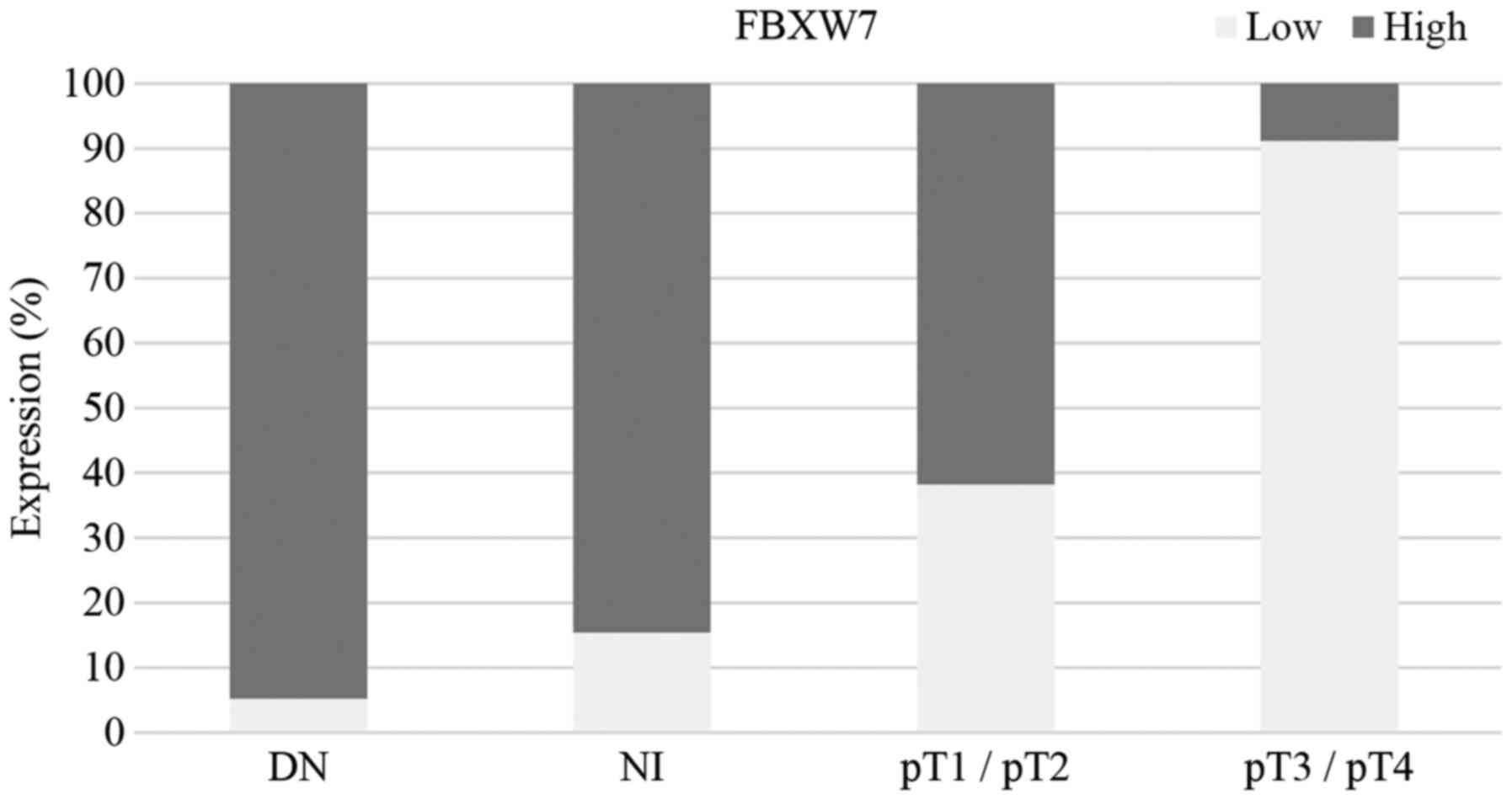
High FBXW7 expression was observed in 53% of
samples, and low expression was observed in 47% of cases (Table I). There was a significant
association between FBXW7 expression and pT stage. There was a
significantly lower level of FBXW7 expression in advanced melanoma
(pT3/pT4) compared with dysplastic nevi, melanoma in situ
and pT1/pT2 stage melanoma (P<0.001; Fisher's exact test;
Table I). Fig. 1 shows the proportion of high/low
FBXW7 expression in dysplastic nevi, melanoma in situ,
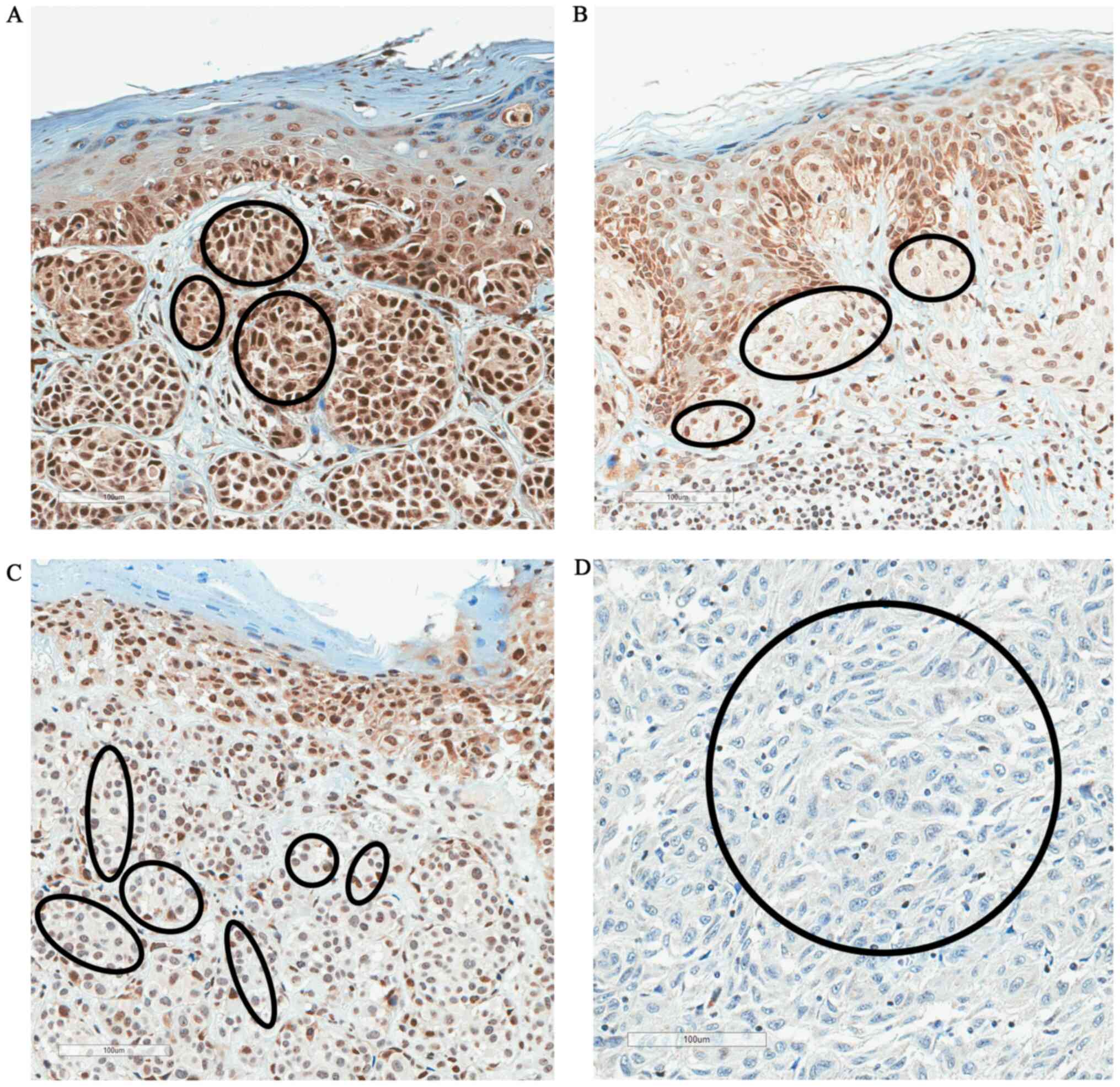
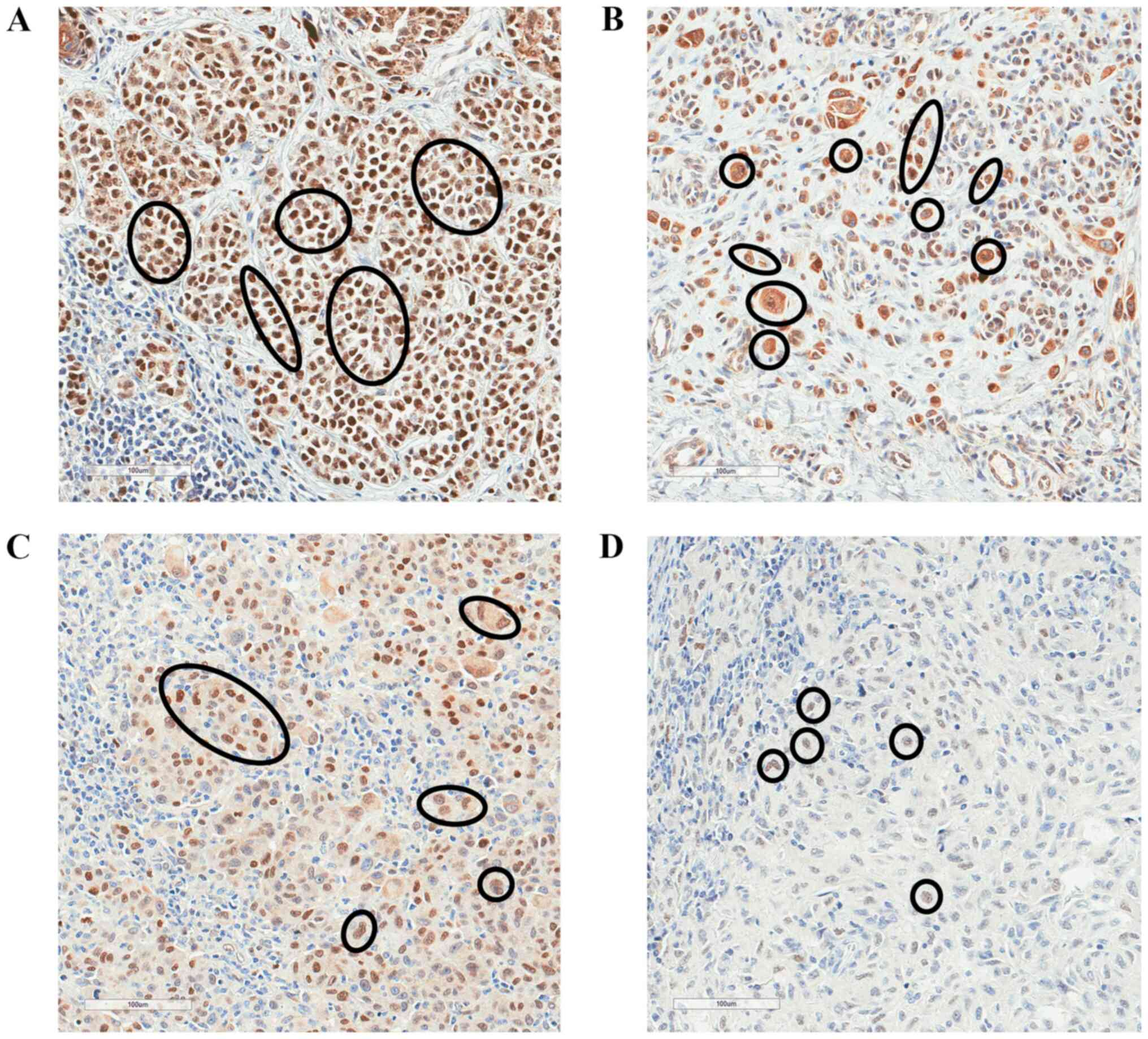
pT1/pT2 and pT3/pT4 melanoma. Fig. 2
shows the changes of immunostaining of FBXW7 protein depending on
pT stage (pT1-pT4). There was strong FBXW7 immunostaining in pT1
melanoma, moderate staining in pT2, weak staining in pT3 and
negative staining in pT4 melanoma (Fig.
2).
Additionally, there was a statistically significant
association between FBXW7 expression and the morphological type of
the tumor (P<0.001); high FBXW7 expression was observed in 93.7%
of dysplastic nevus tissues, 77.8% of lentigomaligna cases and
54.2% of cases of superficial spreading melanoma, whereas 81.5% of
nodular melanomas exhibited low FBXW7 expression (Table I). Furthermore, there was a
statistically significant association between FBXW7 expression and
the tumor invasion (P<0.001), with a depth ≤1 mm observed in
76.9% of cases with high FBXW7 expression, whereas 90.5% of cases
with melanoma invasion >1 mm exhibited low FBXW7 expression
(Table I). There was no
statistically significant association between FBXW7 expression and
sex, age or tumor localization (Table
I).
p53 expression is associated with the
depth of melanoma invasion and the morphological type of the
tumor
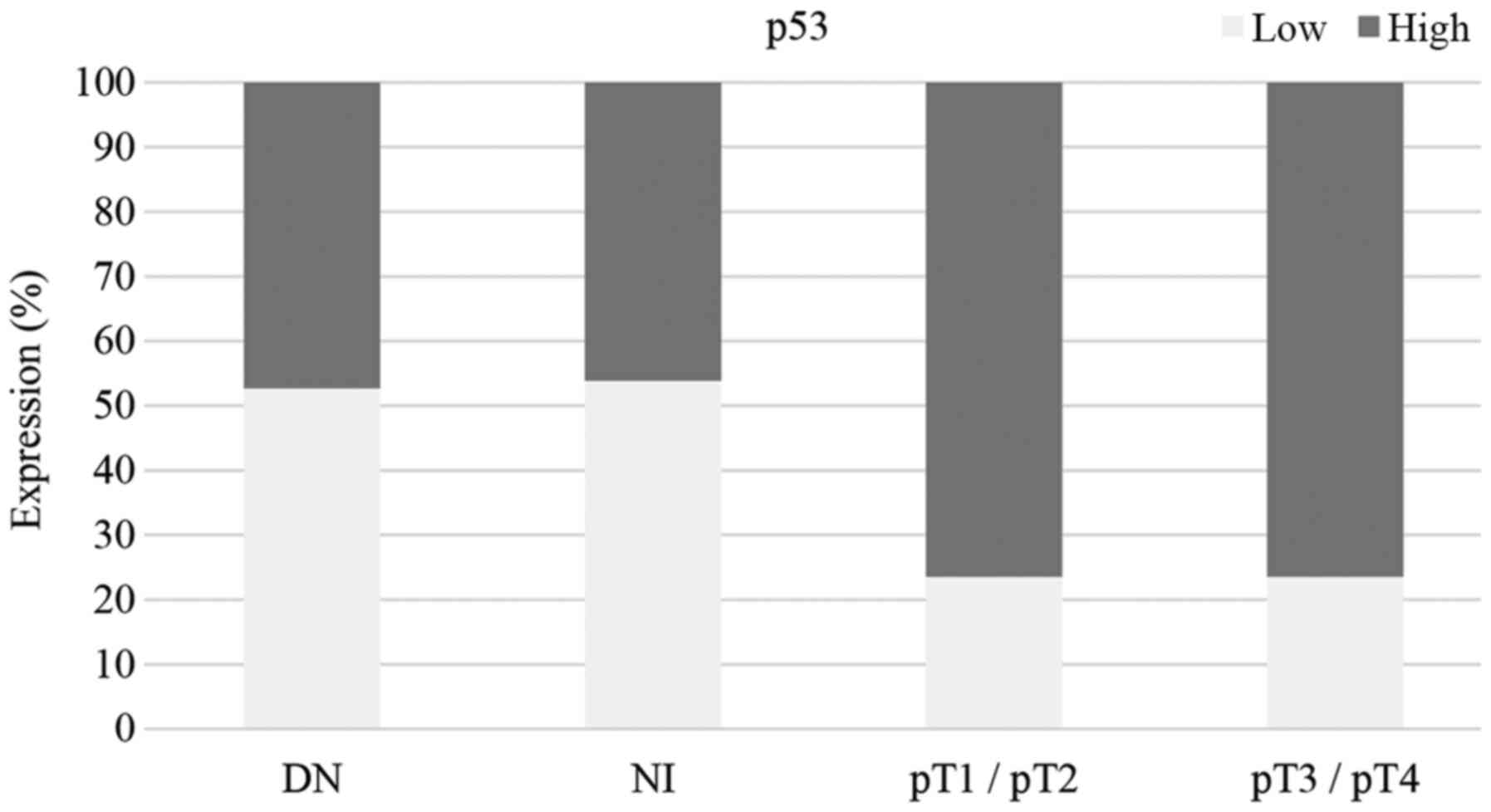
There was no statistically significant association
between p53 expression and melanoma pT stage. Almost equal
proportions of high and low expression levels of p53 were observed
in dysplastic nevi and melanoma in situ. Higher proportions
of high p53 expression were observed in stage pT1/pT2 and pT3/pT4
melanoma samples compared with high p53 expression in dysplastic
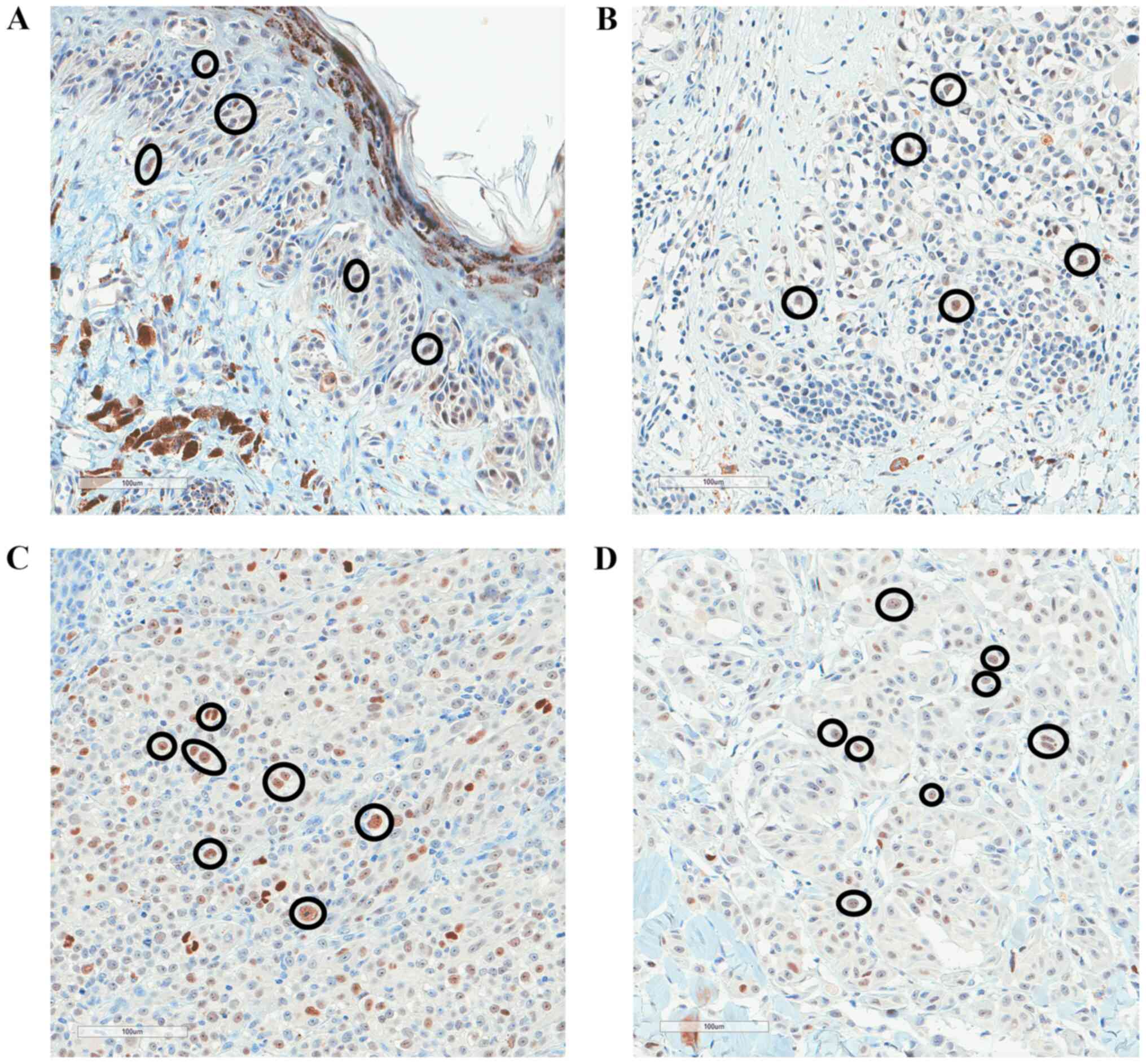
nevi and melanoma in situ tissues (Fig. 3). High p53 expression was observed in
70–80% of melanoma samples ranging from stage pT1 to pT4, but p53
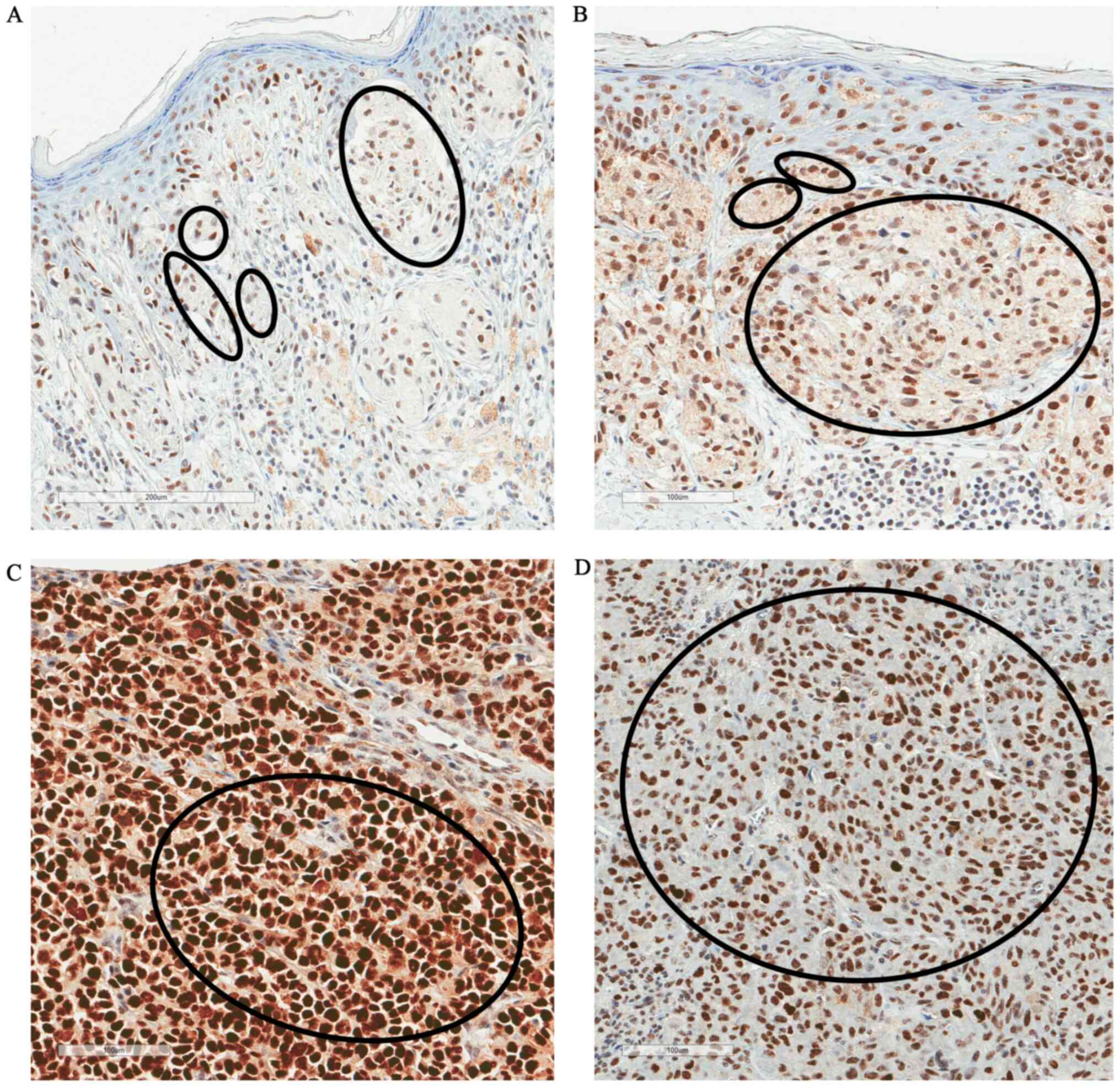
expression was not associated with pT stage (P=0.06; Table II). Fig.
4 shows the immunostaining of p53 protein in different pT
melanoma stages (pT1-pT4). Additionally, there was no association
between p53 expression with sex, age and tumor localization.
 | Table II.Association between p53 protein
expression and clinicopathological variables. |
Table II.
Association between p53 protein
expression and clinicopathological variables.
|
|
| p53 expression, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
variables | N | High | Low | P-value |
|---|
| All cases | 100 | 53 (53.0) | 47 (47.0) | – |
| Sex |
|
|
| 0.20 |
|
Male | 39 | 23 (59.0) | 16 (41.0) |
|
|
Female | 61 | 44 (72.1) | 17 (27.9) |
|
| Age, years |
|
|
| 0.52 |
|
≤58 | 44 | 31 (70.5) | 13 (29.5) |
|
|
>58 | 56 | 36 (64.3) | 20 (35.7) |
|
| pT stage |
|
|
| 0.06 |
|
Dysplastic nevi | 16 | 8 (50.0) | 8 (50.0) |
|
|
pTis | 16 | 7 (43.7) | 9 (56.3) |
|
|
pT1 | 17 | 14 (82.3) | 3 (17.7) |
|
|
pT2 | 17 | 12 (70.6) | 5 (29.4) |
|
|
pT3 | 17 | 12 (70.6) | 5 (29.4) |
|
|
pT4 | 17 | 14 (82.3) | 3 (17.7) |
|
| Depth of invasion,
mm |
|
|
| 0.02a |
| ≤1 | 26 | 20 (76.9) | 6 (23.1) |
|
|
>1 | 42 | 32 (76.2) | 10 (23.8) |
|
|
Non-invasive | 32 | 15 (46.9) | 17 (53.1) |
|
| Morphology |
|
|
| 0.01a |
|
Superficial spreading | 48 | 33 (68.8) | 15 (31.2) |
|
|
Lentigomaligna | 9 | 3 (33.3) | 6 (66.7) |
|
|
Nodular | 27 | 23 (85.2) | 4 (14.8) |
|
|
Dysplastic nevi | 16 | 8 (50.0) | 8 (50.0) |
|
| Site |
|
|
| 0.35 |
|
Sun-protected | 13 | 11 (84.6) | 2 (15.4) |
|
|
Sun-exposed | 77 | 49 (63.6) | 28 (36.4) |
|
|
Unknown | 10 | 7 (70.0) | 3 (30.0) |
|
There was a statistically significant association
between p53 expression and depth of melanoma invasion (P=0.02);
when melanoma depth was ≤1 and >1 mm, high p53 expression was
observed in 76.9 and 76.2% of cases, respectively, whereas a low
p53 protein expression was detected in 53.1% of non-invasive tumors
(Table II). There was also a
statistically significant association between p53 expression and
the morphological type of the tumor (P=0.01), with high p53
expression observed in 85.2% of nodular melanomas and in 68.8% of
superficial spreading melanomas, while p53 expression in
lentigomaligna tissues was mainly low (Table II).
c-Myc protein expression is not
associated with advanced melanoma
Examination of c-Myc protein expression (Fig. 5) revealed that its expression was
high in 36% of cases and low in 64% of cases. c-Myc protein
expression was not significantly associated with advanced melanoma.
Similarly, c-Myc expression was not associated with any
clinicopathological parameters, including sex, age, morphological
type of the tumor, depth of invasion and localization (Table III).
 | Table III.Association between c-Myc protein
expression and clinicopathological variables. |
Table III.
Association between c-Myc protein
expression and clinicopathological variables.
|
|
| c-Myc expression, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
variables | N | High | Low | P-value |
|---|
| All cases | 100 | 36 (36.0) | 64 (64.0) | – |
| Sex |
|
|
| 0.29 |
|
Male | 39 | 17 (43.6) | 22 (56.4) |
|
|
Female | 61 | 19 (31.1) | 42 (68.9) |
|
| Age, years |
|
|
| 0.53 |
|
≤58 | 44 | 14 (31.8) | 30 (68.2) |
|
|
>58 | 56 | 22 (39.3) | 34 (60.7) |
|
| pT stage |
|
|
| 0.75 |
|
Dysplastic nevus | 16 | 8 (50.0) | 8 (50.0) |
|
|
pTis | 16 | 6 (37.5) | 10 (62.5) |
|
|
pT1 | 17 | 7 (41.2) | 10 (58.8) |
|
|
pT2 | 17 | 5 (29.4) | 12 (70.6) |
|
|
pT3 | 17 | 5 (29.4) | 12 (70.6) |
|
|
pT4 | 17 | 5 (29.4) | 12 (70.6) |
|
| Depth of invasion,
mm |
|
|
| 0.37 |
| ≤1 | 26 | 10 (38.5) | 16 (61.5) |
|
|
>1 | 42 | 12 (28.6) | 30 (71.4) |
|
|
Non-invasive | 32 | 14 (43.7) | 18 (56.3) |
|
| Morphology |
|
|
| 0.57 |
|
Superficial spreading | 48 | 17 (35.4) | 31 (64.6) |
|
|
Lentigomaligna | 9 | 3 (33.3) | 6 (66.7) |
|
|
Nodular | 27 | 8 (29.6) | 19 (70.4) |
|
|
Dysplastic nevus | 16 | 8 (50.0) | 8 (50.0) |
|
| Site |
|
|
| 0.59 |
|
Sun-protected | 13 | 5 (38.5) | 8 (61.5) |
|
|
Sun-exposed | 77 | 29 (37.7) | 48 (62.3) |
|
|
Unknown | 10 | 2 (20.0) | 8 (80.0) |
|
Decreased c-Myc expression was observed in 56% of
melanoma cases, and in 71.4% of these cases, decreased expression
was observed at melanoma depths >1 mm. There were no changes in
c-Myc expression in dysplastic nevus, as both increased and
decreased expression was observed in 50% of cases. There was a
slight decrease in c-Myc expression observed in cases with melanoma
in situ and stage pT1 melanoma, and low c-Myc protein
expression levels were observed in 70.6% of melanoma cases in
stages pT2, pT3 and pT4. However, there was no statistically
significant association between c-Myc expression levels and the
pathological stage of melanoma (P=0.75).
MDM2 expression levels are not
associated with advanced melanoma
The present study revealed that there was low MDM2
expression in 97% of all investigated tumor samples; therefore,
MDM2 expression did not exhibit any statistically significant
association with advanced melanoma or any other clinicopathological
parameters (Table IV and Fig. 6).
 | Table IV.Association between MDM2 protein
expression and clinicopathological variables. |
Table IV.
Association between MDM2 protein
expression and clinicopathological variables.
|
|
| MDM2 expression, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
variables | N | High | Low | P-value |
|---|
| All cases | 100 | 3 (3.0) | 97 (97.0) | – |
| Sex |
|
|
| 0.56 |
|
Male | 39 | 2 (5.1) | 37 (94.9) |
|
|
Female | 61 | 1 (1.1) | 60 (98.9) |
|
| Age, years |
|
|
| 0.58 |
|
≤58 | 44 | 2 (4.5) | 42 (95.5) |
|
|
>58 | 56 | 1 (1.8) | 55 (98.2) |
|
| pT stage |
|
|
| 0.99 |
|
Dysplastic nevus | 16 | 0 (0.0) | 16 (100.0) |
|
|
pTis | 16 | 1 (6.3) | 15 (93.7) |
|
|
pT1 | 17 | 1 (5.9) | 16 (94.1) |
|
|
pT2 | 17 | 0 (0.0) | 17 (100.0) |
|
|
pT3 | 17 | 1 (5.9) | 16 (94.1) |
|
|
pT4 | 17 | 0 (0.0) | 17 (100.0) |
|
| Depth of invasion,
mm |
|
|
| 0.99 |
| ≤1 | 26 | 1 (3.8) | 25 (96.2) |
|
|
>1 | 42 | 1 (2.4) | 41 (97.6) |
|
|
Non-invasive | 32 | 1 (3.1) | 31 (96.9) |
|
| Morphology |
|
|
| 0.34 |
|
Superficial spreading | 48 | 1 (2.1) | 47 (97.9) |
|
|
Lentigomaligna | 9 | 1 (11.1) | 8 (88.9) |
|
|
Nodular | 27 | 1 (3.7) | 26 (96.3) |
|
|
Dysplastic nevus | 16 | 0 (0.0) | 16 (100.0) |
|
| Site |
|
|
| 0.99 |
|
Sun-protected | 13 | 0 (0.0) | 13 (100.0) |
|
|
Sun-exposed | 77 | 3 (3.9) | 74 (96.1) |
|
|
Unknown | 10 | 0 (0.0) | 10 (100.0) |
|
FBXW7 protein expression is associated
with c-Myc protein expression
Fisher's exact test was performed to evaluate the
association between FBXW7, c-Myc, MDM2 and p53 expression. The
results revealed that there was a statistically significant
association between FBXW7 and c-Myc expression, revealing that
76.6% of cases with low FBXW7 expression had also low c-Myc
expression (P=0.02; Table V). There
was no other statistically significant association between protein
expression.
 | Table V.Association between the changes in
the protein expression levels of FBXW7, c-Myc, MDM2 and p53. |
Table V.
Association between the changes in
the protein expression levels of FBXW7, c-Myc, MDM2 and p53.
|
|
| p53, n (%) |
| c-Myc, n (%) |
| MDM2, n (%) |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Proteins | Expression
levels | High | Low | P-value | High | Low | P-value | High | Low | P-value |
|---|
| c-Myc | High | 27 (75.0) | 9 (25.0) | 0.27 |
|
|
|
|
|
|
|
| Low | 40 (62.5) | 24 (37.5) |
|
|
|
|
|
|
|
| MDM2 | High | 2 (66.7) | 1 (33.3) | 0.99 | 2 (66.7) | 1 (33.3) | 0.29 |
|
|
|
|
| Low | 34 (35.0) | 36 (65.0) |
| 34 (35.0) | 36 (65.0) |
|
|
|
|
| FBXW7 | High | 33 (62.3) | 20 (37.7) | 0.30 | 25 (47.2) | 28 (52.8) | 0.02a | 2 (3.8) | 51 (96.2) | 0.99 |
|
| Low | 34 (72.3) | 13 (27.7) |
| 11 (23.4) | 36 (76.6) |
| 1 (2.1) | 46 (97.9) |
|
High FBXW7 expression is positively
associated with survival
A univariate Cox regression analysis was used to
determine which of the analyzed clinicopathological indicators may
be associated with survival. The results revealed that sex, age,
morphological type and the localization of tumors were not
significantly associated with mortality. However, tumor ulceration
was found to increase the risk of death by 2.79 times, although
this rate was not statistically significant (P=0.06). Patient
survival was significantly influenced by pT stage, where the risk
of death increased by 5.6 times with advanced stage (P=0.03).
Taking into account the impact of changes in the expression levels
of FBXW7, c-Myc and p53 on the risk of death, the results revealed
that high FBXW7 expression decreased the risk of death and improved
survival (P=0.08), and high expression levels of p53 increased the
risk of death by 2.48 times (P=0.10). However, these rates were not
statistically significant. The results revealed that c-Myc
expression was not significantly associated with mortality
(Table VI). A multivariate Cox
regression analysis revealed no statistically significant results
(Table VII).
 | Table VI.Univariate Cox regression analysis of
protein expression and clinicopathological variables predicting the
survival of patients with cutaneous melanoma. |
Table VI.
Univariate Cox regression analysis of
protein expression and clinicopathological variables predicting the
survival of patients with cutaneous melanoma.
| Variables | HR (95% CI) | P-value |
|---|
| Sex, males vs.
females | 2.01
(0.56–7.22) | 0.29 |
| Age, ≤58 vs. >58
years | 1.16
(0.39–3.49) | 0.79 |
| pT stage, pT1/pT2
vs. pT3/pT4 | 5.60
(1.24–25.2) |
0.03a |
| Morphology,
superficial spreading vs. other | 2.48
(0.83–7.43) | 0.10 |
| Ulceration, absent
vs. present | 2.79
(0.95–8.08) | 0.06 |
| Site, sun-protected
vs. sun-exposed | 0.57
(0.16–2.11) | 0.40 |
| p53 expression, low
vs. high | 2.48
(0.83–7.43) | 0.10 |
| c-Myc expression,
low vs. high | 0.36
(0.08–1.63) | 0.19 |
| FBXW7 expression,
low vs. high | 0.16
(0.02–1.23) | 0.08 |
 | Table VII.Multivariate Cox regression analysis
of protein expression and clinicopathological variables predicting
the survival of patients with cutaneous melanoma. |
Table VII.
Multivariate Cox regression analysis
of protein expression and clinicopathological variables predicting
the survival of patients with cutaneous melanoma.
| Variables | HR (95% CI) | P-value |
|---|
| Sex, males vs.
females | 1.47
(0.31–7.07) | 0.48 |
| Age, ≤58 vs. >58
years | 0.69
(0.13–3.55) | 0.66 |
| pT stage, pT1/pT2
vs. pT3/pT4 | 3.13
(0.38–25.7) | 0.29 |
| Morphology,
superficial spreading vs. other | 1.37
(0.28–6.60) | 0.69 |
| Ulceration, absent
vs. present | 2.33
(0.60–9.09) | 0.23 |
| Site, sun-protected
vs. sun-exposed | 0.42
(0.09–1.98) | 0.27 |
| p53 expression, low
vs. high | 0.65
(0.16–2.70) | 0.55 |
| c-Myc expression,
low vs. high | 0.35
(0.07–1.72) | 0.19 |
| FBXW7 expression,
low vs. high | 0.65
(0.05–9.12) | 0.75 |
Discussion
FBXW7 is a tumor suppressor that controls the
protein expression levels of several oncogenes, including c-Myc,
Notch, Cyclin E, c-Jun, Mcl-1 and m-TOR (5,38,39).
However, little is known regarding the regulation of FBXW7 in
tumors. Regulation of FBXW7 expression may occur at the
transcriptional or protein levels, as well as by post-translational
modifications, such as phosphorylation (40). p53 molecules, NUMB4, NF-κB1,
microRNA-27 and microRNA-223 are known to be important in FBXW7
regulation (10).
Previous studies have revealed decreased FBXW7
activity in melanoma cells (3,9). The
present study demonstrated that FBXW7 expression was lower in
primary melanoma compared with dysplastic nevi. FBXW7 expression in
metastatic melanoma was lower compared with in primary melanoma,
and its decreased expression was associated with a less favorable
5-year survival rate (9).
Furthermore, in vitro experiments have demonstrated that
FBXW7 suppresses the migration of melanoma cells via the MAPK/ERK
signaling pathway; therefore, suppression of FBXW7 in melanoma
cells results in increased cell migration and stress fiber
formation (9).
The results of the present study revealed that FBXW7
expression decreased in advanced melanoma, and a statistically
significant association was found between the decrease in FBXW7
expression and the increasing pT stage of melanoma. Additionally, a
trend was observed between decreased FBXW7 expression and an
increased risk of death in patients, although this association was
not significant.
Previous studies have demonstrated that decreased
FBXW7 expression is associated with melanoma progression and the
accumulation of c-Myc protein (3,9,41). c-Myc is one of the major targets of
FBXW7, and c-Myc regulates the expression of >15% of the genes
involved in processes of cell differentiation, proliferation,
protein synthesis, metabolism and apoptosis; thus, impaired c-Myc
function may underlie tumor formation (5,42). FBW7α
promotes ubiquitination of Myc in proteasomes, whereas FBW7γ
ubiquitinates Myc in the nucleus and thus suppresses the ability of
Myc to promote cell growth (41,43–46).
Therefore, a decrease in FBXW7 expression results in increased
c-Myc expression (10).
In the present study, 17 cases of stage pT2
melanoma, 17 cases of stage pT3 melanoma and 17 cases of stage pT4
melanoma were examined, and low c-Myc protein expression was
detected in 12 cases (70.6%) in each group. According to a
comparison of these groups with melanoma in situ and stage
pT1 melanoma, the changes in c-Myc protein expression were not
statistically significant. In addition, there was a strong direct
association observed between the changes in FBXW7 and c-Myc
expression with a decrease in FBXW7 expression, and a decrease in
c-Myc expression was also observed. These results differ from those
of previously published studies (41,45,47). The
results of the present study may be influenced by the sample size.
In addition, melanoma is a heterogeneous tumor and its development
and progression is affected by the interaction of multiple genes
and various signaling pathways (48). In some types of cancer, c-Myc may
acquire loss-of-function mutations, while in the majority of cases,
c-Myc expression is upregulated (49–52). In
both cases, altered c-Myc expression results in tumor formation
through disrupted transcription, translation or differentiation
(42,49).
One of the most important regulators of FBXW7 is
p53; p53 is a major tumor suppressor protein and is frequently
mutated in several types of cancer, such as breast cancer, bone and
soft tissue sarcoma, brain tumor, adrenocortical carcinoma,
leukemia, stomach cancer and colorectal cancer (12–17).
Previous studies have demonstrated that FBXW7 is a p53-dependent
tumor suppressor gene involved in tumor development (10,11).
Additionally, studies have revealed that FBXW7 expression may be
restored via targeting the p53 signaling pathway (10,11). The
increased expression levels of wild-type p53 have been previously
demonstrated in melanoma (53).
However, considering the malignant nature and resistance to
treatment in cases of melanoma (54,55), p53
does not appear to be effective as a tumor suppressor in melanoma.
Although the mechanisms are not yet fully understood, certain p53
targets have been shown to be downregulated (56,57).
Increased expression levels of the MDM2 oncoprotein
have been reported in several types of human cancer, including
sarcoma, glioma, hematologic malignancies, melanoma and carcinoma,
carrying the wild-type p53 allele (35,58).
High levels of MDM2 are associated with a worse prognosis in
several types of cancer, such as sarcoma, glioma and pediatric
acute lymphoblastic leukemia (58,59).
Malignant melanoma is characterized by increased MDM2 expression
(60). Rajabi et al (61) revealed that there was an association
between MDM2 expression with tumor thickness and invasion in
primary cutaneous malignant melanoma. In 50% of melanoma cases,
strong MDM2 expression is detected, leading to enhanced degradation
of p53, and thus resulting in tumor cell proliferation (56,60,62).
In the present study, upregulated p53 expression was
observed in primary and invasive melanoma. In cases of melanoma
with a thickness >1 mm, p53 expression was increased compared
with in non-invasive tumors, whereas FBXW7 expression decreased in
advanced melanoma. Previous studies have demonstrated that the
first exon of FBXW7 has a p53 binding site (11,63).
Additionally, decreased FBXW7 expression following genotoxic stress
may be activated by p53 (6,42). However, in the present study, there
was no statistically significant association between p53 expression
and the changes in FBXW7 expression.
p53 and MDM2 interact and form a negative
autoregulatory loop in which elevated p53 transcriptional levels
activate MDM2, which in turn decreases the levels of p53 (15,30,36). The
present study revealed that there was a decrease in MDM2 expression
in almost all cases assessed (97%). In contrast to published
studies (60–62,64), the
results of the present study did not identify a significant
association between MDM2 expression and melanoma invasion, and
there was no association between the decrease in MDM2 expression
and p53 expression.
The main limitation of the present study consists in
a relatively small number of subjects in the analyzed groups,
assembled according to the stage of melanoma by depth. The sample
size may have influenced the results obtained and the assessment of
the effect of low FBXW7 protein expression on patient survival.
Future studies should continue to analyze the expression levels of
E3 ubiquitin ligases in melanoma tissues, since it is important to
identify new potential targets for the treatment of melanoma, as
well as to evaluate their prognostic significance. Future studies
should investigate the expression levels of E3 ligases and their
substrates at the mRNA level, as well as other genes involved in
the development of melanoma, and should evaluate their
interactions, their changes in expression associated with clinical
characteristics and their prognostic significance.
In conclusion, the present study demonstrated that
FBXW7 exhibited the most statistically significant prognostic value
and associations with advanced melanoma. As most of the FBXW7
substrates are oncoproteins, their degradation by FBXW7 may
underlie the mechanism by which decreased FBXW7 expression results
in tumor progression and may highlight these proteins as potential
targets for the treatment of melanoma.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Cancer
Institute (grant no. ESFA Nr.09.3.3-V-711-01-0001) and the project
‘Development of doctoral studies’ realization according to the
2014–2020 European Union Funds Programme of Investment Activity,
priority 9 ‘Education of society and increasing of human source
potential’ (grant no. ESFA-09.3.3-V-711) and ‘Support for
scientific research by scientists and other researchers’.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM selected the patients, collected the
clinicopathological data, interpreted the results and prepared the
manuscript. ZG was involved in drafting and revising the
manuscript, and made substantial contributions to the analysis and
interpretation of data. IV performed the statistical analysis,
interpreted the results and created the graphs and tables. AL was
involved in performing the tissue microarray and
immunohistochemical analysis, drafted and revised the manuscript.
JP performed the histological examination of tissue samples of
melanoma and dysplastic nevi, assisted in the tissue microarray
preparation and evaluated the immunohistochemical staining. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Vilnius
Regional Committee of Biomedical Research (approval no.
158200-16-878-387, approval date 2016-12-13, and addition no.
158200-878-PP1-05, 2017-03-17). All patients signed informed
consent forms to participate in the present study (approval no.
II-2016-5, 2016-12-07, version 4, and approval no. II-2016-5,
2017-02-14, version 5).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morrow JK, Lin HK, Sun SC and Zhang S:
Targeting ubiquitination for cancer therapies. Future Med Chem.
7:2333–2350. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma J, Guo W and Li C: Ubiquitination in
melanoma pathogenesis and treatment. Cancer Med. 6:1362–1377. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aydin IT, Melamed RD, Adams SJ,
Castillo-Martin M, Demir A, Bryk D, Brunner G, Cordon-Cardo C,
Osman I, Rabadan R, et al: FBXW7 mutations in melanoma and a new
therapeutic paradigm. J Natl Cancer Inst. 106:dju1072014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie CM, Wei W and Sun Y: Role of
SKP1-CUL1-F-box-protein (SCF) E3 ubiquitin ligases in skin cancer.
J Genet Genomics. 40:97–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Welcker M and Clurman BE: FBW7 ubiquitin
ligase: A tumour suppressor at the crossroads of cell division,
growth and differentiation. Nat Rev Cancer. 8:83–93. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Inuzuka H, Zhong J, Wan L,
Fukushima H, Sarkar FH and Wei W: Tumor suppressor functions of
FBW7 in cancer development and progression. FEBS Lett.
586:1409–1418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang HL, Weng HY, Wang LQ, Yu CH, Huang
QJ, Zhao PP, Wen JZ, Zhou H and Qu LH: Triggering Fbw7-mediated
proteasomal degradation of c-Myc by oridonin induces cell growth
inhibition and apoptosis. Mol Cancer Ther. 11:1155–1165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu H, Wang K, Fu H and Song J: Low
expression of the ubiquitin ligase FBXW7 correlates with poor
prognosis of patients with colorectal cancer. Int J Clin Exp
Pathol. 11:413–419. 2018.PubMed/NCBI
|
|
9
|
Cheng Y, Chen G, Martinka M, Ho V and Li
G: Prognostic significance of Fbw7 in human melanoma and its role
in cell migration. J Invest Dermatol. 133:1794–1802. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Ye X, Liu Y, Wei W and Wang Z:
Aberrant regulation of FBW7 in cancer. Oncotarget. 5:2000–2015.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mao JH, Perez-Losada J, Wu D, Delrosario
R, Tsunematsu R, Nakayama KI, Brown K, Bryson S and Balmain A:
Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor
gene. Nature. 432:775–779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pei D, Zhang Y and Zheng J: Regulation of
p53: A collaboration between Mdm2 and Mdmx. Oncotarget. 3:228–235.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Madan E, Gogna R, Bhatt M, Pati U,
Kuppusamy P and Mahdi AA: Regulation of glucose metabolism by p53:
Emerging new roles for the tumor suppressor. Oncotarget. 2:948–957.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Muller PAJ and Vousden KH: p53 mutations
in cancer. Nat Cell Biol. 15:2–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wade M, Li Y and Wahl G: MDM2, MDMX and
p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 13:83–96.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Olivier M, Goldgar DE, Sodha N, Ohgaki H,
Kleihues P, Hainaut P and Eeles RA: Li-Fraumeni and related
syndromes: Correlation between tumor type, family structure, and
TP53 genotype. Cancer Res. 63:6643–6650. 2003.PubMed/NCBI
|
|
17
|
Birch JM, Alston RD, McNally RJ, Evans DG,
Kelsey AM, Harris M, Eden OB and Varley JM: Relative frequency and
morphology of cancers in carriers of germline TP53 mutations.
Oncogene. 20:4621–4628. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Felsher DW and Bishop JM: Reversible
tumorigenesis by MYC in hematopoietic lineages. Mol Cell.
4:199–207. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marinkovic D, Marinkovic T, Mahr B, Hess J
and Wirth T: Reversible lymphomagenesis in conditionally c-MYC
expressing mice. Int J Cancer. 110:336–342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jain M, Arvanitis C, Chu K, Dewey W,
Leonhardt E, Trinh M, Sundberg CD, Bishop JM and Felsher DW:
Sustained loss of a neoplastic phenotype by brief inactivation of
MYC. Science. 297:102–104. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gabay M, Li Y and Felsher DW: MYC
activation is a hallmark of cancer initiation and maintenance. Cold
Spring Harb Perspect Med. 4:1–14. 2014. View Article : Google Scholar
|
|
22
|
Shachaf CM, Kopelman AM, Arvanitis C,
Karlsson A, Beer S, Mandl S, Bachmann MH, Borowsky AD, Ruebner B,
Cardiff RD, et al: MYC inactivation uncovers pluripotent
differentiation and tumour dormancy in hepatocellular cancer.
Nature. 431:1112–1117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sato M, Rodriguez-Barrueco R, Yu J, Do C,
Silva JM and Gautier J: MYC is a critical target of FBXW7.
Oncotarget. 6:3292–3305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meyer N and Penn LZ: Reflecting on 25
years with MYC. Nat Rev Cancer. 8:976–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ross DA and Wilson GD: Expression of c-myc
oncoprotein represents a new prognostic marker in cutaneous
melanoma. Br J Surg. 85:46–51. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Greulich KM, Utikal J, Peter RU and Krähn
G: c-MYC and nodular malignant melanoma. A case report. Cancer.
89:97–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kraehn GM, Utikal J, Udart M, Greulich KM,
Bezold G, Kaskel P, Leiter U and Peter RU: Extra c-myc oncogene
copies in high risk cutaneous malignant melanoma and melanoma
metastases. Br J Cancer. 84:72–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanzler MH and Mraz-Gernhard S: Primary
cutaneous malignant melanoma and its precursor lesions: Diagnostic
and therapeutic overview. J Am Acad Dermatol. 45:260–276. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin X, Sun R, Zhao X, Zhu D, Zhao X, Gu Q,
Dong X, Zhang D, Zhang Y, Li Y, et al: C-myc overexpression drives
melanoma metastasis by promoting vasculogenic mimicry via
c-myc/snail/Bax signaling. J Mol Med (Berl). 95:53–67. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nag S, Zhang X, Srivenugopal KS, Wang MH,
Wang W and Zhang R: Targeting MDM2-p53 interaction for cancer
therapy: Are we there yet? Curr Med Chem. 21:553–574. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Freedman DA, Wu L and Levine AJ: Functions
of the MDM2 oncoprotein. Cell Mol Life Sci. 55:96–107. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Momand J, Wu HH and Dasgupta G: MDM2 -
master regulator of the p53 tumor suppressor protein. Gene.
242:15–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oliner JD, Pietenpol JA, Thiagalingam S,
Gyuris J, Kinzler KW and Vogelstein B: Oncoprotein MDM2 conceals
the activation domain of tumour suppressor p53. Nature.
362:857–860. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Momand J, Zambetti GP, Olson DC, George D
and Levine AJ: The mdm-2 oncogene product forms a complex with the
p53 protein and inhibits p53-mediated transactivation. Cell.
69:1237–1245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Q and Lozano G: Molecular pathways:
Targeting Mdm2 and Mdm4 in cancer therapy. Clin Cancer Res.
19:34–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vassilev LT: MDM2 inhibitors for cancer
therapy. Trends Mol Med. 13:23–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual, 8th edition.
Springer; 2017
|
|
38
|
Cheng Y and Li G: Role of the ubiquitin
ligase Fbw7 in cancer progression. Cancer Metastasis Rev. 31:75–87.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Busino L, Millman SE, Scotto L, Kyratsous
CA, Basrur V, OConnor O, Hoffmann A, Elenitoba-Johnson KS and
Pagano M: Fbxw7α- and GSK3-mediated degradation of p100 is a
pro-survival mechanism in multiple myeloma. Nat Cell Biol.
14:375–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Min SH, Lau AW, Lee TH, Inuzuka H, Wei S,
Huang P, Shaik S, Lee DY, Finn G, Balastik M, et al: Negative
regulation of the stability and tumor suppressor function of Fbw7
by the Pin1 prolyl isomerase. Mol Cell. 46:771–783. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yada M, Hatakeyama S, Kamura T, Nishiyama
M, Tsunematsu R, Imaki H, Ishida N, Okumura F, Nakayama K and
Nakayama KI: Phosphorylation-dependent degradation of c-Myc is
mediated by the F-box protein Fbw7. EMBO J. 23:2116–2125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Davis RJ, Welcker M and Clurman BE: Tumor
suppression by the Fbw7 ubiquitin ligase: Mechanisms and
opportunities. Cancer Cell. 26:455–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bonetti P, Davoli T, Sironi C, Amati B,
Pelicci PG and Colombo E: Nucleophosmin and its AML-associated
mutant regulate c-Myc turnover through Fbw7γ. J Cell Biol.
182:19–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Grim JE, Gustafson MP, Hirata RK, Hagar
AC, Swanger J, Welcker M, Hwang HC, Ericsson J, Russell DW and
Clurman BE: Isoform- and cell cycle-dependent substrate degradation
by the Fbw7 ubiquitin ligase. J Cell Biol. 181:913–920. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Welcker M, Orian A, Grim JE, Eisenman RN
and Clurman BE: A nucleolar isoform of the Fbw7 ubiquitin ligase
regulates c-Myc and cell size. Curr Biol. 14:1852–1857. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Welcker M, Orian A, Jin J, Grim JE, Harper
JW, Eisenman RN and Clurman BE: The Fbw7 tumor suppressor regulates
glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein
degradation. Proc Natl Acad Sci USA. 101:9085–9090. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Reavie L, Buckley SM, Loizou E, Takeishi
S, Aranda-Orgilles B, Ndiaye-Lobry D, et al: Regulation of c-Myc
ubiquitination controls CML initiation and progression. Cancer
Cell. 23:362–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Niezgoda A, Niezgoda P and Czajkowski R:
Novel approaches to treatment of advanced melanoma: A review on
targeted therapy and immunotherapy. Biomed Res Int. 2015:851382015.
View Article : Google Scholar
|
|
49
|
Kalkat M, De Melo J, Hickman KA, Lourenco
C, Redel C, Resetca D, Tamachi A, Tu WB and Penn LZ: MYC
deregulation in primary human cancers. Genes (Basel). 8:2–30. 2017.
View Article : Google Scholar
|
|
50
|
Cancer Genome Atlas Network: Comprehensive
molecular portraits of human breast tumors. Nature. 490:61–70.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Witkiewicz AK, McMillan EA, Balaji U, Baek
G, Lin WC, Mansour J, Mollaee M, Wagner KU, Koduru P, Yopp A, et
al: Whole-exome sequencing of pancreatic cancer defines genetic
diversity and therapeutic targets. Nat Commun. 6:67442015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cancer Genome Atlas Research Network, .
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Houben R, Hesbacher S, Schmid CP, Kauczok
CS, Flohr U, Haferkamp S, Müller CS, Schrama D, Wischhusen J and
Becker JC: High-level expression of wild-type p53 in melanoma cells
is frequently associated with inactivity in p53 reporter gene
assays. PLoS One. 6:e220962011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Winder M and Virós A: Mechanisms of drug
resistance in melanoma. Handb Exp Pharmacol. 249:91–108. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Webster MR, Fane ME, Alicea GM, Basu S,
Kossenkov AV, Marino GE, Douglass SM, Kaur A, Ecker BL,
Gnanapradeepan K, et al: Paradoxical role for wild-type p53 in
driving therapy resistance in melanoma. Mol Cell. 77:633–644.e5.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Soengas MS, Capodieci P, Polsky D, Mora J,
Esteller M, Opitz-Araya X, McCombie R, Herman JG, Gerald WL,
Lazebnik YA, et al: Inactivation of the apoptosis effector Apaf-1
in malignant melanoma. Nature. 409:207–211. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Karst AM, Dai DL, Martinka M and Li G:
PUMA expression is significantly reduced in human cutaneous
melanomas. Oncogene. 24:1111–1116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Onel K and Cordon-Cardo C: MDM2 and
prognosis. Mol Cancer Res. 2:1–8. 2004.PubMed/NCBI
|
|
59
|
Sun Y: E3 ubiquitin ligases as cancer
targets and biomarkers. Neoplasia. 8:645–654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Polsky D, Bastian B, Hazan C, Melzer K,
Pack J, Houghton A, Busam K, Cordon-Cardo C and Osman I: HDM2
protein overexpression, but not gene amplification, is related to
tumorigenesis of cutaneous melanoma. Cancer Res. 61:7642–7646.
2001.PubMed/NCBI
|
|
61
|
Rajabi P, Karimian P and Heidarpour M: The
relationship between MDM2 expression and tumor thickness and
invasion in primary cutaneous malignant melanoma. J Res Med Sci.
17:452–455. 2012.PubMed/NCBI
|
|
62
|
Polsky D, Melzer K, Hazan C, Panageas KS,
Busam K, Drobnjak M, Kamino H, Spira JG, Kopf AW, Houghton A, et
al: HDM2 protein overexpression and prognosis in primary malignant
melanoma. J Natl Cancer Inst. 94:1803–1806. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kimura T, Gotoh M, Nakamura Y and Arakawa
H: hCDC4b, a regulator of cyclin E, as a direct transcriptional
target of p53. Cancer Sci. 94:431–436. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Muthusamy V, Hobbs C, Nogueira C,
Cordon-Cardo C, McKee PH, Chin L and Bosenberg MW: Amplification of
CDK4 and MDM2 in malignant melanoma. Genes Chromosomes Cancer.
45:447–454. 2006. View Article : Google Scholar : PubMed/NCBI
|