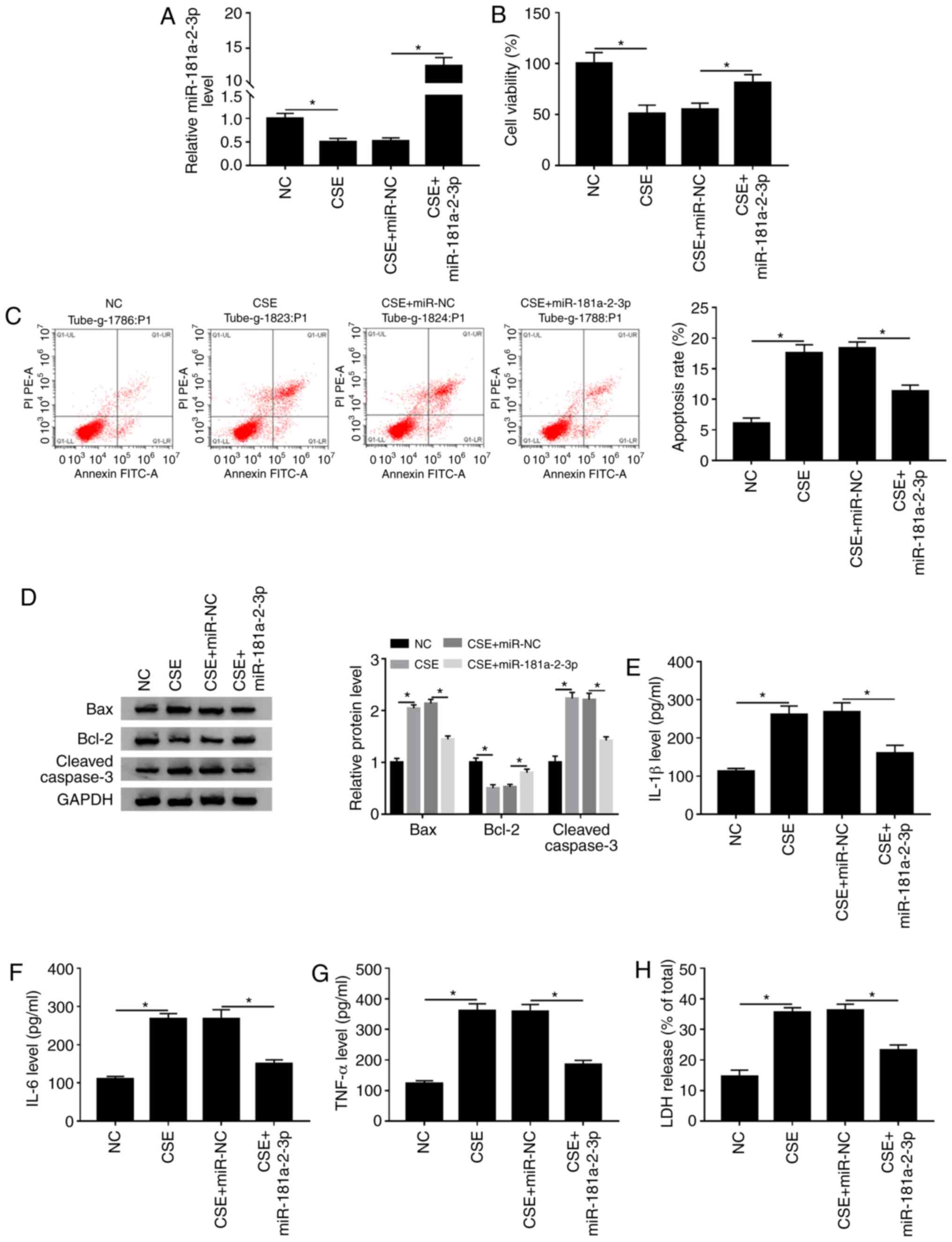
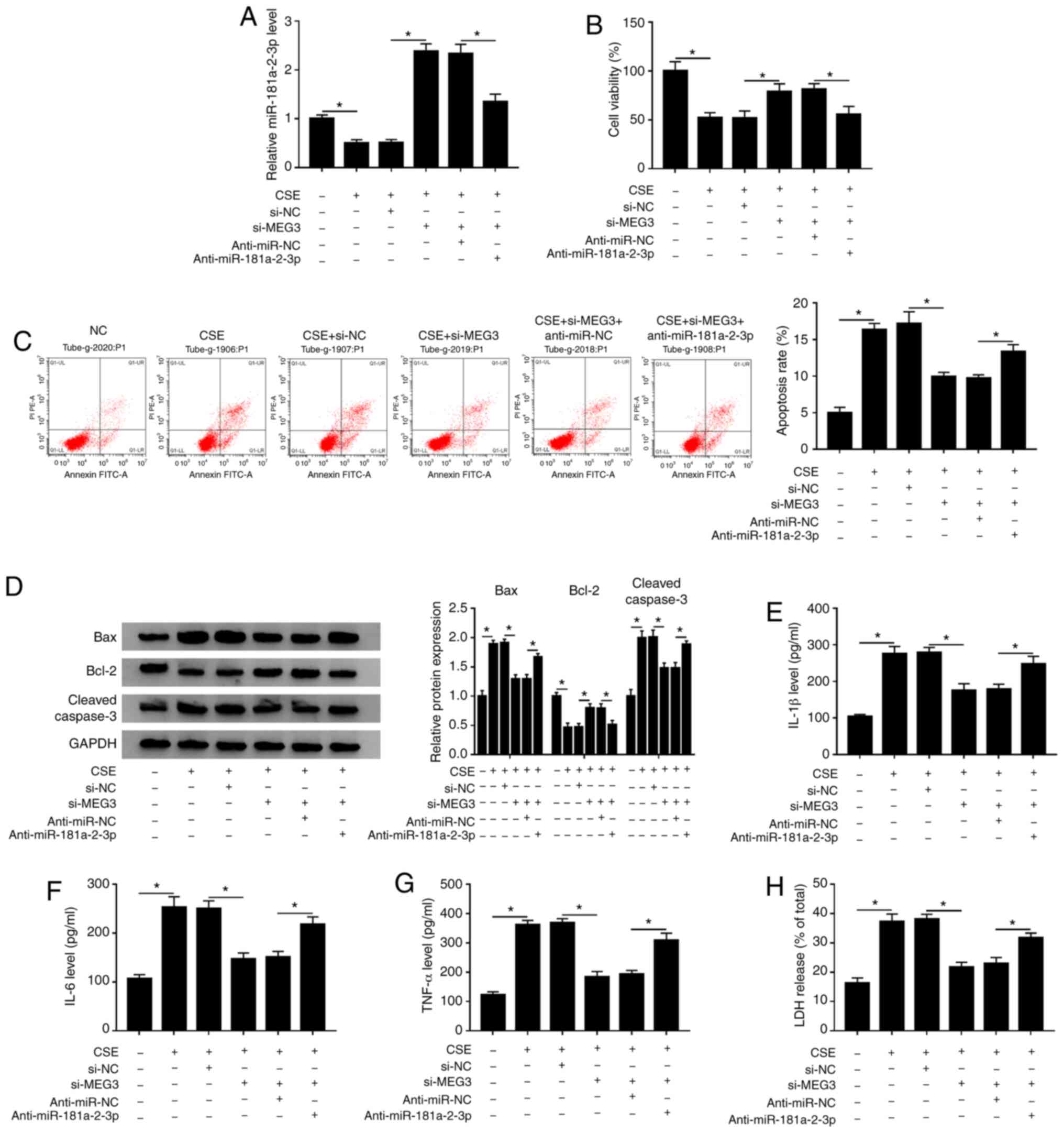
|
1
|
Pauwels RA and Rabe KF: Burden and
clinical features of chronic obstructive pulmonary disease (COPD).
Lancet. 364:613–620. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nussbaumer-Ochsner Y and Rabe KF: Systemic
manifestations of COPD. Chest. 139:165–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanni SE, Pelegrino NR, Angeleli AY,
Correa C and Godoy I: Smoking and tumor necrosis factor-alpha
mediated systemic inflammation in COPD patients. J Inflamm (Lond).
7:292010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rusznak C, Mills PR, Devalia JL, Sapsford
RJ, Davies RJ and Lozewicz S: Effect of cigarette smoke on the
permeability and IL-1 β and sICAM-1 release from cultured human
bronchial epithelial cells of never-smokers, smokers, and patients
with chronic obstructive pulmonary disease. Am J Respir Cell Mol
Biol. 23:530–536. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu H, Yang S, Wu X, Zhao J, Zhao J, Ning
Q, Xu Y and Xie J: Interleukin-33/ST2 signaling promotes production
of interleukin-6 and interleukin-8 in systemic inflammation in
cigarette smoke-induced chronic obstructive pulmonary disease mice.
Biochem Biophys Res Commun. 450:110–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilusz JE, Hongjae S and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lalevée S and Feil R: Long noncoding RNAs
in human disease: Emerging mechanisms and therapeutic strategies.
Epigenomics. 7:877–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu W, Yuan Y, Wang L, Yang H, Li S, Tang Z
and Li Q: Long non-coding RNA TUG1 promotes airway remodelling by
suppressing the miR-145-5p/DUSP6 axis in cigarette smoke-induced
COPD. J Cell Mol Med. 23:7200–7209. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li N, Liu Y and Cai J: LncRNA MIR155HG
regulates M1/M2 macrophage polarization in chronic obstructive
pulmonary disease. Biomed Pharmacother. 117:1090152019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Bian EB, He XJ, Ma CC, Zong G, Wang
HL and Zhao B: Epigenetic repression of long non-coding RNA MEG3
mediated by DNMT1 represses the p53 pathway in gliomas. Int J
Oncol. 48:723–733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng W, Si S, Zhang Q, Li C, Zhao F, Wang
F, Yu J and Ma R: Long non-coding RNA MEG3 functions as a competing
endogenous RNA to regulate gastric cancer progression. J Exp Clin
Cancer Res. 34:792015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song B, Ye L, Wu S and Jing Z: Long
non-coding RNA MEG3 regulates CSE-induced apoptosis and
inflammation via regulating miR-218 in 16HBE cells. Biochem Biophys
Res Commun. 521:368–374. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar MS, Armenteros-Monterroso E, East P,
Chakravorty P, Matthews N, Winslow MM and Downward J: HMGA2
functions as a competing endogenous RNA to promote lung cancer
progression. Nature. 505:212–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ardekani AM and Naeini MM: The role of
microRNAs in human diseases. Avicenna J Med Biotechnol. 2:161–179.
2010.PubMed/NCBI
|
|
17
|
Hobbs BD and Tantisira KG: MicroRNAs in
COPD: Small molecules with big potential. Eur Respir J.
53:19005152019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Diao X, Zhou J, Wang S and Ma X:
Upregulation of miR-132 contributes to the pathophysiology of COPD
via targeting SOCS5. Exp Mol Pathol. 105:285–292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim J, Kim DY, Heo HR, Choi SS, Hong SH
and Kim WJ: Role of miRNA-181a-2-3p in cadmium-induced inflammatory
responses of human bronchial epithelial cells. J Thorac Dis.
11:3055–3069. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Richter A, O'Donnell RA, Powell RM,
Sanders MW, Holgate ST, Djukanovic R and Davies DE: Autocrine
ligands for the epidermal growth factor receptor mediate
interleukin-8 release from bronchial epithelial cells in response
to cigarette smoke. Am J Respir Cell Mol Biol. 27:85–90. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Danpure C: Lactate dehydrogenase and cell
injury. Cell Biochem Funct. 2:144–148. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophys Res Commun.
500:26–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin B, Jin H, Wu HB, Xu JJ and Li B: Long
non-coding RNA SNHG15 promotes CDK14 expression via miR-486 to
accelerate non-small cell lung cancer cells progression and
metastasis. J Cell Physiol. 233:7164–7172. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rabe KF, Hurd S, Anzueto A, Barnes PJ and
Zielinski J: Global strategy for the diagnosis, management, and
prevention of chronic obstructive pulmonary disease: GOLD executive
summary. Am J Respir Crit Care Med. 176:532–555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manel E: Non-coding RNAs in human disease.
Nat Rev Genet. 12:861–874. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan B, Wang ZH, Liu JY, Tao ZF, Li XM and
Qin J: Long noncoding RNAs: Versatile players in biologcial
processes and human disorders. Epigenomics. 6:375–379. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu KH, Li W, Liu XH, Sun M, Zhang ML, Wu
WQ, Xie WP and Hou YY: Long non-coding RNA MEG3 inhibits NSCLC
cells proliferation and induces apoptosis by affecting p53
expression. BMC Cancer. 13:4612013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiu GZ, Tian W, Fu HT, Li CP and Liu B:
Long noncoding RNA-MEG3 is involved in diabetes mellitus-related
microvascular dysfunction. Biochem Biophys Res Commun. 471:135–141.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang W, Shen Z, Guo J and Sun S: Screening
of long non-coding RNA and TUG1 inhibits proliferation with TGF-β
induction in patients with COPD. Int J Chron Obstruct Pulmon Dis.
11:2951–2964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Zheng M, Pu J, Zhou Y, Hong W, Fu X,
Peng Y, Zhou W, Pan H, Li B and Ran P: Identification of abnormally
expressed lncRNAs induced by PM2.5 in human bronchial epithelial
cells. Biosci Rep. 38:BSR201715772018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Militello G, Weirick T, John D, Döring C,
Dimmeler S and Uchida S: Screening and validation of lncRNAs and
circRNAs as miRNA sponges. Brief Bioinform. 18:780–788.
2017.PubMed/NCBI
|
|
34
|
Momen-Heravi F and Bala S: Emerging role
of non-coding RNA in oral cancer. Cell Signal. 42:134–143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng Z, Li Z, Ma K, Li X, Tian N, Duan J,
Xiao X and Wang Y: Long non-coding RNA XIST promotes glioma
tumorigenicity and angiogenesis by acting as a molecular sponge of
miR-429. J Cancer. 8:4106–4116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang G, Li S, Lu J, Ge Y, Wang Q, Ma G,
Zhao Q, Wu D, Gong W, Du M, et al: LncRNA MT1JP functions as a
ceRNA in regulating FBXW7 through competitively binding to
miR-92a-3p in gastric cancer. Mol Cancer. 17:872018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y and Kong D: Knockdown of lncRNA
MEG3 inhibits viability, migration, and invasion and promotes
apoptosis by sponging miR-127 in osteosarcoma cell. J Cell Biochem.
119:669–679. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Z, Yang L, Liu X, Nie Z and Luo J: Long
noncoding RNA MEG3 inhibits proliferation of chronic myeloid
leukemia cells by sponging microRNA21. Biomed Pharmacother.
104:181–192. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ravindresh C: let-7i-5p, miR-181a-2-3p and
EGF/PI3K/SOX2 axis coordinate to maintain cancer stem cell
population in cervical cancer. Sci Rep. 8:78402018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nabhan M, Louka ML, Khairy E, Tash F,
Ali-Labib R and El-Habashy S: MicroRNA-181a and its target Smad 7
as potential biomarkers for tracking child acute lymphoblastic
leukemia. Gene. 628:253–258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gorur A, Balci Fidanci S, Dogruer Unal N,
Ayaz L, Akbayir S, Yildirim Yaroglu H, Dirlik M, Serin MS and Tamer
L: Determination of plasma microRNA for early detection of gastric
cancer. Mol Biol Rep. 40:2091–2096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xie W, Li Z, Li M, Xu N and Zhang Y:
miR-181a and inflammation: miRNA homeostasis response to
inflammatory stimuli in vivo. Biochem Biophys Res Commun.
430:647–652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weidong X, Mengnan L, Naihan X, Qing L,
Nunu H, Jie H, Yaou Z and Tobias E: miR-181a regulates inflammation
responses in monocytes and macrophages. PLoS One. 8:e586392013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kirschner MB, Edelman JJ, Kao SC, Vallely
MP, van Zandwijk N and Reid G: The impact of hemolysis on cell-free
microRNA biomarkers. Front Genet. 4:942013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Myklebust MP, Rosenlund B, Gjengstø P,
Bercea BS, Karlsdottir Á, Brydøy M and Dahl O: Quantitative PCR
measurement of miR-371a-3p and miR-372-p is influenced by
hemolysis. Front Genet. 10:4632019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kirschner MB, Kao SC, Edelman JJ,
Armstrong NJ, Vallely MP, van Zandwijk N and Reid G: Haemolysis
during sample preparation alters microRNA content of plasma. PLoS
One. 6:e241452011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 13:215–233. 2009. View Article : Google Scholar
|