Introduction
The global burden of cancer continues to increase
due to a variety of factors (1).
Gastric cancer is the fourth most common cancer worldwide and is
the second most common cause of cancer-associated mortality
(2). The treatment of gastric cancer
includes surgery, chemotherapy, radiotherapy and molecular targeted
therapy, among which surgery combined with chemoradiotherapy is the
most effective treatment regimen (3). However, the treatment has entered a
bottleneck period due to congenital or acquired drug resistance and
postoperative recurrence (4).
Therefore, identification of new targets and signaling pathways
related to the progression of gastric cancer may be beneficial for
the treatment of gastric cancer.
Tumor occurrence and development are closely
associated with uncontrolled cell proliferation, while malignant
cells often escape apoptosis to obtain unlimited proliferation
capacity (5). In this case, three
types of evasion mechanisms of apoptosis exist, including the
weakening of caspase function, the damage of the death receptor
pathway, and the destruction of the balance between anti-apoptotic
and pro-apoptotic proteins (6).
Therefore, targeting cancer cell apoptosis by modulating key
proteins or enzymes in the apoptosis-related signaling pathway has
become an area of focus in cancer research (7). Notably, a number of natural products
have been shown to act in apoptosis signaling pathways involved in
cancer cell death. Camptothecin, a quinoline alkaloid, induced
apoptosis of human myeloid leukemia cells by upregulating
proapoptotic proteins and downregulating cyclins (8). Matrine, an alkaloid derived from the
Sophora flavescensait, promoted apoptosis of gastric cancer cells
by boosting the pro-apoptotic proteins, altering the ratio of
Fas/FasL and activating caspase-3 (9,10). These
findings have led researchers to focus on developing new potential
anticancer agents.
Demethylzeylasteral is a triterpene monomer
extracted from tripterygium wilfordii, which has been widely used
in the study of anti-inflammatory immune regulation, antifertility
and estrogen metabolism regulation (11–14). In
recent years, the anticancer properties of demethylzeylasteral have
been continuously studied. Li et al (15) reported that demethylzeylasteral
significantly impeded the invasion of triple-negative breast cancer
by blocking the TGF-β signaling pathway (15). Meanwhile, this compound may suppress
glioma growth by mediating the miR-30e-5p/MYBL2 axis (16). Notably, demethylzeylasteral was found
to evoke the apoptosis of melanoma cells by downregulating the
level of MCL1 (17). However, the
anticancer activity of demethylzeylasteral on gastric cancer cells
and its underlying mechanism have not been investigated.
The present study aimed to determine and
characterize the anticancer properties of demethylzeylasteral on
human gastric cancer cells. The present study demonstrated that
demethylzeylasteral impeded the viability and migration of gastric
cancer cells, while inducing cancer cell apoptosis. Furthermore,
treatment with demethylzeylasteral attenuated the ERK1/2 pathway,
as well as a decreasing the levels of phosphorylated Akt (p-Akt)
and phosphorylated GSK3β (p-GSK3β) in the cancer cells. The present
study demonstrated that demethylzeylasteral has a therapeutic
potential for gastric cancer.
Materials and methods
Reagents
The reagents used in this study were purchased as
follows: Demethylzeylasteral (CAS:107316-88-1) from Target Molecule
Corp.; fetal bovine serum (FBS) from Shanghai Nuova Pharmaceutical
Technology Co., Ltd.; 0.25% Trypsin-EDTA, RPMI-1640 medium and
penicillin/streptomycin from Gibco; Thermo Fisher Scientific, Inc.;
dimethyl sulfoxide (DMSO) and enhanced chemiluminescent (ECL)
substrate from Thermo Fisher Scientific, Inc.; Wright-Giemsa Stain
it from Jian Cheng Technology Company; crystal violet from Sangon
Biotechnology; Annexin V-FITC/PI apoptosis detection kit from Multi
Sciences (Lianke) Biotech, Co., Ltd.; phosphate-buffered solution
(PBS), mitochondrial membrane potential (MMP) assay kit with JC-1,
and Cell Counting Kit-8 (CCK-8) from Beyotime Institute of
Biotechnology; methanol and ethanol from Macklin Reagent Co., Ltd.;
primary antibodies against Bax, cleaved PARP, caspase-9, cleaved
caspase-3, Bax, c-Myc, GSK-3β, p-GSK-3β (Ser9), ERK1/2,
phosphorylated ERK1/2 (p-ERK1/2), Akt, p-Akt and β-actin from Cell
Signaling Technology, Inc.; horseradish peroxidase-conjugated
secondary antibodies against rabbit and mice from Jackson
ImmunoResearch Inc.
Demethylzeylasteral liquid
preparation
A total of 10 mg demethylzeylasteral was dissolved
in DMSO for preparation of 100 mmol/ml solution, and the solution
was kept at −20°C for a long-term storage. For the experiments, the
solution was diluted with DMSO to the indicated concentrations.
Cell culture
Human gastric cancer MKN-45 cells were purchased
from American Type Culture Collection. Human normal gastric mucosal
GES-1 cells were obtained from the Cell Bank of the Chinese Academy
of Sciences. The cells were grown in a 37°C incubator with 5%
CO2 and cultured in RPMI-1640 medium supplemented with
10% FBS and 1% penicillin/streptomycin for 24 h.
Cytotoxicity assay
CCK-8 was used to assess the cytotoxicity of the
compound. In brief, MKN-45 cells were seeded onto 96-well plates
(5,000 cells/well) and incubated with 0.2 µl DMSO or
demethylzeylasteral at various concentrations (50 nM, 100 nM, 500
nM, 1 µM, 5 µM, 10 µM, 30 µM, 50 µM or 100 µM) for 24 h. Next, 10
µl CCK-8 solution was applied to each well, followed by incubation
for 2 h at 37°C. Finally, the absorbance at a wavelength of 450 nm
was measured using a microplate reader. Similarly, the effects of
different concentrations of demethylzeylasteral on the activity of
human normal gastric mucosa GES-1 cells were also investigated
using the same method.
Colony formation assay
A colony formation assay was utilized to assess cell
proliferation. MKN-45 cells in a single cell suspension state were
seeded onto a 6-well plate at a density of 500 cells per well, and
then treated with 2 µl DMSO or demethylzeylasteral at different
concentrations (100 nM, 500 nM, 1 µM or 10 µM). The cells were
grown in RPMI-1640 medium containing 10% FBS for 1 week until each
colony reached 100–200 cells. Colonies were fixed in 100% methanol
at room temperature for 20 min and stained using Giemsa staining at
room temperature for 20 min. Finally, the plate was washed
moderately with running tap water, and the colony with at least 100
cells was counted. Digital photography of colonies was conducted,
and the data was analyzed using Prism software 7.0 (GraphPad
Software, Inc.).
Transwell assay
The Transwell assay was used to assess the effect of
demethylzeylasteral on gastric cancer cell migration. A total of
200 µl serum-free cell suspension containing 2×105
MKN-45 cells and 600 µl RPMI-1640 medium with 5% FBS were added to
the upper chamber of the transwell chambers (8-µm pore size,
Corning Inc.) and lower compartment of the Transwell chamber,
respectively. Next, 2 µl DMSO or demethylzeylasteral at different
concentrations (1, 3 or 10 µM) were applied to the upper chamber.
After growth for 24 h, the cells migrating to the submembrane
surface were fixed with methanol for 20 min at room temperature and
stained with 0.5% crystal violet for 20 min at room temperature.
Finally, the stained cells were photographed under a Leica optical
microscope at ×100 magnification and ImageJ computer software 1.6
(National Institutes of Health) was used to calculate the area of
cell migration.
Measurement of MMP
Alteration in MMP in apoptosis was investigated
using an MMP-specific fluorescent probe, 5, 5′, 6,
6′-tetrachloro-1, 1′, 3, 3′-tetraethyl-benzimidazole-carbocyanide
iodine (JC-1; Beyotime Institute of Biotechnology). In brief,
MKN-45 cells were treated with 2 µl DMSO or demethylzeylasteral at
different concentrations (1, 3 or 10 µM) for 24 h and centrifuged
for 5 min at −4°C and 500 × g. The cells were stained with JC-1
staining solution for 20 min in the dark at 37°C. After being
washed twice with JC-1 buffer, the MKN-45 cells were re-suspended
in JC-1 buffer and analyzed on a flow cytometer (BD
FACSVerse™).
Apoptosis assay
Annexin V-FITC/PI apoptosis detection kit was used
to assay apoptosis in MKN-45 cells treated with
demethylzeylasteral, according to the manufacturer's protocol.
MKN-45 cells were seeded onto 12-well plates (5×105
cells/well) and grown at 37°C overnight. Next, the cells were
incubated with 2 µl DMSO or demethylzeylasteral at different
concentrations (1, 3 or 10 µM) for 24 h. Following treatment, the
cells were harvested by centrifugation (5 min at −4°C and 500 × g),
rinsed twice with PBS and re-suspended in 500 µl Annexin-binding
buffer. Finally, the cells were stained with 5 µl Annexin-V-FITC
and 10 µl PI for 15 min at 4°C, and the fluorescence was determined
using a flow cytometer (BD FACSVerse™; BD Biosciences). FlowJo 7.6
computer software (BD Biosciences) was used to analyze the
data.
Western blot analysis
Protein expression was detected using western blot
analysis. MKN-45 cells were seeded onto 6-well plates
(1×106 cells/well) and treated with 2 µl DMSO or
demethylzeylasteral at different concentrations for 24 h at 37°C.
Following treatment, MKN-45 cells were washed twice with cold PBS,
mixed with the loading buffer in each well, and then heated at 99°C
for 10 min. Protein lysates were separated by electrophoresis on 12
or 15% SDS-PAGE, and then transferred onto nitrocellulose
membranes. After being blocked with fresh 5% skimmed milk at room
temperature for 2 h, the membranes were incubated with primary
antibodies (Cell Signaling Technology, Inc.; 1:1,000) against Bax
(cat. no. 5023), cleaved PARP (cat. no. 5625), cleaved caspase-9
(cat. no. 52873), cleaved caspase-3 (cat. no. 9669), Bcl-2 (cat.
no. 15071), c-Myc (cat. no. 13987), GSK-3β (cat. no. 9315),
p-GSK-3β (Ser9; cat. no. 5558), ERK1/2 (cat. no. 4695), p-ERK1/2
(cat. no. 4376), Akt (cat. no. 4685), p-Akt (cat. no. 9563) and
β-actin (cat. no. 8457) at 4°C overnight. The membranes were
subsequently incubated with horseradish peroxidase-conjugated
secondary antibodies against rabbit and mice at room temperature
for 2 h. The target proteins in the membrane were detected and
visualized using the Chemiluminescence Luminol kit. ImageJ software
(National Institutes of Health) was used to measure the intensity
of the bands.
Statistical analysis
Demethylzeylasteral structure was drawn using
ChemDraw Professional 16.0 software (PerkinElmer, Inc.). All
statistical data are expressed as the mean ± standard error of the
mean, and all experiments were repeated at least three times.
GraphPad Prism 7.0 was used to perform Student's t-tests or one-way
analysis of variance with a Dunnett's post hoc test to analyze the
significance of the results. P<0.05 was considered to indicate a
statistically significant difference.
Results
Demethylzeylasteral has cytotoxic
effects on human gastric cancer cells
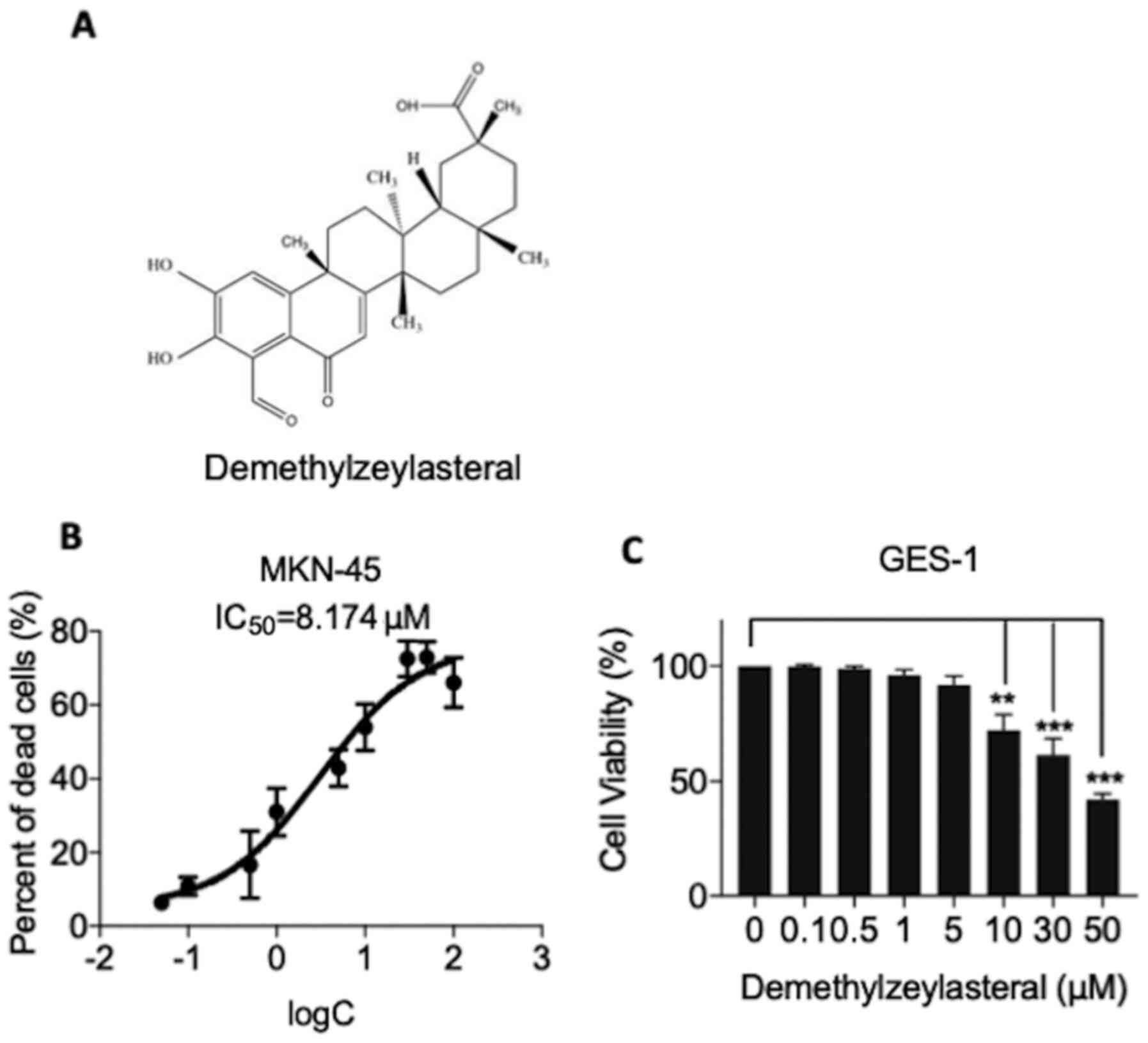
The chemical structure of demethylzeylasteral was
depicted in Fig. 1A. CCK-8 assay was
applied to test whether demethylzeylasteral has a cytotoxic effect
on human gastric cancer MKN-45 cells. As shown in Fig. 1B, demethylzeylasteral exhibited a
concentration-dependent cytotoxic effect on MKN-45 cells.
Additionally, the 50% inhibitory concentration (IC50)
values were found to be 8.174 µM for the MKN-45 cells. In the
experiments, human gastric mucosa GES-1 cells were included as a
control. As shown in Fig. 1C, the
CCK-8 assay revealed that demethylzeylasteral at concentrations
below 10 µM (0.1, 0.5, 1 or 5 µM) had no significant influence on
the survival rate of GES-1 cells, while cytotoxicity was evident in
the control cells treated with 10 µM or higher concentrations of
demethylzeylasteral (10, 30 or 50 µM).
Demethylzeylasteral inhibits gastric
cancer cell proliferation
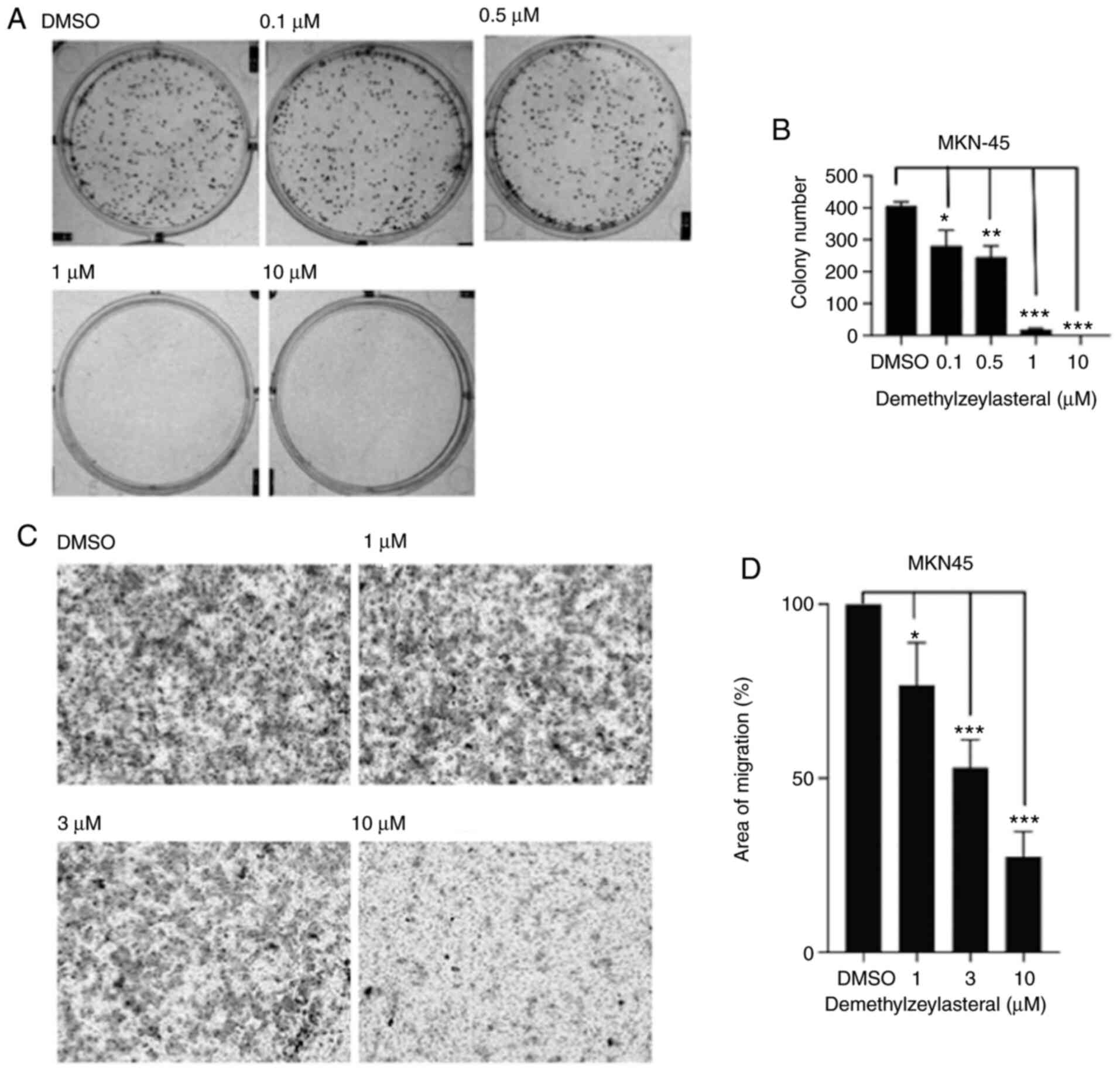
In the present study, MKN-45 cells were treated with
various concentrations of demethylzeylasteral for 1 week. As shown
in Fig. 2A, the treatment decreased
colony formation in all tested gastric cancer cells. Furthermore,
quantitative analysis of the colonies indicated that
demethylzeylasteral decreased the proliferation of gastric cancer
cells in a dose-dependent manner.
Demethylzeylasteral suppresses cancer
cell migration
The effects of demethylzeylasteral on the migration
of human gastric cancer cells were investigated using an in
vitro Transwell assay. As shown in Fig. 2C, a significant decrease in the
number of cells migrating to the lower surface of the filter was
observed in the group of MKN-45 cells treated with
demethylzeylasteral, compared with that in the DMSO-treated group.
Further statistical analysis revealed that treatment with
demethylzeylasteral at various concentrations suppressed the
migration of MKN-45 cells in a dose-dependent manner (Fig. 2D).
Demethylzeylasteral induces apoptosis
of MKN-45 cells
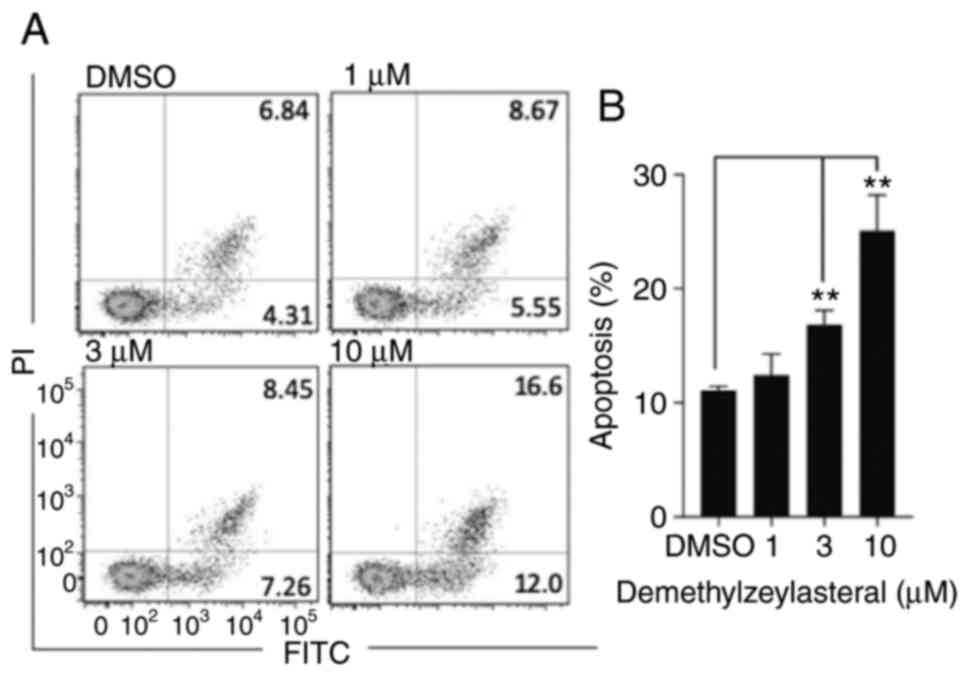
It has been reported that numerous compounds inhibit
tumor cell growth by inducing apoptosis (18). To determine whether the
anti-proliferative effect of demethylzeylasteral is associated with
apoptosis, Annexin V-FITC/PI double staining and flow cytometry
were used to detect the number of apoptotic cells. As shown in
Fig. 3A and B, compared with the
control group, treatment with demethylzeylasteral at various
concentrations for 24 h resulted in a significant increase in the
rate of apoptosis from 11.15% (DMSO) to 28.6% (10 µM). Notably, the
number of apoptotic cells was gradually increased along with
increasing concentrations of demethylzeylasteral (Fig. 3B). Furthermore, western blot analysis
revealed that markedly upregulated expression of pro-apoptotic
proteins, including Bax, cleaved caspase-9, cleaved caspase-3 and
cleaved PARP, as well as downregulated expression of an
anti-apoptotic protein, Bcl-2, were detected in MKN-45 cells
treated with demethylzeylasteral (Fig.
4). These observations indicated that demethylzeylasteral may
induce apoptosis of MKN-45 cells.
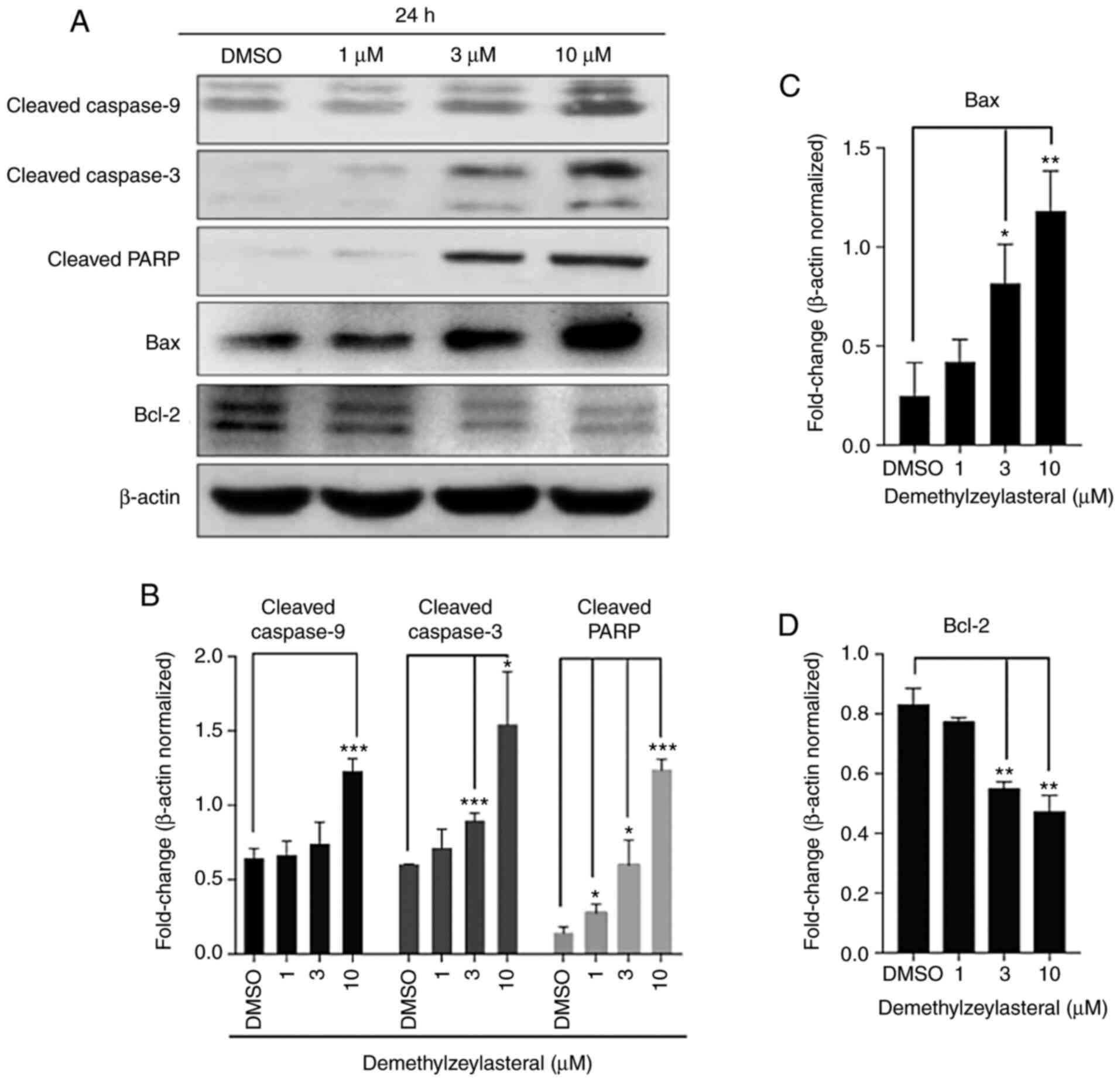 | Figure 4.Demethylzeylasteral increases the
expression of Bax, Bcl-2, cleaved Caspase-9, cleaved Caspase-3 and
cleaved PARP. (A) The expression of Bax, Bcl-2, cleaved Caspase-9,
cleaved Caspase-3, and cleaved PARP in MKN-45 cells treated with
DMSO or various concentrations of demethylzeylasteral. β-actin was
used as a loading control. (B-D) ImageJ software-based quantitative
analysis of cleaved PARP, cleaved Caspase-9, cleaved Caspase-3,
Bcl-2 and Bax. P<0.05 was considered to indicate a statistically
significant difference. *P<0.05, **P<0.01, and ***P<0.001
compared with the DMSO group. DMSO, dimethyl sulfoxide. |
Demethylzeylasteral affects the MMP in
MKN-45 cells
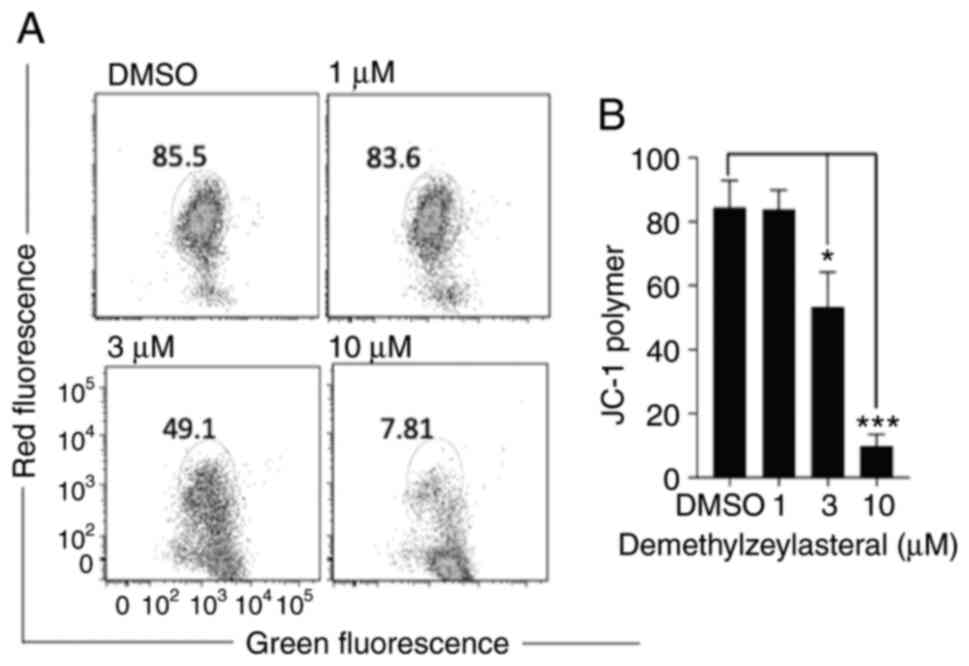
It has been shown that the cleavage of caspase-3,
and caspase-9 and PARP is increased during the
mitochondria-dependent apoptosis (19). JC-1 staining was used to investigate
whether demethylzeylasteral promotes apoptosis via the
mitochondrial-dependent pathway. As shown in Fig. 5A and B, the JC-1 polymer was
significantly decreased in MKN-45 cells treated with
demethylzeylasteral at various indicated concentrations for 24 h.
Furthermore, the change in JC-1 from red to green fluorescence
indicated a loss of the MMP (Fig.
5A). Taken together, these results suggested that
demethylzeylasteral-induced apoptosis in the cancer cells may
involve the mitochondria-dependent pathway.
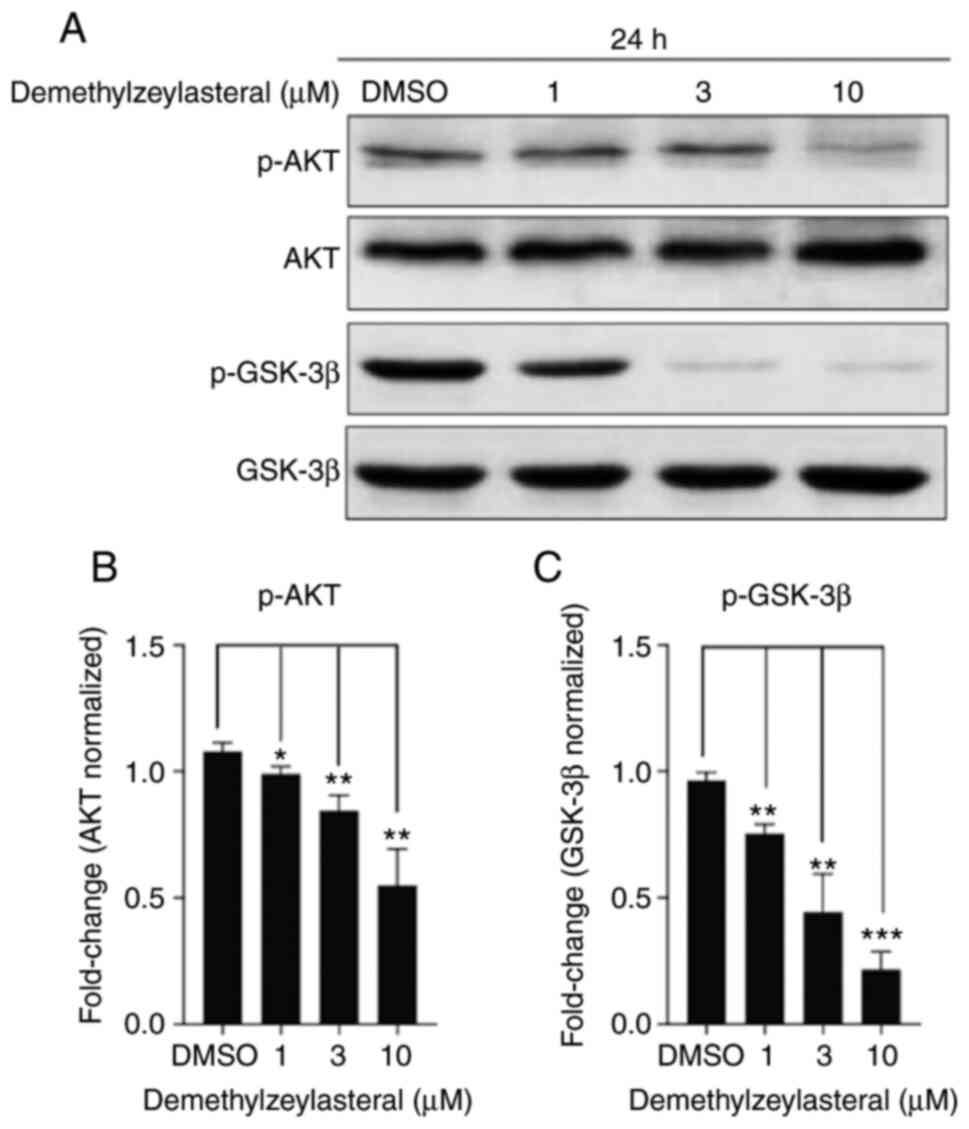
Demethylzeylasteral treatment
downregulates Akt/GSK3β pathway
Akt is a canonical oncogenic kinase serving a
prominent role in tumor progression (20). To improve understanding of the
molecular mechanism underlying the antitumor effect of
demethylzeylasteral on the cancer cells, the levels of total Akt,
total GSK-3β, p-Akt and p-GSK-3β (ser9) in MKN-45 cells treated
with demethylzeylasteral were investigated. As depicted in Fig. 6A, treatment with increasing
concentrations of demethylzeylasteral led to a stepwise decrease in
the levels of p-Akt and p-GSK-3β, while the levels of total Akt and
GSK-3β remained unchanged. Quantitative analysis revealed a
dose-dependent decrease in the levels of p-Akt and p-GSK-3β
(Fig. 6B and C).
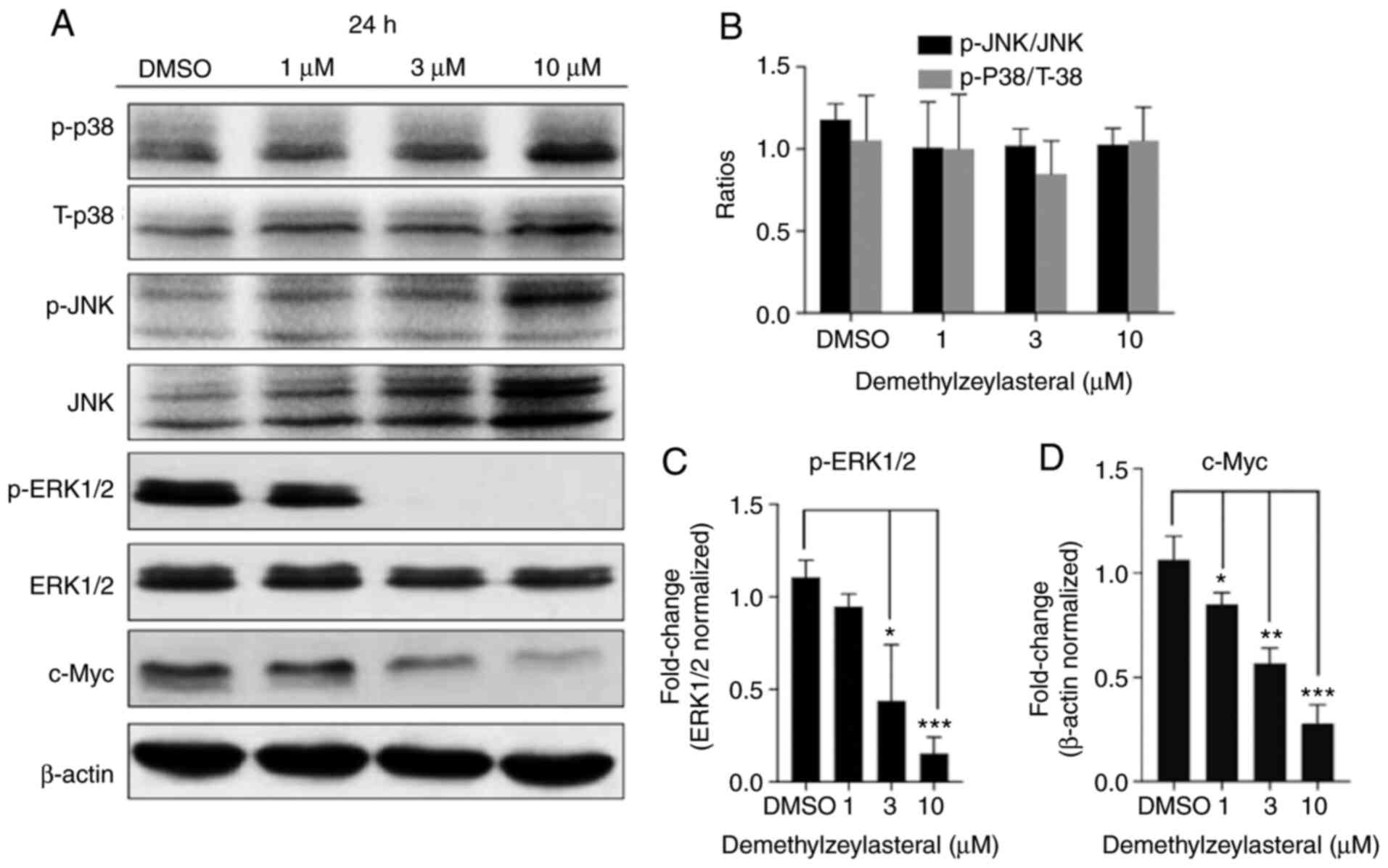
Demethylzeylasteral attenuates the
ERK1/2 pathway
It has been documented that the activation of the
MAPK pathway contributes toward the development of gastric cancer.
To clarify whether the anticancer properties of demethylzeylasteral
are associated with MAPK signaling pathways in gastric cancer
cells, the expression of JNK, ERK1/2 and p38 protein in
demethylzeylasteral-treated cells was measured using western blot
analysis. The analysis revealed that, compared with the
DMSO-treated cells, the level of p-ERK1/2 was decreased in the
cancer cells treated with demethylzeylasteral, while no significant
change in the levels of phosphorylated-JNK and phosphorylated-p38
was detected in the cells treated with various concentrations of
demethylzeylasteral (Fig. 7). ERK1/2
serves a pro-tumorigenic role in numerous cancer types and has
hundreds of substrates (21), some
of which control cell growth, differentiation, survival and death
by regulating the phosphorylation and activation of transcription
factors. Given that c-Myc is a downstream target of ERK1/2
(22,23), the present study aimed to analyze the
expression of c-Myc protein in MKN-45 cells incubated with
demethylzeylasteral. As shown in Fig.
7A, treatment with demethylzeylasteral decreased the expression
of c-Myc, while downregulating p-ERK1/2 in the cancer cells. Taken
together, these data suggested that the ERK pathway may be involved
in demethylzeylasteral-mediated cell proliferation and apoptosis in
MKN-45 cells.
Discussion
Gastric cancer is the second leading cause of
cancer-associated mortality after lung cancer. Although the rate of
early diagnosis has been improved, the therapeutic outcomes of
gastric cancer requires improvement due to postoperative
recurrence, acquired drug resistance and late metastasis (4,24).
Therefore, developing novel drugs for the treatment of gastric
cancer may contribute toward promoting the therapeutic outcomes.
Demethylzeylasteral is a natural compound from tripterygium
wilfordii, which possesses multiple pharmacological effects.
Although this compound has been shown to be associated with breast
cancer, glioma and malignant melanoma, determining whether it has a
broad-spectrum antitumor effect requires further investigation. The
present study aimed to investigate the potential anticancer effect
of demethylzeylasteral on gastric cancer cells.
The present study investigated the
anti-proliferative activity of demethylzeylasteral by using gastric
cancer MKN-45 cell line. The CCK-8 assay demonstrated that
demethylzeylasteral had a dose-dependent cytotoxic effect on cancer
MKN-45 cells. Demethylzeylasteral was found to downregulate the
Akt/GSK-3β pathway, as well as the ERK1/2 pathway. Furthermore, it
was found that treatment with demethylzeylasteral decreased MMP,
while downregulating Bcl-2 expression and upregulating Bax, cleaved
caspase-9, cleaved caspase-3 and cleaved PARP expression.
Apoptosis or programmed cell death is mediated by
the endogenous mitochondrial pathway and the exogenous death
receptor pathway (25). The complex
mechanism involves numerous signaling pathways (6); among them, the intrinsic pathway of
mitochondria-induced apoptosis is dependent on the expression of a
series of proteins, including the Bcl-2 family, which contains
anti-apoptotic and pro-apoptotic proteins. Bcl-2 family proteins
mediate the activation of cysteine aspartic acid specific proteases
(caspases) for inducing apoptosis (26–28).
Caspases are the core of apoptosis mechanism, as they act as both
the promoters and the executors of cell death (29). Once activated, the initiator caspases
will cleave and activate executioner caspases, which subsequently
perform critical cleavage on specific cell substrates, ultimately
leading to apoptosis (30).
Activated Caspase-9 in the endogenous mitochondria-dependent
pathway in turn results in the activation of Caspase-3 (31). Caspase-3 is a key component in the
caspase-dependent apoptosis pathway, which triggers the cleavage of
downstream substrate PARP (32),
leading to chromatin lysis and apoptosis (18). In the present study, treatment of
MKN-45 cells with demethylzeylasteral caused an upregulation in the
levels of cleaved Caspase-9, cleaved Caspase-3 and cleaved PARP; a
decreased expression of anti-apoptotic protein Bcl-2; increased
expression of pro-apoptotic protein Bax; and disruption of MMP. All
these data suggested that demethylzeylasteral-elicited apoptosis in
MKN-45 cells may involve the activation of the caspase cascade in
the endogenous death pathway (mitochondrial pathway).
Akt, a serine/threonine kinase, is highly amplified
in gastric cancer and regulates numerous biological and
pathological processes, including apoptosis, cell proliferation and
glucose usage (33,34). Apatinib is known to be significant
for the treatment of advanced gastric cancer (35). Compound Astragalus polysaccharide
(AsPs) works with apatinib against gastric cancer by inhibiting the
Akt pathway in AGS cells (36). In
addition, helicobacter pylori (HP) has been highlighted due
to its association with and involvement in the occurrence of
gastric cancer associated with the Akt/GSK-3β signaling pathway
(37). Additionally, clinical
studies have demonstrated that Akt phosphorylation is involved in
tumor invasion, and p-Akt status may be associated with early
recurrence and poor prognosis (38).
Akt promotes survival and cell cycle by phosphorylating cellular
proteins, including GSK-3α and GSK-3β (39); GSK-3α and GSK-3β constitute two main
isoforms of GSK-3, a multifunctional serine/threonine protein
kinase. It has been reported that GSK-3 is regulated by activated
Akt, and activated GSK-3 (non-phosphorylated state) regulates cell
cycle and apoptosis (40). It is
worth noting that GSK-3β is inactivated once phosphorylated on
serine 9. Multiple studies have demonstrated that Dioscin,
β-sitosterol and Isobavachalcone, respectively, induced apoptosis
of osteosarcoma, pancreatic cancer and colon cancer cells by
downregulating Akt/GSK-3β pathway (41–43). The
present study demonstrated that the treatment of MKN-45 cells with
demethylzeylasteral in induced cell growth inhibition and
apoptosis, while suppressing the Akt/GSK-3β pathway. These findings
suggested that the anti-proliferative and apoptosis-inducing
effects of demethylzeylasteral on gastric cancer cells are
associated with the Akt/GSK-3β signaling pathway.
The MAPK signaling pathway includes JNK, ERK and
p38, and serves a considerable role in cell proliferation, survival
and apoptosis (44). The present
study demonstrated that significantly decreased p-ERK1/2, but minor
changes in the phosphorylation of JNK or p38, were detected in the
cancer cells treated with demethylzeylasteral. ERK 1/2 is a key
modulator of cell proliferation, and inhibitors of the ERK pathway
are currently used as potential anticancer agents in clinical
trials (45). Furthermore, ERK1/2
phosphorylation-targeting compounds were found to serve a vital
role in the survival of gastric cancer. Wang et al (46) reported that 1,4-naphthoquinone
derivatives evoked apoptosis of gastric cancer cells by
downregulating the ERK pathway (46). An extract from Triptolide has been
identified to have antitumor effects on gastric cancer cells
(47). Furthermore, recent studies
have shown that Triptolide may prevent the proliferation and
metastasis of esophageal cancer cells via the ERK1/2 pathway
(48). The aforementioned two
compounds differ from demethylzeylasteral in chemical properties
and are greatly limited in clinical application due to their
undesirable side effects. Once translocated to the nucleus,
p-ERK1/2 activates multiple transcription factors, including CREB,
NF-κB and c-Myc, thereby regulating various cellular processes,
including cell survival and proliferation (22,23). The
present study observed a dose-dependent inhibitory effect of
demethylzeylasteral on the expression of p-ERK 1/2 in cancer cells.
Meanwhile, treatment with demethylzeylasteral significantly
decreased the expression level of c-Myc. We hypothesize that
demethylzeylasteral inhibits gastric cancer cell proliferation and
induces apoptosis of cancer cells by modulating the ERK1/2 pathway
and downregulating c-Myc expression.
Toxicity has long been a key factor limiting the
clinical application of novel research drugs. The growing
advancement in technology suggests that the toxicity of
demethylzeylasteral may be decreased through either increasing its
solubility via modification of the chemical structure or
administration combined with other chemotherapy drugs. More
importantly, structural and chemical modification of
demethylzeylasteral will enable more in vivo experiments to
be conducted in future studies. Therefore, decreased toxicity may
make it possible for demethylzeylasteral to become a novel drug for
the treatment of gastric cancer.
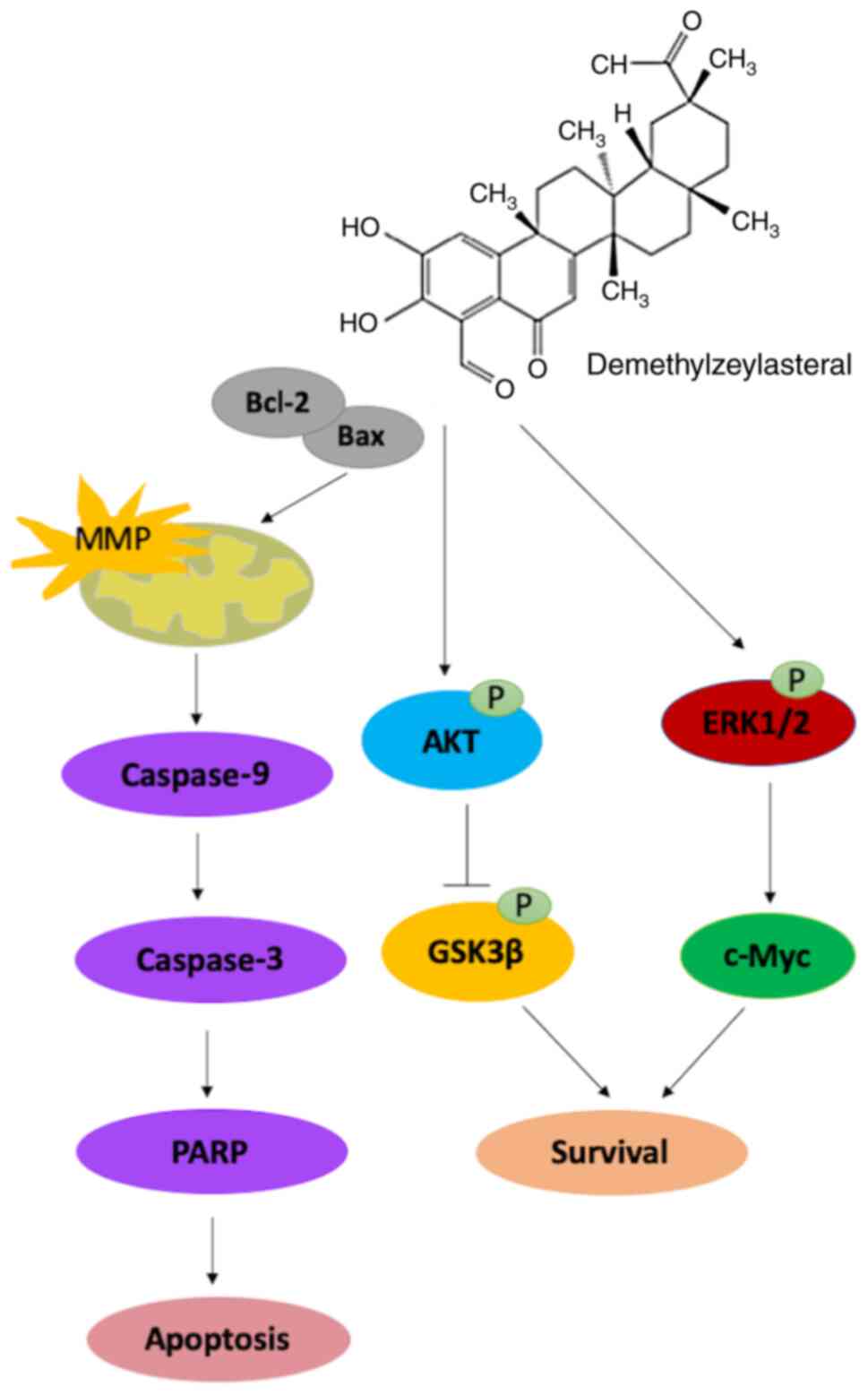
In conclusion, the results of the present study
suggested that demethylzeylasteral inhibited the proliferation and
migration of gastric cancer cells, while promoting the apoptosis of
cancer cells via the mitochondria-dependent pathway. Further
investigation suggested that the Akt/GSK-3β and ERK1/2 signaling
pathways may be involved in the demethylzeylasteral-induced
antitumor effects on the cancer cells (Fig. 8). These results suggested that
demethylzeylasteral has therapeutic potential for the treatment of
gastric cancer.
Acknowledgements
Not applicable.
Funding
This work was supported by The Natural Science
Research Key Project of Education Office of Anhui Province (grant
no. KJ2019A0329) and Research and Innovation Team of Bengbu Medical
College (grant no. BYKC201908).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YY, ZW and FQ designed the study. YY, MZ and TH
collected experimental materials and carried out the experiments.
FS performed the statistical analyses. YY wrote the manuscript. FQ
and ZW made critical revisions to the manuscript. ZW given final
approval of the version to be published. All authors read and
approved the final version.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ang TL and Fock KM: Clinical epidemiology
of gastric cancer. Singapore Med J. 55:621–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JY, von Karsa L and Herrero R:
Prevention strategies for gastric cancer: A global perspective.
Clin Endosc. 47:478–489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klener P Jr, Andera L, Klener P, Necas E
and Zivny J: Cell death signalling pathways in the pathogenesis and
therapy of haematologic malignancies: Overview of therapeutic
approaches. Folia Biol (Praha). 52:119–136. 2006.PubMed/NCBI
|
|
6
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bianco R, Melisi D, Ciardiello F and
Tortora G: Key cancer cell signal transduction pathways as
therapeutic targets. Eur J Cancer. 42:290–294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ullmannova V and Haskovec C: Gene
expression during camptothecin-induced apoptosis in human myeloid
leukemia cell line ML-2. Neoplasma. 51:175–180. 2004.PubMed/NCBI
|
|
9
|
Luo C, Zhu Y, Jiang T, Lu X, Zhang W, Jing
Q, Li J, Pang L, Chen K, Qiu F, et al: Matrine induced gastric
cancer MKN45 cells apoptosis via increasing pro-apoptotic molecules
of Bcl-2 family. Toxicology. 229:245–252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai ZJ, Gao J, Ji ZZ, Wang XJ, Ren HT, Liu
XX, Wu WY, Kang HF and Guan HT: Matrine induces apoptosis in
gastric carcinoma cells via alteration of Fas/FasL and activation
of caspase-3. J Ethnopharmacol. 123:91–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Q, Xiao Y, Liu T, Yuan H and Li C:
Demethylzeylasteral ameliorates inflammation in a rat model of
unilateral ureteral obstruction through inhibiting activation of
the NF-κB pathway. Mol Med Rep. 16:373–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Q, Yang C, Wang Q, Zeng H and Qin W:
Demethylzeylasteral (T-96) treatment ameliorates mice lupus
nephritis accompanied by inhibiting activation of NF-κB pathway.
PLoS One. 10:e1337242015.
|
|
13
|
Zhao JW, Wang GH, Chen M, Cheng LH and Ji
XQ: Demethylzeylasteral exhibits strong inhibition towards
UDP-glucuronosyltransferase (UGT) 1A6 and 2B7. Molecules.
17:9469–9475. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bai JP, Shi YL, Fang X and Shi QX: Effects
of demethylzeylasteral and celastrol on spermatogenic cell
Ca2+ channels and progesterone-induced sperm acrosome
reaction. Eur J Pharmacol. 464:9–15. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Ji Y, Fan J, Li F, Li Y, Wu M, Cheng
H and Xu C: Demethylzeylasteral (T-96) inhibits triple-negative
breast cancer invasion by blocking the canonical and non-canonical
TGF-β signaling pathways. Naunyn Schmiedebergs Arch Pharmacol.
392:593–603. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang K, Fu G, Pan G, Li C, Shen L, Hu R,
Zhu S, Chen Y and Cui H: Demethylzeylasteral inhibits glioma growth
by regulating the miR-30e-5p/MYBL2 axis. Cell Death Dis.
9:10352018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao Y, He J, Li J, Peng X, Wang X, Dong
Z, Zhao E, Liu Y, Wu Z and Cui H: Demethylzeylasteral inhibits cell
proliferation and induces apoptosis through suppressing MCL1 in
melanoma cells. Cell Death Dis. 8:e31332017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, An R, Umanah GK, Park H, Nambiar
K, Eacker SM, Kim B, Bao L, Harraz MM, Chang C, et al: A nuclease
that mediates cell death induced by DNA damage and poly(ADP-ribose)
polymerase-1. Science. 354:aad68722016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HJ, Lee HJ, Lee EO, Ko SG, Bae HS, Kim
CH, Ahn KS, Lu J and Kim SH: Mitochondria-cytochrome C-caspase-9
cascade mediates isorhamnetin-induced apoptosis. Cancer Lett.
270:342–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao C, Yuan X, Jiang Z, Gan D, Ding L, Sun
Y, Zhou J, Xu L, Liu Y and Wang G: Regulation of AKT
phosphorylation by GSK3β and PTEN to control chemoresistance in
breast cancer. Breast Cancer Res Treat. 176:291–301. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sammons RM, Perry NA, Li Y, Cho EJ,
Piserchio A, Zamora-Olivares DP, Ghose R, Kaoud TS, Debevec G,
Bartholomeusz C, et al: A novel class of common docking domain
inhibitors that prevent ERK2 activation and substrate
phosphorylation. ACS Chem Biol. 14:1183–1194. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chambard JC, Lefloch R, Pouyssegur J and
Lenormand P: ERK implication in cell cycle regulation. Biochim
Biophys Acta. 1773:1299–1310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu F, Yang X, Geng M and Huang M:
Targeting ERK, an Achilles' Heel of the MAPK pathway, in cancer
therapy. Acta Pharm Sin B. 8:552–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
den Hoed CM and Kuipers EJ: Gastric
cancer: How can we reduce the incidence of this disease? Curr
Gastroenterol Rep. 18:342016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heo SK, Yun HJ, Park WH and Park SD:
Emodin inhibits TNF-alpha-induced human aortic smooth-muscle cell
proliferation via caspase- and mitochondrial-dependent apoptosis. J
Cell Biochem. 105:70–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guerra F, Arbini AA and Moro L:
Mitochondria and cancer chemoresistance. Biochim Biophys Acta
Bioenerg. 1858:686–699. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akl H, Vervloessem T, Kiviluoto S,
Bittremieux M, Parys JB, De Smedt H and Bultynck G: A dual role for
the anti-apoptotic Bcl-2 protein in cancer: Mitochondria versus
endoplasmic reticulum. Biochim Biophys Acta. 1843:2240–2252. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Birkinshaw RW and Czabotar PE: The BCL-2
family of proteins and mitochondrial outer membrane
permeabilisation. Semin Cell Dev Biol. 72:152–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van Opdenbosch N and Lamkanfi M: Caspases
in cell death, inflammation, and disease. Immunity. 50:1352–1364.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stennicke HR and Salvesen GS:
Caspases-controlling intracellular signals by protease zymogen
activation. Biochim Biophys Acta. 1477:299–306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hill MM, Adrain C and Martin SJ: Portrait
of a killer: The mitochondrial apoptosome emerges from the shadows.
Mol Interv. 3:19–26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhola PD and Letai A: Mitochondria-Judges
and executioners of cell death sentences. Mol Cell. 61:695–704.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Revathidevi S and Munirajan AK: Akt in
cancer: Mediator and more. Semin Cancer Biol. 59:80–91. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Staal PS: Molecular cloning of the akt
oncogene and its human homologues AKT1 and AKT2: Amplification of
AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci
USA. 84:5034–5037. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roviello G, Ravelli A, Fiaschi AI,
Cappelletti MR, Gobbi A, Senti C, Zanotti L, Polom K, Reynolds AR,
Fox SB and Generali D: Apatinib for the treatment of gastric
cancer. Expert Rev Gastroenterol Hepatol. 10:887–892.
2016.PubMed/NCBI
|
|
36
|
Wu J, Yu J, Wang J, Zhang C, Shang K, Yao
X and Cao B: Astragalus polysaccharide enhanced antitumor effects
of Apatinib in gastric cancer AGS cells by inhibiting AKT
signalling pathway. Biomed Pharmacother. 100:176–183. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Geng W and Zhang HY: Research on the
mechanism of HP mediated PI3K/AKT/GSK3β pathways in gastric cancer.
Eur Rev Med Pharmacol Sci. 21 (3 Suppl):S33–S37. 2017.
|
|
38
|
Nakanishi K, Sakamoto M, Yamasaki S, Todo
S and Hirohashi S: Akt phosphorylation is a risk factor for early
disease recurrence and poor prognosis in hepatocellular carcinoma.
Cancer. 103:307–312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Namba T, Kodama R, Moritomo S, Hoshino T
and Mizushima T: Zidovudine, an anti-viral drug, resensitizes
gemcitabine-resistant pancreatic cancer cells to gemcitabine by
inhibition of the Akt-GSK3β-Snail pathway. Cell Death Dis.
6:e17952015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cao ZQ, Wang XX, Lu L, Xu JW, Li XB, Zhang
GR, Ma ZJ, Shi AC, Wang Y and Song YJ: β-sitosterol and gemcitabine
exhibit synergistic anti-pancreatic cancer activity by modulating
apoptosis and inhibiting epithelial-mesenchymal transition by
deactivating Akt/GSK-3β signaling. Front Pharmacol. 9:15252019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li Y, Qin X, Li P, Zhang H, Lin T, Miao Z
and Ma S: Isobavachalcone isolated from Psoralea corylifolia
inhibits cell proliferation and induces apoptosis via inhibiting
the AKT/GSK-3β/β-catenin pathway in colorectal cancer cells. Drug
Des Devel Ther. 13:1449–1460. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu W, Zhao Z, Wang Y, Li W, Su Q, Jia Q,
Zhang J, Zhang X, Shen J and Yin J: Dioscin inhibits stem-cell-like
properties and tumor growth of osteosarcoma through
Akt/GSK3/β-catenin signaling pathway. Cell Death Dis. 9:3432018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kohno M and Pouyssegur J: Pharmacological
inhibitors of the ERK signaling pathway: Application as anticancer
drugs. Prog Cell Cycle Res. 5:219–224. 2003.PubMed/NCBI
|
|
46
|
Wang H, Luo YH, Shen GN, Piao XJ, Xu WT,
Zhang Y, Wang JR, Feng YC, Li JQ, Zhang Y, et al: Two novel
1,4-naphthoquinone derivatives induce human gastric cancer cell
apoptosis and cell cycle arrest by regulating reactive oxygen
species-mediated MAPK/Akt/STAT3 signaling pathways. Mol Med Rep.
20:2571–2582. 2019.PubMed/NCBI
|
|
47
|
Chang HJ, Kim MH, Baek MK, Park JS, Chung
IJ, Shin BA, Ahn BW and Jung YD: Triptolide inhibits tumor
promoter-induced uPAR expression via blocking NF-kappaB signaling
in human gastric AGS cells. Anticancer Res. 27:3411–3417.
2007.PubMed/NCBI
|
|
48
|
Yanchun M, Yi W, Lu W, Yu Q, Jian Y,
Pengzhou K, Ting Y, Hongyi L, Fang W, Xiaolong C, et al: Triptolide
prevents proliferation and migration of esophageal squamous cell
cancer via MAPK/ERK signaling pathway. Eur J Pharmacol. 851:43–51.
2019. View Article : Google Scholar : PubMed/NCBI
|