Introduction
Small-cell lung cancer (SCLC), which accounted for
~15% of all cases of lung cancer in the United States in 2020
(1), is a highly aggressive lung
cancer tumor. The 5-year survival rate was <7% in the United
States in 2017 (2). The survival
rate is poor for a number of reasons. Firstly, SCLC is
characterized by rapid growth and metastasis and the lack of early
detection means few cases can be treated by surgery (3). Secondly, the initial effects of
chemotherapy quickly diminish, and the majority of patients relapse
within the first year, worldwide (1), developing multidrug resistance.
Finally, a lack of adequate surgical tissues and re-biopsy for
molecular profiling results in few therapeutic options for SCLC
compared with that in non-small-cell lung cancer (NSCLC).
Sulforaphane (SFN) is an isothiocyanate derived from
cruciferous vegetables, particularly broccoli sprouts (4). SFN has been reported to protect against
the development of numerous types of cancer, such as liver,
prostate, and colon cancer, by inhibiting phase I enzymes, that
activate carcinogens, and by inducing nuclear factor erythroid
2-related factor 2 (Nrf2)-regulated genes of phase II
detoxification enzymes (4–7). The anticancer effects of SFN in various
types of cancer have been reported, in both in vitro and
in vivo studies. For example, SFN has been reported to
induce apoptotic cell death in NSCLC, as well as in pancreatic,
breast and prostate cancer (8–11). Other
angiogenic activities of SFN are mediated by the suppression of
vascular endothelial growth factor and matrix metalloproteinase-2
(12). Antimetastatic effects of SFN
were also achieved by inhibiting cell migration and invasion
(13,14). However, the anticancer effects of SFN
against SCLC have not yet been fully elucidated.
Ferroptosis is a type of programmed cell death, that
is non-apoptotic and has been described as an iron- and reactive
oxygen species (ROS)-dependent form of regulated cell death
(15). The morphological features of
ferroptotic cells include alterations in the mitochondrial
structure, accompanied by the absence of nuclear shrinkage and
ruptured plasma membranes (15,16).
This process is triggered by two mechanisms. The first mechanism is
the inhibition of system xc- (e.g. induced by erastin) (17). System xc- is the cystine/glutamate
antiporter that imports extracellular cystine in exchange for
intracellular glutamate (18). The
cystine/glutamate antiporter xCT (SLC7A11) is a key component of
system xc- (19). Intracellular
cystine starvation leads to the depletion of glutathione (GSH)
levels and subsequent inactivation of GSH peroxidase 4 (GPX4)
function (20). The second mechanism
of ferroptosis is direct blocking of GPX4 [e.g. induced by
Ras-selective lethal (RSL)3] (21,22).
GPX4 is an enzyme that reduces lipid hydroperoxides to lipid
alcohols (15). The loss of GPX4
activity leads to the formation of high lipid ROS (15,22). In
addition, excessive iron also contributes to ferroptotic cell death
by producing ROS via the Fenton reaction (23,24). A
previous report demonstrated that siramesine and lapatinib induced
ferroptosis in breast cancer cells (25). The newly discovered ‘ferroptosis’
form of programmed cell death (17)
may provide a novel target for cancer treatment.
The present study investigated the cell death
effects of SFN treatment and demonstrated the anticancer effects of
SFN in SCLC cells.
Materials and methods
Reagents
SFN (R,S-sulforaphane; cat. no. S8044) was purchased
from LKT Laboratories, Inc.. SFN was dissolved in DMSO and was
subsequently used for each assay. Z-vad (cat. no. 4800-520), an
apoptosis inhibitor, was purchased from Medical and Biological
Laboratories Co., Ltd. Necrostatin-1 (cat. no. N9037), a
necroptosis inhibitor, ferrostatin-1 (cat. no. SML0583), a
ferroptosis inhibitor, and deferoxamine (DFO; cat. no. D9533), an
iron chelator, were purchased from Sigma-Aldrich (Merck KGaA). The
optimum concentration of each reagent (z-vad, necrostatin-1 and
ferrostatin-1) was determined as previously described (17,26,27). The
optimum concentration of DFO was determined from preliminary
experiments (data not shown). The following concentrations were
used: z-vad, 10 µM; necrostatin-1, 50 µM; ferrostatin-1, 1 µM; and
DFO, 10 µM. Amrubicinol (AMR), an anthracycline anticancer agent
and active metabolite derived from amrubicin, was obtained from
Dainippon Sumitomo Pharma Co., Ltd.
Cell culture
The human SCLC cell lines NCI-H69 (H69), NCI-H82
(H82) and NCI-H69AR (H69AR) were purchased from American Type
Culture Collection. The H69AR cell line are cross-resistant to
anthracycline analogs. The normal bronchial epithelial cell line,
16HBE14o- (16HBE) were kindly provided by Dr Gruenert (Head and
Neck Stem Cell Laboratory, University of California, USA). The H69,
H82 and H69AR cells were cultured in RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA), supplemented with 10% FBS
(Sigma-Aldrich; Merck KGaA) and 1% penicillin-streptomycin (Nacalai
Tesque, Inc.) while, the 16HBE cells were cultured in minimum
essential medium (Sigma-Aldrich; Merck KGaA) supplemented with 10%
FBS (Sigma-Aldrich; Merck KGaA) and 1% penicillin-streptomycin
(Nacalai Tesque, Inc.). The cells were maintained at 37°C in a
humidified atmosphere with 5% CO2. Stocks of these cells were
prepared within five passages of receipt; cells used for
experiments were passaged for <6 months.
MTT assay
H69 cells (5,000 cells/well) were treated with
increasing concentrations (1, 5, 10, 20 µM) of SFN and seeded into
each well of 96-well plates. Control cells were left untreated.
Cells were incubated for 24, 48 and 96 h at 37°C in a humidified
atmosphere with 5% CO2. Cell viability was measured
using an MTT Cell Viability Assay kit (cat. no. 30006; Biotium,
Inc.). The signal absorbances were measured at 570 nm using a
Multiskan™ GO Microplate Spectrophotometer (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions, and
the background absorbance at 630 nm was subtracted.
Lactate dehydrogenase (LDH) assay
To determine if cell death was induced by SFN in the
H69, H82, H69AR and 16HBE cell lines, the release of LDH into the
medium was measured using a Cytotoxicity LDH Assay kit-WST (LDH
assay) (Dojindo Molecular Technologies, Inc.). The cells were
treated with z-vad (10 µM), necrostatin-1 (50 µM), ferrostatin-1 (1
µM), and DFO (10 µM) 1 h before SFN treatment at 37°C. Each sample
(5,000 cells/well) treated with SFN was incubated in a 96-well
plate for 48 or 96 h at 37°C, and the LDH assay was performed
according to the manufacturer's instructions. H69 and H69AR cells
(5,000 cells/well) treated with AMR were incubated in a 96-well
plate for 96 h at 37°C, and the LDH assay was performed. The
absorbance of each sample was determined at 490 nm, using a
Multiskan GO Microplate Spectrophotometer (Thermo Fisher
Scientific, Inc.). LDH release (cytotoxicity) was calculated by
comparing the absorbance with maximum LDH release (value measured
upon adding the lysis buffer included in the kit) of control cells
(28). Representative images of the
H69 cell line undergoing cell death in SFN-treated (20 µM) and
untreated controls were obtained using Fluorescent Cell Imager
(Bio-Rad Laboratories, Inc.).
Live and dead assay
The H69 cells treated with SFN (20 µM) and untreated
control cells were seeded into 24-well plates, at a density of
1×105 cells/well at 37°C for 96 h. Cytotoxicity was
measured using a LIVE/DEAD™ Viability/Cytotoxicity kit for
mammalian cell analysis (Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions. Calcein-AM is retained within
live cells, producing green fluorescence, whereas ethidium
homodimer-1 (EthD-1) enters the dead cells with damaged membranes
and binds to nucleic acids, producing red fluorescence (29). A concentration of 50 µM calcein-AM
and 4 µM EthD-1 solution was used according to preliminary
experiments (data not shown). Flow cytometry analysis was performed
using a flow cytometer (Gallios; Beckman Coulter, Inc.) and
analyzed with Kaluza software (v1.0; Beckman Coulter, Inc.).
Iron assay
Intracellular levels of ferrous (Fe2+)
iron were determined using an iron assay kit from Sigma-Aldrich
(cat. no. MAK025; Merck KGaA), according to the manufacturer's
instructions. The H69 cells (2×106 cells), treated with
20 µM SFN and untreated control cells were cultured at 37°C for 96
h. The absorbance of each sample was measured at 593 nm using a
Multiskan GO Microplate Spectrophotometer (Thermo Fisher
Scientific, Inc.). The results were analyzed using Skanit Software
(v3.2; Research Edition for Multiskan GO; Thermo Fisher Scientific,
Inc.).
Analysis of lipid peroxidation
The H69 cells, treated with SFN (20 µM) and
untreated control cells, were seeded into 6- or 24-well plates, at
a density of 2×105 cells/well at 37°C for 48, 72, 96 or
120 h. DFO (10 µM) and ferrostatin-1 (1 µM) were added to the cells
at 37°C, 1 h before they were treated with SFN. Lipid peroxidation
was measured using an Image-iT Lipid Peroxidation kit for live cell
analysis (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Flow cytometry analysis was performed
using a flow cytometer (FACSCalibur; BD Biosciences) and mean
fluorescence intensity (MFI) was analyzed using FlowJo software
(v10.7.1; BD Biosciences).
Detection of intracellular ROS
The intracellular levels of ROS were detected using
a fluorescence plate reader (Infinite 200Pro MPlex; Tecan Group,
Ltd.), using 6-carboxy-2′,7′-dichlorofluorescein diacetate dye
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. The H69 cells (3×105 cells), treated with
20 µM SFN, and untreated control cells were cultured at 37°C for 4,
8, 12, 16 or 24 h before subsequent analysis.
Confocal fluorescence imaging of
mitochondria
The H69 cells, treated with 20 µM SFN or untreated
control cells, were seeded into plates, at a density of
5×105 cells/ml, and incubated at 37°C for 96 h. To
determine the mitochondrial morphology, the H69 cells were cultured
in glass-bottom dishes (AGC Techno Glass Co., Ltd.) and transfected
with a modified baculovirus vector (BacMam 2.0; Thermo Fisher
Scientific, Inc.) encoding mitochondria-targeted green fluorescent
protein according to the manufacturer's instructions. The vector (2
µl/10,000 cells) was transfected into the cells for 16 h at 37°C.
The cells were incubated at 37°C in a humidified incubator with 5%
CO2. The cell nuclei were stained with Hoechst33342 (5
µg/ml; Thermo Fisher Scientific, Inc.), at room temperature for 5
min in the dark box. Images of the cells were captured with an
Olympus FV1000 confocal laser scanning microscope, using an α
Plan-Apochromatic X ×100/1.46 NA objective with 3X digital zoom
(Carl Zeiss AG).
Reverse transcription-quantitative
(RT-q) PCR
SFN (20 µM) 96 h-treated and untreated control cells
(1×104 cells/sample) were seeded into 96-well plates. To
investigate the changes in mRNA expression levels of SLC7A11,
RT-qPCR was performed using the TaqMan® Gene Expression
Cells-to-CT™ kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The following temperature conditions were
used: Incubation at at 37°C for 60 min then at 95°C for 5 min.
RT-qPCR was performed using an Applied Biosystems 7500 Fast
Real-Time PCR System (Thermo Fisher Scientific, Inc.). The target
mRNA was amplified using a TaqMan gene expression master mix
(Thermo Fisher Scientific, Inc.) and the following TaqMan gene
expression assay (probe and primer): SLC7A11, Hs00921938_m1 and
GAPDH, Hs02758991_g1 (both from Thermo Fisher Scientific,
Inc.).
The RT-qPCR thermocycling conditions were as
follows: Initial denaturation at 50°C for 2 min, 95°C for 10 min
and 40 cycles of 95°C for 15 sec and 60°C for 1 min. The expression
levels of target mRNA were calculated using the 2−∆∆Cq
method and normalized to those of GAPDH (30).
GSH assay
Total GSH levels in SFN (20 µM)-treated for 96 h and
untreated cells (5×106 cells/sample) were determined
using a GSSG/GSH Quantification kit (Dojindo Molecular
Technologies, Inc.) according to the manufacturer's instructions.
The absorbance of each sample was measured at 405 nm using a
Multiskan GO microplate spectrophotometer (Thermo Fisher
Scientific, Inc.). The glutathione concentration was obtained from
the calibration curve using GraphPad Prism software (v8; GraphPad
Software, Inc.). A total of three independent repeats was
performed.
Western blot analysis
The H69 cells treated with 20 µM SFN for 48, 72 or
96 h were subsequently prepared for analysis using western blot
analysis. The whole cell lysates were collected at the indicated
times and lysed using RIPA buffer [25 mM Tris-HCl (pH 7.6), 150 mM
NaCl, 1% NP-40, 1% sodium deoxycholate and 0.1% SDS]. The protein
levels were quantified with a Bio-Rad Protein Assay Dye Reagent
Concentrate (Bio-Rad Laboratories, Inc.), according to the
manufacturer's instructions. The total proteins (20 µg) were
separated on 4–15% gradient gels (Criterion™ TGX™ Precast Gel;
Bio-Rad Laboratories, Inc.) using SDS-PAGE and subsequently
transferred onto PVDF membranes (Merck Millipore), then blocked
with 3% BSA (Iwai Chemicals Company) mixed with TBS-Tween-20
(0.05%; TBST) at room temperature for 1 h. After washing three
times with TBS, in 0.1% Tween-20, the membranes were incubated
overnight at 4°C with anti-human xCT/SLC7A11 (cat. no. ab175186;
1:1,000; Abcam) and β-actin (cat. no. 4967; 1:1,000) (Cell
Signaling Technology, Inc.) antibodies diluted in TBST. The
membranes were subsequently incubated at room temperature for 1 h
with a goat anti-rabbit-HRP secondary antibody (cat. no. 7074;
1:3,000; Cell Signaling Technology, Inc.). The proteins were then
detected with an Amersham™ ECL™ Prime Western Blotting Detection
Reagent (Cytiva) and quantified using ImageJ software (v1.53a;
National Institutes of Health).
Detection of apoptotic cell death
The H69 cells (3×105 cells), treated with
20 µM SFN for 96 h and untreated control cells, were subsequently
prepared for detection of cellular apoptosis using flow cytometry.
Apoptosis was detected by analyzing the sub-G1 peaks (DNA
fragmentation). The cells were stained using a Propidium Iodide
Flow Cytometry kit (Abcam), according to the manufacturer's
instructions. Flow cytometry was performed using a Gallios flow
cytometer and analyzed using the Kaluza (v1.0) Flow Cytometry
Analysis software (both Beckman Coulter, Inc.).
Statistical analysis
Data are presented as the mean ± SD (n≥3). The
statistical significance of differences between two groups was
determined using a paired or unpaired Student's t-test or
Mann-Whitney U test. The statistical significance of differences
among >2 groups was determined using one-way or two-way ANOVA
followed by Tukey's multiple comparisons post hoc test. The
statistical analysis was performed using GraphPad Prism software
(v8; GraphPad Software, Inc.). P<0.05 was considered to indicate
a statistically significant difference; P<0.01, P<0.001 and
P<0.0001 were considered as further thresholds of
significance.
Results
SFN inhibits growth and induces cell
death in the SCLC cells
The effect of SFN on growth inhibition of the H69
SCLC cells was investigated using a MTT assay (Fig. 1A). Significant inhibition of cell
growth was observed in H69 cells treated with >10 µM SFN over 48
h compared with that in the control group.
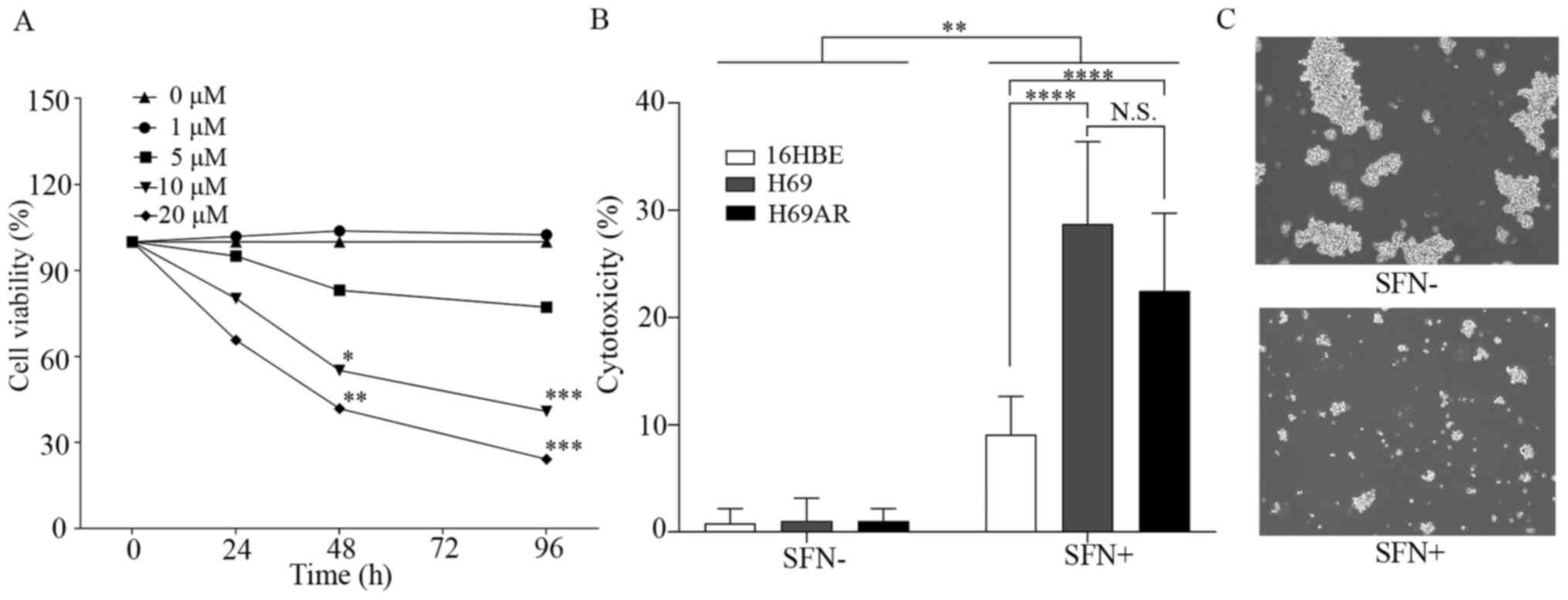 | Figure 1.SFN induces growth inhibition and
cell death in SCLC cells. (A) H69 cells were treated with 0, 1, 5,
10 and 20 µM SFN for 0, 24, 48 and 96 h then, cell viability was
determined using a MTT assay. (B) The H69 and H69AR SCLC and 16HBE
cell lines were treated with 20 µM SFN for 96 h then, cell death
was determined using a lactate dehydrogenase assay. (C)
Representative images of H69 cells in the presence or absence of
SFN (20 µM, 96 h) treatment. Magnification, ×20. Data are presented
as the mean ± SD (n≥3). *P<0.05, **P<0.01, ***P<0.001,
****P<0.0001. SFN, sulforaphane; SCLC, small cell lung cancer;
N.S., not significant. |
The cell death effects of SFN in the SCLC H69 and
H69AR, and the normal bronchial epithelial cell line, 16HBE cell
lines were initially observed to occur in a dose-dependent manner
(data not shown). The cell death effects (cytotoxicity) of SFN (20
µM) in the H69, H69AR and 16HBE cell lines, after 96 h were
measured using LDH assays (Fig. 1B).
A significant cell death effect was shown in the SCLC and 16HBE
cell lines following 20 µM SFN treatment compared with that in the
untreated cells. However, the cell death effect in the 16HBE cell
line was significantly less compared with that in the SCLC cell
lines. Furthermore, the cell death effect was not significantly
different between the H69 and H69AR cell lines. Similar results
were observed using the SCLC H82 cell line. Significant inhibition
of cell growth was observed at 24 h following >20 µM SFN
treatment, at 48 h with >10 µM SFN treatment, and at 96 h with
>5 µM SFN treatment. Cell death effects of SFN in H82 small cell
lung cancer cells were observed to occur in a dose-dependent manner
(Fig. S1). Fig. 1C shows representative images of H69
cell death in SFN-treated (20 µM) and untreated controls from the
LDH assay experiments.
Flow cytometric cell sorting analysis of the live
and dead assays demonstrated that cell death was significantly
induced in SFN-treated (20 µM; 96 h) SCLC cells compared with that
in the untreated control cells (Fig.
S2).
SFN exhibits anticancer effects
against SCLC cells via induction of ferroptosis
Apoptosis, necroptosis and ferroptosis were
investigated as potential cell death mechanisms caused by SFN
treatment in the SCLC cells using the cell death inhibitors, z-vad,
necrostatin-1 and ferrostatin-1. The results demonstrated that
ferrostatin-1, which inhibits ferroptosis, significantly inhibited
the effects of SFN-induced cell death. However, necrostatin-1,
which inhibits necroptosis, and z-vad, which is a caspase
inhibitor, did not inhibit SFN-induced cell death (Fig. 2A). In the sub G1 peak assay, the
percentage of cells in the sub G1 phase (<2N), in the cells
treated with SFN was 6.56% compared with that in the control group
2.73% (Fig. S3).
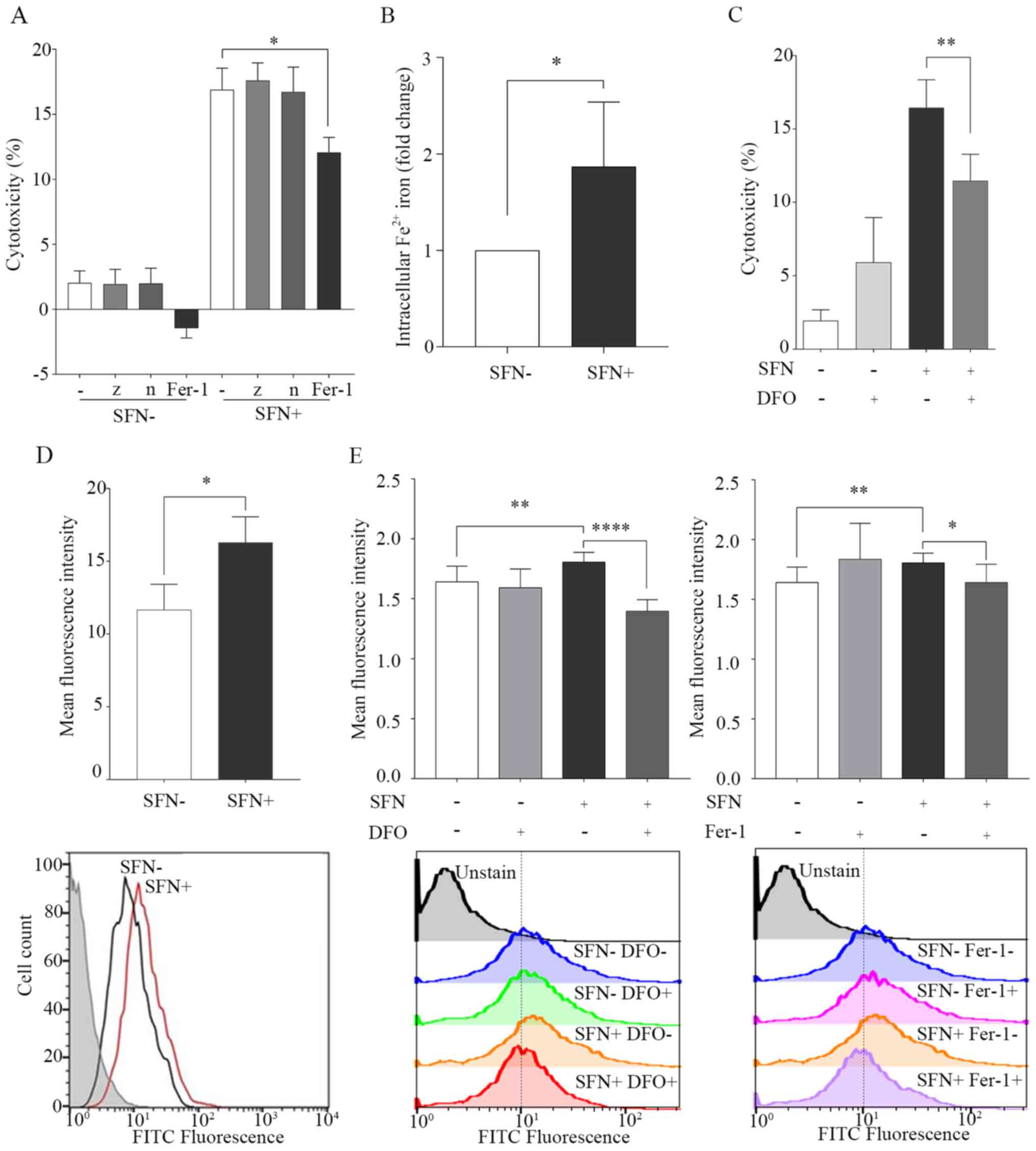 | Figure 2.SFN induces cell death by
ferroptosis. H69 cells were treated with SFN (20 µM) for 96 h or
untreated, as a control. (A) Inhibition of cell death by SFN using
inhibitors of apoptosis, necroptosis and ferroptosis. Cell death
was investigated using a LDH assay. (B) Intracellular levels of
Fe2+ were determined using an iron assay and the 2
groups were statistically analyzed. (C) Inhibition of SFN-induced
cell death in the presence of DFO (10 µM; 1 h). Cell death was
investigated using a LDH assay. (D) Lipid peroxidation by SFN in
H69 cells. FITC fluorescence in SFN-treated H69 cells (red line),
untreated (black line) and background controls (gray filled). Lipid
peroxidation was quantified using flow cytometry. (E) Inhibition of
lipid peroxidation by SFN in the presence of DFO and Fer-1.
Following treatment with DFO (10 µM) or Fer-1 (1 µM) for 1 h, lipid
peroxidation was quantified using flow cytometry. Data are
presented as the mean ± SD (n≥3). *P<0.05, **P<0.01,
****P<0.0001. SFN, sulforaphane; LDH, lactate dehydrogenase;
DFO, deferoxamine; -, no inhibitor; z, z-vad; n, necrostatin-1;
Fer-1, ferrostatin-1. |
Ferroptosis is a type of iron-dependent cell death
and is known to be inhibited by the iron chelator, DFO (17). The intracellular levels of
Fe2+ were significantly elevated in SFN-treated cells
compared with that in untreated cells at 96 h (Fig. 2B). It was next determined whether DFO
inhibited cell death induced by SFN in the H69 cell line.
SFN-induced cell death was significantly decreased in DFO-treated
cells compared with that in the control cells (Fig. 2C).
Ferroptosis is characterized by cell death leading
to the accumulation of lipid peroxidation (17). The effects of SFN on lipid
peroxidation in the H69 cells were determined using FACS analysis.
MFI was significantly increased in SFN-treated cells compared with
that in the untreated cells at 96 h (Figs. 2D and S4A). Maximum increase in the production of
ROS induced by SFN was observed at 12 h (Fig. S4B). The effect of lipid peroxidation
induced by SFN was reversed by pretreatment with DFO and
ferrostatin-1 (Fig. 2E). Confocal
fluorescence imaging indicated that the levels of mitochondria were
decreased; however, there were no changes in nuclear morphology,
which are characteristic features of ferroptosis (15,16)
(Fig. S5).
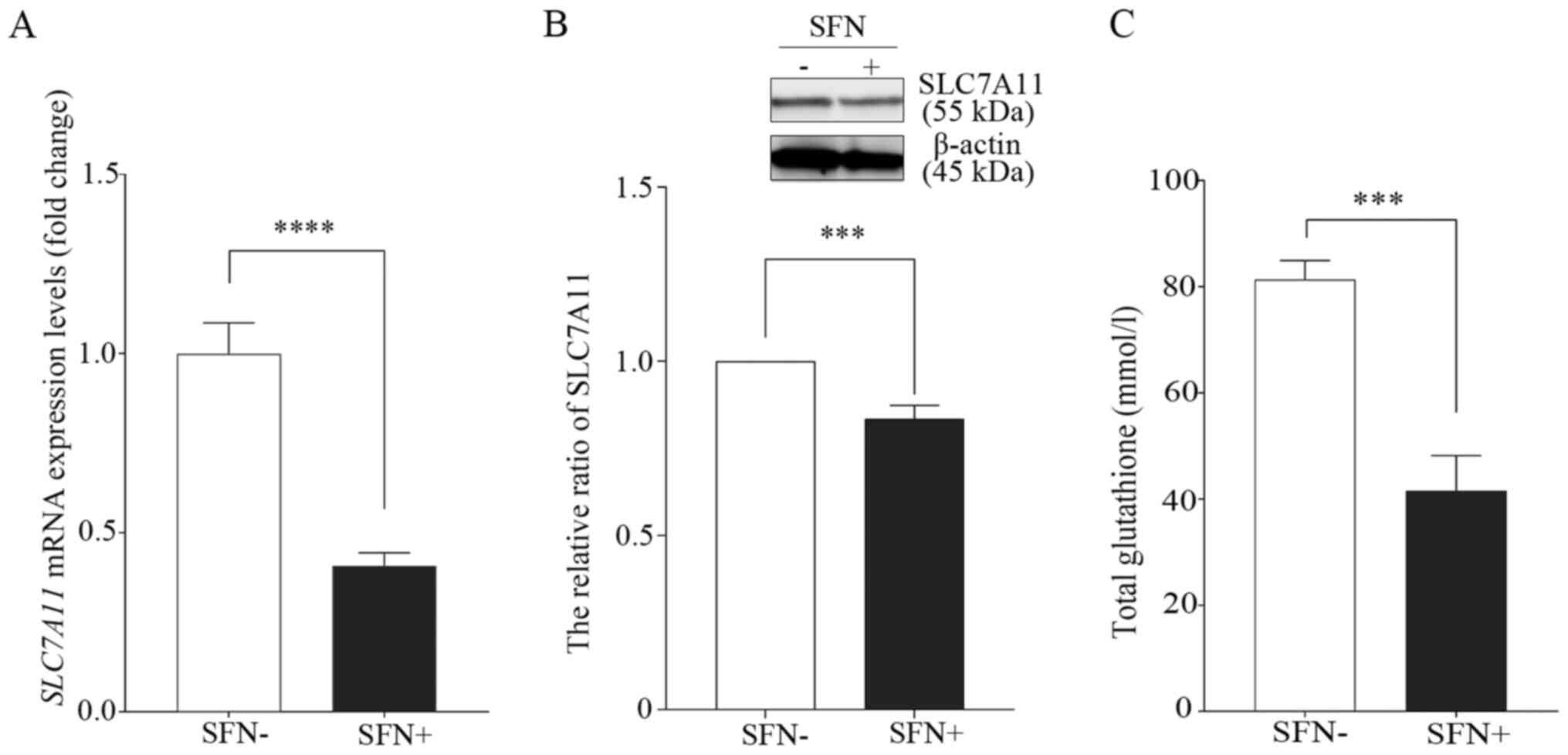
SFN inhibits SLC7A11 expression
levels
Previous studies have showed that ferroptosis was
initiated by the inhibition of system xc-, which leads to a
depletion of GSH levels (17,20).
RT-qPCR was performed to determine the mRNA expression levels of
SLC7A11, a member of system xc-, following treatment of the
H69 cell line with SFN (Fig. 3A).
The mRNA expression levels of SLC7A11 were 1.00±0.21 in the
untreated control cells; however, they were 0.41±0.09 in
SFN-treated cells (20 µM, 96 h). Thus, the mRNA expression levels
of SLC7A11 were significantly lower in the SFN-treated cells
compared with that in the untreated cells. In addition, the protein
expression levels of SLC7A11 in the SFN-treated cells were
significantly lower compared with that in the untreated control
group (Fig. 3B).
Furthermore, intracellular total GSH levels were
significantly lower in the SFN-treated H69 cells compared with that
in the untreated control cells (Fig.
3C).
Cytotoxic effects of SFN in the
anticancer drug-resistant H69AR cell line
The effect of SFN was analyzed in the H69AR cell
lines. First, the minimum dose of AMR, that was found to be
ineffective in the H69AR cell line, when compared with that in the
H69 cell line, was investigated. At a minimum AMR concentration of
20 µM, the rate of cell death was significantly lower in the H69AR
cell line compared with that in the H69 cell line (Fig. S6A). Thereafter, the effects of SFN
were investigated in the H69AR cell line compared with that in AMR.
A significant increase in induced cell death was observed in the
presence of SFN (20 µM) when compared with that in cells treated
with AMR (20 µM) in the H69AR cells (Fig. S6B). Furthermore, the SFN-induced
cell death was significantly inhibited by ferrostatin-1 in the
H69AR cell line (Fig. S6C).
Discussion
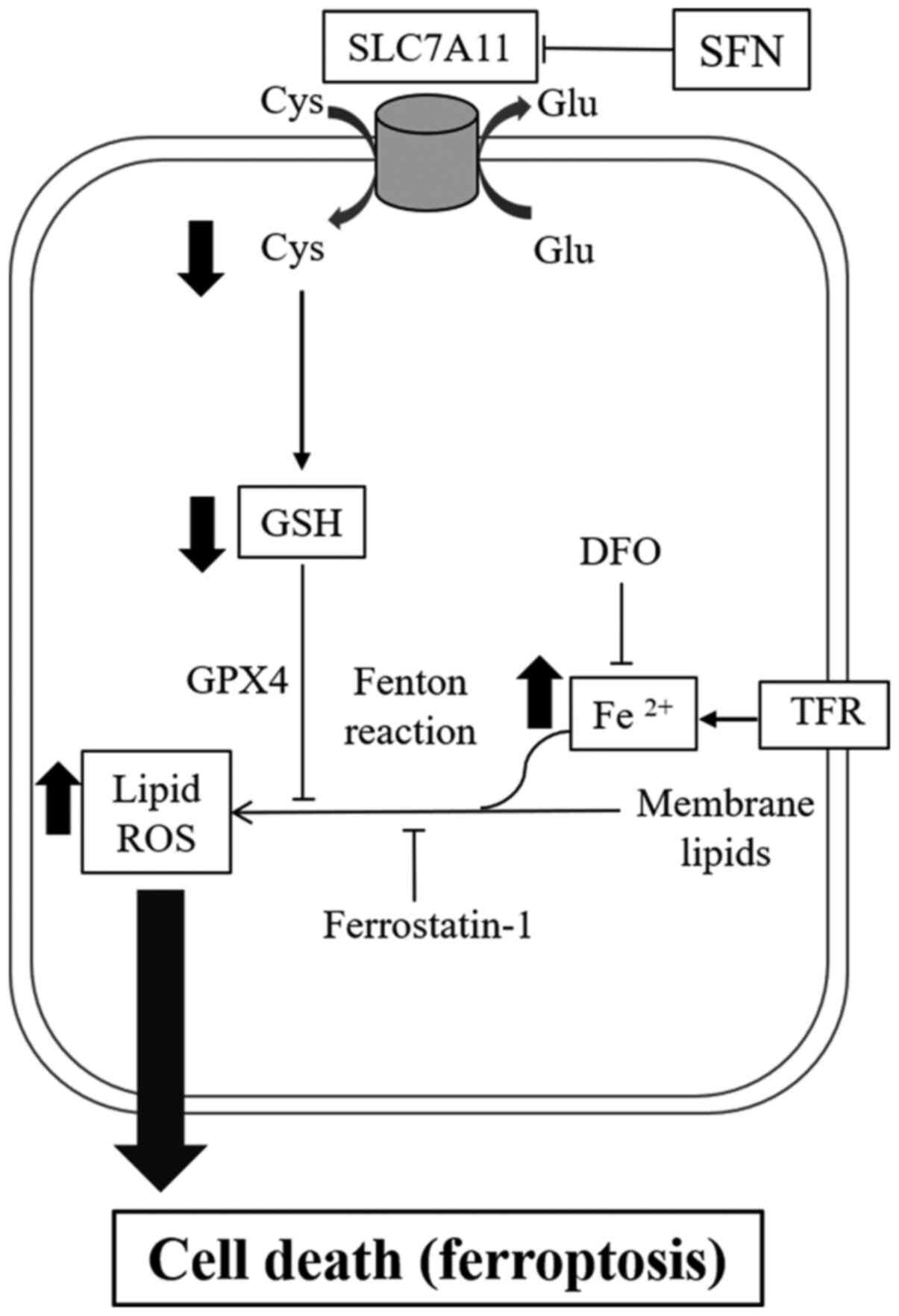
The present study demonstrated that SFN exhibited
anticancer effects by inducing cell death via ferroptosis in the
SCLC cells.
Ferroptosis is triggered by the inactivation of
cellular GSH-dependent antioxidant defenses, leading to
iron-dependent accumulation of toxic lipid ROS (20,31). It
has been reported that ferroptosis is an iron-dependent
non-apoptotic regulated form of cell death that can be inhibited by
ferrostatin-1 and the iron chelator, DFO, but not by apoptosis or
necroptosis inhibitor (z-vad and necrostatin-1, respectively)
(17,20). However, the effect of ferroptosis in
the SCLC cell is still unclear. The present study observed
inhibition of SFN-induced cell death by ferrostatin-1 and DFO, GSH
depletion, elevated intracellular levels of iron and the
accumulation of lipid ROS, which indicated that SFN induced
ferroptosis in SCLC cells.
A number of mechanisms have been reported for
ferroptosis inducers. For example, erastin and sorafenib induced
ferroptosis by inhibition of system xc- (17). RSL3 has also been reported to
directly inhibit GPX4 activity (22). The present study investigated the
mechanisms of SFN-induced ferroptosis in 2 SCLC cell lines.
According to the RT-qPCR and western blot analysis results, the
mRNA and protein expression levels of SLC7A11, a component of
system xc-, were significantly decreased following treatment with
SFN. The present results demonstrated that inhibition of SLC7A11
expression levels, which was triggered by SFN-induced ferroptosis,
resulted in decreased intracellular cystine and GSH and
iron-dependent lipid peroxidation (Fig.
4).
Cytotoxic chemotherapy (e.g. cisplatin, etoposide
and amrubicin) is currently a standard therapy for the treatment of
advanced stage SCLC (32). These
agents induce apoptotic cell death in SCLC cells (33). However, these agents also induce cell
death in non-malignant cells as one of their side effects, which
also include severe bone marrow suppression and pneumonia (34,35).
Therefore, selective and specific drugs for tumor cells are
required for SCLC treatment. A previous report demonstrated that
SFN (15 µM) selectively induced cell cycle arrest and apoptosis in
cancerous prostate epithelial cells (LnCap and PC3), but not in
normal prostate epithelial cells, under the same stimulation
(36). SFN may exhibit different
effects on cell proliferation and death between cancer cells and
non-cancerous cells. It was also confirmed that the cell death
activity was significantly lower in normal bronchial cells (16HBE)
compared with that in SCLC tumor cells (Fig. 1B). The present results indicated that
SFN may exhibit specific anticancer effects in tumor cells, but not
in normal non-cancerous cells in SCLC. Numerous types of cancer
cell, such as breast and prostate cancer, exhibit higher expression
levels of the cystine/glutamate antiporter system xc- compared with
that in non-cancerous cells (18,37,38).
Cystine is an essential amino acid, and its uptake from the
microenvironment is required for growth and progression in cancer
(18). Furthermore, excess iron has
been found to contribute to tumor growth (39), and previous studies have reported
that cancer cells exhibited increased expression levels of
transferrin receptors and iron uptake (40,41). A
previous study reported that serum iron levels were elevated in
smokers (42); the majority of
patients with SCLC were previous or are current smokers (2). Given the high expression levels of
system xc- and excess intracellular iron, we hypothesized that
cancer cells have a greater tendency to undergo ferroptosis
compared with that in non-cancer cells.
Furthermore, it has previously been reported that
tumor cells which exhibit an epithelial-to-mesenchymal transition
(EMT)-like signature were more sensitive to ferroptosis compared
with that in tumor cells, which are not sensitive (43). Zinc finger E-box binding homeobox 1,
which suppresses E-cadherin, has been associated with changes in
lipid accumulation and an increase in sensitivity to ferroptosis
(43). SCLC is highly metastatic and
develops chemoresistance via the EMT process (44). Therefore, ferroptosis may be induced
in SCLC tumor cells by SFN.
The present results indicated that SFN may be used
in novel anticancer strategies for SCLC. Furthermore, SFN
significantly induced cell death compared to AMR in H69AR cells.
SFN-induced cell death in multidrug-resistant H69AR SCLC cells was
not significantly different from that induced in H69 cells. It has
been reported that ferroptosis overcame cisplatin resistance in the
head and neck cancer cells via pharmacological and genetic
inhibition of the cystine/glutamate antiporter (45). Therefore, SFN may be a new potential
adjuvant therapy for patients with SCLC, who have few effective
treatment options, even after resistance to cytotoxic anticancer
drugs occurs.
In the present study, the newly discovered
programmed cell death ‘ferroptosis’ was induced by SFN treatment in
SCLC cells. However, it is still unclear whether the anticancer
effects of SFN was more effective in terms of cell death or cell
cycle arrest. The effect on cell cycle arrest in SCLC cells with
SFN treatment should be investigated further. In addition, although
z-vad did not inhibit SFN-induced cell death, SFN may have induced
caspase-independent apoptosis, as indicated by the increase in the
percentage of SFN-treated cells in the sub-G1 phase, compared with
that in the untreated control cells (Fig. S6). Further studies are required to
investigate the association between SFN-induced ferroptosis and
caspase-independent apoptosis. In addition, SFN-induced detoxifying
effects, that activate Nrf2-driving detoxifying genes have been
previously reported (4). However,
Nrf2 has been shown to be a negative regulator of ferroptosis in
hepatocellular carcinoma (46) and
SFN has been reported to induce different effects in prostate
cancer and non-cancerous cells compared with that in Nrf2 (47). The role of Nrf2 in SFN-induced
ferroptosis in SCLC cells is still unclear. Future studies will
investigate the cell death effect of SFN by considering the
involvement of Nrf2 in SCLC cells.
In conclusion, SFN treatment of SCLC cells led to
ferroptotic cell death via the inhibition of SLC7A11, leading to
decreased GSH and increased lipid ROS levels. These results
indicated that SFN may be a novel potential anticancer agent for
the treatment SCLC via its underlying mechanism of ferroptosis.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Mariko
Esumi (Division of Biochemistry, Department of Biomedical Sciences,
Nihon University School of Medicine, Tokyo, Japan) for offering
advice on the manuscript, and Ms. Ikuko Takeshita, Ms. Kaori Soda
and Ms. Eriko Tsuboi (Division of Respiratory Medicine, Department
of Internal Medicine, Nihon University School of Medicine, Tokyo,
Japan) for their technical assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YI, SMar and YG conceived and designed the study.
YI, TI and MOK performed the experiments. YI, MOK, KM and YN
performed the statistical analysis. YI, MOK, SMar and YN wrote the
paper. SS, MH, MT, KT, SO, TS, NT, SMas and SH contributed to the
analysis and interpretation of data. TS, NT and SMas reviewed the
data. GY and SH supervised the study. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tjong MC, Mak DY, Shahi J, Li GJ, Chen H
and Louie AV: Current Management and Progress in Radiotherapy for
Small Cell Lung Cancer. Front Oncol. 10:11462020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang S, Tang J, Sun T, Zheng X, Li J, Sun
H, Zhou X, Zhou C, Zhang H, Cheng Z, et al: Survival changes in
patients with small cell lung cancer and disparities between
different sexes, socioeconomic statuses and ages. Sci Rep.
7:13392017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takenaka T, Takenoyama M, Inamasu E,
Yoshida T, Toyokawa G, Nosaki K, Hirai F, Yamaguchi M, Shimokawa M,
Seto T, et al: Role of surgical resection for patients with limited
disease-small cell lung cancer. Lung Cancer. 88:52–56. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clarke JD, Dashwood RH and Ho E:
Multi-targeted prevention of cancer by sulforaphane. Cancer Lett.
269:291–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Myzak MC and Dashwood RH: Chemoprotection
by sulforaphane: Keep one eye beyond Keap1. Cancer Lett.
233:208–218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riedl MA, Saxon A and Diaz-Sanchez D: Oral
sulforaphane increases Phase II antioxidant enzymes in the human
upper airway. Clin Immunol. 130:244–251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prochaska HJ, Santamaria AB and Talalay P:
Rapid detection of inducers of enzymes that protect against
carcinogens. Proc Natl Acad Sci USA. 89:2394–2398. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kallifatidis G, Rausch V, Baumann B, Apel
A, Beckermann BM, Groth A, Mattern J, Li Z, Kolb A, Moldenhauer G,
et al: Sulforaphane targets pancreatic tumour-initiating cells by
NF-kappaB-induced antiapoptotic signalling. Gut. 58:949–963. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Żuryń A, Litwiniec A, Safiejko-Mroczka B,
Klimaszewska-Wiśniewska A, Gagat M, Krajewski A, Gackowska L and
Grzanka D: The effect of sulforaphane on the cell cycle, apoptosis
and expression of cyclin D1 and p21 in the A549 non-small cell lung
cancer cell line. Int J Oncol. 48:2521–2533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SH, Park HJ and Moon DO: Sulforaphane
sensitizes human breast cancer cells to paclitaxel-induced
apoptosis by downregulating the NF-κB signaling pathway. Oncol
Lett. 13:4427–4432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh SV, Srivastava SK, Choi S, Lew KL,
Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, et
al: Sulforaphane-induced cell death in human prostate cancer cells
is initiated by reactive oxygen species. J Biol Chem.
280:19911–19924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jackson SJ, Singletary KW and Venema RC:
Sulforaphane suppresses angiogenesis and disrupts endothelial
mitotic progression and microtubule polymerization. Vascul
Pharmacol. 46:77–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jee HG, Lee KE, Kim JB, Shin HK and Youn
YK: Sulforaphane inhibits oral carcinoma cell migration and
invasion in vitro. Phytother Res. 25:1623–1628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shan Y, Zhang L, Bao Y, Li B, He C, Gao M,
Feng X, Xu W, Zhang X and Wang S: Epithelial-mesenchymal
transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail,
ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J
Nutr Biochem. 24:1062–1069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao M, Yi J, Zhu J, Minikes AM, Monian P,
Thompson CB and Jiang X: Role of mitochondria in ferroptosis. Mol
Cell. 73:354–363.e3. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lo M, Wang YZ and Gout PW: The x(c)-
cystine/glutamate antiporter: A potential target for therapy of
cancer and other diseases. J Cell Physiol. 215:593–602. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao JY and Dixon SJ: Mechanisms of
ferroptosis. Cellular and molecular life sciences : CMLS.
73:2195–2209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu B, Chen XB, Ying MD, He QJ, Cao J and
Yang B: The role of ferroptosis in cancer development and treatment
response. Front Pharmacol. 8:9922017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Louandre C, Ezzoukhry Z, Godin C, Barbare
J-C, Mazière J-C, Chauffert B and Galmiche A: Iron-dependent cell
death of hepatocellular carcinoma cells exposed to sorafenib. Int J
Cancer. 133:1732–1742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shin D, Lee J, You JH, Kim D and Roh JL:
Dihydrolipoamide dehydrogenase regulates cystine
deprivation-induced ferroptosis in head and neck cancer. Redox
Biol. 30:1014182020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma S, Henson ES, Chen Y and Gibson SB:
Ferroptosis is induced following siramesine and lapatinib treatment
of breast cancer cells. Cell Death Dis. 7:e23072016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Slee EA, Zhu H, Chow SC, MacFarlane M,
Nicholson DW and Cohen GM: Benzyloxycarbonyl-Val-Ala-Asp (OMe)
fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the
processing of CPP32. Biochem J. 315:21–24. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Degterev A, Huang Z, Boyce M, Li Y, Jagtap
P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA and Yuan J:
Chemical inhibitor of nonapoptotic cell death with therapeutic
potential for ischemic brain injury. Nat Chem Biol. 1:112–119.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukami T, Iida A, Konishi K and Nakajima
M: Human arylacetamide deacetylase hydrolyzes ketoconazole to
trigger hepatocellular toxicity. Biochem Pharmacol. 116:153–161.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahn CH, Hong KO, Jin B, Lee WW, Jung YC,
Lee H, Shin J-A, Cho S-D and Hong SD: Contribution of p38 MAPK
pathway to norcantharidin-induced programmed cell death in human
oral squamous cell carcinoma. Int J Mol Sci. 20:34872019.
View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Torii S, Shintoku R, Kubota C, Yaegashi M,
Torii R, Sasaki M, Suzuki T, Mori M, Yoshimoto Y, Takeuchi T, et
al: An essential role for functional lysosomes in ferroptosis of
cancer cells. Biochem J. 473:769–777. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Demedts IK, Vermaelen KY and van Meerbeeck
JP: Treatment of extensive-stage small cell lung carcinoma: Current
status and future prospects. Eur Respir J. 35:202–215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang JY: Apoptosis-based anticancer
drugs. Nat Rev Drug Discov. 1:101–102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fisher MD and D'Orazio A; CIG Media Group,
: Irinotecan and cisplatin versus etoposide and cisplatin in
small-cell lung cancer: JCOG 9511. Clin Lung Cancer. 2:23–24. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Erasmus JJ, McAdams HP and Rossi SE:
Drug-induced lung injury. Semin Roentgenol. 37:72–81. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Clarke JD, Hsu A, Yu Z, Dashwood RH and Ho
E: Differential effects of sulforaphane on histone deacetylases,
cell cycle arrest and apoptosis in normal prostate cells versus
hyperplastic and cancerous prostate cells. Mol Nutr Food Res.
55:999–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. Oct 1–2020.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lo M, Ling V, Wang YZ and Gout PW: The xc-
cystine/glutamate antiporter: A mediator of pancreatic cancer
growth with a role in drug resistance. Br J Cancer. 99:464–472.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Manz DH, Blanchette NL, Paul BT, Torti FM
and Torti SV: Iron and cancer: Recent insights. Ann N Y Acad Sci.
1368:149–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Daniels TR, Bernabeu E, Rodríguez JA,
Patel S, Kozman M, Chiappetta DA, Holler E, Ljubimova JY, Helguera
G and Penichet ML: The transferrin receptor and the targeted
delivery of therapeutic agents against cancer. Biochim Biophys
Acta. 1820:291–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shibata Y, Inoue S, Igarashi A, Yamauchi
K, Abe S, Aida Y, Nunomiya K, Sato M, Nakano H, Sato K, et al:
Elevated serum iron is a potent biomarker for spirometric
resistance to cigarette smoke among Japanese males: The Takahata
study. PLoS One. 8:e740202013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Viswanathan VS, Ryan MJ, Dhruv HD, Gill S,
Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada
K, Aguirre AJ, et al: Dependency of a therapy-resistant state of
cancer cells on a lipid peroxidase pathway. Nature. 547:453–457.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Krohn A, Ahrens T, Yalcin A, Plönes T,
Wehrle J, Taromi S, Wollner S, Follo M, Brabletz T, Mani SA, et al:
Tumor cell heterogeneity in Small Cell Lung Cancer (SCLC):
Phenotypical and functional differences associated with
Epithelial-Mesenchymal Transition (EMT) and DNA methylation
changes. PLoS One. 9:e1002492014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Roh JL, Kim EH, Jang HJ, Park JY and Shin
D: Induction of ferroptotic cell death for overcoming cisplatin
resistance of head and neck cancer. Cancer Lett. 381:96–103. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sestili P and Fimognari C: Cytotoxic and
antitumor activity of sulforaphane: The role of reactive oxygen
species. BioMed Res Int. 2015:4023862015. View Article : Google Scholar : PubMed/NCBI
|