Introduction
Primary liver cancer is a common malignant tumor in
China (1). Liver cancer can be
divided into three types as follows: Hepatocellular carcinoma, bile
duct cell carcinoma and a mixture of both types. Hepatocellular
carcinoma represents 70–90% of all primary liver cancers worldwide.
Primary liver cancer is the second leading cause of
tumor-associated mortality worldwide and in China. The main cause
is chronic infection of hepatitis B virus (HBV) and hepatitis C
virus (HCV) (2).
The tumor microenvironment is the major site for
tumor cell proliferation and differentiation. Previous studies have
demonstrated that the tumor microenvironment significantly affects
tumorigenesis and development, metastasis, and response to
immunotherapy and chemotherapy (3,4). The
hepatocellular carcinoma tumor microenvironment is divided into
cellular and non-cellular components (5). The cellular components of this
microenvironment include liver sinusoidal endothelial cells and
hepatic stellate cells (6,7). In addition, infiltrating immune cells
are present in hepatocellular carcinoma, and mainly include
neutrophils, lymphocytes, tumor-associated macrophages, dendritic
cells and myeloid-derived suppressor cells (8–10).
Myeloid-derived suppressor cells (MDSCs) form a
group of heterogeneous cells composed of bone marrow progenitor
cells and immature bone marrow cells. These cells are precursors of
dendritic cells, granulocytes and macrophages. In healthy
individuals, immature bone marrow cells produced by the bone marrow
can rapidly differentiate into mature dendritic cells, macrophages
and granulocytes (11). However,
under certain pathological conditions, such as cancer, infection,
inflammation, sepsis, trauma, transplantation or autoimmune
diseases, the differentiation of immature bone marrow cells into
mature bone marrow cells is blocked (12,13).
This phenomenon eventually leads to the expansion of MDSCs.
In humans, MDSCs express CD11b and CD33 on their
surface, whereas in mouse, MDSCs express CD11b and glucocorticoid
receptor 1 Gr-1, which includes two types, Ly6G and Ly6C (14,15).
MDSCs express high levels of the immunosuppressive molecule
arginase 1 (Arg-1) and inducible nitric oxide synthase (iNOS) that
exert immunosuppressive functions in humans and mice (16,17).
MDSCs are one of the main components of the tumor microenvironment.
They migrate to peripheral lymphoid organs, such as the spleen, to
infiltrate into the tumor, contributing therefore to the formation
of the tumor microenvironment (18,19).
Fibrinogen-like protein 2 (FGL2) comprises two
forms, a membrane-associated form and a secreted form (20,21). The
cleavage of the amino-terminal hydrophobic segment results in the
secreted form of FGL2 that has immunoregulatory functions. The
carboxyl terminus of FGL2 contains a fibrinogen-related domain
(FRED) that is similar to the β and γ chains of fibrinogen
(22). This domain has been
demonstrated to have immunoregulatory effects (23). The immunoregulatory function of FGL2
serves crucial roles in certain diseases, including viral
hepatitis, transplant rejection and cancers (24). Previous studies have reported that
FGL2 is involved in the promotion of tumor progression by
regulating the cellular components of the tumor microenvironment in
some types of cancer, including renal clear cell carcinoma, gliomas
and lung cancer (25–27). However, the expression of FGL2 in
hepatocellular carcinomas and its effect on the tumor
microenvironment remain unknown.
Materials and methods
Clinical samples and
clinicopathological data
A total of 40 pairs of hepatocellular carcinoma
cancer and peritumor fresh and FFPE tissue samples were collected
between October 2017 and December 2017 from patients who were
pathologically diagnosed with hepatocellular carcinoma at The First
Hospital of China Medical University. The clinicopathological
characteristics of patients are presented in Table I. The age of the patients ranged from
39 to 72 years, and the mean age was 54 years. All clinical samples
were collected after obtaining written informed consent from
patients. The study was approved by the Ethics Committee of the
China Medical University.
 | Table I.Association between FGL2 expression
level and the clinicopathological characteristics of patients with
hepatocellular carcinoma. |
Table I.
Association between FGL2 expression
level and the clinicopathological characteristics of patients with
hepatocellular carcinoma.
| Clinicopathological
characteristics | Number of
patients | Low/medium
expression of FGL2 | High expression of
FGL2 | P-value |
|---|
| Total cases | 40 | 23 | 17 |
|
| Age, years |
|
≤53 | 22 | 11 | 11 | 0.348 |
|
>53 | 18 | 12 | 6 |
|
| Sex |
|
Male | 32 | 20 | 12 | 0.250 |
|
Female | 8 | 3 | 5 |
|
| Tumor size, cm |
| ≤5 | 23 | 17 | 6 | 0.024a |
|
>5 | 17 | 6 | 11 |
|
| Number of
tumors |
|
Single | 28 | 16 | 12 | 1.000 |
|
Multiple | 12 | 7 | 5 |
|
| HBsAg
expression |
| + | 30 | 16 | 14 | 0.471 |
| – | 10 | 7 | 3 |
|
| Cirrhosis |
| + | 32 | 19 | 13 | 0.702 |
| – | 8 | 4 | 4 |
|
| AFP level,
ng/dl |
|
≤200 | 19 | 13 | 6 | 0.216 |
|
>200 | 21 | 10 | 11 |
|
| TNM stage |
| I | 23 | 14 | 9 | 0.749 |
|
II–IV | 17 | 9 | 8 |
|
Immunohistochemistry
Serial pathological tissue sections (5 µm-thick)
were dewaxed using xylene and rinsed with gradient ethanol (100,
95, 90, 80 and 70%, 5 min each). Antigen retrieval was performed
using citrate buffer at high pressure. Sections were incubated with
rabbit anti-FGL2 polyclonal antibody (1:100; cat. no. ab198029;
Abcam) overnight at 4°C, washed with PBS and incubated with
secondary antibody (goat anti-rabbit; ready to use; MaxVision; cat.
no. KIT-5004) for 30 min at room temperature.
Streptavidin-peroxidase solution (ready to use; MaxVision; cat. no.
KIT-5004) was then added and incubated for 30 min at room
temperature. Sections were color developed using DAB, stained with
hematoxylin for 5 min at room temperature and dried. Transparent
sections were neutral resin counterstained and imaged using a
microscope (magnification, ×200; Nikon Eclipse Ti-S; Nikon
Corporation). The expression level of FGL2 was evaluated using the
Engers scoring method (28). In
addition, tumor tissue cases were divided into high and low/medium
groups according to the median expression level using the Engers
scoring method.
Reverse transcription quantitative
(RT-q) PCR
TRIZOL (Invitrogen; Thermo Fisher Scientific, Inc.)
were used to extract total RNA from human hepatocellular carcinoma
tissues. cDNA was generated using 5× Prime Script RT Master Mix
(Takara Bio, Inc.) on PCR System 9700 (GeneAmp) under the following
conditions: 37°C for 15 min, 85°C for 5 sec and 4°C. The sequences
of the primers were as follows: FGL2, forward,
5′-ACAAAGGTGTCCGTAATGGG-3′ and reverse,
5′-GTTTGAAGGAGGACTTGTAGCC-3′; CD11b, forward,
5′-CTGTTTACCTGTTTCACGGAAC-3′ and reverse,
5′-GATTGCCTTGACTCTCAGTACT-3′; and CD33, forward,
5′-CGATCTTCTCCTGGTTGTCAG-3′ and reverse,
5′-GATGGTTCTCTCCGCGTAGTCAC-3′. Human GAPDH Endogenous Reference
Genes Primers (cat. no. B662104; Sangon Biotech Co., Ltd.) and
Mouse GAPDH Endogenous Reference Genes Primers (cat. no. B662304;
Sangon Biotech Co, Ltd.) were used as the internal controls. The
mRNA expression was determined using TB Green Premix Ex TaqII
(Takara Bio, Inc.) on Applied Biosystems® 7500 (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
following thermocycling conditions: 95°C for 30 sec, followed by 40
cycles of 95°C for 3 sec and 60°C for 30 sec. The relative
expression levels were normalized to endogenous control and were
expressed as 2−ΔCt or 2−ΔΔCt (29).
Cell line experiments
The C57BL/6 mouse-derived Hepa1-6 hepatocellular
carcinoma cell line was purchased from The Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences. Cells were
cultured in DMEM medium (cat. no. SH30243.01; HyClone; Cytiva)
supplemented with 10% FBS (cat. no. VS500T; Ausbian) and
penicillin-streptomycin (100 U/ml penicillin and 100 µg/ml
streptomycin; Gibco; Thermo Fisher Scientific, Inc.; cat. no.
15070063) and placed at 37°C in a humidified incubator containing
5% CO2. Cells were transfected with LV5-FGL2
[overexpressing (OE) FGLV2] and LV5-Negative Control (NC)
lentivirus (Shanghai GenePharma Co., Ltd.) using standard
protocols. The transfection efficiency was verified in 293T cells.
The MOI of Hepa1-6 was 100, and 1×108 lentivirus were
used to transfect 1×106 Hepa1-6 cells for 24 h. After 48
h of transfection, light and green fluorescence microscopy
(magnification ×4; Nikon Eclipse Ti-S) images were taken to verify
the proportion of transfected cells. After 1 week of transfection,
the mRNA and protein expression of FGL2 was tested by RT-qPCR,
western blotting and ELISA. Transfected cells were subsequently
used for further experiments. To generate conditioned media (CM)
for MDSC culture, ~1×106 Hepa1-6 cells were cultured in
serum-free DMEM medium for 48 h. CM was then collected, centrifuged
at 300 × g at 37°C and for 5 min to remove any debris and was
freshly used.
Western blotting and ELISA assay
Western blotting was performed to detect the FGL2
expression in Hepa1-6 cells transfected with LV5-FGL2 and
LV5-NC.
Hepa1-6 cells were washed with ice-cold PBS and
lysed by RIPA (Beyotime Institute of Biotechnology). Protein
concentration was determined using BCA Protein Assay Kit (Beyotime
Institute of Biotechnology). Proteins (25 µg per lane) were
separated by 10% SDS-PAGE and transferred onto PVDF membranes. The
membranes were blocked by 5% skimmed milk dissolved in TBST for 60
min at room temperature and were incubated with rabbit anti-FGL2
polyclonal (1:900; cat. no. ab198029; Abcam) or anti-GAPDH
(1:10,000; cat. no. ab8245; Abcam) antibodies overnight at 4°C.
Membranes were washed with TBST and incubated with the secondary
antibodies goat anti-rabbit IgG (1:50,000; cat. no. ab205718;
Abcam) or goat anti-mouse IgG (1:20,000; cat. no. ab205719; Abcam)
for 60 min at room temperature. Enhanced chemiluminescence reagent
(Pierce; Thermo Fisher Scientific, Inc.) was used to detect the
signal on the membrane.
ELISA assay was performed to detect the FGL2
expression in the CM of Hepa1-6 cells transfected with LV5-FGL2 and
LV5-NC. After centrifugation of the CM to remove cell debris as
aforementioned, FGL2 expression level in CM was measured using an
ELISA kit (cat. no. CSB-EL008654MO; Cusabio Biotech Co., Ltd.)
according to the manufacturers protocol.
Isolation of bone marrow cells from
mice
A total of 8 male C57BL/6 mice (5–6 weeks old) were
purchased from the Experimental Animal Center of China Medical
University. Animal experiments were approved by the Laboratory
Animal Management and Use Committee (approval no. 2020016). Mice
were euthanized by cervical dislocation by trained certified
people. Mice were placed in 75% alcohol for 1–2 min and were
positioned on a sterile bench. Skin and muscles were excised, the
epiphysis was cut at both ends of the femur and bone marrow was
extracted. Briefly, 1 ml of DMEM was aspirated into a syringe and
inserted into the bone marrow cavity. The bone marrow cavity was
rinsed to wash out the bone marrow tissues that was filtered using
a 200-mesh nylon strainer (Beijing Solarbio Science &
Technology Co., Ltd.) to obtain fresh bone marrow cells. After
centrifugation (300 × g; 4°C; 5 min), the fresh bone marrow cells
were cultured in CM collected from cell line culture.
Flow cytometry
Two flow cytometry tubes containing single-cell
suspensions (from bone marrow cells, tumor or spleen) in cell
staining buffer (CSB; cat. no. 00-4222-57; Invitrogen; Thermo
Fisher Scientific, Inc.) were prepared per sample, one serving as
the negative control. Each tube contained ~1×106 cells,
and 400 µl CSB solution was added to the tube 1 that was stored on
ice. Mouse Fc Block (2 µl; BD Biosciences) was added to the tube 2
of each sample. Then, APC rat anti-mouse CD11b (1 µl; BD
Pharmingen; BD Biosciences; cat. no. 557657) and PE rat anti-mouse
Ly-6G and Ly-6C (1 µl; BD Pharmingen; BD Biosciences; cat. no.
561084) were added to the tube 2 of each sample. After incubation
on ice for 20 min in the dark, cells were washed twice with CSB
solution, diluted in 500 µl CSB and analyzed by flow cytometry (BD
FACSAria III; BD Biosciences) and data were analyzed by Flowjo
(v10.0.7; BD Biosciences).
Establishment of the hepatocellular
carcinoma orthotopic mouse model
FGL2-OE Hepa1-6 cells and NC Hepa1-6 cells were
harvested, washed twice with PBS, resuspended in PBS and counted.
Cells were diluted in PBS to the density of 5×107/ml. A
total of 12 C57BL/6 mice (age, 5–6 weeks) purchased from the
Experimental Animal Center of China Medical University were
anesthetized using an intraperitoneal injection of 1% sodium
pentobarbital (60 mg/kg). Using sterile ophthalmic scissors, the
abdomen was opened using a 1 cm incision under the xiphoid. Tumor
cell suspension (FGL2-OE Hepa1-6 cells or NC Hepa1-6 cells; n=6
mice per group; 20 µl, ~1×106 cells) was then injected
at a 20° angle from the liver surface into the liver parenchyma of
the left lobe. The abdomen was closed layer by layer using surgical
suture. Mice were placed in the incubator immediately after the
operation and had free access to food and water for 2 weeks
(temperature 20–22°C, humidity 40–60% and 12/12 h light/dark cycle,
before the euthanasia by cervical dislocation. Animal health and
behavior were monitored twice a day. No unplanned death occurred
before the humane endpoints. Tumor size and volume were measured
with a caliper using the following formula: Length × width × height
× 0.52 (mm3).
Preparation of single-cell
suspensions
A total of 12 tumor-bearing C57BL/6 mice (6 per
group) were euthanized by cervical dislocation. For tumor
single-cell suspension, liver tumors in situ were excised
and washed with PBS. Tumors were cut into small pieces and washed
with PBS. Tumor tissues were then digested with 0.1% collagenase
solution in a 37°C water bath for 30 min. Cell suspensions were
filtered using a 200-mesh nylon strainer, washed twice with PBS and
resuspended into CSB solution.
For spleen single-cell suspension, spleens were
excised and placed in a 24-well plate containing 2 ml PBS. Tissues
were crushed using a 1 ml syringe core and filtered with a 200-mesh
nylon strainer. Erythrocytes were lysed using erythrocyte-lysing
solution, and the cell suspension was washed twice with PBS
solution and resuspended with CSB solution.
Statistical analysis
SPSS 22.0 (IBM Corp.) was used for statistical
analysis. χ2 test was used for clinical-pathological
data analysis, linear Pearson correlation coefficient was used for
correlation analysis, and unpaired t-test was used to compare means
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
FGL2 expression level in
hepatocellular carcinoma and analysis of clinicopathological
characteristics
Immunohistochemistry was used to determine the
expression level of FGL2 in 40 pairs of hepatocellular carcinoma
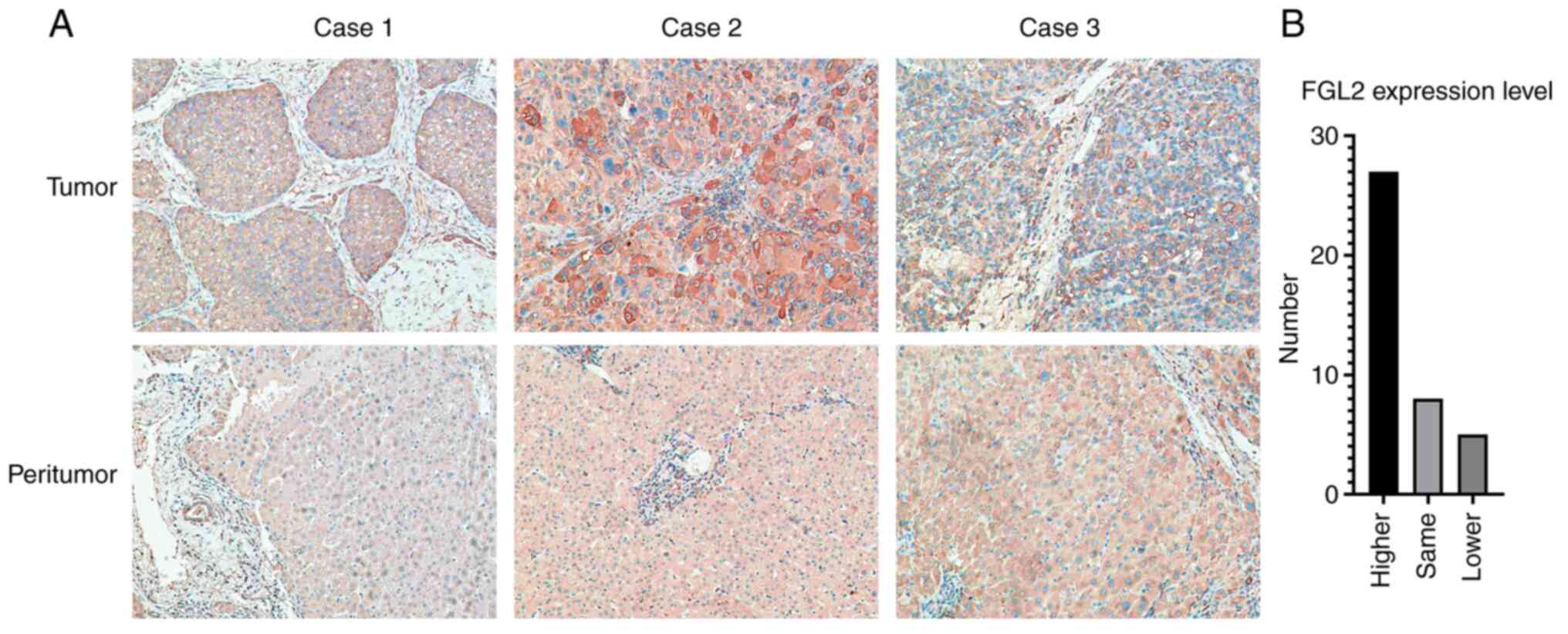
tumor tissues and peritumor tissue samples. In tumor tissues, FGL2
was mainly expressed in the cancer nest and cytoplasm. In peritumor
tissues, FGL2 is mainly expressed in hepatocytes and in some
vascular endothelial cells (Fig.
1A). Among the 40 pairs of samples, 27 pairs of tumor tissues
had higher expression level of FGL2 relative to peritumor tissues,
eight pairs of tumor tissues expressed the same level of FGL2
relative to peritumor tissues, whereas five pairs of tumor tissues
had lower FGL2 expression level relative to peritumor tissues
(Fig. 1B). Furthermore, the results
from association analysis between FGL2 expression level and the
clinicopathological characteristics of patients demonstrated that
FGL2 expression level was only associated with hepatocellular
carcinoma tumor size (P<0.05; Table
I).
Positive correlation between FGL2
expression and expression of MDSC surface markers in hepatocellular
carcinoma
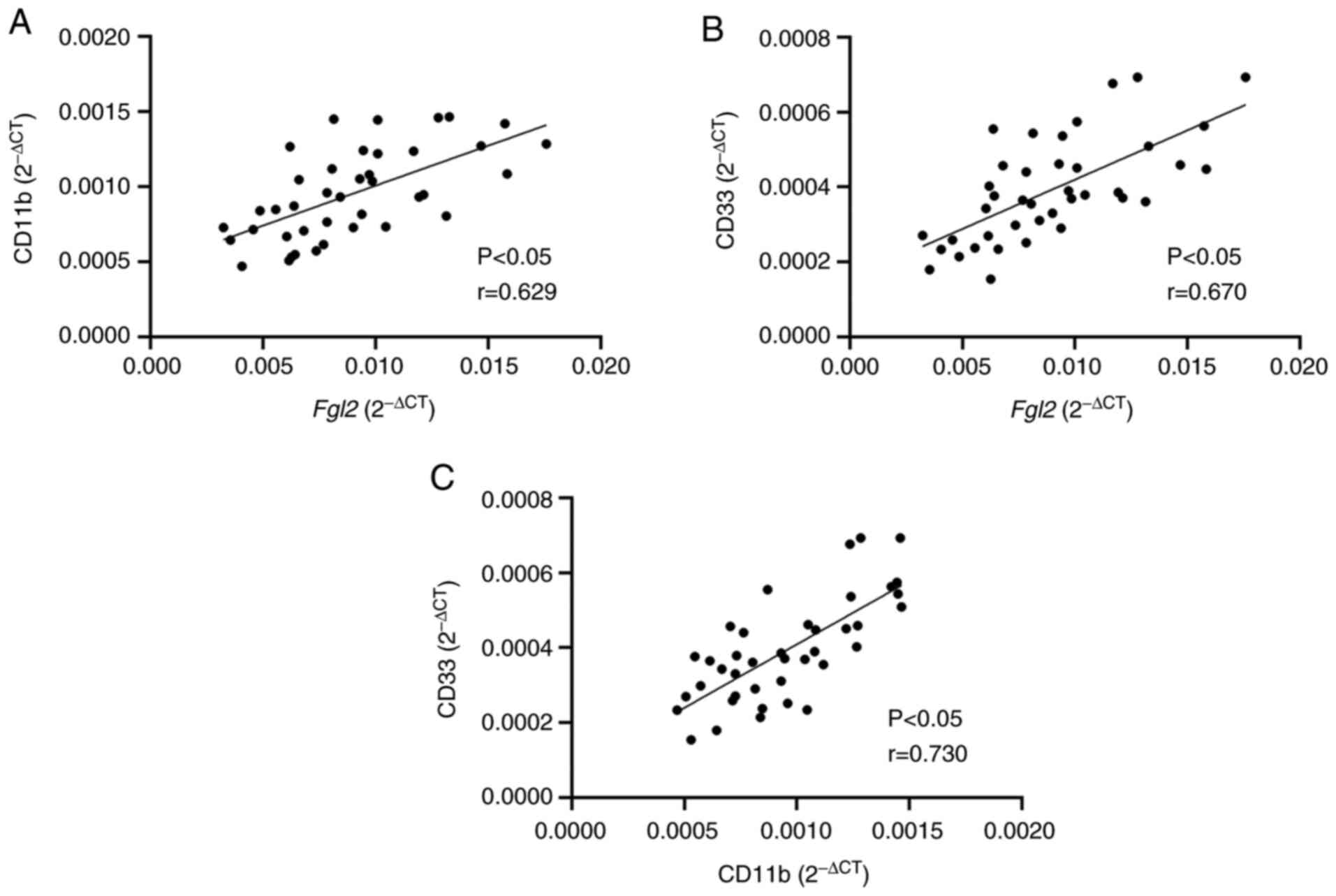
RT-qPCR was performed to determine the mRNA
expression of FGL2 and of CD11b and CD33, which are surface markers
of human MDSCs, in the hepatocellular carcinoma tumor tissue
samples. Linear Pearson correlation coefficient was used to
determine the correlation between the expression levels of FGL2 and
CD11b/CD33. The results demonstrated that FGL2 expression level was
positively correlated with CD11b and CD33 expression levels
(r=0.629 and r=0.670, respectively; P<0.05; Fig. 2A). In addition, a positive
correlation between the expression levels of CD11b and CD33 was
observed (r=0.730; P<0.05; Fig.
2B). These findings suggested that FGL2 expression level may be
correlated with the number of MDSCs in the tumor microenvironment
of hepatocellular carcinoma.
Conditioned media from FGL2-OE Hepa1-6
cells increases the in vitro proportion of MDSCs in mouse bone
marrow cells
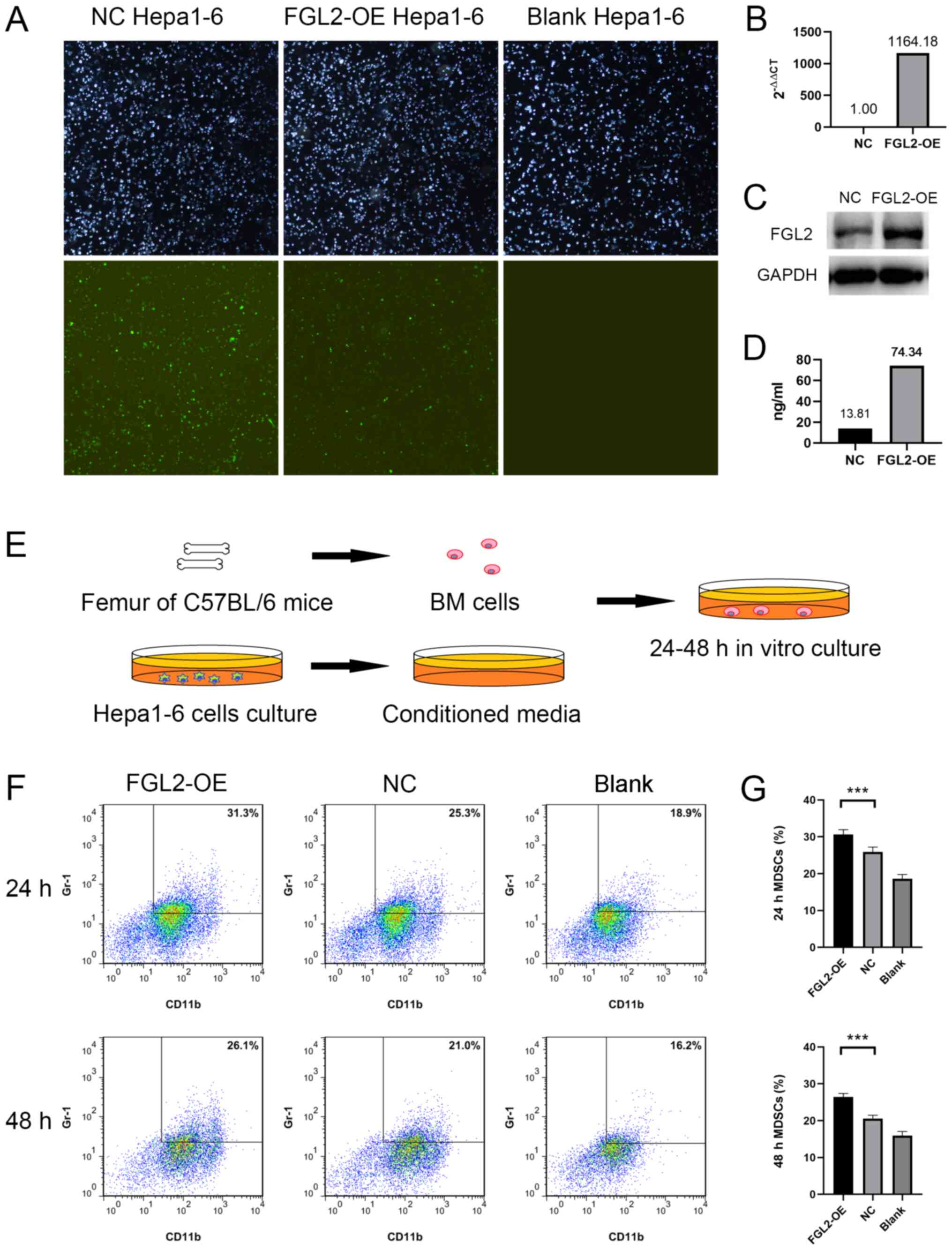
FGL2-OE Hepa1-6 and NC Hepa1-6 cell lines were
established by lentivirus transfection. Light and green
fluorescence microscopy images demonstrated the transfection
efficiency (Fig. 3A). One week post
LV5-FGL2 lentivirus transfection, FGL2 mRNA expression level in
FGL2-OE Hepa1-6 cells was significantly upregulated (Fig. 3B). FGL2 protein expression was
increased in FGL2-OE Hepa1-6 cells and in the culture supernatants
compared with NC Hepa1-6 cells (Fig. 3C
and D). Bone marrow cells harvested from the femurs of C57BL/6
mice were cultured in vitro using CM from either FGL2-OE
Hepa1-6 or NC Hepa1-6 cells for 24–48 h (Fig. 3E). Bone marrow cells cultured in
serum-free DMEM medium were used as control. Flow cytometry was
used to determine the proportion of
CD11b+/Gr-1+ cells in the three groups. The
results demonstrated that the proportions of
CD11b+/Gr-1+ cells in bone marrow cells
cultured using FGL2-OE Hepa1-6 CM for 24 and 48 h were
significantly higher compared with cells cultured in NC Hepa1-6 CM
(n=8, P<0.001; t-test; Fig. 3F and
G).
FGL2-OE Hepa1-6 cells promote tumor
growth and MDSC accumulation in tumors and spleen
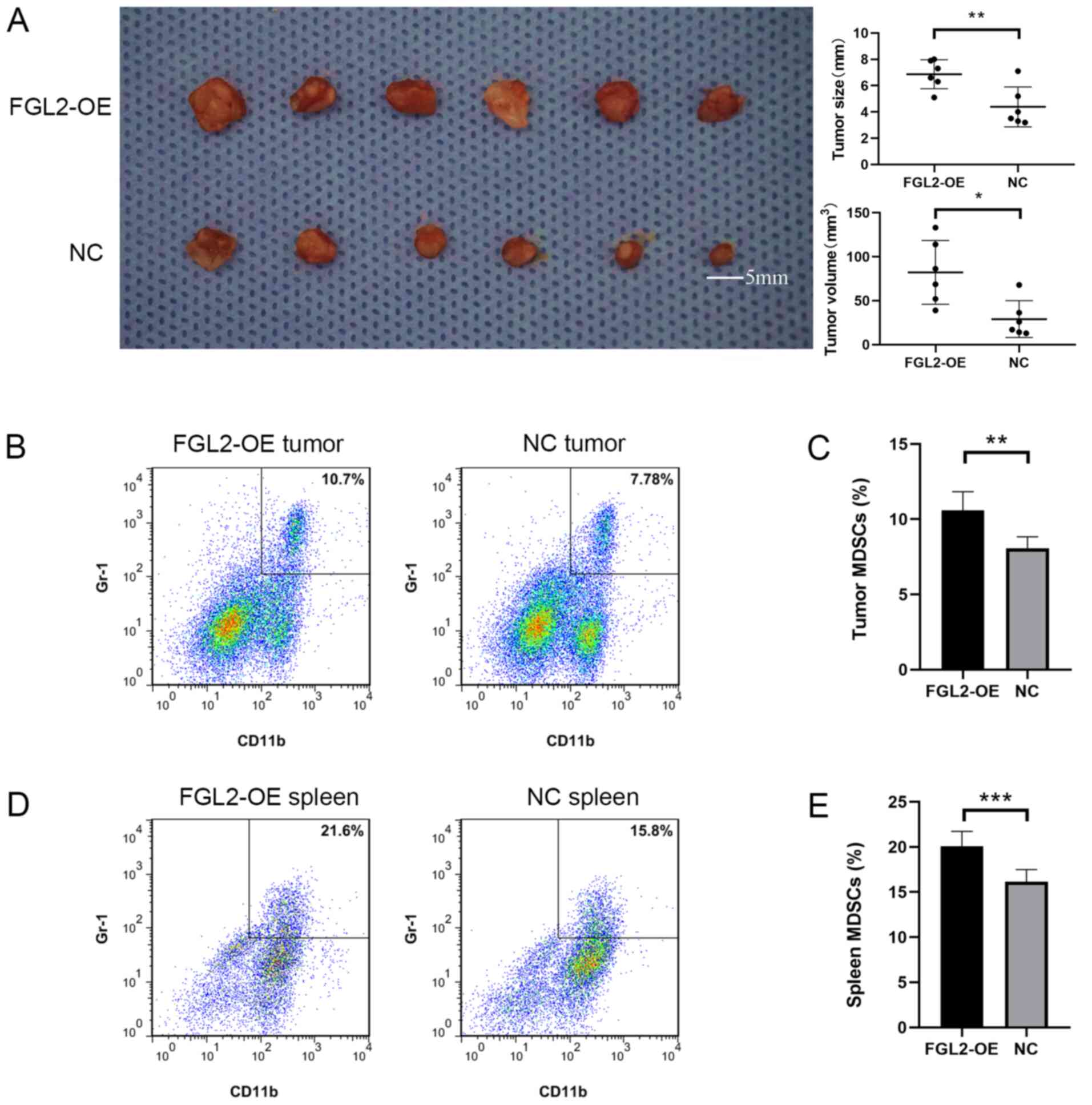
The FGL2-OE Hepa1-6 and NC Hepa1-6 cell lines were
used to establish an orthotopic hepatocellular carcinoma model in
C57BL/6 mice. Two weeks post-inoculation, the size and volume of
the tumors in the FGL2-OE Hepa1-6 group were significantly larger
compared with tumors in the NC Hepa1-6 group (Fig. 4A). The diameter of the tumors in the
FGL2-OE Hepa1-6 group ranged from 5.1 mm to 8.0 mm, and the
diameter of the tumors in the NC Hepa1-6 group ranged from 3.2 mm
to 7.1 mm. Single-cell suspensions from the harvested tumors were
used to determine the proportion of
CD11b+/Gr-1+ cells by flow cytometry. The
results demonstrated that the proportion of
CD11b+/Gr-1+ cells in the tumors of the
FGL2-OE Hepa1-6 group was significantly higher compared with the NC
Hepa1-6 group (n=6; P<0.001; t-test; Fig. 4B and C). In addition, the proportion
of CD11b+/Gr-1+ cells in the spleen of
FGL2-OE Hepa1-6 tumor-bearing C57BL/6 mice was significantly higher
compared with mice in the NC Hepa1-6 group (n=6; P<0.001;
t-test; Fig. 4D and E).
Discussion
Liver cancer is the second leading cause of
cancer-associated mortality worldwide and in China (1,2). Most
primary liver cancers are hepatocellular carcinomas (2). FGL2 has been reported to play a role in
promoting tumor progression by regulating the cellular components
of the tumor microenvironment (25–27). In
the present study, the expression level of FGL2 in hepatocellular
carcinomas was associated with tumor size. It was therefore
hypothesized that FGL2 may promote tumor progression by affecting
certain cellular components in the tumor microenvironment. The
present study demonstrated a positive correlation between the
expression level of FGL2 and surface markers of MDSCs in
hepatocellular carcinomas. These results suggested that FGL2 may
promote tumor progression by promoting the accumulation of MDSCs in
hepatocellular carcinoma. Immunohistochemistry will be performed in
the future to determine CD11b and CD33 expression in human
hepatocellular carcinoma tissues.
MDSCs express high levels of the immunosuppressive
molecules Arg-1 and iNOS, which can significantly inhibit the
function of CD4+ and CD8+ T lymphocytes
(30) and inhibit NK cell activation
(31), in addition to suppressing
antitumor immune response through direct and indirect mechanisms
(32). MDSCs are some of the main
components of the tumor microenvironment, and can also migrate to
peripheral lymphoid organs, such as the spleen (33). Previous studies reported that the
suppression of T cells in peripheral lymphoid organs by MDSCs
requires direct cell contact (19,34).
Furthermore, the number of MDSCs in the peripheral blood and tumors
of patients with hepatocellular carcinoma is significantly higher
compared with healthy people (35)
and is associated with tumor stage, size and Child-Pugh
classification (36). In addition,
MDSCs presence has been associated with poor overall survival and
early relapse in patients with hepatocellular carcinoma (37–40).
In the present study, a mouse hepatocellular
carcinoma cell line overexpressing FGL2 was established by
lentivirus stable transfection. Furthermore, fresh bone marrow
cells harvested from the femurs of C57BL/6 mice were cultured in
vitro using conditioned media from hepatocellular carcinoma
cells overexpressing FGL2. The results demonstrated that FGL2 could
maintain the undifferentiated state of bone marrow cells in
vitro, thereby promoting the accumulation of MDSCs. In
vivo, the overexpression of FGL2 could promote tumor growth. In
addition, MDSC levels in the tumors and spleen of mouse
overexpressing FGL2 were significantly increased. It was therefore
hypothesized that FGL2 may promote the accumulation of MDSCs to
stimulate tumor growth. The original low expression of FGL2 in
mouse hepatocellular carcinoma cell line makes it not suitable for
silencing FGL2. Therefore, FGL2 was only overexpressed in Hepa1-6
cells. FGL2 knockdown in mice will be performed in the future.
Previous studies have demonstrated that FGL2 could specifically
bind to FcγR receptors, including FcγRIIB (41–43).
FcγRIIB is a transmembrane protein that is abundantly expressed on
the surface of myeloid cells (44,45).
FGL2 in hepatocellular carcinoma may help maintaining the
undifferentiated state of bone marrow cells directly via FcγRIIB,
and therefore promote the accumulation of MDSCs required for tumor
growth. FGL2 may also upregulate the expression of several
cytokines, including tumor growth factor β and interleukin 6
(46,47), in the human hepatocellular carcinoma
tumor microenvironment in order to promote the maintenance and
recruitment of MDSCs. FGL2 may also induce the accumulation of
MDSCs by direct and indirect mechanisms. Further investigation is
required to test this hypothesis.
The liver is exposed to large amounts of antigen
from the gastrointestinal tract via the portal vein blood flow. To
prevent continuous immune stimulation and autoimmune damage induced
by antigen exposure, the liver undergoes innate and adaptive immune
responses and develops an intrinsic tolerance mechanism (48,49). The
immune characteristics of the tumor microenvironment, the immune
checkpoint molecules and the prognosis differ between patients with
hepatocellular carcinoma (50,51).
Immunotherapy for hepatocellular carcinoma has been rapidly
developed due to the crucial role that immunosuppressive cells play
in anti-tumor immune response (52).
Recently, immunotherapy has become the first-line treatment method
to treat solid tumors. This includes anti-cytotoxic T lymphocyte
antigen 4 (CTLA-4) and anti-programmed cell death 1 (PD-1/PD-L1)
monoclonal antibodies (53,54). Targeted therapy for tumor-associated
macrophages has rapidly progressed in the recent years (55), and targeted therapy for MDSCs shows
great potential (56). Since the
accumulation of MDSCs in tumors could increase resistance to
anti-CTLA-4/anti-PD-1 therapy, combined targeted therapy with
anti-MDSCs may significantly increase the efficacy of this therapy
(57). Because of the unique
immunosuppressive microenvironment of the liver and hepatocellular
carcinoma insensitivity to traditional chemotherapy and
radiotherapy, precise cell therapy and associated cytokines
targeted to the immunosuppressive microenvironment may represent a
successful therapeutic strategy for the treatment of hepatocellular
carcinoma. Further investigation is required to better understand
the role of FGL2 on the tumor microenvironment of hepatocellular
carcinomas.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81373173).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors contributions
BQL performed the main experimental work and wrote
the manuscript. ZYB performed in vitro experiments. JYZ
contributed to animal experiments. HL performed the important
experimental design work and contributed to the writing and
revising of the manuscript. All authors read and approved the final
version.
Ethics approval and consent to
participate
All clinical samples were collected after obtaining
written informed consent from patients. The study was approved by
the Ethics Committee of the China Medical University. Animal
experiments were approved by the Laboratory Animal Management and
Use Committee of the China Medical University (approval no.
2020016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
An L, Zeng HM, Zheng RS, Zhang SW, Sun KX,
Zou XN, Chen R, Wang SM, Gu XY, Wei WW and He J: Liver cancer
epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi. 41:721–727.
2019.(In Chinese). PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eggert T and Greten TF: Tumor regulation
of the tissue environment in the liver. Pharmacol Ther. 173:47–57.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu T and Dai Y: Tumor microenvironment and
therapeutic response. Cancer Lett. 387:61–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sevic I, Spinelli FM, Cantero MJ, Reszegi
A, Kovalszky I, García MG and Alaniz L: The role of the tumor
microenvironment in the development and progression of
hepatocellular carcinoma. Hepatocellular Carcinoma [Internet].
Tirnitz-Parker J: Codon Publications; Brisbane (AU): Chapter 2.
2019, simplehttps://www.ncbi.nlm.nih.gov/books/NBK549192/
View Article : Google Scholar
|
|
6
|
Yin Z, Dong C, Jiang K, Xu Z, Li R, Guo K,
Shao S and Wang L: Heterogeneity of cancer-associated fibroblasts
and roles in the progression, prognosis, and therapy of
hepatocellular carcinoma. J Hematol Oncol. 12:1012019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thomas H: LSEC stretch promotes fibrosis
during hepatic vascular congestion. Nat Rev Gastroenterol Hepatol.
16:262–263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tesi RJ: MDSC; the most important cell you
have never heard of. Trends Pharmacol Sci. 40:4–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tahmasebi Birgani M and Carloni V: Tumor
microenvironment, a paradigm in hepatocellular carcinoma
progression and therapy. Int J Mol Sci. 18:4052017. View Article : Google Scholar
|
|
10
|
Novikova MV, Khromova NV and Kopnin PB:
Components of the hepatocellular carcinoma microenvironment and
their role in tumor progression. Biochemistry (Mosc). 82:861–873.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Condamine T, Mastio J and Gabrilovich DI:
Transcriptional regulation of myeloid-derived suppressor cells. J
Leukoc Biol. 98:913–922. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Porembka MR, Mitchem JB, Belt BA, Hsieh
CS, Lee HM, Herndon J, Gillanders WE, Linehan DC and Goedegebuure
P: Pancreatic adenocarcinoma induces bone marrow mobilization of
myeloid-derived suppressor cells which promote primary tumor
growth. Cancer Immunol Immunother. 61:1373–1385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sinha P, Okoro C, Foell D, Freeze HH,
Ostrand-Rosenberg S and Srikrishna G: Proinflammatory S100 proteins
regulate the accumulation of myeloid-derived suppressor cells. J
Immunol. 181:4666–4675. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Talmadge JE and Gabrilovich DI: History of
myeloid-derived suppressor cells. Nat Rev Cancer. 13:739–752. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ugel S, De Sanctis F, Mandruzzato S and
Bronte V: Tumor-induced myeloid deviation: When myeloid-derived
suppressor cells meet tumor-associated macrophages. J Clin Invest.
125:3365–3376. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bergenfelz C and Leandersson K: The
generation and identity of human myeloid-derived suppressor cells.
Front Oncol. 10:1092020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsieh CC, Hung CH, Lu L and Qian S:
Hepatic immune tolerance induced by hepatic stellate cells. World J
Gastroenterol. 21:11887–11892. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Fang F, Jiao H, Zheng X, Huang L, Yi
X and Zhao W: Activated hepatic stellate cells regulate MDSC
migration through the SDF-1/CXCR4 axis in an orthotopic mouse model
of hepatocellular carcinoma. Cancer Immunol Immunother.
68:1959–1969. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar V, Patel S, Tcyganov E and
Gabrilovich DI: The nature of myeloid-derived suppressor cells in
the tumor microenvironment. Trends Immunol. 37:208–220. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuwaraj S, Ding J, Liu M, Marsden PA and
Levy GA: Genomic characterization, localization, and functional
expression of FGL2, the human gene encoding fibroleukin: A novel
human procoagulant. Genomics. 71:330–338. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marazzi S, Blum S, Hartmann R, Gundersen
D, Schreyer M, Argraves S, von Fliedner V, Pytela R and Rüegg C:
Characterization of human fibroleukin, a fibrinogen-like protein
secreted by T lymphocytes. J Immunol. 161:138–147. 1998.PubMed/NCBI
|
|
22
|
Yang G and Hooper WC: Physiological
functions and clinical implications of fibrinogen-like 2: A review.
World J Clin Infect Dis. 3:37–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chan CW, Kay LS, Khadaroo RG, Chan MW,
Lakatoo S, Young KJ, Zhang L, Gorczynski RM, Cattral M, Rotstein O
and Levy GA: Soluble fibrinogen-like protein 2/fibroleukin exhibits
immunosuppressive properties: Suppressing T cell proliferation and
inhibiting maturation of bone marrow-derived dendritic cells. J
Immunol. 170:4036–4044. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Yang PS, Zhu T, Manuel J, Zhang J,
He W, Shalev I, Zhang L, Cybulsky MI, Grant DR, et al:
Characterization of fibrinogen-like protein 2 (FGL2): Monomeric
FGL2 has enhanced immunosuppressive activity in comparison to
oligomeric FGL2. Int J Biochem Cell Biol. 45:408–418. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang M, Cao X, Li P, Zhang K, Li Y, Zheng
QY, Li GQ, Chen J, Xu GL and Zhang KQ: Increased expression of
fibrinogen-like protein 2 is associated with poor prognosis in
patients with clear cell renal cell carcinoma. Sci Rep.
7:126762017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan J, Kong LY, Hu J, Gabrusiewicz K,
Dibra D, Xia X, Heimberger AB and Li S: FGL2 as a multimodality
regulator of tumor-mediated immune suppression and therapeutic
target in gliomas. J Natl Cancer Inst. 107:djv1372015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu Y, Zhang L, Zha H, Yang F, Hu C, Chen
L, Guo B and Zhu B: Stroma-derived fibrinogen-like protein 2
activates cancer-associated fibroblasts to promote tumor growth in
lung cancer. Int J Biol Sci. 13:804–814. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Engers R, Mueller M, Walter A, Collard JG,
Willers R and Gabbert HE: Prognostic relevance of Tiam1 protein
expression in prostate carcinomas. Br J Cancer. 95:1081–1086. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vetsika EK, Koukos A and Kotsakis A:
Myeloid-derived suppressor cells: Major figures that shape the
immunosuppressive and angiogenic network in cancer. Cells.
8:16472019. View Article : Google Scholar
|
|
31
|
Law AMK, Valdes-Mora F and Gallego-Ortega
D: Myeloid-derived suppressor cells as a therapeutic target for
cancer. Cells. 9:5612020. View Article : Google Scholar
|
|
32
|
Tian X, Shen H, Li Z, Wang T and Wang S:
Tumor-derived exosomes, myeloid-derived suppressor cells, and tumor
microenvironment. J Hematol Oncol. 12:842019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Andreu P, Johansson M, Affara NI, Pucci F,
Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, et al:
FcRgamma activation regulates inflammation-associated squamous
carcinogenesis. Cancer Cell. 17:121–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gabrilovich DI, Ostrand-Rosenberg S and
Bronte V: Coordinated regulation of myeloid cells by tumours. Nat
Rev Immunol. 12:253–268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hoechst B, Ormandy LA, Ballmaier M, Lehner
F, Krüger C, Manns MP, Greten TF and Korangy F: A new population of
myeloid-derived suppressor cells in hepatocellular carcinoma
patients induces CD4+CD25+Foxp3+ T
cells. Gastroenterology. 135:234–243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang D, An G, Xie S, Yao Y and Feng G: The
clinical and prognostic significance of CD14(+) HLA-DR(−/low)
myeloid-derived suppressor cells in hepatocellular carcinoma
patients receiving radiotherapy. Tumour Biol. 37:10427–10433. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang S, Ma X, Zhu C, Liu L, Wang G and
Yuan X: The role of myeloid-derived suppressor cells in patients
with solid tumors: A meta-analysis. PLoS One. 11:e01645142016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao XH, Tian L, Wu J, Ma XL, Zhang CY,
Zhou Y, Sun YF, Hu B, Qiu SJ, Zhou J, et al: Circulating
CD14+ HLA-DR−/low myeloid-derived suppressor
cells predicted early recurrence of hepatocellular carcinoma after
surgery. Hepatol Res. 47:1061–1071. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mizukoshi E, Yamashita T, Arai K,
Terashima T, Kitahara M, Nakagawa H, Iida N, Fushimi K and Kaneko
S: Myeloid-derived suppressor cells correlate with patient outcomes
in hepatic arterial infusion chemotherapy for hepatocellular
carcinoma. Cancer Immunol Immunother. 65:715–725. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang X, Fu X, Li T and Yan H: The
prognostic value of myeloid derived suppressor cell level in
hepatocellular carcinoma: A systematic review and meta-analysis.
PLoS One. 14:e02253272019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu H, Shalev I, Manuel J, He W, Leung E,
Crookshank J, Liu MF, Diao J, Cattral M, Clark DA, et al: The
FGL2-FcgammaRIIB pathway: A novel mechanism leading to
immunosuppression. Eur J Immunol. 38:3114–3126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Morris AB, Farley CR, Pinelli DF, Adams
LE, Cragg MS, Boss JM, Scharer CD, Fribourg M, Cravedi P, Heeger PS
and Ford ML: Signaling through the inhibitory Fc receptor
FcgammaRIIB induces CD8+T cell apoptosis to limit T cell
immunity. Immunity. 52:136–150.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Selzner N, Liu H, Boehnert MU, Adeyi OA,
Shalev I, Bartczak AM, Xue-Zhong M, Manuel J, Rotstein OD,
McGilvray ID, et al: FGL2/fibroleukin mediates hepatic reperfusion
injury by induction of sinusoidal endothelial cell and hepatocyte
apoptosis in mice. J Hepatol. 56:153–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takai T, Li M, Sylvestre D, Clynes R and
Ravetch JV: FcR gamma chain deletion results in pleiotrophic
effector cell defects. Cell. 76:519–529. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ravetch JV and Bolland S: IgG Fc
receptors. Annu Rev Immunol. 19:275–290. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pan G, Zhao Z, Tang C, Ding L, Li Z, Zheng
D, Zong L and Wu Z: Soluble fibrinogen-like protein 2 ameliorates
acute rejection of liver transplantation in rat via inducing
Kupffer cells M2 polarization. Cancer Med. 7:3168–3177. 2018.
View Article : Google Scholar
|
|
47
|
Jin SJ, Liu Y, Deng SH, Liao LH, Lin TL,
Ning Q and Luo XP: Neuroprotective effects of activated protein C
on intrauterine inflammation-induced neonatal white matter injury
are associated with the downregulation of fibrinogen-like protein
2/fibroleukin prothrombinase and the inhibition of pro-inflammatory
cytokine expression. Int J Mol Med. 35:1199–1212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Buonaguro L, Mauriello A, Cavalluzzo B,
Petrizzo A and Tagliamonte M: Immunotherapy in hepatocellular
carcinoma. Ann Hepatol. 18:291–297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Brown ZJ and Greten TF: Hepatocellular
carcinoma: Translational precision medicine approaches (Internet).
Hoshida Y: Immune Therapies Cham (CH): Humana Press. Chapter 12.
2019
|
|
50
|
Ma L, Hernandez MO, Zhao Y, Mehta M, Tran
B, Kelly M, Rae Z, Hernandez JM, Davis JL, Martin SP, et al: Tumor
cell biodiversity drives microenvironmental reprogramming in liver
cancer. Cancer Cell. 36:418–430.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li W, Wang H, Ma Z, Zhang J, Ou-Yang W, Qi
Y and Liu J: Multi-omics analysis of microenvironment
characteristics and immune escape mechanisms of hepatocellular
carcinoma. Front Oncol. 9:10192019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lu C, Rong D, Zhang B, Zheng W, Wang X,
Chen Z and Tang W: Current perspectives on the immunosuppressive
tumor microenvironment in hepatocellular carcinoma: Challenges and
opportunities. Mol Cancer. 18:1302019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liao H, Chen W, Dai Y, Richardson JJ, Guo
J, Yuan K, Zeng Y and Xie K: Expression of programmed cell
death-ligands in hepatocellular carcinoma: Correlation with immune
microenvironment and survival outcomes. Front Oncol. 9:8832019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hilmi M, Vienot A, Rousseau B and
Neuzillet C: Immune therapy for liver cancers. Cancers (Basel).
12:772019. View Article : Google Scholar
|
|
55
|
van der Heide D, Weiskirchen R and Bansal
R: Therapeutic targeting of hepatic macrophages for the treatment
of liver diseases. Front Immunol. 10:28522019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lu LC, Chang CJ and Hsu CH: Targeting
myeloid-derived suppressor cells in the treatment of hepatocellular
carcinoma: Current state and future perspectives. J Hepatocell
Carcinoma. 6:71–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chesney JA, Mitchell RA and Yaddanapudi K:
Myeloid-derived suppressor cells-a new therapeutic target to
overcome resistance to cancer immunotherapy. J Leukoc Biol.
102:727–740. 2017. View Article : Google Scholar : PubMed/NCBI
|