Introduction
It is estimated that there were ~570,000 cases of
cervical cancer and 311,000 deaths worldwide in 2018, making it the
fourth most common cancer and the fourth leading cause of female
cancer-associated mortality (1).
There are no symptoms typically seen in the early stages of
disease; therefore, novel biomarkers with high sensitivity and
specificity for early detection and diagnosis are urgently required
to ensure patients can receive timely treatment. Liquid biopsies,
which include circulating tumor (ct)DNA, circulating tumor cells
(CTCs), platelets and exosomes, are promising for early cancer
detection and real time monitoring of cancer progression, response
to treatment and cancer metastasis (2). Compared with CTCs and ctDNA, exosomes
are advantageous in terms of stability, abundance and
accessibility; exosomes are abundant in plasma and are broadly
distributed in body fluids, and can be easily acquired (3). Exosomes are nano-sized and
membrane-enclosed extracellular vesicles with a diameter of 30–150
nm (4). Almost all mammalian cells,
including dendritic cells, adipocytes, endothelial and epithelial
cells, can secrete exosomes under normal or pathological conditions
(5). Exosomes carry numerous types
of biological molecules, including proteins, RNAs, DNAs and lipids.
Previous studies have indicated that miRNA is stably present in the
exosomes, suggesting that exosomal miRNAs could be investigated as
biomarkers for early diagnosis, prognosis and prediction of
treatment in cancer (6). In patients
with cervical cancer, homosapiens (hsa)-microRNA (miR/miRNA)-21 and
hsa-miR-164a have been reported to be upregulated and associated
with the high levels of cervical cancer-derived exosomes (7). It has also been reported that long
non-coding RNAs HOTAIR, MALAT1 and MEG3 in exosomes can be isolated
from cervicovaginal lavage and are differentially expressed in
patients with cervical cancer and control patients (8).
miR-125a-5p was previously identified in normal
tissue and exhibited a decreased expression in breast cancer
(9). Additionally, upregulation of
miR-125a-5p induced cancer cell apoptosis through p53 activation
(10). In hepatocellular carcinoma,
inhibiting miR-125a-5p increases MMP11 and vascular endothelial
growth factor A protein expression, while restoring miR-125a-5p can
inhibit cancer cell proliferation and metastasis (11). Circulating plasma exosomal
miR-125a-3p is accessible as a diagnostic biomarker for early-stage
colon cancer, which can also improve diagnostic power when combined
with the serum serological tumor marker carcinoembryonic antigen
(12). The aim of the present study
was to determine whether cervical cancer exosomes contain low
levels of miR-125a-5p by using a larger number of samples, and to
evaluate whether exosomal miR-125a-5p can potentially serve as a
biomarker for cervical cancer diagnosis.
Materials and methods
Patients and samples
A total of 72 individuals, including 44 patients
with cervical cancer (age range, 42–65 years; median age, 56 years)
and 28 healthy controls (age range, 41–69 years; median age, 58
years), were recruited between July 2017 and November 2018 from the
First Affiliated Hospital of Hainan University (Haikou, Hainan),
the 940th Hospital of Joint Logistics Support Force of Chinese
People's Liberation Army (Lanzhou, Gansu) and the First Hospital of
Shanxi Medical University (Taiyuan, Shanxi). The clinical
characteristics of the patients with cervical cancer were obtained
through the hospital medical systems, including age, histological
type, tumor size, the International Federation of Gynecology and
Obstetrics (FIGO) stage (13),
metastasis and HPV status. All cases of cervical cancer were
confirmed by pathological diagnosis. The exclusion criteria were as
follows: Other types of cancer besides cervical cancer, anemia,
active infections, liver or kidney dysfunction, mental or
psychological diseases, autoimmune disease and severe heart
failure. Firstly, 12 samples were used for the exosomal small RNA
sequencing, which included 6 patients with cervical cancer and 6
healthy control subjects. Next, the remaining 60 subjects, which
included 22 healthy individuals and 38 patients with cervical
cancer, were investigated using quantitative PCR (qPCR) to confirm
the differential exosomal miRNAs. Whole blood (10 ml) was collected
by venipuncture into EDTA tubes (Guangzhou Improve Medical
Instruments Co., Ltd.), centrifuged at 800 × g for 15 min at 4°C,
and the isolated plasma samples were stored at −80°C until RNA
isolation.
Exosomal RNA extraction and sequencing
library preparation
Exosomes were isolated using the exoEasy Maxi kit
(cat. no. 76064; Qiagen, Inc.) according to the manufacturer's
instructions. Total RNA was extracted from plasma using the
miRNeasy Serum/Plasma kit (cat. no. 77064; Qiagen, Inc.) according
to the manufacturer's protocol and information on the label.
Extracted exosomal RNA was used to prepare miRNA next-generation
sequencing (NGS) libraries with the QIAseq miRNA Library kit (cat.
no. 331505; Qiagen, Inc.). The sequencing was performed by HaploX
Biotechnology Co., Ltd. on an Illumina HiSeq 2500 instrument. NGS
data analysis was performed using the GeneReader system (Qiagen,
Inc.) according to manufacturer's instructions (14).
Reverse transcription-qPCR
Total RNA was extracted from exosomes using a
miRNeasy kit (cat. no. 217184; Qiagen, Inc.). A total of 200 µl
plasma was mixed with QIAzol Lysis Reagent (cat. no. 79306; Qiagen,
Inc.) according to the manufacturer's instructions. Exosomal RNA
was reverse transcribed using a Qiagen miRNA Reverse Transcription
kit (Qiagen, Inc.) according to the manufacturer's instructions.
The levels of miRNA 125-5p were determined in triplicate and
analyzed using a Real-time PCR detection system (LineGene K Plus;
Hangzhou Bioer Co., Ltd.) using SYBR green qPCRmaster mix (Qiagen,
Inc.). PCR was performed in 40 cycles with each cycle consisting of
30 sec at 94°C and 30 sec at 62°C. The PCR data were normalized
using the 2−ΔΔCq value method (15), using hsa-miR-16-5p for normalization
(16,17). The primers used for the amplification
were as follows: hsa-miR-125a-5p, forward,
5′-ACACTCCAGCTGGGTCCCTGAGACCCTTTAAC-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′; hsa-miR-16-5p, forward,
5′-TAGCAGCACGTAAATATTGGCG-3′ and reverse,
5′-TGCGTGTCGTGGAGTC-3′.
ZetaView nanoparticle tracking
analysis (NTA)
The present study measured exosome particle size and
concentration with a ZetaView PMX v110 (Particle Metrix GmbH) using
the settings recommended by the manufacturer's software manual. For
each exosomal sample, 2 ml of the sample was diluted using 1X PBS
buffer, the NTA measurement was recorded and analyzed, and the
mean, median and mode, as well as the concentration of the sample,
were calculated using ZetaView software (version 8.02.28; Particle
Metrix GmbH).
Transmission electron microscopy
(TEM)
For TEM, 20 µl of the exosomal sample was fixed to
an electron microscopy mesh grid with 2% glutaraldehyde at room
temperature, so that the dropping liquid remained on the copper net
for 20 min. Subsequently, 2% aqueous solution of uranyl acetate was
used to stain samples for 5 min at room temperature, and the filter
paper was air-dried at room temperature and observed under a 120 kv
biotransmission electron microscope (magnification, ×60,000).
Statistical analysis
Statistical analysis was performed using R software
(version 3.5.0; http://www.R-project.org). The miRNA experiments were
performed in triplicate and the results are presented as the mean ±
standard deviation. Associations between exosomal miR-125a-5p
expression and clinicopathological characteristics were analyzed
using unpaired Student's t-test. The heat map was created using the
pheatmap R package (version 1.0.12; http://www.rdocumentation.org/packages/pheatmap)
and the receiver operating characteristic (ROC) curve analyses were
plotted using the ggplot2 R package (version 3.3.2; CRAN.R-project.org/package=ggplot2). The
cut-off values were calculated using Youden's index. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of isolated
exosomes
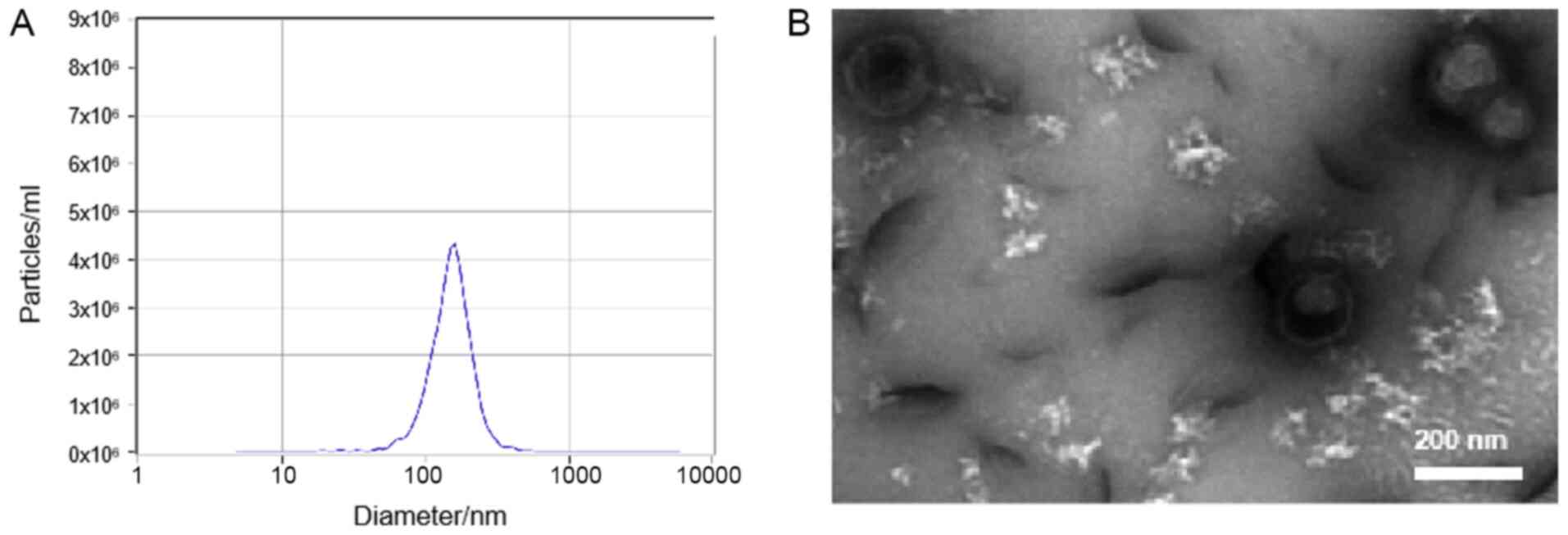
The results of the present study demonstrated that
exosomes had a spherical shape with a diameter of 30–200 nm, as
indicated by TEM, and the size distribution of exosomes ranged from
50 to 180 nm in diameter, with a mode value of 140–150 nm, as
indicated by NTA (Fig. 1).
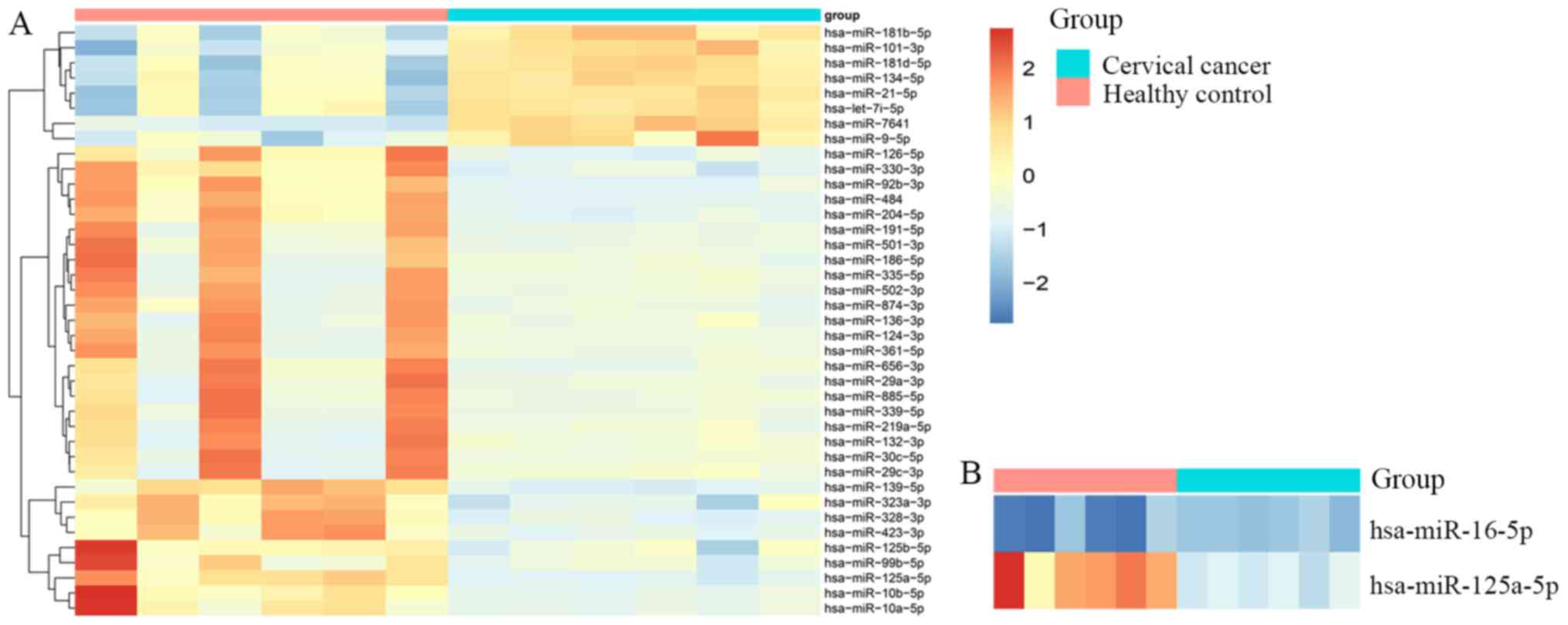
Exosomal miRNA profiling
The present study performed plasma exosomal miRNA
profiling for 6 patients with cervical cancer and 6 matched healthy
controls. A total of 1,725 unique mature miRNAs were identified
from exosome miRNA sequencing data, and 167 miRNAs were included
for statistical analysis after removing the miRNAs with <100
average reads per sample. The results of the statistical analysis
revealed that 39 miRNAs were differentially expressed between
patients with cervical cancer and healthy controls, among which, 8
miRNAs were downregulated and 31 miRNAs were upregulated
(P<0.001; fold-change>2.0; Fig.
2A). Considering these exosomal miRNA expression patterns, and
evidence obtained from previous studies (7,8),
miR-125a-5p was selected for the further analysis of its prognostic
values in cervical cancer. In the present study, miR-16a-5p had a
small standard deviation, and was also used as an endogenous
reference in previously published studies (15,16)
(Fig. 2B). Thus, miR-16a-5p was
selected as an endogenous reference in the subsequent qPCR
assay.
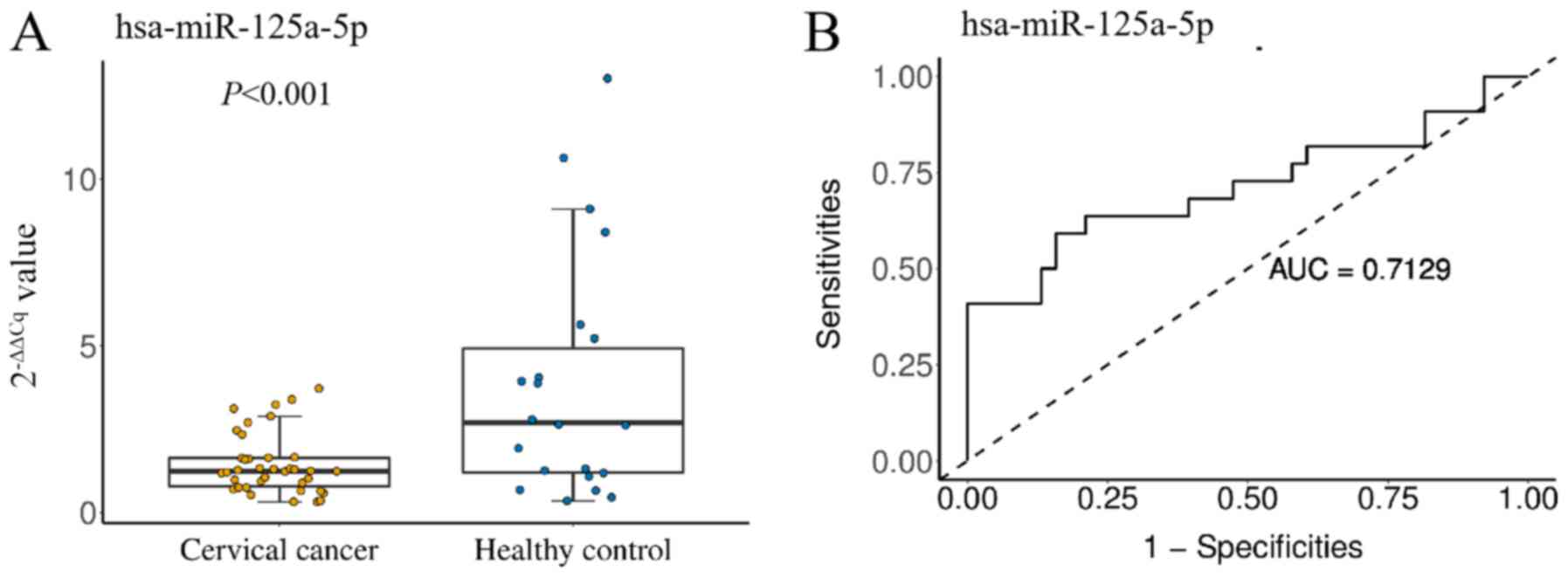
Prognostic role of plasma exosomal
miR-125a-5p expression levels in patients with cervical cancer
To assess the potential prognostic role of exosomal
miRNA expression levels, miR-125a-5p was quantified in the plasma
of 60 subjects, which included 22 healthy individuals and 38
patients with cervical cancer. The results revealed that exosomal
miR-125a-5p expression levels in patients with cervical cancer were
significantly lower than those in healthy controls (P<0.001).
ROC curve analyses were performed to evaluate the diagnostic value
of exosomal miR-125a-5p for cervical cancer. These analyses
revealed that the level of plasma exosomal miR-125a-5p was a
potential marker for differentiating between non-cervical and
cervical cancer, with an ROC area under the curve value of 0.7129
[95% confidence interval (CI), 0.561–0.865] (Fig. 3B). At the cut-off value of 2.537 for
miR-125a-5p, cervical cancer diagnostic sensitivities and
specificities were 59.1 and 84.2%, respectively.
Clinicopathological characteristics
and miR-125a-5p profile
miR-125a-5p was significantly associated with age,
tumor size and FIGO stage. Compared with HPV-negative patients,
miR-125a-5p expression in HPV-positive patients was significantly
decreased. However, there was no significant difference between
miR-125a-5p expression and lymph node metastasis or histological
types (Table I).
 | Table I.Association between exosomal
miR-125a-5p expression levels and the clinicopathological
characteristics of patients with cervical cancer. |
Table I.
Association between exosomal
miR-125a-5p expression levels and the clinicopathological
characteristics of patients with cervical cancer.
| Characteristic | Number of
patients | miR-125a-5p
expressiona | T-value | P-value |
|---|
| Age, years |
|
| 3.461 | 0.0032 |
|
<50 | 6 | 0.76±0.41 |
|
|
| ≥50 | 32 | 1.57±0.92 |
|
|
| Histological
type |
|
| 0.989 | 0.3361 |
| SCC | 28 | 1.53±0.93 |
|
|
| SDC | 10 | 1.22±0.84 |
|
|
| Tumor size, cm |
|
| 3.155 | 0.0039 |
|
<4 | 29 | 0.88±0.48 |
|
|
| ≥4 | 9 | 1.62±0.94 |
|
|
| FIGO stage |
|
| 2.475 | 0.0217 |
|
IA-IIA | 29 | 0.95±0.59 |
|
|
|
IIB-IVB | 9 | 1.60±0.94 |
|
|
| Lymph node
metastasis |
|
| 1.312 | 0.1978 |
|
Yes | 22 | 1.60±0.99 |
|
|
| No | 16 | 1.23±0.73 |
|
|
| HPV status |
|
| 2.111 | 0.0418 |
|
Positive | 30 | 1.29±0.83 |
|
|
|
Negative | 8 | 2.02±0.88 |
|
|
Discussion
Cervical cancer is a common tumor in gynecology. The
early screening techniques for cervical cancer include high-risk
human papillomavirus (HR-HPV) testing and cervical exfoliation cell
examination (18). As certain
persistent HR-HPV subtype infections can develop into cervical
cancer, the HR-HPV test is highly sensitive and has low specificity
in screening for cervical cancer (19). By contrast, cytology has an improved
specificity for cervical cancer screening, and cervical screening
strategies for women based on cytology are safe and effective
(20). Exosomes are a subvesicle
structure secreted by a variety of cells in the body; they carry a
variety of proteins and nucleic acid substances from cells, and can
be easily obtained from peripheral blood and other body fluids,
such as joint cavity effusion and pleural effusion (21,22).
Thus, in recent years, exosomes have been used as biomarkers for
disease diagnosis (23).
It has been demonstrated that miRNAs are likely to
be transported through exosomes in body fluids (24). When exosomes are released from the
original cells, they fuse with the recipient cells and control the
gene expression, which increases the possibility of potential
tumorigenicity of exosomal miRNA (25,26). In
a clinical study (4), exosomal
miR-1290 and miR-375 have been reported as promising prognostic
biomarkers for castration-resistant prostate cancer. Liu et
al (27) determined that
exosomal circ_0047921, circ_0056285 and circ_0007761 were promising
biomarkers for the diagnosis of non-small cell lung carcinoma,
including the early stages of disease; in addition, exosomal
circ_0056285 was associated with clinical stage and lymph node
metastasis. However, serum exosome-derived miRNAs are different
from the total serum miRNAs. A previous study determined that there
were no correlations between serum miR-126 and exosomal miR-126
(28), and another study
demonstrated that serum miR-122 and miR-199a were potential
biomarkers that reflect antiviral therapy efficacy in patients with
hepatitis C (29); however, to the
best of our knowledge, there is no evidence that exosomal miR-122
and miR-199a have the same predictive effect.
A previous study has revealed that miR-125a-5p
expression was decreased in clinical gastric cancer tissue samples
and gastric cancer cell lines, and was associated with
tumorigenesis and poor prognosis (30). Furthermore, an experimental study
involving molecular mechanisms have demonstrated that miR-125a-5p
inhibited the proliferation and invasion of ovarian cancer cells by
targeting GALNT14 (31), and
inhibited the invasion and migration of hepatoma cells by
regulating the activity of the PI3K/AKT/mTOR signaling pathway
(32). In addition, a previous study
revealed that HPV suppresses miR-125a-5p by inhibiting p53
expression in cervical carcinogenesis, and it is speculated that
miR-125a-5p may be a therapeutic target for cervical cancer
(33). However, to the best of our
knowledge, there are no studies on miR-125a-5p in cervical
cancer.
The present study performed plasma exosomal miRNA
sequencing, the results of which revealed that a total of 39 miRNAs
were differentially expressed between patients with cervical cancer
and healthy controls, among which, 8 miRNAs were downregulated and
31 miRNAs were upregulated with the statistical criteria of
P<0.001, fold-change >2.0. Based on both NGS and PCR
experiments, exosomal miR-125a-5p appears to be a promising
biomarker. Diagnostic accuracy was calculated based on the PCR
results (0.7129; 95% CI, 0.561–0.865). The present study revealed
that miR-125a-5p was significantly associated with age; 6 patients
<50 years of age had a lower miR-125a-5p level compared with 32
patients ≥50 years of age. In addition, tumor size and FIGO stage
were associated with miR-125a-5p level, but no differences were
observed between cervical squamous cell carcinoma and
adenocarcinoma/adenosquamous carcinoma. However, it should be noted
that exosomal miRNAs do not necessarily represent cellular levels,
since the miRNA sorting mechanism may affect the incorporation of
miRNA into exosomes. It should also be noted that the present study
contained a small number of samples and more samples are required
to support the conclusions of the present study.
As previously reported, miR-125a-5p could be a
potential biomarker of certain types of cancer, such as breast
(9), hepatocellular (34) and colon cancer (12), which indicates that miR-125a-5p is
not a cervical cancer specific biomarker. The present study
suggested that when miR-125a-5p expression is decreased,
miR-125a-5p cannot be relied on to determine which type of cancer,
but it may provide auxiliary diagnostic value for clinical samples
with an uncertain diagnosis. Liquid biopsy is a new area of
research, and it is therefore not appropriate for them to replace
traditional methods in diagnosing cancer, including exosome-based
diagnosis and screening programs. However, it is still of great
scientific value to investigate novel approaches to cancer
diagnosis, including exosomal miRNAs. However, the present study
has several limitations. Different histological types of cervical
cancer may have different molecular mechanisms and different
circulating exosomal miRNAs. Future studies should focus on one
histological subtype, squamous cell carcinoma or adenocarcinoma,
and include more samples to further confirm the present
results.
In conclusion, the results of the present study
revealed that plasma exosomal miR-125a-5p is poorly expressed in
cervical cancer and that miR-125a-5p could be regarded as a
diagnostic marker for cervical cancer. Further research with larger
sample sizes is required to confirm these results.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Major Science
and Technology Program of Hainan Province (grant no. ZDKJ2017007),
the Natural Science Foundation of China (grant no. 81560244), and
the Key Research and Development Program of Hainan Province (grant
no. ZDYF2019158)
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BX and YH designed the study. AL and ZT collected
the clinical data and participated the writing work. YH, AL and WL
were responsible for the RNA-sequencing data and PCR processing and
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the First Affiliated
Hospital of Hainan University approved the present study. All
samples were anonymized during the analysis. Written informed
consent of blinded individuals was obtained from each
participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Rubis G, Rajeev Krishnan S and Bebawy
M: Liquid biopsies in cancer diagnosis, monitoring, and prognosis.
Trends Pharmacol Sci. 40:172–186. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baassiri A, Nassar F, Mukherji D,
Shamseddine A, Nasr R and Temraz S: Exosomal Non Coding RNA in
LIQUID Biopsies as a Promising Biomarker for Colorectal Cancer. Int
J Mol Sci. 21:13982020. View Article : Google Scholar
|
|
4
|
Huang X, Yuan T, Liang M, Du M, Xia S,
Dittmar R, Wang D, See W, Costello BA, Quevedo F, et al: Exosomal
miR-1290 and miR-375 as prognostic markers in castration-resistant
prostate cancer. Eur Urol. 67:33–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Camussi G, Deregibus MC, Bruno S,
Cantaluppi V and Biancone L: Exosomes/microvesicles as a mechanism
of cell-to-cell communication. Kidney Int. 78:838–848. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang L, Gu Y, Du Y and Liu J: Exosomes:
Diagnostic biomarkers and therapeutic delivery vehicles for cancer.
Mol Pharm. 16:3333–3349. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Sun H, Wang X, Yu Q, Li S, Yu X and
Gong W: Increased exosomal microRNA-21 and microRNA-146a levels in
the cervicovaginal lavage specimens of patients with cervical
cancer. Int J Mol Sci. 15:758–773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Liu SC, Luo XH, Tao GX, Guan M,
Yuan H and Hu DK: Exosomal long noncoding RNAs are differentially
expressed in the cervicovaginal lavage samples of cervical cancer
patients. J Clin Lab Anal. 30:1116–1121. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang L, Huang Q, Chang J, Wang E and Qiu
X: MicroRNA HSA-miR-125a-5p induces apoptosis by activating p53 in
lung cancer cells. Exp Lung Res. 37:387–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bi Q, Tang S, Xia L, Du R, Fan R, Gao L,
Jin J, Liang S, Chen Z, Xu G, et al: Ectopic expression of MiR-125a
inhibits the proliferation and metastasis of hepatocellular
carcinoma by targeting MMP11 and VEGF. PLoS One. 7:e401692012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Yan F, Zhao Q, Zhan F, Wang R,
Wang L, Zhang Y and Huang X: Circulating exosomal miR-125a-3p as a
novel biomarker for early-stage colon cancer. Sci Rep. 7:41502017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuo K, Machida H, Mandelbaum RS,
Konishi I and Mikami M: Validation of the 2018 FIGO cervical cancer
staging system. Gynecol Oncol. 152:87–93. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koitzsch U, Heydt C, Attig H, Immerschitt
I, Merkelbach-Bruse S, Fammartino A, Büttner RH, Kong Y and
Odenthal M: Use of the GeneReader NGS System in a clinical
pathology laboratory: A comparative study. J Clin Pathol.
70:725–728. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shahid S, Shaheen J, Shahid W, Akhtar MW
and Sadaf S: mir-16-5p as a suitable reference gene for
normalization of quantitative real time PCR in acute lymphoblastic
leukemia. Pak J Zool. 51:747–754. 2019. View Article : Google Scholar
|
|
17
|
Lange T, Stracke S, Rettig R, Lendeckel U,
Kuhn J, Schlüter R, Rippe V, Endlich K and Endlich N:
Identification of miR-16 as an endogenous reference gene for the
normalization of urinary exosomal miRNA expression data from CKD
patients. PLoS One. 12:e01834352017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Vuyst H, Chung MH, Baussano I, Mugo NR,
Tenet V, van Kemenade FJ, Rana FS, Sakr SR, Meijer CJLM, Snijders
PJF, et al: Comparison of HPV DNA testing in cervical exfoliated
cells and tissue biopsies among HIV-positive women in Kenya. Int J
Cancer. 133:1441–1446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benevolo M, Vocaturo A, Caraceni D, French
D, Rosini S, Zappacosta R, Terrenato I, Ciccocioppo L, Frega A and
Giorgi Rossi P: Sensitivity, specificity, and clinical value of
human papillomavirus (HPV) E6/E7 mRNA assay as a triage test for
cervical cytology and HPV DNA test. J Clin Microbiol. 49:2643–2650.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dillner J, Rebolj M, Birembaut P, Petry
KU, Szarewski A, Munk C, de Sanjose S, Naucler P, Lloveras B, Kjaer
S, et al Joint European Cohort Study, : Long term predictive values
of cytology and human papillomavirus testing in cervical cancer
screening: Joint European cohort study. BMJ. 337:(oct13 1).
a17542008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bard MP, Hegmans JP, Hemmes A, Luider TM,
Willemsen R, Severijnen LAA, van Meerbeeck JP, Burgers SA,
Hoogsteden HC and Lambrecht BN: Proteomic analysis of exosomes
isolated from human malignant pleural effusions. Am J Respir Cell
Mol Biol. 31:114–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keller S, Ridinger J, Rupp AK, Janssen JW
and Altevogt P: Body fluid derived exosomes as a novel template for
clinical diagnostics. J Transl Med. 9:862011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jalalian SH, Ramezani M, Jalalian SA,
Abnous K and Taghdisi SM: Exosomes, new biomarkers in early cancer
detection. Anal Biochem. 571:1–13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Salido-Guadarrama I, Romero-Cordoba S,
Peralta-Zaragoza O, Hidalgo-Miranda A and Rodríguez-Dorantes M:
MicroRNAs transported by exosomes in body fluids as mediators of
intercellular communication in cancer. Onco Targets Ther.
7:1327–1338. 2014.PubMed/NCBI
|
|
25
|
Ahmadi M and Rezaie J: Tumor cells
derived-exosomes as angiogenenic agents: Possible therapeutic
implications. J Transl Med. 18:2492020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ghaemmaghami AB, Mahjoubin-Tehran M,
Movahedpour A, Morshedi K, Sheida A, Taghavi SP, Mirzaei H and
Hamblin MR: Role of exosomes in malignant glioma: microRNAs and
proteins in pathogenesis and diagnosis. Cell Commun Signal.
18:1202020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu L, Su W, Xian J, Wang Y, Rao B, Lin M
and Chen J: Identification of Three Circular RNAs Cargo in Serum
Exosomes as Diagnostic Biomarkers of Non-Small Cell. Lung Cancer.
2018.
|
|
28
|
Chen F, Du Y, Esposito E, Liu Y, Guo S,
Wang X, Lo EH, Xing C and Ji X: Effects of focal cerebral ischemia
on exosomal versus serum miR126. Transl Stroke Res. 6:478–484.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiao X, Fan Z, Chen H, He P, Li Y, Zhang Q
and Ke C: Serum and exosomal miR-122 and miR-199a as a biomarker to
predict therapeutic efficacy of hepatitis C patients. J Med Virol.
89:1597–1605. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao Y, Tan S, Tu Y, Zhang G, Liu Y, Li D,
Xu S, Le Z, Xiong J, Zou W, et al: MicroRNA-125a-5p inhibits
invasion and metastasis of gastric cancer cells by targeting BRMS1
expression. Oncol Lett. 15:5119–5130. 2018.PubMed/NCBI
|
|
31
|
Yang J, Li G and Zhang K: MiR-125a
regulates ovarian cancer proliferation and invasion by repressing
GALNT14 expression. Biomed Pharmacother. 80:381–387. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang S, Dong SM, Kim BR, Park MS, Trink B,
Byun HJ and Rho SB: Thioridazine induces apoptosis by targeting the
PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells.
Apoptosis. 17:989–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fan Z, Cui H, Xu X, Lin Z, Zhang X, Kang
L, Han B, Meng J, Yan Z, Yan X, et al: MiR-125a suppresses tumor
growth, invasion and metastasis in cervical cancer by targeting
STAT3. Oncotarget. 6:25266–25280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim JK, Noh JH, Jung KH, Eun JW, Bae HJ,
Kim MG, Chang YG, Shen Q, Park WS, Lee JY, et al: Sirtuin7
oncogenic potential in human hepatocellular carcinoma and its
regulation by the tumor suppressors MiR-125a-5p and MiR-125b.
Hepatology. 57:1055–1067. 2013. View Article : Google Scholar : PubMed/NCBI
|