Introduction
Malignant lymphoma (ML), a type of hematological
malignancy, occurs in the lymph nodes or lymphoid tissues outside
the lymph nodes (1). Non-Hodgkin's
lymphoma (NHL) is the most common type of lymphoma and is about 4%
of all cancers in the United States (2). Approximately 85–90% of all NHL cases
are of B cell origin (3). The
incidence rate of lymphoma continues to increase globally, causing
serious harm to human health (3).
However, the pathogenesis of NHL remains unclear.
CD40, a 48-kDa type 1 transmembrane cell surface
receptor, was initially identified in B lymphocytes and was found
to trigger numerous key processes (4). CD40 is a member of the tumor necrosis
factor (TNF) receptor superfamily and is widely expressed in
several hematological cancer types and solid tumors, including
leukemias (5), gastric cancer and
bladder cancer (6,7). The natural ligand of the CD40 receptor
is CD40L, also known as CD154 or gp39, which belongs to the TNF
family and is a type II transmembrane protein with a relative
molecular mass of 39 kDa. In the 1990s, Mach et al (8) demonstrated that atheroma of mice
treated with anti-CD40L antibody contained significantly fewer
macrophages (64%) and T lymphocytes (70%) and exhibited decreased
expression of vascular cell adhesion molecule-1. This indicated
that CD40 served an important role in atherogenesis (8). Additionally, membrane-bound CD40L may
promote senescence and initiate senescence-associated secretory
phenotype via NF-κB activation in lung adenocarcinoma (9). Activated CD40, through CD40L, serves a
central role in regulating the proliferation of CD4 (+) and CD8 (+)
T cells, as well as T cell and B cell activity (10–12).
Under certain conditions, CD40L may bind to the receptor protein
CD40 on the surface of tumor cells, thereby activating the CD40
relative downstream signaling pathway to regulate the proliferation
of tumor cells (13). The
suppression of CD40L expression in T cells has also been
demonstrated in B cell chronic lymphocytic leukemia (14). Our previous study demonstrated that
the upregulation of CD40L expression attenuated drug resistance in
Adriamycin-resistant THP-1 cells (15). Furthermore, recent studies have
demonstrated that CD40L may significantly inhibit the cell
proliferation and promote the cell apoptosis of cancer cells,
including colon cancer and ovarian cancer cells (16,17). A
previous study reported that CD40 may induce apoptosis of carcinoma
cells through a mechanism involving TRAF3 and JNK/AP-1 activation
(18). At present, the effect of
CD40L on tumors has become a popular topic in the field of tumor
pathogenesis (19–21). Additionally, CD40 activation has
anti-apoptotic or apoptotic effects in follicular lymphoma (FL)
cell lines (PMID: 28610909) (22).
However, the function and mechanism of CD40/CD40L in NHL are rarely
reported.
In the present study, the NHL cells were treated
with soluble CD40 ligand (sCD40L). By conducting Cell Counting
kit-8 (CCK-8) assays, cell flow cytometry and western blot
analysis, the present study confirmed that exogenous CD40L
inhibited the proliferation and promoted the apoptosis of NHL cells
by activating the JNK signaling pathway. The present study serves
as a basis for examining CD40L in the clinical treatment of
NHL.
Materials and methods
Cells and reagents
Human Burkitt lymphoma (NHL) Raji (no. bncc338283)
and CA46 (no. bncc337642) cell lines were purchased from BeNa
Culture Collection. Antibodies against Bax (cat. no. SC-7480) and
Bcl-2 (cat. no. SC-7382) were purchased from Santa Cruz
Biotechnology, lnc. Antibodies against ERK (cat. no. 4695T), p-ERK
(cat. no. 4370T), p38 (cat. no. 8690T), p-p38 (cat. no. 4511S), JNK
(cat. no. 9252T), p-JNK (cat. no. 9255S), c-JUN (cat. no. 9165T)
and GAPDH (cat. no. 5174T) were purchased from CST Biological
Reagents Co., Ltd. CCK-8 kit (kit no. C0037) was purchased from
Beyotime Institute of Biotechnology. sCD40L (no. cyt-245) was
purchased from Prospec-Tany TechnoGene, Ltd. JNK inhibitor SP600125
was purchased from Selleck Chemicals.
CCK-8 assay
Cells were cultured in RPIM-1640 medium (SH30809.01;
Hyclone; GE Healthcare Life Sciences) which supplemented with 10%
fetal bovine serum (cat. no. 900-108; Gemini Bio Products) in a
cell incubator (5% CO2, 37°C). When the confluence of
the cells reached 95%, the cells were digested, collected and
resuspended in PRIM-1640 medium. The cells were then transferred
onto 96-well plates (5×104 cells/well). Following
incubation for 24 h, the cells were further treated with different
concentrations of sCD40L (0, 2, 4, 6, 8 and 10 µg/ml) for 48 h or
10 µmol/l SP600125 for 48 h, each group had five replicates. Next,
cells were incubated for 48 h in a cell incubator (5%
CO2, 37°C). A total of ~10 µl CCK-8 solution was added
into each well and incubated at 37°C for 3 h. The absorbance of the
reaction solution at 450 nm was measured.
Cell apoptosis assay using flow
cytometry
The cells were cultured in six-well plates
(1×106 cells/well) and incubated for 24 h. Next, the
cells were treated with sCD40L and SP600125 and incubated for
another 48 h. The cells were then collected and centrifuged at 300
× g for 3 min at 25°C. Furthermore, the cells were washed twice
with precooled phosphate-buffered saline (PBS), and subsequently, 1
ml 1× binding buffer was used to resuspend the cells. Approximately
100 µl cell resuspension solution was added to the new tubes, along
with 5 µl fluorescein isothiocyanate (FITC)-labelled Annexin V and
5 µl PI and incubated in the dark for 15 min at room temperature.
Finally, 400 µl 1X binding buffer was added, and the reaction
solution was detected using a CytoFLEX flow cytometer (Beckman
Coulter Inc.) with CytExpert v.2.0 software (Beckman Coulter
Inc.).
Western blotting
The cells were treated with sCD40L and SP600125 and
incubated for 48 h, prior to being harvested and washed with
precooled PBS. Radio immunoprecipitation assay lysate [150 mM NaCl,
0.1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl
sulfate (SDS), 50 mm Tris HCl (pH 8.0) and protease inhibitor] was
used to extract the total protein, and was kept on ice and then
centrifuged at 16,000 × g for 5 min at 4°C. The protein
concentrations were determined by conducting a bicinchoninic acid
protein assay. For each sample, protein (40 µg) were separated
using 10% SDS-PAGE and then transferred onto polyvinylidene
fluoride or polyvinylidene difluoride membranes. The membranes were
blocked with 5% skimmed milk powder for 1 h at room temperature and
then incubated overnight with protein antibodies BAX (1:1,000);
Bcl-2 (1:1,000); ERK (1:1,000); p-ERK(1:2,000); p38(1:1,000); p-p38
(1:1,000); JNK(1:1,000); p-JNK (1:2,000); c-JUN (1:1,000) and GAPDH
(1:1,000) at 4°C. The membranes were washed with tris-buffered
saline (TBST) three times. The second antibody solution goat
anti-rabbit IgG (H+L)-HRP (cat. no. SA009; Auragene) or goat
anti-mouse IgG (H+L)-HRP (cat. no. SA001; Auragene) was added, and
the membranes were incubated with the membranes at room temperature
for 1 h. Following washing with TBST three times, the proteins were
examined using an enhanced chemiluminescent kit (Auragene).
Statistical analysis
SPSS 22.0 (IBM, Corp.) and GraphPad Prism 7.0
(GraphPad Software, Inc.) were used for data processing and
statistical analysis. All experiments were performed three times.
Data are expressed as the mean ± standard deviation and were
analyzed using analysis of variance, followed by the least
significant difference post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Exogenous sCD40L significantly
inhibits the proliferation and promotes the apoptosis of lymphoma
Raji and CA46 cells
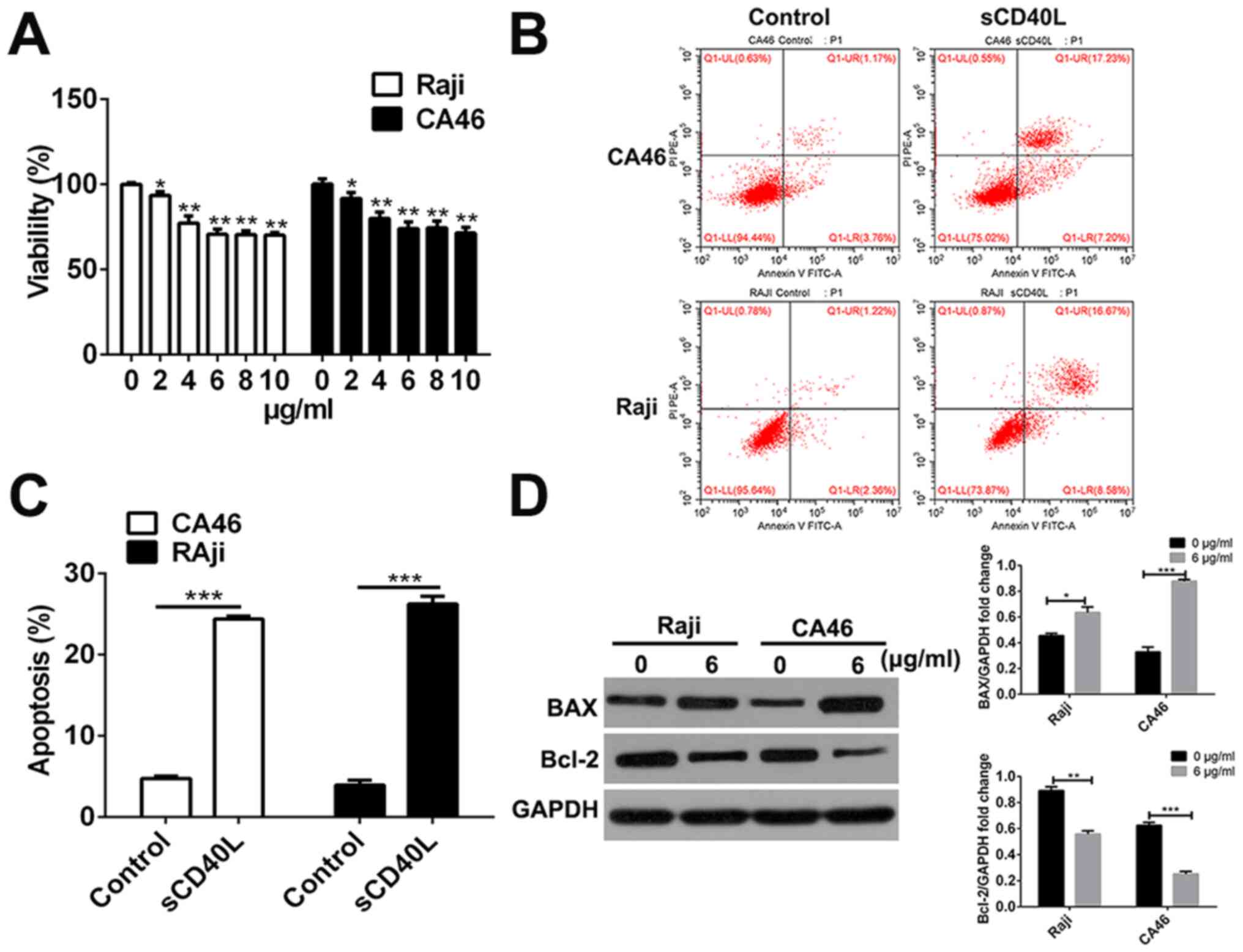
The Raji and CA46 cells were initially treated with
different concentrations of sCD40L (0, 2, 4, 6, 8 and 10 µg/ml) for
48 h. Different concentrations of sCD40L significantly inhibited
the viability of Raji and CA46 cells, and there were no notable
differences in inhibitory effects among groups when the
concentration of sCD40L was higher than 6 µg/ml (Fig. 1A). Therefore, 6 µg/ml sCD40L was used
to stimulate the Raji and CA46 cells for further study. To
elucidate the apoptosis-inducing effect of sCD40L on Raji and CA46
cells, flow cytometry was used to detect the apoptosis of Raji and
CA46 cells and treatment with 6 µg/ml sCD40L was found to
significantly increase the proportion of apoptosis (Fig. 1B and C). To further confirm the
function of sCD40L in Raji and CA46 cells, the protein expression
levels of the pro-apoptotic protein, BAX, and the anti-apoptotic
protein, Bcl-2, were determined by conducting western blot analysis
following treatment with 6 µg/ml sCD40L for 48 h. Compared with
that in the untreated control, the stimulation of cells with sCD40L
significantly upregulated the BAX protein expression levels and
downregulated the Bcl-2 expression levels in Raji and CA46 cell
lines (Fig. 1D). These results
indicated that sCD40L significantly inhibited the cell
proliferation and promoted the cell apoptosis of lymphoma Raji and
CA46 cells.
Exogenous CD40L activates the JNK
signaling pathway
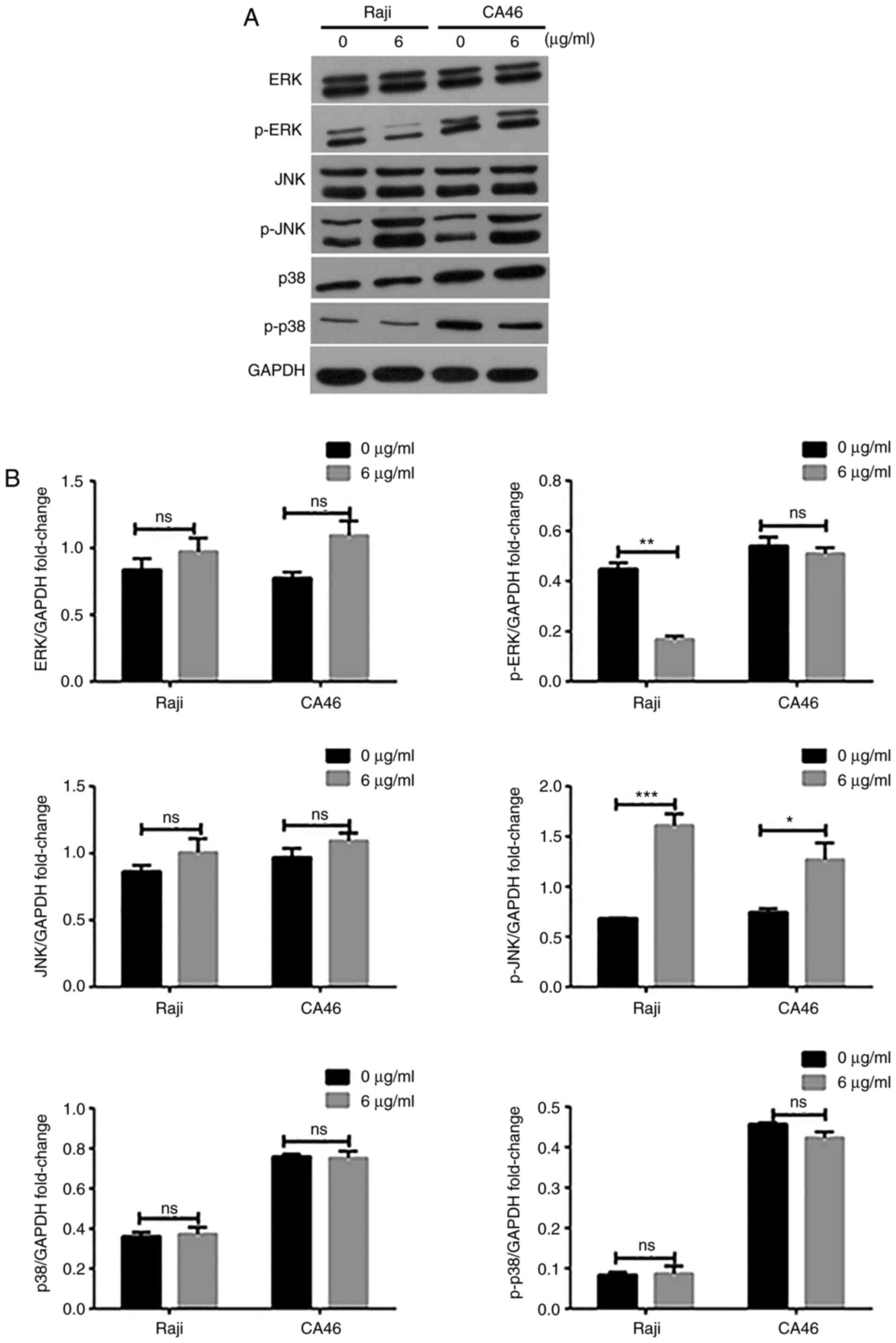
To further investigate the regulated mechanism of
sCD40L in lymphoma Raji cells, western blotting was used to detect
the protein expression levels of ERK, P38 and JNK, which were the
classical pathway factors of the MAPK signaling pathway. As shown
in Fig. 2, the expression levels of
p-JNK in sCD40L-treated lymphoma cells increased significantly,
while the P38 and ERK activity did not increase, suggesting that
sCD40L activated the JNK signaling pathway, thereby promoting the
apoptosis of lymphoma cells.
Inhibition of the JNK signaling
pathway rescuing the sCD40L-induced apoptosis of lymphoma
cells
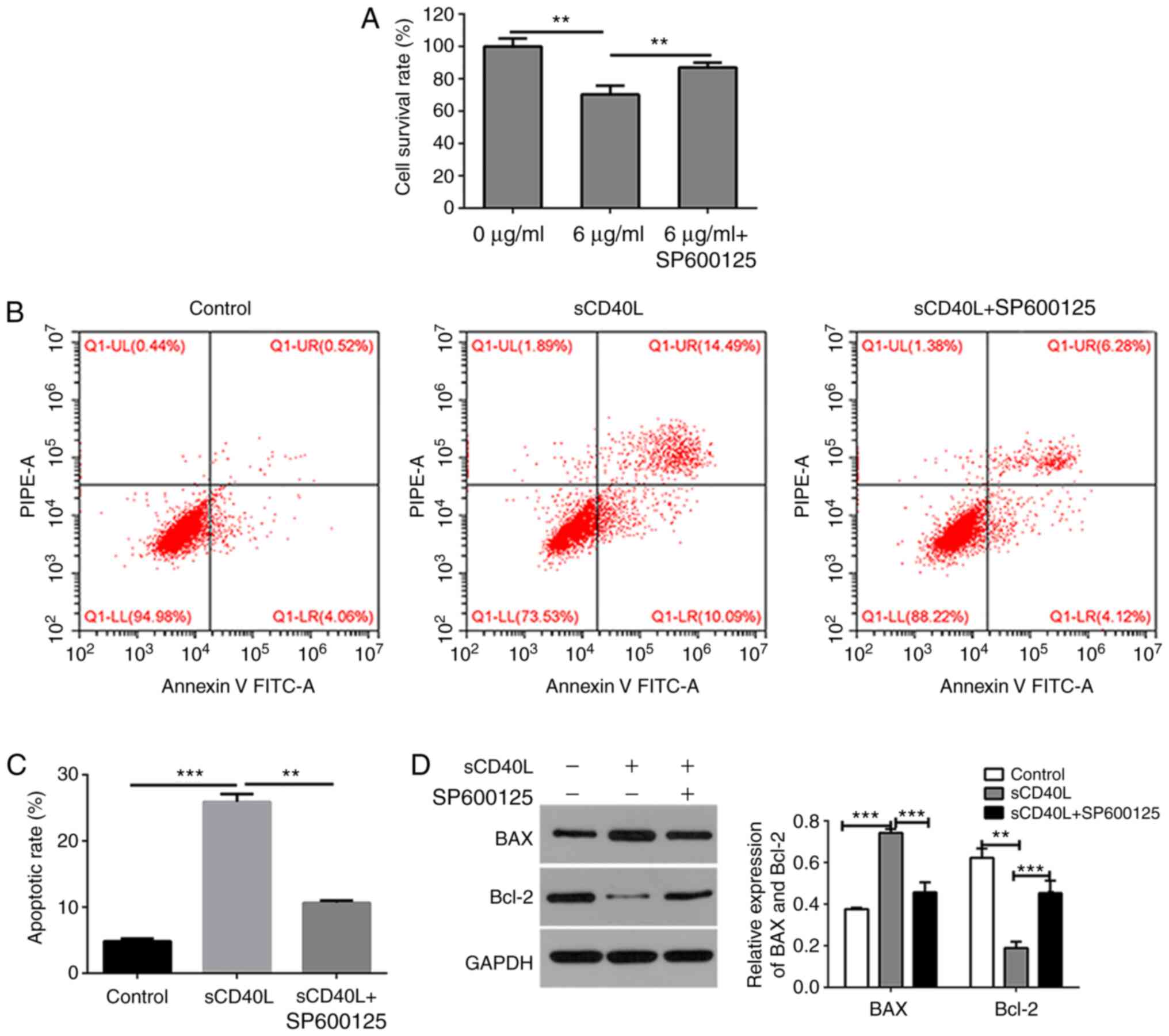
To further determine whether sCD40L induces
apoptosis of lymphoma cells by activating the JNK signaling
pathway, the lymphoma cells were treated with SP600125 (10 µM), a
specific inhibitor of the JNK signaling pathway. The results
demonstrated that co-treatment with sCD40L and SP600125
significantly increased the cell viability (Fig. 3A) and decreased the cell apoptosis
(Fig. 3B and C), compared with
treatment with sCD40L alone, as detected by CCK-8 assay and flow
cytometry, respectively. Western blotting was conducted and
confirmed that SP600125 downregulated the expression of
sCD40L-regulated BAX and upregulated the expression of
sCD40L-regulated Bcl-2 (Fig. 3D). To
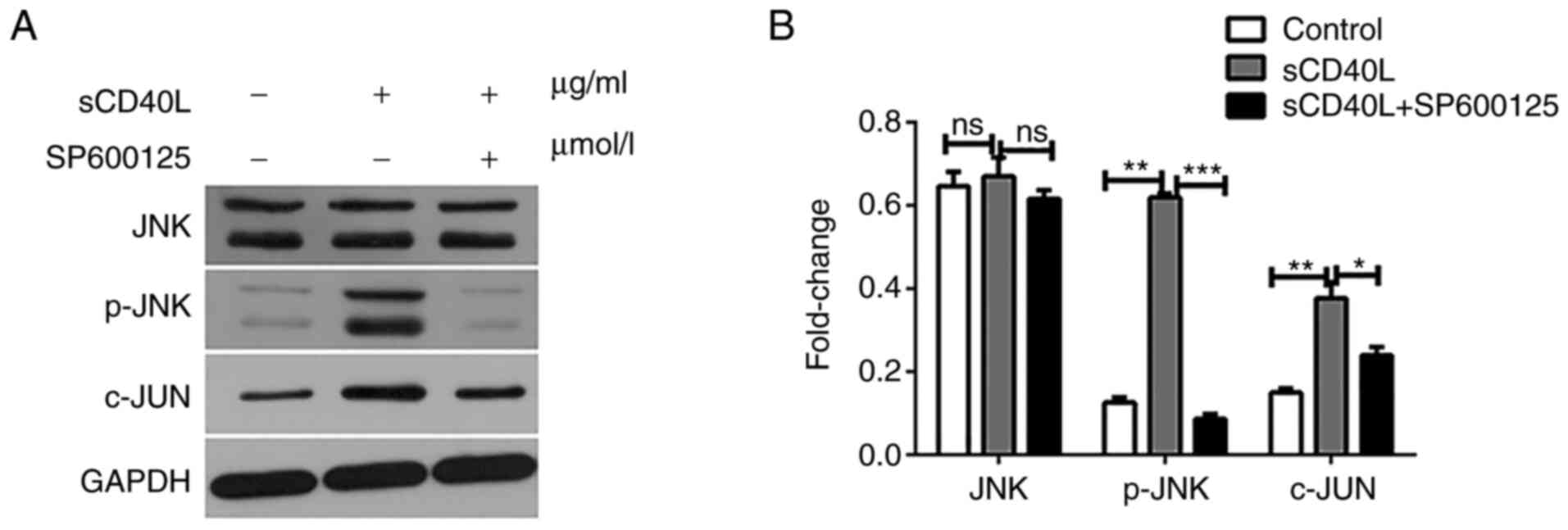
further confirm the mechanism underlying the sCD40L-regulated
activity through the JNK signaling pathway, the JNK, p-JNK and
c-JUN expression levels were determined using western blot analysis
and it was found that SP600125 significantly inhibited the
sCD40L-regulated activity of JNK (Fig.
4A and B). These results indicated that sCD40L promotes
lymphoma cell apoptosis through the activation of JNK signals.
Discussion
Lymphoma originates from malignant tumors of the
lymphoid hematopoietic system, invading several tissues and organs
outside the lymph nodes, including the bone marrow, causing
abnormal bone marrow hematopoiesis (23,24).
Lymphomas can be classified into NHL and Hodgkin's lymphoma
according to the tumor histopathology (25). NHL is a lymphatic malignant
hyperplasia disease, with extremely strong heterogeneity. The
incidence of NHL is high in China, accounting for 90% of all solid
tumors of the immune system (26).
However, the exact pathogenesis of NHL remains unknown.
CD40L (CD154) is a CD40 ligand that belongs to the
TNF family (27). CD40L is expressed
on the surface of normal cells and cancer cells, including bladder
cancer (28), primary bone cancer
(29), lung cancer and ovarian
cancer cells (30). CD40L is
associated with tumors, immunity and inflammation (31,32).
Additionally, CD40L is highly expressed in numerous types of
cancer, but the tumorigenic role of CD40L remain controversial
(33). Certain studies have
suggested that CD40L has a tumorigenic effect (7,34), but
others have suggested that CD40L has an antitumor effect (16,35,36). The
CD40 signal has direct and indirect antitumor effects (35). CD40L has a significant inhibitory
effect on AGS cells (36).
There are several shorter, stable, soluble forms of
CD40L in addition to the full-length transmembrane protein. sCD40L
retains the trimeric structure of the full-length protein and its
biological functions (37). The
sCD40L was able to bind and internalize into B cells that expressed
the CD40 receptor and specifically and efficiently induced
apoptotic death (38). In the 1990,
MacDonald et al (39)
reported that trimeric sCD40L was able to inhibit apoptosis induced
by the combination of agonists to a certain degree, but such rescue
proved to be short-lived.
Therefore, to further study the influence of CD40L
on lymphoma cells, the effect of sCD40L on the proliferation and
apoptosis in lymphoma cells was investigated. The results of the
present study demonstrated that the proliferation of Raji and CA46
cells was significantly inhibited by different concentrations of
sCD40L investigated by CCK-8 assays. Our previous study
demonstrated that the proliferation inhibition rate of cells
treated with different concentrations of sCD40L after 48 and 72 h
was higher than that at 24 h (40);
therefore, 48 h was selected as an incubation time for the CCK-8
assay. The results demonstrated that as the concentration is
increased, the inhibitory ability became more evident, and when the
sCD40L concentration was higher than 6 µg/ml, the inhibitory
effects exhibited a steady trend. The follow-up studies were
conducted using flow cytometry, and it was found that 6 µg/ml
sCD40L treatment significantly increased the proportion of
apoptosis. Although quantitative polymerase chain reaction (qPCR)
was not used to detect the expression of the apoptotic factor, BAX,
and the anti-apoptotic factor, Bcl-2, in the present study, the
expression of Bcl-2 and BAX were detected by western blot analysis,
and the results demonstrated that the expression of BAX increased
and the expression of Bcl-2 decreased following Raji and CA46 cells
being treated with 6 µg/ml sCD40L. The expression of BAX and Bcl-2
should be detected by qPCR in the future. Taken together, these
results suggested that sCD40L inhibited the cell proliferation and
induced the apoptosis of lymphoma Raji and CA46 cell lines.
The present study verified that sCD40L inhibits the
growth of lymphoma, but the relative molecular mechanism of sCD40L
in lymphoma cells remained uncertain. The earliest detectable
events following CD40 activation were the activation of protein
tyrosine kinases, phosphoinositide-3 kinase (PI3k) and
phospholipase Cg2; the activation of cAMP modulated both positive
and negative CD40-induced responses (41). CD40L mediated the alternative NFκB
signaling pathway in mantle cell lymphoma to induce resistance to
BCR inhibitors (42). Recent studies
have concentrated on the involvement of JNK/SAPK, p38 MAPK and ERK
signaling pathways (4,43–46).
However, the conclusions of several associated studies were
controversial, and general conclusions should be interpreted with
caution because these studies often used different cellular
models.
Considering the important role of the MAPK signaling
pathway in lymphomas (47,48) and that the JNK signaling pathway is a
well-known pathway that induces cell apoptosis (49), the present study investigated the
molecular mechanism by which sCD40L induces apoptosis in lymphoma
cell lines. The results demonstrated that the phosphorylation level
of JNK was significantly increased, while the phosphorylation level
of p38 and ERK did not change following Raji and CA46 cells being
treated with sCD40L. Therefore, sCD40L may significantly induce the
activation of the JNK signaling pathway but not p38 signaling
pathway or ERK signaling pathway. Therefore, the data indicated
that sCD40L induced the apoptosis of lymphoma Raji and CA46 cell
lines through the JNK signaling pathway. Furthermore, the results
showed that sCD40L has the same effect on the apoptosis of Raji and
CA46 cells. Therefore, in order to verify these results, the Raji
cell line was selected for further research. SP600125, an inhibitor
of JNK signaling pathway, was used to treat Raji cells, and the
results demonstrated that SP600125 may promote the proliferation
and inhibited the apoptosis of Raji cells, which were induced by
sCD40. Although the study also obtained a similar conclusion that
sCD40L induced the apoptosis of Raji through the JNK signaling
pathway in vitro, in vivo experiments need to be performed
in future studies.
Although the present study confirmed that sCD40L may
inhibit the proliferation and induce the apoptosis of Raji and CA46
cell lines, CD40 expression in Raji and CA46 cells was not
investigated. This is a limitation of the present study, which will
be considered in future studies.
In summary, sCD40L inhibited the proliferation and
promoted the apoptosis of the lymphoma cell lines. Additionally,
sCD40L inhibited the growth of lymphoma Raji and CA46 cell lines
and induced apoptosis by activating the JNK signaling pathway.
These results suggested that sCD40L may be a potential therapeutic
drug for suppressing NHL.
Acknowledgements
Not applicable.
Funding
The present study was supported by funds from the
Joint Fund of Science and Technology Department of Guizhou Province
(Qiankehe LH [2017]7092).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZF designed the study, analyzed data, performed
experiments and wrote the manuscript. JW collected funding,
researched the literature, managed the project and collected the
data. Both authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
c-JUN
|
oncoprotein c-JUN
|
|
ML
|
malignant lymphoma
|
|
NHL
|
non-Hodgkin's lymphoma
|
|
p38
|
p38 mitogen-activated protein
kinases
|
|
p-ERK
|
phosphorylated ERK
|
|
p-JNK
|
phosphorylated JNK
|
|
p-p38
|
phosphorylated p38
|
|
sCD40L
|
soluble CD40 ligand
|
References
|
1
|
Jiang M, Bennani NN and Feldman AL:
Lymphoma classification update: T-cell lymphomas, Hodgkin
lymphomas, and histiocytic/dendritic cell neoplasms. Expert Rev
Hematol. 10:239–249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pratap S and Scordino TS: Molecular and
cellular genetics of non-Hodgkin lymphoma: Diagnostic and
prognostic implications. Exp Mol Pathol. 106:44–51. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muto R, Miyoshi H, Sato K, Furuta T, Muta
H, Kawamoto K, Yanagida E, Yamada K and Ohshima K: Epidemiology and
secular trends of malignant lymphoma in Japan: Analysis of 9426
cases according to the world health organization classification.
Cancer Med. 7:5843–5858. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Kooten C and Banchereau J: CD40-CD40
ligand. J Leukoc Biol. 67:2–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carbone A, Gloghini A, Zagonel V,
Aldinucci D, Gattei V, Degan M, Improta S, Sorio R, Monfardini S
and Pinto A: The expression of CD26 and CD40 ligand is mutually
exclusive in human T-cell non-Hodgkin's lymphomas/leukemias. Blood.
86:4617–4626. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hussain SA, Ganesan R, Hiller L, Murray
PG, el-Magraby MM, Young L and James ND: Proapoptotic genes BAX and
CD40L are predictors of survival in transitional cell carcinoma of
the bladder. Br J Cancer. 88:586–592. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li R, Chen WC, Pang XQ, Hua C, Li L and
Zhang XG: Expression of CD40 and CD40L in gastric cancer tissue and
its clinical significance. Int J Mol Sci. 10:3900–3917. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mach F, Schönbeck U, Sukhova GK, Atkinson
E and Libby P: Reduction of atherosclerosis in mice by inhibition
of CD40 signaling. Nature. 394:200–203. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu W, Li Y, Yuan WW, Yin Y, Song WW, Wang
Y, Huang QQ, Zhao WH and Wu JQ: Membrane-Bound CD40L promotes
senescence and initiates senescence-associated secretory phenotype
via NF-κB activation in lung adenocarcinoma. Cell Physiol Biochem.
48:1793–1803. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buhmann R, Nolte A, Westhaus D, Emmerich B
and Hallek M: CD40-activated B-cell chronic lymphocytic leukemia
cells for tumor immunotherapy: Stimulation of allogeneic versus
autologous T cells generates different types of effector cells.
Blood. 93:1992–2002. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsubata T, Wu J and Honjo T: B-cell
apoptosis induced by antigen receptor crosslinking is blocked by a
T-cell signal through CD40. Nature. 364:645–648. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Kooten C, Gaillard C, Galizzi JP,
Hermann P, Fossiez F, Banchereau J and Blanchard D: B cells
regulate expression of CD40 ligand on activated T cells by lowering
the mRNA level and through the release of soluble CD40. Eur J
Immunol. 24:787–792. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chatzigeorgiou A, Seijkens T, Zarzycka B,
Engel D, Poggi M, van den Berg S, van den Berg S, Soehnlein O,
Winkels H, Beckers L, et al: Blocking CD40-TRAF6 signaling is a
therapeutic target in obesity-associated insulin resistance. Proc
Natl Acad Sci USA. 111:2686–2691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cantwell M, Hua T, Pappas J and Kipps TJ:
Acquired CD40-ligand deficiency in chronic lymphocytic leukemia.
Nat Med. 3:984–989. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng Z and Chen Q: Raised CD40L expression
attenuates drug resistance in adriamycin-resistant THP-1 cells. Exp
Ther Med. 19:2188–2194. 2020.PubMed/NCBI
|
|
16
|
Pang X, Zhang L, Wu J, Ma C, Mu C, Zhang G
and Chen W: Expression of CD40/CD40L in colon cancer, and its
effect on proliferation and apoptosis of SW48 colon cancer cells. J
BUON. 22:894–899. 2017.PubMed/NCBI
|
|
17
|
Qin L, Qiu H, Zhang M, Zhang F, Yang H,
Yang L, Jia L, Qin K, Jia L, Dou X, et al: Soluble CD40 ligands
sensitize the epithelial ovarian cancer cells to cisplatin
treatment. Biomed Pharmacother. 79:166–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Georgopoulos NT, Steele LP, Thomson MJ,
Selby PJ, Southgate J and Trejdosiewicz LK: A novel mechanism of
CD40-induced apoptosis of carcinoma cells involving TRAF3 and
JNK/AP-1 activation. Cell Death Differ. 13:1789–1801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ara A, Ahmed KA and Xiang J: Multiple
effects of CD40-CD40L axis in immunity against infection and
cancer. Immunotargets Ther. 7:55–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deng N, Chen Q, Guo X, Liu L, Chen S, Wang
A, Li R, Huang Y, Ding X, Yu H, et al: Blockade of CD40L inhibits
immunogenic maturation of lung dendritic cells: Implications for
the role of lung iNKT cells in mouse models of asthma. Mol Immunol.
121:167–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lima PMA, Torres LC, Martins MR, da Matta
MC, Lima JTO, de Mello MJG, da Silva LM, Cintra EB Jr, Lira CCR, da
Fonte EJA and Forones NM: Soluble levels of sCD40L and s4-1BB are
associated with a poor prognosis in elderly patients with
colorectal cancer. J Surg Oncol. 121:901–905. 2020.PubMed/NCBI
|
|
22
|
Adem J, Eray M, Eeva J, Nuutinen U and
Pelkonen J: RIP1 has a role in CD40-mediated apoptosis in human
follicular lymphoma cells. Immunobiology. 222:998–1003. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Armitage JO, Gascoyne RD, Lunning MA and
Cavalli F: Non-Hodgkin lymphoma. Lancet. 390:298–310. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeudy J, Burke AP and Frazier AA: Cardiac
lymphoma. Radiol Clin North Am. 54:689–710. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mugnaini EN and Ghosh N: Lymphoma. Prim
Care. 43:661–675. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bowzyk Al-Naeeb A, Ajithkumar T, Behan S
and Hodson DJ: Non-Hodgkin lymphoma. BMJ. 362:k32042018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seigner J, Basilio J, Resch U and de
Martin R: CD40L and TNF both activate the classical NF-κB pathway,
which is not required for the CD40L induced alternative pathway in
endothelial cells. Biochem Biophys Res Commun. 495:1389–1394. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu W, Li Y, Wang X, Wang C, Zhao W and Wu
J: Anti-tumor activity of gene transfer of the membrane-stable
CD40L mutant into lung cancer cells. Int J Oncol. 37:935–941.
2010.PubMed/NCBI
|
|
29
|
Solooki S, Khozaei A, Shamsdin SA, Emami
MJ and Khademolhosseini F: sCD30 and sCD40L detection in patients
with osteosarcoma, chondrosarcoma and Ewing sarcoma. Iran J
Immunol. 10:229–237. 2013.PubMed/NCBI
|
|
30
|
Gallagher NJ, Eliopoulos AG, Agathangelo
A, Oates J, Crocker J and Young LS: CD40 activation in epithelial
ovarian carcinoma cells modulates growth, apoptosis, and cytokine
secretion. Mol Pathol. 55:110–120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hausding M, Jurk K, Daub S, Kröller-Schön
S, Stein J, Schwenk M, Oelze M, Mikhed Y, Kerahrodi JG, Kossmann S,
et al: CD40L contributes to angiotensin II-induced pro-thrombotic
state, vascular inflammation, oxidative stress and endothelial
dysfunction. Basic Res Cardiol. 108:3862013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walker PR and Migliorini D: The CD40/CD40L
axis in glioma progression and therapy. Neurooncology.
17:1428–1430. 2015.
|
|
33
|
Hassan GS, Stagg J and Mourad W: Role of
CD154 in cancer pathogenesis and immunotherapy. Cancer Treat Rev.
41:431–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pan W, Gong J, Yang C, Feng R, Guo F, Sun
Y and Chen H: Peripheral blood CD40-CD40L expression in human
breast cancer. Ir J Med. 182:719–721. 2013. View Article : Google Scholar
|
|
35
|
Ottaiano A, Pisano C, De Chiara A,
Ascierto PA, Botti G, Barletta E, Apice G, Gridelli C and Iaffaioli
VR: CD40 activation as potential tool in malignant neoplasms.
Tumori. 88:361–366. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qi CJ, Qian KQ, Ning YL, Ma HB, Wang SZ
and Zhang XG: Ligation or cross-linking of CD40 has different
effects on AGS gastric cancer cells. Cell Immunol. 259:135–140.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mazzei GJ, Edgerton MD, Losberger C,
Lecoanet-Henchoz S, Graber P, Durandy A, Gauchat JF, Bernard A,
Allet B and Bonnefoy JY: Recombinant soluble trimeric CD40 ligand
is biologically active. J Biol Chem. 270:7025–7028. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kedar R, Sabag O, Licthenstein M and
Lorberboum-Galski H: Soluble CD40 ligand (sCD40L) provides a new
delivery system for targeted treatment: SCD40L-caspase 3 chimeric
protein for treating B-cell malignancies. Cancer. 118:6089–6104.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
MacDonald I, Wang H, Grand R, Armitage RJ,
Fanslow WC, Gregory CD and Gordon J: Transforming growth
factor-beta 1 cooperates with anti-immunoglobulin for the induction
of apoptosis in group I (biopsy-like) Burkitt lymphoma cell lines.
Blood. 87:1147–1154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mingqiang R and Xinging M: Effects of
soluble CD40 ligand on proliferation and apoptosis of Human B cell
lymphoma cell line BJAB and its mechanism. Shandong Med J.
56:16–19. 2016.(In Chinese).
|
|
41
|
Goldstein MD, Cochrane A and Watts TH:
Cyclic-AMP modulates downstream events in CD40-mediated signal
transduction, but inhibition of protein kinase A has no direct
effect on CD40 signaling. J Immunol. 159:5871–5880. 1997.PubMed/NCBI
|
|
42
|
Rauert-Wunderlich H, Rudelius M, Berberich
I and Rosenwald A: CD40L mediated alternative NFκB-signaling
induces resistance to BCR-inhibitors in patients with mantle cell
lymphoma. Cell Death Dis. 9:862018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Berberich I, Shu G, Siebelt F, Woodgett
JR, Kyriakis JM and Clark EA: Cross-linking CD40 on B cells
preferentially induces stress-activated protein kinases rather than
mitogen-activated protein kinases. EMBO J. 15:92–101. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Grammer AC, Swantek JL, McFarland RD,
Miura Y, Geppert T and Lipsky PE: TNF receptor-associated factor-3
signaling mediates activation of p38 and Jun N-terminal kinase,
cytokine secretion, and Ig production following ligation of CD40 on
human B cells. J Immunol. 161:1183–1193. 1998.PubMed/NCBI
|
|
45
|
Mintz MA, Felce JH, Chou MY, Mayya V, Xu
Y, Shui JW, An J, Li Z, Marson A, Okada T, et al: The HVEM-BTLA
axis restrains T cell help to germinal center B cells and functions
as a cell-extrinsic suppressor in lymphomagenesis. Immunity.
51:310–323 e317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Purkerson JM and Parker DC: Differential
coupling of membrane Ig and CD40 to the extracellularly regulated
kinase signaling pathway. J Immunol. 160:2121–2129. 1998.PubMed/NCBI
|
|
47
|
Huang Y, Zou Y, Lin L, Ma X and Zheng R:
miR101 regulates the cell proliferation and apoptosis in diffuse
large Bcell lymphoma by targeting MEK1 via regulation of the
ERK/MAPK signaling pathway. Oncol Rep. 41:377–386. 2019.PubMed/NCBI
|
|
48
|
Moriguchi M, Watanabe T, Kadota A and
Fujimuro M: Capsaicin induces apoptosis in KSHV-positive primary
effusion lymphoma by suppressing ERK and p38 MAPK signaling and
IL-6 expression. Front Oncol. 9:832019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Akhter R, Sanphui P, Das H, Saha P and
Biswas SC: The regulation of p53 up-regulated modulator of
apoptosis by JNK/c-Jun pathway in β-amyloid-induced neuron death. J
Neurochemistry. 134:1091–1103. 2015. View Article : Google Scholar
|