Introduction
Lung cancer is the leading cause of cancer-related
mortality. Non-small cell lung carcinoma accounts 85% of lung
cancer, and adenocarcinoma is the most common histological type
(1). Lung adenocarcinoma has various
histologic subtypes, such as lepidic, acinar, papillary,
micropapillary, solid, invasive mucinous adenocarcinoma, and so on.
Histologic subtypes are a prognostic factor; lepidic subtype
harbors the best prognostic course, whereas micropapillary and
solid patterns have a more aggressive behavior. Moreover, most lung
adenocarcinomas demonstrate a mixture of different histologic
patterns. The combination of histologic subtypes is important for
prognosis (2).
Trefoil factor 3 (TFF3) is a small secreting protein
and a member of trefoil factor family, which is involved in mucosal
stabilization and repair through mitogenic and antiapoptotic
activities (3,4). TFF3 is distributed mainly in goblet
cells of intestine and lung (5).
Overexpression of TFF3 has been reported to be associated with
several types of cancer, such as stomach, uterus, and breast
(6–8). In lung cancer, TFF3 is expressed
significantly in adenocarcinoma and is a useful biomarker to
distinguish between adenocarcinoma and squamous cell carcinoma
(9). However, the relationship
between TFF3 expression and histologic subtypes in lung
adenocarcinoma has not been examined. Here we revealed the
relationship between TFF3 expression and histologic subtypes in
lung adenocarcinoma. By immunohistochemical analysis of 93 lung
adenocarcinoma cases, we showed TFF3 was highly expressed not only
in invasive mucinous adenocarcinoma but also in papillary and
acinar adenocarcinoma. The expression level of TFF3 was higher in
papillary/acinar subtype than in lepidic subtype; the former was
more aggressive subtype than the latter. Moreover, we generated
TFF3-knockdown lung adenocarcinoma cells and showed that the
depletion of TFF3 attenuated invasion. TFF3 expression is
correlated to invasiveness in lung adenocarcinoma.
Materials and methods
Patients
We examined 93 cases undergoing surgery for
adenocarcinoma of the lung at Osaka University Hospital from 2013
to 2018. No prior therapy was administered in any case. Due to the
short observation period, only 3 cases died of the underlying
disease, and then we focused the relation of TFF3 expression to
histological subtypes rather than prognosis. Histologic subtypes
were classified according to WHO criteria (10). The histological subtypes were lepidic
(n=20), acinar (n=15), papillary (n=22), solid adenocarcinoma
(n=19), and invasive mucinous adenocarcinoma (n=17) (Table I). Lepidic adenocarcinoma included 3
cases of adenocarcinoma in situ (AIS) and 3 cases of
minimally invasive adenocarcinoma (MIA). Resected specimens were
fixed in 10% formalin and processed for paraffin embedding.
Specimens were stored at room temperature in a dark room. Specimens
for evaluation were sectioned at 4 µm thickness and stained with
hematoxylin and eosin (H&E). The study was approved by the
Ethical Review Board of the Graduate School of Medicine, Osaka
University (approval no. 16293). Informed consent was obtained from
all patients.
 | Table I.Histologic subtypes of 93 cases of
lung adenocarcinoma. |
Table I.
Histologic subtypes of 93 cases of
lung adenocarcinoma.
| Histologic
subtypes | Number of
patients |
|---|
| Lepidic
adenocarcinomaa | 20 |
| Acinar
adenocarcinoma | 15 |
| Papillary
adenocarcinoma | 22 |
| Solid
adenocarcinoma | 19 |
| Invasive mucinous
adenocarcinoma | 17 |
Immunohistochemistry for TFF3 and
evaluation with histological score (H-score)
Expression of TFF3 was examined with the primary
rabbit anti-TFF3 monoclonal antibody (dilution 1:2,000 cat. no.
ab108599; Abcam). Subsequent to deparaffinization with xylene and
rehydration with graded alcohol treatment, sections were heated to
121°C in the Pascal Pressurized Heating Chamber (Agilent
Technologies, Inc.). After cooling, the sections were washed in
phosphate-buffered saline, blocked with blocking solution (cat. no.
X0909; Agilent Technologies, Inc.) and incubated with anti-TFF3
antibody. Next, the sections were treated with a ChemMate EnVision
kit (Agilent Technologies, Inc.) that contains a polymerized
secondary antibody to increase detection sensitivity for the
primary antibody. Diaminobenzidine (DAB) (Agilent Technologies,
Inc.) was used as a chromogen. Sections were counterstained with
hematoxylin and observed by microscopy. As the negative control,
staining was carried out in the absence of primary antibody.
Staining intensity (0, 1+, 2+ or 3+) was determined for each sample
independently by two pathologists (S.T. and E.M.). H-score was
calculated using the following formula: [1× (% tumor cells of 1+) +
2× (% tumor cells of 2+) + 3× (% tumor cells of 3+)].
Cell line
The human lung adenocarcinoma cell line, A549 was
obtained from ATCC. Cells were cultured in DMEM supplemented with
10% FBS (Biosera) and in a humidified 5% CO2 incubator
at 37°C.
Immunoblotting
Cells were lysed in buffer containing 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 10 mM KCl, 1 mM
ethylenediaminetetraacetic acid, 1 mM dithiothreitol and 0.1%
Nonidet P-40. Electrophoresis was performed in 5–20% gradient
sodium dodecyl sulphate-polyacrylamide gels (ATTO), and proteins
were transferred to polyvinylidene fluoride membranes (Merck KGaA).
We used the primary anti-TFF3 antibody at 1:500 and it was detected
using a horseradish peroxidase-conjugated anti-rabbit IgG (H+L
chain) (1:5,000; MBL). We quantified the results using ImageJ
(https://imagej.nih.gov/ij/).
Generation of TFF3-knockdown cells
using siRNA-mediated silencing
A549 cells (1×105) seeded into six-well
culture plates were transfected with TFF3-targeting siRNA (Silencer
Select s14039, s14040 and s14041; Thermo Fisher Scientific, Inc.)
or non-targeting control siRNA (AM4611; Thermo Fisher Scientific,
Inc.) using Lipofectamine RNAiMAX Reagent (Thermo Fisher
Scientific, Inc.) at a final concentration of 50 nM. Cells were
subjected to the immunoblotting analysis and matrigel invasion
assay 72 h after siRNA transfection.
Matrigel invasion assay
Tumor cell invasion was examined using the Corning
BioCoat Matrigel Invasion Chamber (Corning, Inc.). Tumor cells were
placed in the upper chamber in DMEM without FBS and incubated at
37°C for 24 h. The lower chamber contained DMEM with 10% FBS.
Invasive cells, which migrated to the lower side of the upper
chamber, were stained with Diff-Quik (Sysmex). The number of
invasive cells was counted in five random fields per chamber at
high magnification.
Statistical analysis
Statistical analyses were performed using JMP Pro
v14 software (SAS Institute Inc.). Results were shown as the means
± standard error (SE). Differences in results were determined using
Student's t-test, Wilcoxon signed-rank test, and analysis of
variance (ANOVA) followed by Dunnett's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of TFF3 with
immunohistochemical analysis
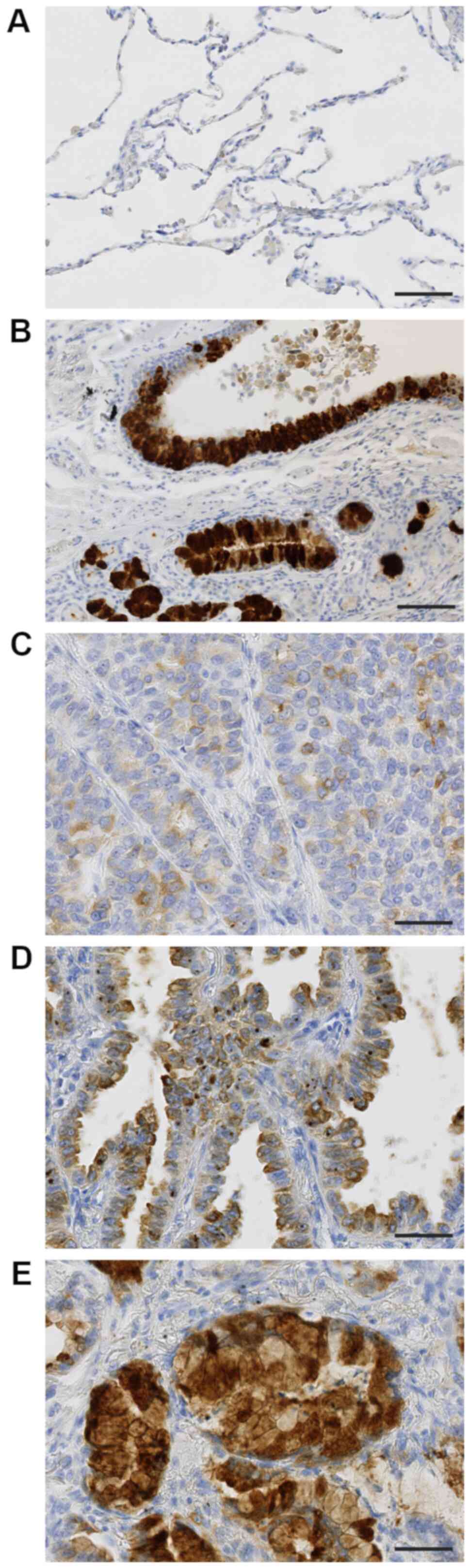
TFF3 was hardly expressed in non-cancerous alveolar
epithelial cells (Fig. 1A), but
strongly in normal bronchial glands and bronchial epithelium
(Fig. 1B). Lung adenocarcinoma cells
expressed TFF3 in their cytoplasm, the expression level of which
was various among cases. The typical staining patterns were shown
in Fig. 1C-E; weakly in Fig. 1C, moderately in Fig. 1D, and strongly in Fig. 1E.
Association between TFF3 expression
and histologic subtypes
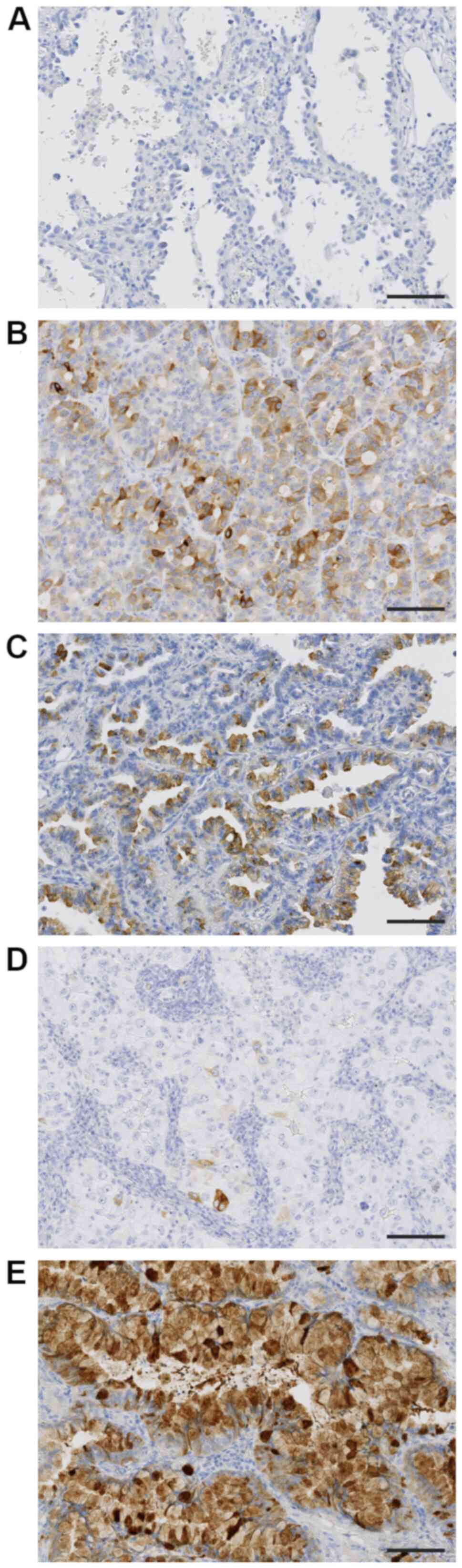
The associations between TFF3 expression level
(H-score) and histologic subtypes were evaluated (Table II). The expression of TFF3 was
hardly detected in lepidic subtype (Fig.
2A), moderately in papillary and acinar subtypes (Fig. 2B and C), and weakly in solid subtype
(Fig. 2D). The highest expression
level of TFF3 was detected in invasive mucinous carcinoma (Fig. 2E), in which tumor cells showed
diffuse and strong positivity. The rank order of TFF3 expression
level was as follows; invasive mucinous adenocarcinoma >
papillary and acinar subtypes > solid and lepidic subtypes.
 | Table II.TFF3 expression in various histologic
subtypes of lung adenocarcinoma. |
Table II.
TFF3 expression in various histologic
subtypes of lung adenocarcinoma.
| Histologic
subtype | H-scorea |
|---|
| Lepidic
adenocarcinoma | 12.95±4.94 |
| Acinar
adenocarcinoma |
30.87±9.26b |
| Papillary
adenocarcinoma |
36.05±10.74b |
| Solid
adenocarcinoma | 18.47±6.58 |
| Invasive mucinous
adenocarcinoma |
137.35±18.33b |
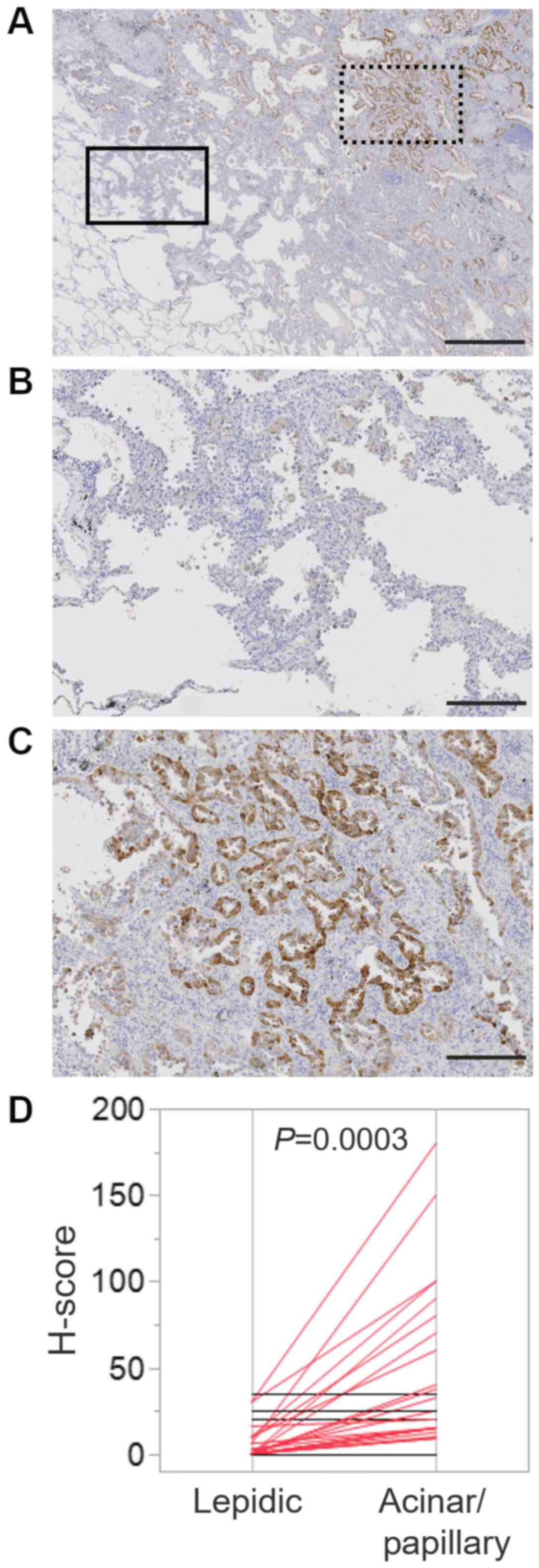
Several lung adenocarcinoma is known to be composed
of mixture of histologic subtypes. In fact, 38 cases showed the
mixture of lepidic subtype and papillary/acinar subtype (Fig. 3A; boxed area with solid line was
lepidic subtype, whereas boxed area with dotted line was papillary
subtype). Then, we compared TFF3 expression level between area of
lepidic subtype (Fig. 3B) and that
of papillary/acinar subtype (Fig.
3C) in an individual case. H-score of papillary/acinar area was
significantly higher than that of lepidic area (Fig. 3D).
Involvement of TFF3 in the invasion of
lung adenocarcinoma cells
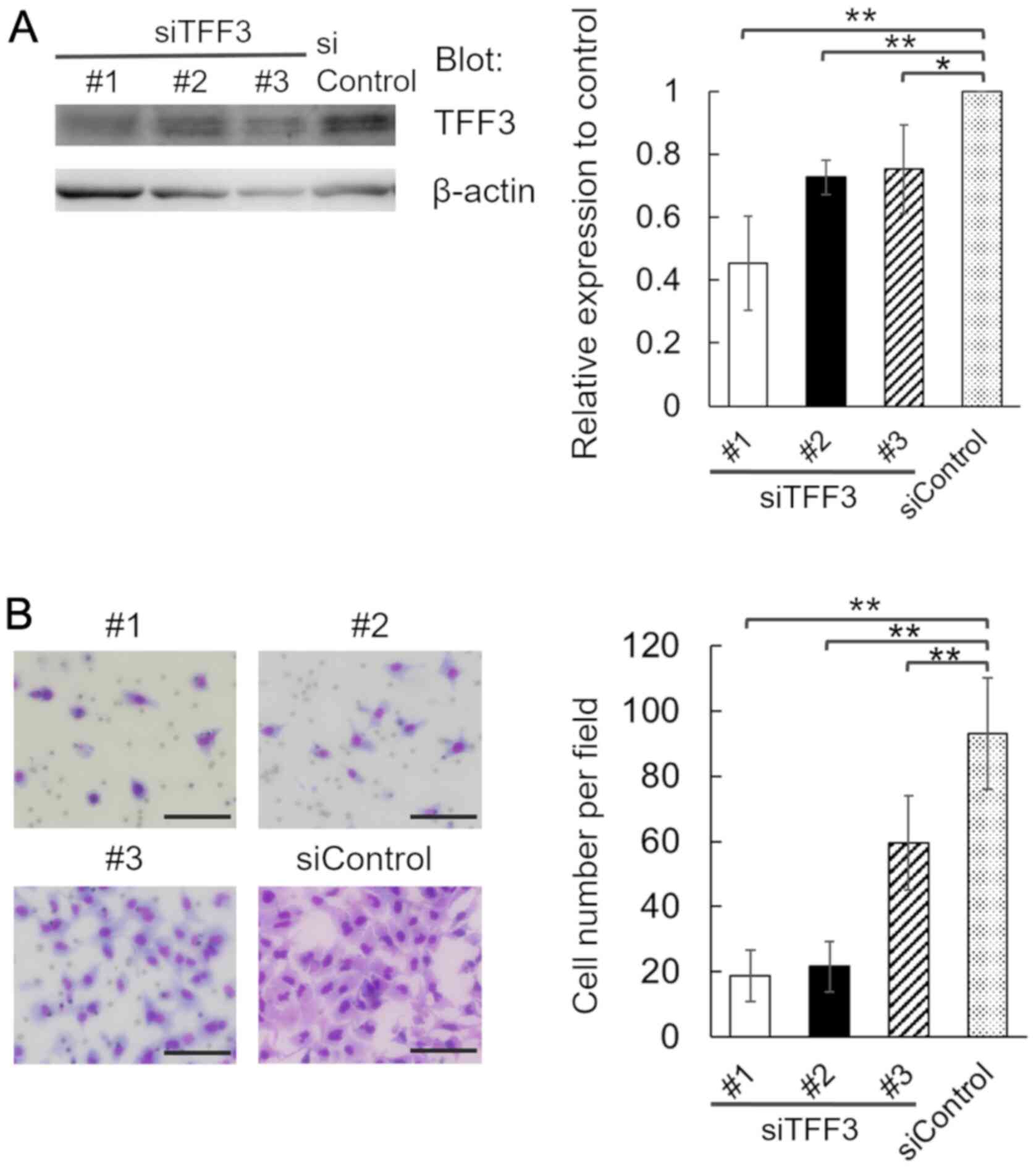
We transfected A549 cells with 3 individual siRNA
duplexes specific for TFF3 (siTFF3 #1, #2 and #3), or a
nontargeting control siRNA (siControl), and confirmed the decrease
in TFF3 protein expression in TFF3-knockdown cells (Fig. 4A). We found that in comparison with
control cells, the invasion of TFF3-knockdown cells was attenuated
(Fig. 4B). Thus, TFF3 is involved in
the invasion of lung adenocarcinoma cells.
Discussion
TFF3 is related to mucosal stabilization in
gastrointestinal tract in normal condition and is upregulated in
various types of cancer (4,6–8). To
date, no studies have compared TFF3 expression in histologic
subtypes of lung adenocarcinoma. In the present study, we pointed
that the expression level of TFF3 in invasive mucinous carcinoma
was the highest, followed by papillary, acinar, solid, and lepidic
subtypes of adenocarcinoma. It's not surprising that the expression
of TFF3 in invasive mucinous carcinoma was overwhelmingly high
because TFF3 is mainly distributed in mucous cells in normal
condition. Besides invasive mucinous carcinoma, the expression of
TFF3 in papillary and acinar adenocarcinoma was significantly
higher than in lepidic adenocarcinoma. We considered that TFF3 was
related to invasiveness in lung adenocarcinoma. To confirm that, we
used 38 cases with both lepidic and papillary/acinar areas. A
subset of lung adenocarcinoma follows a linear multistep
progression, in which a precursor lesion progresses to
adenocarcinoma in situ, which is followed by invasive
adenocarcinoma (11). In the case
with both lepidic and papillary/acinar areas, lepidic area means
non-invasive and papillary/acinar area means invasive area. The
expression of TFF3 in papillary/acinar area was significantly
higher than that of lepidic area in an individual sample. Moreover,
we showed that using lung adenocarcinoma cells, the depletion of
TFF3 attenuated invasion. Therefore, we proved that TFF3 is related
to invasiveness by means of both in vitro and
immunohistochemical assays on clinical samples.
On the other hand, the expression of TFF3 in solid
adenocarcinoma, a highly invasive histologic subtype, was not
significantly high. Further investigation is necessary to detect
the molecular mechanism of invasion by TFF3 in lung
adenocarcinoma.
Collectively, our findings revealed that in lung
adenocarcinoma TFF3 was highly expressed not only in invasive
mucinous carcinoma but also in papillary and acinar adenocarcinoma.
This is, to our knowledge, the first report that TFF3 expression
was related to the histologic subtype in lung adenocarcinoma.
Acknowledgements
The authors would like to thank Ms. Etsuko Maeno,
Ms. Takako Sawamura and Mr. Masaharu Kohara (Department of
Pathology, Osaka University Graduate School of Medicine) for their
technical assistance.
Funding
The present study was supported by the Japan China
Sasakawa Medical Fellowship (grant no. 2019-41-16); Project MEET,
Osaka University Graduate School of Medicine (grant nos.
A19H034520, T17K195550 and T18K150780); The Ministry of Education,
Culture, Sports, Science and Technology, Japan.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
EM proposed and designed the current study. KK, AK
and SN selected patients and collected samples/clinical data. WL
and ST conducted the experiments. ST and EM evaluated
immunohistochemical data. ST and EM wrote the manuscript. WL, ST,
KK, AK, SN and EM reviewed and edited the manuscript. All authors
approved the submitted and published versions.
Ethics approval and consent to
participate
The present study was approved by the Ethical Review
Board of the Graduate School of Medicine, Osaka University
(approval no. 16293). Informed consent was obtained from all study
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zappa C and Mousa SA: Non-small cell lung
cancer: Current treatment and future advances. Transl Lung Cancer
Res. 5:288–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee G, Choi ER, Lee HY, Jeong JY, Ahn JH,
Kim S, Bae J, Kim HK, Choi YS, Kim J, et al: Pathologic
heterogeneity of lung adenocarcinomas: A novel pathologic index
predicts survival. Oncotarget. 7:70353–70363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Plaut AG: Trefoil peptides in the defense
of the gastrointestinal tract. N Engl J Med. 336:506–507. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Emami S, Rodrigues S, Rodrigue CM, Le
Floch N, Rivat C, Attoub S, Bruyneel E and Gespach C: Trefoil
factor family (TFF) peptides and cancer progression. Peptides.
25:885–898. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wiede A, Jagla W, Welte T, Köhnlein T,
Busk H and Hoffmann W: Localization of TFF3, a new mucus-associated
peptide of the human respiratory tract. Am J Respir Crit Care Med.
159:1330–1335. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taniguchi Y, Kurokawa Y, Takahashi T,
Mikami J, Miyazaki Y, Tanaka K, Makino T, Yamasaki M, Nakajima K,
Mori M and Doki Y: Prognostic value of trefoil factor 3 expression
in patients with gastric cancer. World J Surg. 42:3997–4004. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mhawech-Fauceglia P, Wang D, Samrao D, Liu
S, DuPont NC and Pejovic T: Trefoil factor family 3 (TFF3)
expression and its interaction with estrogen receptor (ER) in
endometrial adenocarcinoma. Gynecol Oncol. 130:174–180. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pandey V, Wu ZS, Zhang M, Li R, Zhang J,
Zhu T and Lobie PE: Trefoil factor 3 promotes metastatic seeding
and predicts poor survival outcome of patients with mammary
carcinoma. Breast Cancer Res. 16:4292014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang XN, Wang SJ, Pandey V, Chen P, Li Q,
Wu ZS, Wu Q and Lobie PE: Trefoil factor 3 as a novel biomarker to
distinguish between adenocarcinoma and squamous cell carcinoma.
Medicine (Baltimore). 94:e8602015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 World Health Organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yatabe Y, Borczuk AC and Powell CA: Do all
lung adenocarcinomas follow a stepwise progression? Lung Cancer.
74:7–11. 2011. View Article : Google Scholar : PubMed/NCBI
|