Introduction
Breast cancer is the most common malignancy in women
worldwide, whereby 2,088,849 new cases of invasive breast cancer
and 626,679 mortalities were reported in 2018 (1,2). Tumor
invasion and metastasis affect >90% of patients with breast
cancer, and thus notably contribute to the high mortality rate
(3–5). This metastatic disease remains
incurable, and effective treatment for end-stage metastatic breast
cancer are yet to be determined (6–8). The
aggressiveness of a tumor is closely associated with its ability to
evade natural barriers, to invade adjacent tissues and metastasize
distant sites (9). The metastatic
cascade is a multistep process where cancer cells escape from the
primary tumor site to distant locations, where they can potentially
establish new cancer colonies (10,11).
Under optimal conditions, epithelial cancer cells detach from the
primary tumor site, penetrate and migrate via peripheral
circulation, and invade secondary sites, where they ultimately
undergo extravasation and populate distant organs (11,12).
Proteolytic degradation of the basement membrane and
extracellular matrix (ECM) is considered a crucial aspect of
metastatic growth, which enables low anchorage of neoplastic cells
(13–17). Several cell-secreted proteolytic
enzymes, including matrix metalloproteinases (MMPs) are implicated
in the cleavage of ECM (13,18,19).
Matrix metalloproteinase 9 (MMP9) is a member of the gelatinase
subfamily of MMPs and is secreted by a variety of cell types in an
inactive form that undergoes activation upon cleavage by different
types of extracellular proteases (18,20).
MMP9 activity is modulated via different biochemical molecules,
including growth factors and cytokines (19,21).
Notably, MMP9 is actively involved in the degradation of type IV
collagen, which is a crucial component of the basement membrane
(19,22). In addition, MMP9 facilitates the
dissemination machinery, and is particularly involved in tumour
invasion, tumour-induced angiogenesis, and immunomodulation of the
tumour microenvironment, where it is implicated in the formation of
so-called premetastatic niches (23,24).
Previous studies have focused on the association between high MMP9
expression and the number of distant metastases in patients with
breast cancer (25–27), as well as poor prognosis (28,29). It
has been speculated that circulating tumor cells (CTCs), which are
responsible for distant metastasis formation, use MMPs to form new
metastatic sites (19,30). In addition, a previous study
demonstrated that elevated MMP1 expression is significantly
associated with the presence of CTC_ epithelial-to-mesenchymal
transition (EMT) cells in the peripheral blood of patients with
primary breast cancer (PBC), as well as with poor prognostic
features of their primary tumors (31). The present study aimed to assess MMP9
expression in tumor cells as well as tumor associated stroma of
patients with PBC, and determine its association with the presence
of CTCs in the peripheral blood of these patients and other
clinicopathological characteristics. The prognostic value of MMP9
in patients with PBC was also assessed.
Patients and methods
Study patients
The present study (Protocol TRU-SK 002; Chair:
Michal Mego) enrolled 318 patients with stages I–III PBC who
underwent definitive surgery. The samples were collected from the
National Cancer Institute (Bratislava, Slovakia) between March 2012
and February 2015. The paraphing embedded tumor tissue and CTCs
status in peripheral blood were available for all patients included
in the present study. Complete diagnostic evaluation was performed
in all patients to exclude the presence of distant metastasis.
Patients with concurrent malignancy in the last 5 years, other than
non-melanoma skin cancer, were excluded from the present study. The
clinicopathological data including age, tumor stage, histology,
regional lymph node involvement, hormone receptor status and HER2
status were retrieved and tabulated from the patients' records
after obtaining all the relevant ethical approvals. Breast cancer
subtypes were identified by immunohistochemical staining (see
below) and classified according to the ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up for early breast
cancer (32).
The present study was reviewed and approved by the
Institutional Review Board of the National Cancer Institute of
Slovakia, Bratislava, Slovakia (TRUSK002, 20.6.2011). Written
informed consent was provided by all patients prior to the study
start.
Tumor pathology
Pathological review was performed at the Department
of Patholoχgy, Faculty of Medicine, Comenius University,
(Bratislava, Slovakia) by an experienced pathologist (ZC).
Tumor samples and tissue microarray
construction
Tumor specimens used in the present study were
classified according to the 2019 World Health Organization
classification (33). According to
the tumor histology results, one or two representative areas
containing the most representative part of the hematoxylin and
eosin (H&E) stained tumor tissues were observed under a light
microscope, original magnification×400. The identified sections
were matched to their corresponding wax blocks (donor blocks). The
3-mm diameter cores of the tumors were removed from the donor
blocks using the multipurpose sampling tool HarrisUni-Core
(Sigma-Aldrich; Merck KGaA) and inserted into the recipient master
block. The recipient block was cut into 5-µm-thick sections, which
were transferred onto coated slides.
Immunohistochemical (IHC)
staining
Deparaffinized slides were rehydrated in phosphate
buffered saline solution (10 mM, pH 7.2). Tissue epitopes were
demasked using the automated water bath heating process in Dako PT
Link (Dako; Agilent Technologies, Inc.) and the slides were
incubated in pH 6.0 citrate retrieval buffer at 98°C for 20 min.
The slides were subsequently incubated for 1 h at room temperature
with primary mouse monoclonal antibody against MMP9 (Abcam; MMP9
(SB15c); cat. no. ab51203) diluted 1:200 in Dako REAL antibody
diluent (Dako; Agilent Technologies, Inc.) and immunostained with
anti-mouse/anti-rabbit immuno-peroxidase polymer (EnVision
FLEX/HRP, Dako; Agilent Technologies, Inc.) for 30 min at room
temperature, according to the manufacturer's instructions. The
reaction was visualized using diaminobenzidine substrate-chromogen
solution (Dako; Agilent Technologies, Inc.) for 5 min, and the
slides were counterstained with hematoxylin. The human clone tissue
served as the positive control, and colon tissue subjected to the
same procedure omitting the primary antibody was used as the
negative control.
IHC evaluation
Tumor scores were blindly assessed by a pathologist
(ZC). The results of the IHC analyzes were scored using a weighted
histoscore (WH), assessing both the percentage of positive cells
(PP) and the staining intensity (SI) of the cytoplasm as follows:
The proportion of cells with nuclear staining was multiplied by the
intensity of staining to provide a histoscore ranging from 0–300.
The histoscore was calculated as follows: Score=(0× percentage not
stained) + (1× percentage weakly stained) + (2× percentage
moderately stained) + (3× percentage strongly stained) (34). The mid-point of WH histoscore was
used as the cut-off criterion similary as previosly (35,36).
MMP9 expression was stratified as low vs. high, according to the
cut-off value of WH histoscore (150).
Detecting CTCs in peripheral
blood
CTCs were identified via reverse
transcription-quantitative (RT-q)PCR analysis. Enrichment of CTC
from peripheral blood by depleting CD45+ cells was performed using
the Rossette Sep™ kit (15162; Stemcell Technologies, Inc.), as
previously described (37,38). Briefly, RNA isolated from
CD45-depleted peripheral blood samples were transcribed into cDNA,
which was subjected to RT-qPCR analysis to assess the expression
levels of epithelial-to-mesenchymal transition (EMT-TF) genes,
including TWIST1, SNAIL1, SLUG and ZEB1. Compared with healthy
donors, patient samples with higher EMT-TF gene transcript levels
were classified as CTC EMT positive, based on the preclinical study
and human sample testing. The highest expression values in healthy
donors were used as a cut-off value to determine CTC positivity
(39).
Statistical analysis
Patient characteristics were summarized using the
median (range) values for continuous variables and frequency
(percentage) for categorical variables. The distribution of MMP9
histoscore was significantly different from the normal distribution
(Shapiro-Wilk test), thus non-parametric tests were used for
analyses. Mann-Whitney U test was used to compare the differences
in distributions of MMP9 expression between two groups of patients
with PBC, whereby MMP9 expression was categorized as absent or
present. Fisher's exact test or the χ2 test were used where
appropriate.
The median follow-up period was estimated as a
median observation time among all patients and among those still
alive at the time of their last follow-up. Disease-free survival
(DFS) was calculated from the date of CTCs measurement to the date
of disease recurrence (locoregional or distant), secondary cancer,
death or last follow-up. DFS was estimated using the Kaplan-Meier
product limit method and log-rank test. Two-sided P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using NCSS 11 statistical
software (2016; NCSS, LLC.; ncss.com/software/ncss).
Results
Patient characteristics
The present study enrolled 318 patients with PBC.
The median age of the assessed cohort was 60 years (age range,
25–83 years). The majority of patients had node negative (60.1%)
and hormone positive (83.6%) tumors; 48/318 patients (15.1%) had a
HER-2/neu amplified status. Patient characteristics are summarized
in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | n (%) |
|---|
| All patients | 318 (100.0) |
| Histology |
|
|
Invasive ductal carcinoma | 272 (85.5) |
|
Invasive lobular
carcinoma | 32 (10.1) |
| Other
histological subtypes | 14 (4.4) |
| Grade |
|
| Low and
intermediate | 200 (62.9) |
| High
grade | 110 (34.6) |
|
Unknown | 8 (2.5) |
| T stage |
|
| T1 | 218 (68.6) |
| T2 and
more | 100 (31.4) |
| N stage |
|
| N0 | 191 (60.1) |
|
N1mi | 10 (3.1) |
| N1 | 68 (21.4) |
| N2 | 27 (8.5) |
| N3 | 19 (6.0) |
|
Unknown | 3 (0.9) |
| Hormone receptor
status (cut-off 1%) |
|
|
Negative for both | 52 (16.4) |
|
Positive for either | 266 (83.6) |
| HER2 status |
|
|
Negative | 270 (84.9) |
|
Positive | 48 (15.1) |
| Ki67 status |
|
|
<20% | 189 (59.4) |
| ≥
20% | 128 (40.3) |
|
Unknown | 1 (0.3) |
| Molecular
subtype |
|
| Luminal
A | 166 (52.2) |
| Luminal
B | 99 (31.1) |
|
HER2+ | 13 (4.1) |
| Triple
negative | 39 (12.3) |
|
Unknown | 1 (0.3) |
| P53 status |
|
|
Negative | 193 (60.7) |
|
Positive | 124 (39.0) |
|
Unknown | 1 (0.3) |
| BCL-2 status |
|
|
Negative | 92 (28.9) |
|
Positive | 225 (70.8) |
|
Unknown | 1 (0.3) |
| CTC EP |
|
|
Negative | 235 (73.9) |
|
Positive | 27 (8.5) |
| CTC EMT |
|
|
Negative | 235 (73.9) |
|
Positive | 56 (17.6) |
| CTC any |
|
|
Negative | 235 (73.9) |
|
Positive | 83 (26.1) |
CTCs detection
To establish overexpression of the EMT-inducing TF
gene transcripts in patients with PBC, the expression levels were
compared between patient samples and healthy donors, as previously
described (39). Among the patient
samples, CTCs were detected in 83 patients (26.1%). CTCs with only
epithelial markers were detected in the peripheral blood of 34
patients (10.7%), while CTCs with an EMT phenotype were present in
56 patients (17.6%).
Association between MMP9 expression,
and patients/tumor characteristic and CTCs
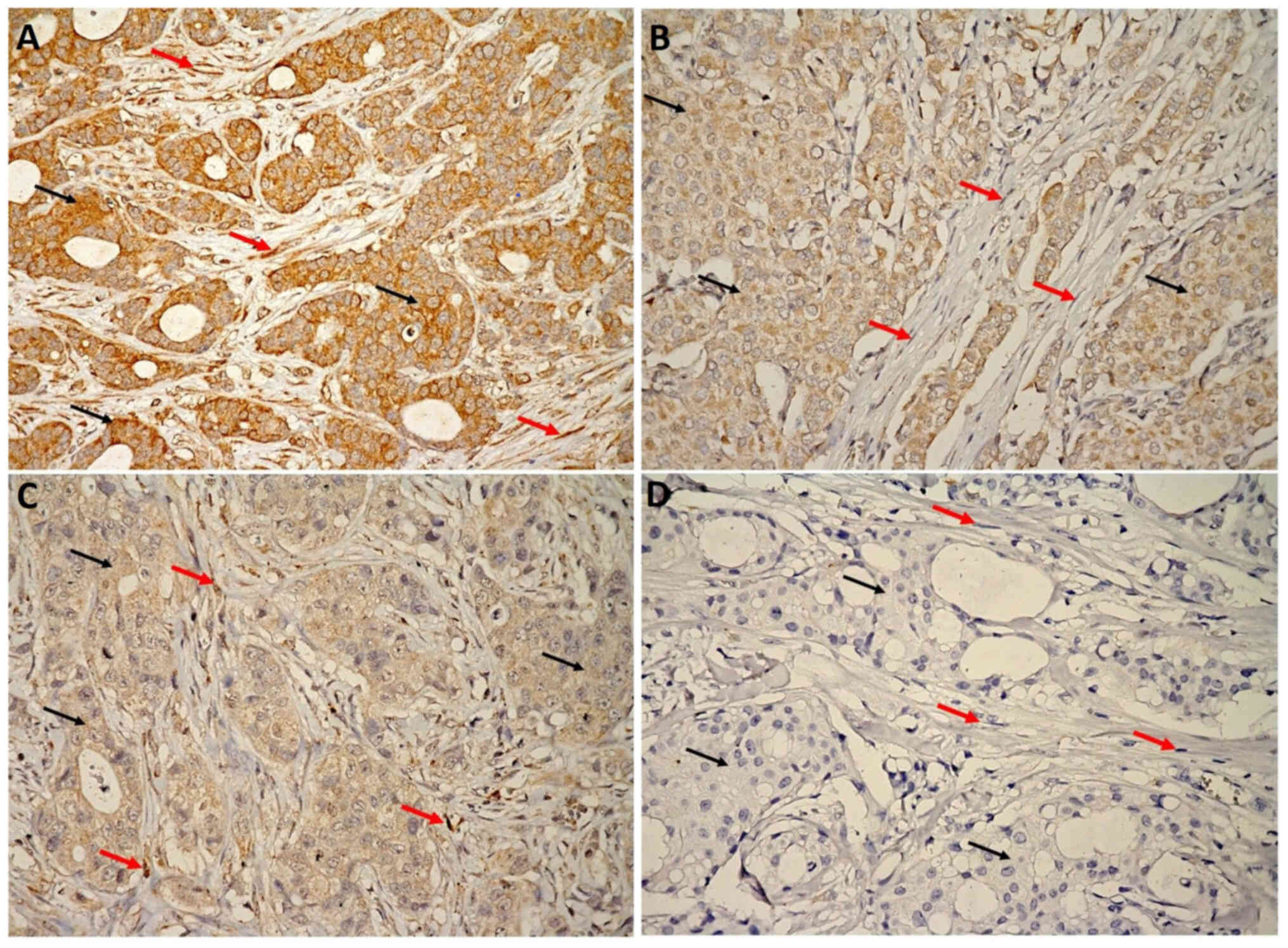
MMP9 protein expression in tumor cells was assessed
in all patients (n=318) (Fig. 1).
However, pathologists were unable to detect stromal cells in 9/318
tumor tissues due to the small sample size, which only constituted
tumor cells. Thus, MMP9 expression in stroma was only assessed in
309 patients. MMP9 expression intensity at least 1+ and higher was
detected in 255 samples (80.2%) in breast tumor cells and in 307
samples (99.4%) of tumor associated stroma (P<0.00001). The mean
WH ± standard error of the mean (SEM) for MMP9 expression was
significantly higher in breast tumor cells compared with tumor
associated stroma (132.0±5.2 vs. 50.8±3.7; P<0.00001). The
association between MMP9 expression in tumor cells and
clinicopathological characteristics, as well as its association
with CTCs are presented in Table
II. The results demonstrated that elevated MMP9 expression was
significantly associated with EP/PR positive breast cancer cells
(mean WH ± SEM=137.6±5.6 vs. 103.4±12.8, P=0.011) and low
proliferating tumors (Ki67 <20%) (mean WH ± SEM=141.1±6.7 vs.
117.9±8.1, P=0.018), while elevated MMP9 expression in tumor
associated stroma was associated with hormone receptor (EP/PR)
status (mean WH ± SEM=54.6±4.0 vs. 30.7±9.1, P=0.021) (Table III). In our analysis, there was
found any association between MMP9 expression in breast cancer
cells, or in tumor associated stroma and CTCs.
 | Table II.Association between MMP9 expression
in tumour cells, patients, tumour characteristics and circulating
tumor cells. |
Table II.
Association between MMP9 expression
in tumour cells, patients, tumour characteristics and circulating
tumor cells.
|
| MMP9 expression in
tumor cells |
|---|
|
|
|
|---|
| Characteristic | N | Mean | SEM | Median | P-value |
|---|
| MMP9
expression |
| weighted
histoscore | 318 | 132.0 | 5.2 | 150 | NA |
| Histology |
|
|
|
| 0.081 |
|
Invasive ductal carcinoma | 272 | 136.0 | 5.6 | 150 |
|
|
Other | 46 | 108.6 | 13.6 | 100 |
|
| Grade |
|
|
|
| 0.242 |
| Low and
intermediate | 200 | 135.1 | 6.5 | 150 |
|
| High
grade | 110 | 130.1 | 8.8 | 110 |
|
|
Unknown | 8 | 80.0 | 32.7 | 0 |
|
| T-stage |
|
|
|
| 0.163 |
| T1 | 218 | 136.4 | 6.3 | 150 |
|
|
>T1 | 100 | 122.6 | 9.3 | 100 |
|
| N stage |
|
|
|
| 0.468 |
| N0 | 201 | 135.4 | 6.5 | 150 |
|
|
N+ | 114 | 127.1 | 8.7 | 150 |
|
|
Unknown | 3 | 90.0 | 53.6 | 100 |
|
| Hormone receptor
status (cut-off 1%) |
|
|
|
| 0.011 |
|
Negative for both | 52 | 103.4 | 12.8 | 100 |
|
|
Positive for either | 266 | 137.6 | 5.6 | 150 |
|
| HER2 status |
|
|
|
| 0.792 |
|
Negative | 270 | 131.4 | 5.6 | 150 |
|
|
Positive | 48 | 135.7 | 13.4 | 150 |
|
| Ki67 status
(cut-off 20%) |
|
|
|
| 0.018 |
|
<20% | 189 | 141.0 | 6.7 | 170 |
|
|
≥20% | 128 | 117.9 | 8.1 | 110 |
|
|
Unknown | 1 | 250.0 | 92.0 | 250 |
|
| Molecular
subtype |
|
|
|
| 0.0711 |
| Luminal
A | 166 | 138.6 | 7.2 | 160 |
|
| Luminal
B | 99 | 134.8 | 9.3 | 150 |
|
|
HER2+ | 13 | 106.2 | 25.6 | 100 |
|
| Triple
negative | 39 | 102.4 | 14.8 | 100 |
|
|
Unknown | 1 | 250.0 | 92.3 | 250 |
|
| P53 status |
|
|
|
| 0.632 |
|
Negative | 193 | 131.1 | 6.7 | 150 |
|
|
Positive | 124 | 134.1 | 8.3 | 150 |
|
|
Unknown | 1 | 50.0 | 92.9 | 50 |
|
| BCL-2 |
|
|
|
| 0.229 |
|
Negative | 92 | 126.0 | 9.7 | 110 |
|
|
Positive | 225 | 134.0 | 6.2 | 150 |
|
|
Unknown | 1 | 250.0 | 92.7 | 250 |
|
| CTC EP |
|
|
|
| 0.851 |
|
Negative | 235 | 134.1 | 6.0 | 150 |
|
|
Positive | 27 | 138.1 | 17.5 | 150 |
|
| CTC EMT |
|
|
|
| 0.300 |
|
Negative | 235 | 134.1 | 6.1 | 150 |
|
|
Positive | 56 | 120.4 | 12.4 | 135 |
|
| CTC any |
|
|
|
| 0.472 |
|
Negative | 235 | 134.1 | 6.1 | 150 |
|
|
Positive | 83 | 126.1 | 10.2 | 150 |
|
 | Table III.Association between MMP9 expression
in stromal cells, patients, tumour characteristics and circulating
tumor cells. |
Table III.
Association between MMP9 expression
in stromal cells, patients, tumour characteristics and circulating
tumor cells.
|
| MMP9 expression in
stromal cells |
|---|
|
|
|
|---|
| Characteristic | N | Mean | SEM | Median | P-value |
|---|
| MMP9
expression |
| weighted
histoscore | 309 | 50.8 | 3.7 | 25 | NA |
| Histology |
|
|
|
| 0.434 |
|
Invasive ductal carcinoma | 266 | 51.9 | 4.0 | 30 |
|
|
Other | 43 | 44.2 | 9.8 | 20 |
|
| Grade |
|
|
|
| 0.489 |
| Low and
intermediate | 194 | 50.9 | 4.6 | 30 |
|
| High
grade | 108 | 51.7 | 6.2 | 20 |
|
|
Unknown | 7 | 34.3 | 24.4 | 0 |
|
| T-stage |
|
|
|
| 0.469 |
| T1 | 213 | 52.6 | 4.4 | 25 |
|
|
>T1 | 96 | 46.8 | 6.6 | 25 |
|
| N stage |
|
|
|
| 0.536 |
| N0 | 197 | 54.9 | 4.6 | 30 |
|
|
N+ | 110 | 43.5 | 6.1 | 20 |
|
|
Unknown | 2 | 50.0 | 45.6 | 50 |
|
| Hormone receptor
status (cut-off 1%) |
|
|
|
| 0.021 |
|
Negative for both | 49 | 30.7 | 9.1 | 5 |
|
|
Positive for either | 260 | 54.6 | 4.0 | 30 |
|
| HER2 status |
|
|
|
| 0.872 |
|
Negative | 263 | 50.7 | 4.0 | 20 |
|
|
Positive | 46 | 51.6 | 9.5 | 30 |
|
| Ki67 status
(cut-off 20%) |
|
|
|
| 0.137 |
|
<20% | 183 | 56.3 | 4.8 | 30 |
|
|
≥20% | 126 | 42.9 | 5.7 | 20 |
|
| Molecular
subtype |
|
|
|
| 0.094 |
| Luminal
A | 163 | 57.0 | 68.7 | 30 |
|
| Luminal
B | 97 | 50.5 | 62.0 | 30 |
|
|
HER2+ | 12 | 15 | 24.3 | 0 |
|
| Triple
negative | 37 | 35.8 | 55.7 | 5 |
|
| P53 status |
|
|
|
| 0.537 |
|
Negative | 188 | 48.1 | 4.7 | 30 |
|
|
Positive | 120 | 55.5 | 5.9 | 20 |
|
|
Unknown | 1 | 0.0 | 64.5 | 0 |
|
| BCL-2 |
|
|
|
| 0.995 |
|
Negative | 88 | 47.9 | 6.9 | 30 |
|
|
Positive | 221 | 52.0 | 4.3 | 20 |
|
| CTC EP |
|
|
|
| 0.350 |
|
Negative | 229 | 54.4 | 4.1 | 30 |
|
|
Positive | 27 | 41.3 | 12.8 | 20 |
|
| CTC EMT |
|
|
|
| 0.400 |
|
Negative | 229 | 54.4 | 4.1 | 30 |
|
|
Positive | 53 | 40.3 | 10 | 10 |
|
| CTC any |
|
|
|
| 0.168 |
|
Negative | 229 | 54.4 | 4.1 | 30 |
|
|
Positive | 80 | 40.6 | 7.2 | 20 |
|
Prognostic value of MMP9 in PBC
The median follow-up time was 54.9 months (range,
0.2–76.6 months). In the assessed cohort, 61 patients (19.2%)
experienced a disease progression during follow-up. Among the
subgroup of patients where MMP9 expression in tumor associated
stroma was assessed (n=309), the median follow-up time was 55.3
months (range, 0.2–76.6), and 59 patients (19.1%) experienced a DFS
event. Due to insufficiency of overall survival data, only DFS data
are presented in the present study.
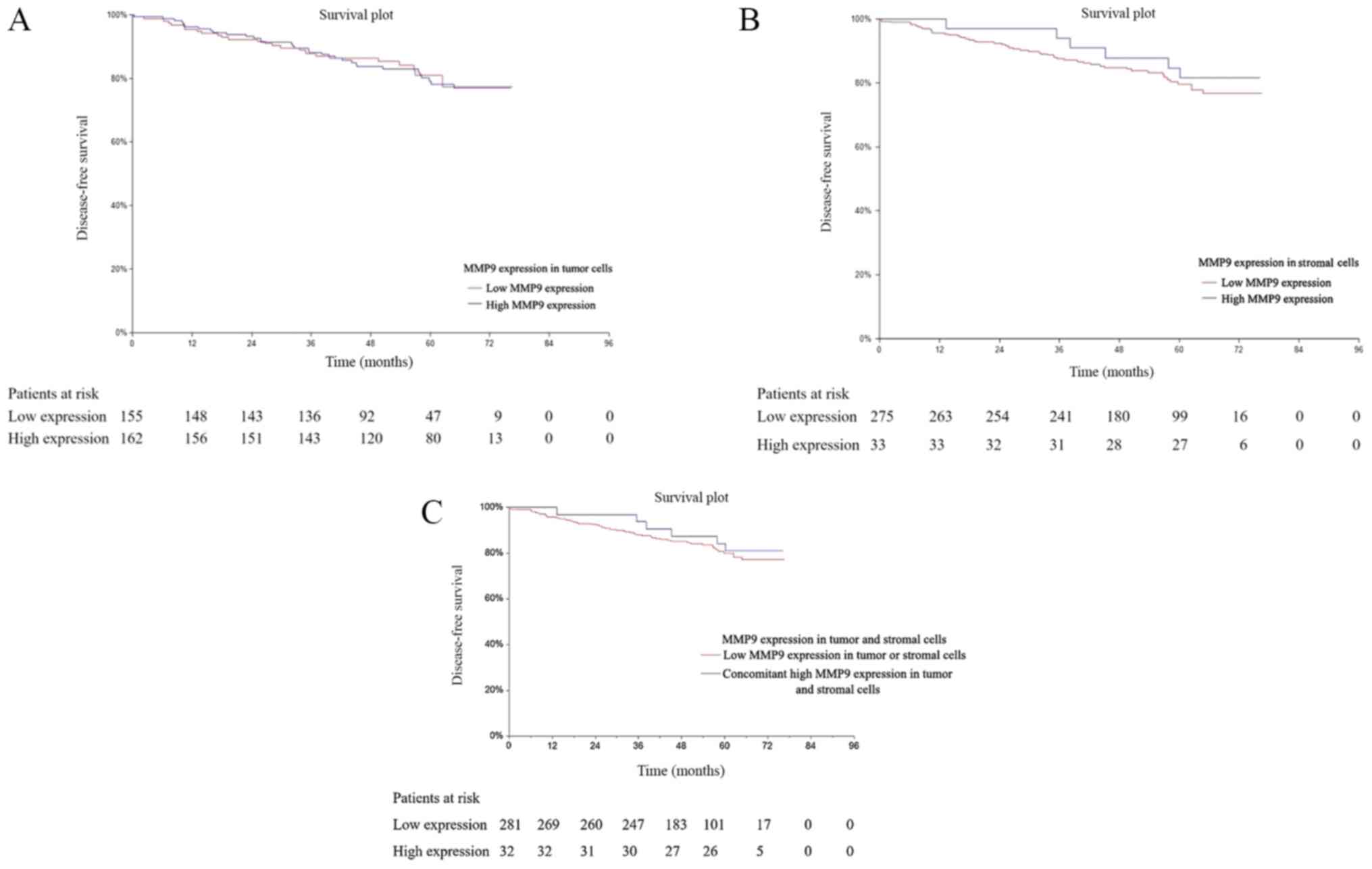
Univariate analysis was performed to determine the
prognostic value of MMP9 in PBC cells [hazard ratio (HR)=0.96; 95%
confidence interval (CI), 0.58–1.59; P=0.864; Fig. 2A], as well as in tumor associated
stroma (HR=1.29; 95% CI, 0.60–2.78; P=0.547; Fig. 2B). Exploratory subgroup analysis was
performed to determine a potential subgroup-related prognostic
value of MMP9 (Tables IV and
V). In addition, also the univariate
analysis in group of patients with concomitant high MMP9 expression
in tumor and stromal cells was carried out. However, no prognostic
value was found using this analysis (HR=1.27, 95% CI 0.59–2.75,
P=0.573) (Fig. 2C). The results
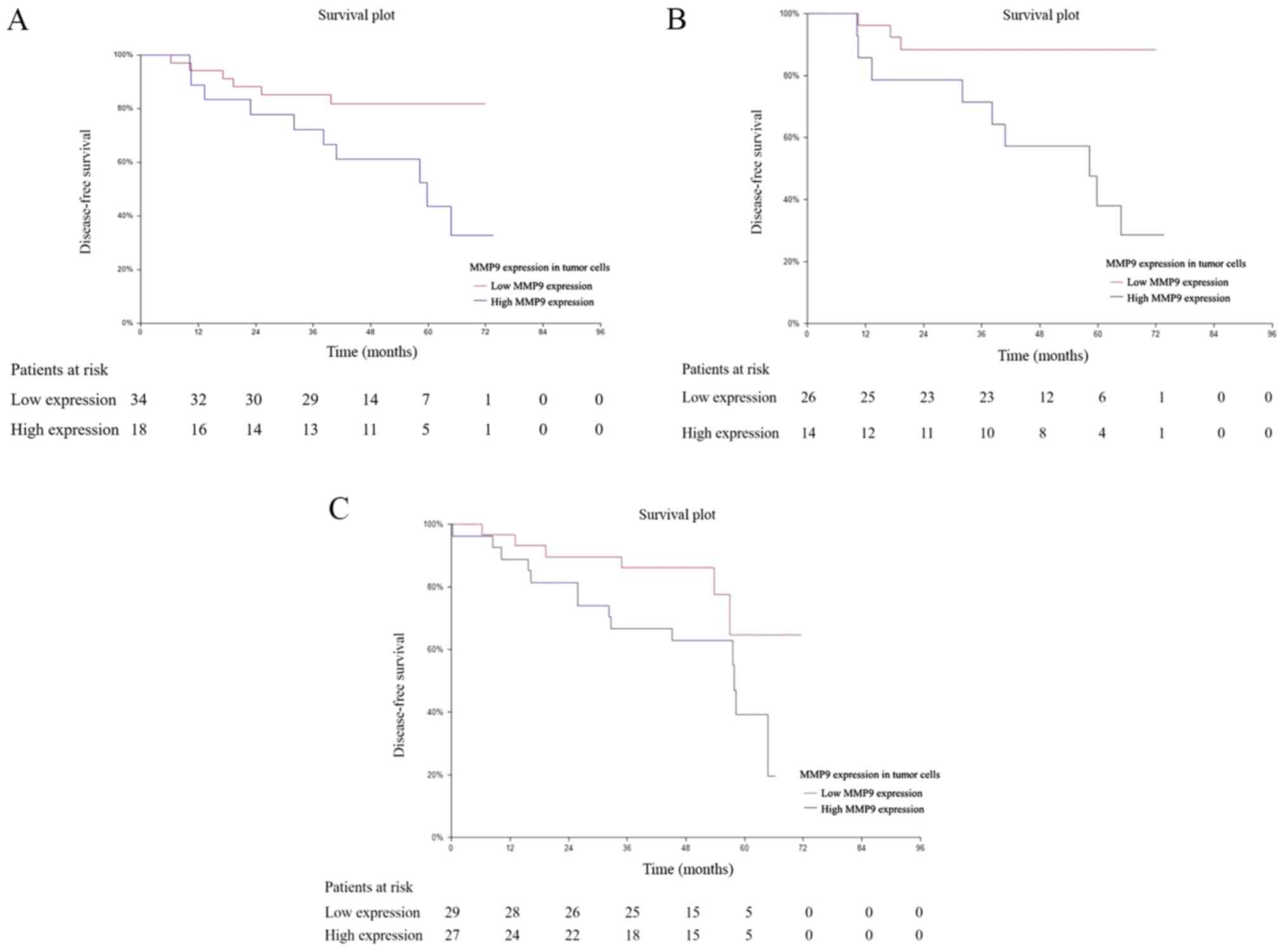
demonstrated that low MMP9 expression in tumor cells was associated
with better DFS in hormone receptor (ER/PR) negative and triple
negative patients with PBC (HR=0.33; 95% CI, 0.12–0.93; P=0.025;
Fig. 3A) and (HR=0.17; 95% CI,
0.05–0.57; P=0.003; Fig. 3B),
respectively. Notably, the prognostic value of MMP9 in tumor cells
was also observed in the CTC_EMT-positive subgroup of patients
(HR=0.40; 95% CI, 0.16–0.95; P=0.047; Fig. 3C). Among the subgroup of patients
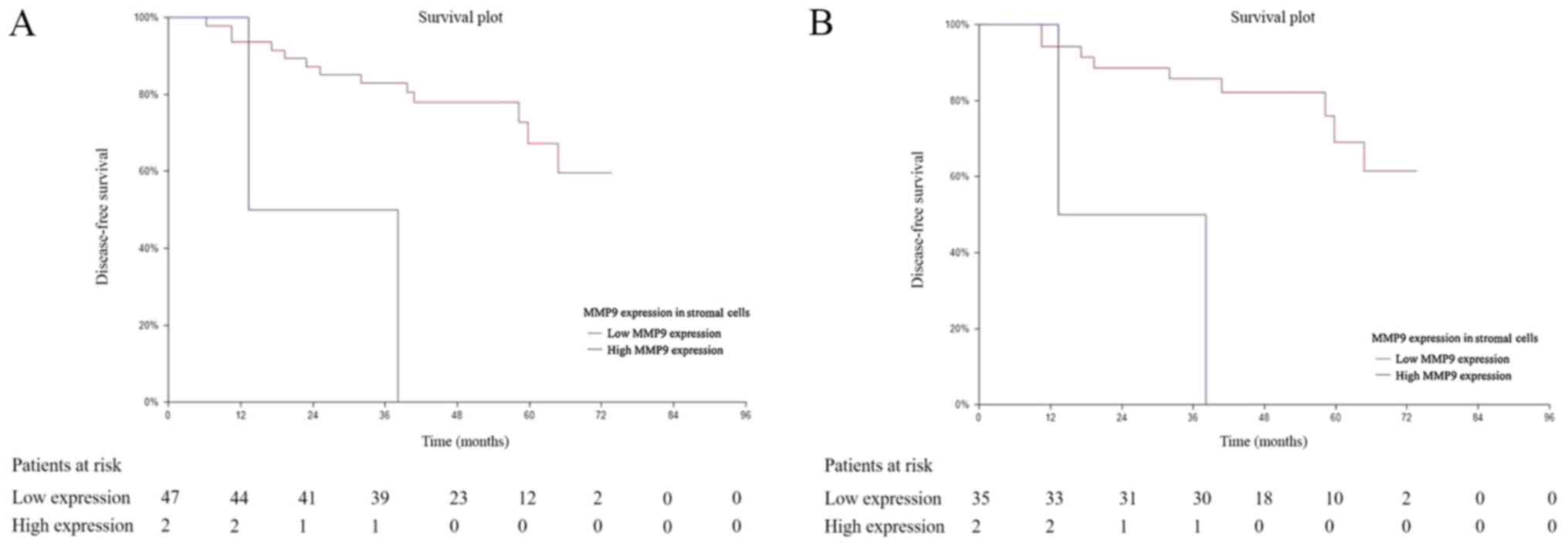
where MMP9 expression in tumor associated stroma was assessed, the
prognostic value of MMP9 was observed in the hormone receptor
(ER/PR) negative subgroup of patients (HR=0.14; 95% CI, 0.00–4.81;
P=0.002; Fig. 4A), triple negative
(HR=0.12; 95% CI, 0.00–4.89; P=0.001; Fig. 4B). In addition, among the subgroup of
CTC_EMT positive patients was progression of the disease documented
in 1 of 2 patients with high MMP9 expression in stromal cells
compared to 22 of 51 patients with low MMP9 expression within
4-years follow up. In subgroup of the CTC_EP positive patients 2 of
4 patient with high MMP9 expression in stromal cells experienced
progression of disease compared to 6 of 22 patient with low MMP9
expression after 4-years follow up.
 | Table IV.Univariate analysis for disease-free
survival according to MMP9 expression in tumor cells. |
Table IV.
Univariate analysis for disease-free
survival according to MMP9 expression in tumor cells.
| Characteristic | N | HR | 95% Low CI | 95% High CI | P-value |
|---|
| Overall |
|
|
|
| 0.864 |
| Low
MMP9 expression | 156 | 0.96 | 0.58 | 1.59 |
|
| High
MMP9 expression | 162 |
|
|
|
|
| Invasive ductal
carcinoma |
|
|
|
| 0.550 |
| Low
MMP9 expression | 126 | 0.84 | 0.48 | 1.47 |
|
| High
MMP9 expression | 146 |
|
|
|
|
| Other
histology |
|
|
|
| 0.335 |
| Low
MMP9 expression | 30 | 2.12 | 0.55 | 8.17 |
|
| High
MMP9 expression | 16 |
|
|
|
|
| Intermediate/low
gradea |
|
|
|
| 0.901 |
| Low
MMP9 expression | 93 | 0.95 | 0.44 | 2.07 |
|
| High
MMP9 expression | 107 |
|
|
|
|
| High grade8
pts NA |
|
|
|
| 0.518 |
| Low
MMP9 expression | 58 | 0.80 | 0.40 | 1.58 |
|
| High
MMP9 expression | 52 |
|
|
|
|
| T1 stage |
|
|
|
| 0.936 |
| Low
MMP9 expression | 99 | 0.97 | 0.48 | 1.98 |
|
| High
MMP9 expression | 119 |
|
|
|
|
| T2 stage and
higher |
|
|
|
| 0.484 |
| Low
MMP9 expression | 57 | 0.77 | 0.37 | 1.60 |
|
| High
MMP9 expression | 43 |
|
|
|
|
| N0
stageb |
|
|
|
| 0.844 |
| Low
MMP9 expression | 99 | 0.92 | 0.41 | 2.09 |
|
| High
MMP9 expression | 102 |
|
|
|
|
| N+
stageb |
|
|
|
| 0.909 |
| Low
MMP9 expression | 55 | 1.04 | 0.54 | 2.00 |
|
| High
MMP9 expression | 59 |
|
|
|
|
| ER/PR positive for
either |
|
|
|
| 0.539 |
| Low
MMP9 expression | 122 | 1.20 | 0.66 | 2.18 |
|
| High
MMP9 expression | 144 |
|
|
|
|
| ER/PR negative for
both |
|
|
|
| 0.025 |
| Low
MMP9 expression | 34 | 0.33 | 0.12 | 0.93 |
|
| High
MMP9 expression | 18 |
|
|
|
|
| HER positive |
|
|
|
| 0.712 |
| Low
MMP9 expression | 22 | 1.22 | 0.42 | 3.49 |
|
| High
MMP9 expression | 26 |
|
|
|
|
| HER negative |
|
|
|
| 0.741 |
| Low
MMP9 expression | 134 | 0.91 | 0.51 | 1.62 |
|
| High
MMP9 expression | 136 |
|
|
|
|
| Ki67 low
(<20%) |
|
|
|
| 0.523 |
| Low
MMP9 expression | 84 | 1.30 | 0.57 | 2.99 |
|
| High
MMP9 expression | 105 |
|
|
|
|
| Ki67 high
(≥20%) |
|
|
|
| 0.149 |
| Low
MMP9 expression | 72 | 0.62 | 0.33 | 1.19 |
|
| High
MMP9 expression | 57 |
|
|
|
|
| Triple
negativec |
|
|
|
| 0.003 |
| Low
MMP9 expression | 26 | 0.17 | 0.05 | 0.57 |
|
| High
MMP9 expression | 13 |
|
|
|
|
| P53
negativec |
|
|
|
| 0.735 |
| Low
MMP9 expression | 96 | 0.90 | 0.49 | 1.65 |
|
| High
MMP9 expression | 97 |
|
|
|
|
| P53
positivec |
|
|
|
| 0.829 |
| Low
MMP9 expression | 59 | 1.11 | 0.43 | 2.82 |
|
| High
MMP9 expression | 65 |
|
|
|
|
| BCL2
negativec |
|
|
|
| 0.124 |
| Low
MMP9 expression | 51 | 0.53 | 0.24 | 1.18 |
|
| High
MMP9 expression | 41 |
|
|
|
|
| BCL2
positivec |
|
|
|
| 0.445 |
| Low
MMP9 expression | 105 | 1.29 | 0.67 | 2.49 |
|
| High
MMP9 expression | 120 |
|
|
|
|
| CTC EP
negative |
|
|
|
| 0.387 |
| Low
MMP9 expression | 115 | 1.33 | 0.69 | 2.57 |
|
| High
MMP9 expression | 120 |
|
|
|
|
| CTC EP
positive |
|
|
|
| 0.675 |
| Low
MMP9 expression | 12 | 1.52 | 0.20 | 11.24 |
|
| High
MMP9 expression | 15 |
|
|
|
|
| CTC EMT
negative |
|
|
|
| 0.387 |
| Low
MMP9 expression | 115 | 1.33 | 0.69 | 2.47 |
|
| High
MMP9 expression | 120 |
|
|
|
|
| CTC EMT
positive |
|
|
|
| 0.047 |
| Low
MMP9 expression | 29 | 0.40 | 0.16 | 0.95 |
|
| High
MMP9 expression | 27 |
|
|
|
|
| CTC any
negative |
|
|
|
| 0.387 |
| Low
MMP9 expression | 115 | 1.33 | 0.69 | 2.57 |
|
| High
MMP9 expression | 120 |
|
|
|
|
| CTC any
positive |
|
|
|
| 0.113 |
| Low
MMP9 expression | 41 | 0.51 | 0.23 | 1.14 |
|
| High
MMP9 expression | 42 |
|
|
|
|
 | Table V.Univariate analysis for disease-free
survival according to MMP9 expression in stromal cells. |
Table V.
Univariate analysis for disease-free
survival according to MMP9 expression in stromal cells.
| Characteristic | N | HR | 95% Low CI | 95% High CI | P-value |
|---|
| Overall |
|
|
|
| 0.547 |
| Low
MMP9 expression | 276 | 1.29 | 0.60 | 2.78 |
|
| High
MMP9 expression | 33 |
|
|
|
|
| Invasive ductal
carcinoma |
|
|
|
| 0.458 |
| Low
MMP9 expression | 237 | 1.41 | 0.63 | 3.18 |
|
| High
MMP9 expression | 29 |
|
|
|
|
| Other
histology |
|
|
|
| 0.825 |
| Low
MMP9 expression | 39 | 0.79 | 0.08 | 7.84 |
|
| High
MMP9 expression | 4 |
|
|
|
|
| Intermediate/low
gradea |
|
|
|
| 0.970 |
| Low
MMP9 expression | 174 | 1.02 | 0.31 | 3.38 |
|
| High
MMP9 expression | 20 |
|
|
|
|
| High
gradea |
|
|
|
| 0.458 |
| Low
MMP9 expression | 96 | 1.56 | 0.57 | 4.24 |
|
| High
MMP9 expression | 12 |
|
|
|
|
| T1 stage |
|
|
|
| 0.974 |
| Low
MMP9 expression | 189 | 1.02 | 0.36 | 2.90 |
|
| High
MMP9 expression | 24 |
|
|
|
|
| T2 stage and
higher |
|
|
|
| 0.457 |
| Low
MMP9 expression | 87 | 1.71 | 0.54 | 5.43 |
|
| High
MMP9 expression | 9 |
|
|
|
|
| N0
stageb |
|
|
|
| 0.460 |
| Low
MMP9 expression | 174 | 1.71 | 0.53 | 5.55 |
|
| High
MMP9 expression | 23 |
|
|
|
|
| N+
stageb |
|
|
|
| 0.871 |
| Low
MMP9 expression | 100 | 0.92 | 0.31 | 2.70 |
|
| High
MMP9 expression | 10 |
|
|
|
|
| ER/PR positive for
either |
|
|
|
| 0.323 |
| Low
MMP9 expression | 229 | 1.67 | 0.71 | 3.88 |
|
| High
MMP9 expression | 31 |
|
|
|
|
| ER/PR negative for
both |
|
|
|
| 0.002 |
| Low
MMP9 expression | 47 | 0.14 | 0.00 | 4.81 |
|
| High
MMP9 expression | 2 |
|
|
|
|
| HER positive |
|
|
|
| 0.242 |
| Low
MMP9 expression | 39 | 3.12 | 0.83 | 11.74 |
|
| High
MMP9 expression | 7 |
|
|
|
|
| HER negative |
|
|
|
| 0.900 |
| Low
MMP9 expression | 237 | 1.06 | 0.43 | 2.64 |
|
| High
MMP9 expression | 26 |
|
|
|
|
| Ki67 low
(<20%) |
|
|
|
| 0.486 |
| Low
MMP9 expression | 159 | 1.67 | 0.50 | 5.53 |
|
| High
MMP9 expression | 24 |
|
|
|
|
| Ki67 high
(≥20%) |
|
|
|
| 0.679 |
| Low
MMP9 expression | 117 | 0.80 | 0.26 | 2.50 |
|
| High
MMP9 expression | 9 |
|
|
|
|
| Triple
negative |
|
|
|
| 0.001 |
| Low
MMP9 expression | 35 | 0.12 | 0.00 | 4.89 |
|
| High
MMP9 expression | 2 |
|
|
|
|
| P53
negativec |
|
|
|
| 0.901 |
| Low
MMP9 expression | 171 | 1.07 | 0.39 | 2.92 |
|
| High
MMP9 expression | 17 |
|
|
|
|
| P53
positivec |
|
|
|
| 0.482 |
| Low
MMP9 expression | 104 | 1.68 | 0.50 | 5.69 |
|
| High
MMP9 expression | 16 |
|
|
|
|
| BCL2 negative |
|
|
|
| 0.078 |
| Low
MMP9 expression | 83 | 0.35 | 0.05 | 2.29 |
|
| High
MMP9 expression | 5 |
|
|
|
|
| BCL2 positive |
|
|
|
| 0.238 |
| Low
MMP9 expression | 193 | 2.00 | 0.81 | 4.96 |
|
| High
MMP9 expression | 28 |
|
|
|
|
| CTC EP
negative |
|
|
|
| 0.143 |
| Low
MMP9 expression | 202 | 2.76 | 1.08 | 7.09 |
|
| High
MMP9 expression | 27 |
|
|
|
|
| CTC EP
positive |
|
|
|
| 0.053 |
| Low
MMP9 expression | 23 | 0.18 | 0.01 | 2.75 |
|
| High
MMP9 expression | 4 |
|
|
|
|
| CTC EMT
negative |
|
|
|
| 0.143 |
| Low
MMP9 expression | 202 | 2.76 | 1.08 | 7.09 |
|
| High
MMP9 expression | 27 |
|
|
|
|
| CTC EMT
positive |
|
|
|
| 0.168 |
| Low
MMP9 expression | 51 | 0.37 | 0.04 | 3.50 |
|
| High
MMP9 expression | 2 |
|
|
|
|
| CTC any
negative |
|
|
|
| 0.143 |
| Low
MMP9 expression | 202 | 2.76 | 1.08 | 7.09 |
|
| High
MMP9 expression | 27 |
|
|
|
|
| CTC any
positive |
|
|
|
| 0.128 |
| Low
MMP9 expression | 74 | 0.44 | 0.10 | 1.91 |
|
| High
MMP9 expression | 6 |
|
|
|
|
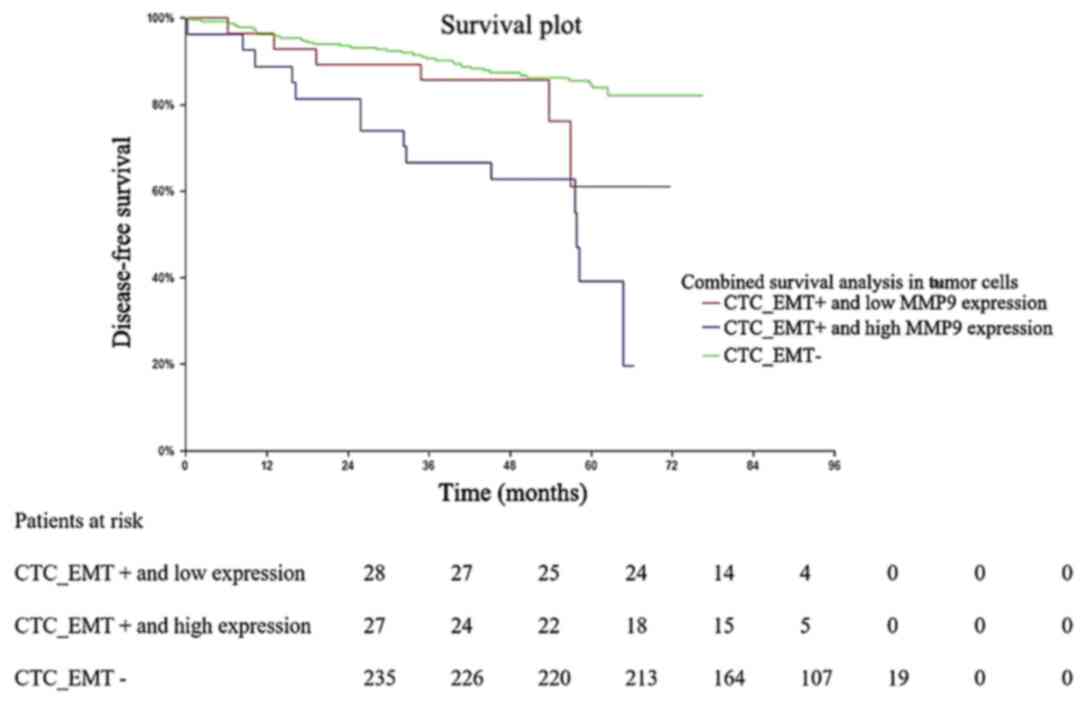
Notably, combinatorial survival analysis of CTC_EMT
and MMP9 expression in tumor cells demonstrated that CTC_EMT
positive patients with high MMP9 expression had a significantly
shorter DFS compared with CTC_EMT negative patients (P<0.00001;
Fig. 5).
Discussion
MMPs represent a large family of proteolytic enzymes
of the extracellular matrix that are involved in extracellular
matrix degradation, tumor cell invasion, metastasis and
angiogenesis (19,40–42). The
results of the present study demonstrated that elevated MMP9
expression levels in tumor cells and tumor associated stroma were
significantly associated with favorable tumor characteristics.
Hormone-positive tumors exhibited significantly higher MMP9
expression in tumor cells, as well as in tumor associated stromal
cells. In addition, the results demonstrated an association between
increased MMP9 expression and low proliferation index of Ki67.
Although the role of MMP9 and its association with breast cancer
has been extensively studied, data regarding the prognostic value
of MMP9 are inconsistent. On one hand, it has been reported that
MMP9 expression is associated with a shorter relapse-free survival
time in patients with primary breast tumours (26,29,43,44). The
association between upregulated MMP9 expression and an increased
risk of overall survival and relapse-free survival in breast cancer
has also been confirmed via meta-analyses by Song et al
(45) and Ren et al (46). Conversely, some studies have
identified MMP-9 as a favourable prognostic marker for breast
cancer (9,47).
The results of the present study demonstrated a
significant association between high MMP9 expression in tumour
cells and poor DFS in hormone receptor negative, triple negative,
as well as in the CTC_EMT-positive subgroup of patients with early
breast cancer. Analysis of stromal cells exhibited this association
in the hormone receptor negative and triple negative subgroups of
patients.
These results are in concordance with previous
studies, confirming the association between MMP9 expression and a
shorter progression time, particularly in patients with basal-like
or triple negative breast cancer (48,49).
Controversy regarding the association between MMP9 expression and
clinical outcomes in different types of malignant tumors, including
breast cancer, suggests the presence of active and inactive forms
of MMP9. MMPs are secreted in the form of inactive proenzymes,
whose activation is mediated via different molecular mechanisms
(20,21). Thus, the level of active MMP9 in
stromal cells and tumor cells may vary, which will subsequently
account for the differences in clinical outcomes (50).
Notably, the results of the present study
demonstrated the prognostic value of MMP9 in the CTC_EMT-positive
subgroup of patients. Generally, ETM is considered a developmental
process, facilitating the resistance to apoptosis and increased
invasion, and is closely associated with development of a cancer
stem cell phenotype (19,51) This machinery can be directly induced
by MMPs in the target epithelial cells. Expression of proteases,
including MMPs is upregulated during reorganization of ECM in EMT.
In addition, the process of MMP-induced EMT has been best
characterized in mammary epithelial cells (52,53).
According to the results of the present study, there was no
significant association between any subpopulations of CTCs and MMP9
expression. Contrary to MMP1, MMP9 does not actively participate in
the release of CTCs into the blood stream of patients with PBC
(31). However, these changes may
result in the resistance to therapy, and development of a cancer
stem cell phenotype closely associated with poor DFS. Given the
limited treatment options for these subgroups of patients
(triple-negative and CTC_EMT-positive PBC), MMP9 may potentially
offer a novel therapeutic target. In addition, the results from the
combinatorial survival analysis demonstrated that CTC_EMT positive
patients with MMP9 expression in tumor cells had a significantly
lower DFS compared with CTC_EMT negative patients, suggesting that
EMT acts as a negative prognostic marker only in subgroups of
patients with high MMP9 expression, while the subgroup of CTC_EMT
positive patients, with low MMP9 expression exhibited no
effects.
The spectrum of synthetized MMP inhibitors (MMPIs)
assessed in clinical trials have demonstrated poor effectiveness
and serious side effects (54,55). The
limited clinical effect of MMPIs may be due to their poor
selectivity. Previous studies have focused on a broad spectrum of
MMPs, most of which exert tumorigenic activity. However, it is
necessary to take into consideration that some MMPs are
characterized by antitumorigenic effects. Another reason for MMPIs
inefficiency can be due to their administration to unselected
groups of patients (44,56).
In conclusion, this prospective translational study
demonstrated the protective role of MMP9 in patients with breast
cancer, whereby its increased expression was associated with
favourable tumour characteristics. Thus, as it has been proposed by
Pozzi et al (57), inhibition
of MMP9 antitumorigenic and antiangiogenic activities may result in
a paradoxical increase of tumor angiogenesis and tumor growth.
Conversely, the results of the present study demonstrated the
association between high MMP9 expression and poor DFS in selected
subgroups of patients with PBC, particularly hormone receptor
negative and triple negative tumors, as well as in CTC_EMT positive
patients. These results suggest that MMP9 exerts different
biological roles in HR positive vs. negative tumors, further
supporting the concept of different biology of breast cancer
subtypes according to their HR status. Thus, assessing MMP9 tumor
expression may help identify individuals with increased risk of
disease recurrence within the aforementioned subgroups of patients
with PBC. However, there were certain limitations to the present
study, such as the retrospective design of the study and
semi-quantitative IHC analysis used for investigating of MMP9
expression. In addition, the study population represent a
homogenous cohort of patients, treatment-naïve, without metastatic
disease, in order to avoid the effect of the metastatic site
heterogeneity factor on analysed variables.
Further studies are required to develop selective
MMPIs against the specific protumorigenic MMPs or protumorigenic
activities of selected MMPs. Another strategy may be anticancer
therapy with antitumorigenic MMPs or with their antitumorigenic
subparts. An example of this phenomenon involves the MMP8 enzyme,
whereby high MMP8 expression supresses metastasis, while MMP8
silencing induces tumour progression and metastasis (58–60).
Acknowledgements
The authors would like to thank Dr Gabriela
Sieberova, Dr Jan Macuch, Dr Michal Majercik, Dr Peter Jani and
Professor Pavel Babal (all from Department of Pathology, Faculty of
Medicine, Comenius University) for their valuable input into IHC
evaluation. The authors would also like to thank Ms. Zlatica Pekova
(2nd Department of Oncology, Faculty of Medicine, Comenius
University, National Cancer Institute) for administrative support,
and Ms. Emilia Klincova and Mr. Ludovit Gaspar (both, Department of
Pathology, Faculty of Medicine, Comenius University) for their
excellent technical assistance.
Funding
The present study was funded by the Slovak Research
and Development Agency (grant no. APVV-16-0010).
Availability of data and materials
All datasets generated and analyzed during the
present study are included in this published article.
Authors' contributions
KK, ZC, JM, MM and GM conceived and designed the
present study. KK performed statistical analysis. ZC and IM
performed immunohistochemical analysis. GM, TS and DK were involved
in CTC detection. MK, JB and DP were involved in patient accrual
and performed breast surgery. KK and MM drafted the initial
manuscript, and all authors reviewed it critically for important
intellectual content. All authors participated in the acquisition,
analysis and interpretation of data. All authors have read and
approved the final version of the manuscript for publication.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Institutional Review Board of the National Cancer Institute of
Slovakia, Bratislava, Slovakia (approval no. TRU-SK 002; Chair:
Professor Michal Mego). Written informed consent was provided by
all patients prior to study commencement.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MMP9
|
matrix metalloproteinase 9
|
|
CTCs
|
circulating tumor cells
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
ER
|
estrogen receptor
|
|
HR
|
hazard ratio
|
|
PBC
|
primary breast cancer
|
|
PR
|
progesterone receptor
|
|
RT-PCR
|
reverse transcription PCR
|
|
NA
|
non-applicable
|
References
|
1
|
Ghoncheh M, Pournamdar Z and Salehiniya H:
Incidence and mortality and epidemiology of breast cancer in the
world. Asian Pac J Cancer Prev. 17:43–46. 2016. View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar
|
|
3
|
Moreau JE, Anderson K, Mauney JR, Nguyen
T, Kaplan DL and Rosenblatt M: Tissue-engineered bone serves as a
target for metastasis of human breast cancer in a mouse model.
Cancer Res. 67:10304–10308. 2007. View Article : Google Scholar
|
|
4
|
Fagan-Solis KD, Schneider SS, Pentecost
BT, Bentley BA, Otis CN, Gierthy JF and Arcaro KF: The RhoA pathway
mediates MMP-2 and MMP-9-independent invasive behavior in a
triple-negative breast cancer cell line. J Cell Biochem.
114:1385–1394. 2013. View Article : Google Scholar
|
|
5
|
Malmgren JA, Mayer M, Atwood MK and Kaplan
HG: Differential presentation and survival of de novo and recurrent
metastatic breast cancer over time: 1990–2010. Breast Cancer Res
Treat. 167:579–590. 2018. View Article : Google Scholar
|
|
6
|
Fan J, Deng X, Gallagher JW, Huang H,
Huang Y, Wen J, Ferrari M, Shen H and Hu Y: Monitoring the
progression of metastatic breast cancer on nanoporous silica chips.
Philos Trans- Royal Soc, Math Phys Eng Sci. 370:2433–2447.
2012.
|
|
7
|
Bottino J, Gelaleti GB, Maschio LB,
Jardim-Perassi BV and de Campos Zuccari DA: Immunoexpression of
ROCK-1 and MMP-9 as prognostic markers in breast cancer. Acta
Histochem. 116:1367–1373. 2014. View Article : Google Scholar
|
|
8
|
Olayide A, Samuel O, Ganiyu R, Moses A,
Gafar O, Abiola D, Dapo K and John A: How effective is the
treatment of locally advanced and metastatic breast cancer in
developing centres?: A retrospective review. Ethiop J Health Sci.
25:337–344. 2015. View Article : Google Scholar
|
|
9
|
Scorilas A, Karameris A, Arnogiannaki N,
Ardavanis A, Bassilopoulos P, Trangas T and Talieri M:
Overexpression of matrix-metalloproteinase-9 in human breast
cancer: A potential favourable indicator in node-negative patients.
Br J Cancer. 84:1488–1496. 2001. View Article : Google Scholar
|
|
10
|
Fidler IJ: Metastasis: Quantitative
analysis of distribution and fate of tumor emboli labeled with 125
I-5-iodo-2-deoxyuridine. J Natl Cancer Inst. 45:773–782. 1970.
|
|
11
|
Lambert AW, Pattabiraman DR and Weinberg
RA: Emerging biological principles of metastasis. Cell.
168:670–691. 2017. View Article : Google Scholar
|
|
12
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar
|
|
13
|
Hsiao KC, Shih NY, Fang HL, Huang TS, Kuo
CC, Chu PY, Hung YM, Chou SW, Yang YY, Chang GC, et al: Surface
α-enolase promotes extracellular matrix degradation and tumor
metastasis and represents a new therapeutic target. PLoS One.
8:e693542013. View Article : Google Scholar
|
|
14
|
Wang X, Lu H, Urvalek AM, Li T, Yu L,
Lamar J, DiPersio CM, Feustel PJ and Zhao J: KLF8 promotes human
breast cancer cell invasion and metastasis by transcriptional
activation of MMP9. Oncogene. 30:1901–1911. 2011. View Article : Google Scholar
|
|
15
|
Ortíz-López L, Morales-Mulia S,
Ramírez-Rodríguez G and Benítez-King G: ROCK-regulated cytoskeletal
dynamics participate in the inhibitory effect of melatonin on
cancer cell migration. J Pineal Res. 46:15–21. 2009. View Article : Google Scholar
|
|
16
|
Paolillo M and Schinelli S: Extracellular
matrix alterations in metastatic processes. Int J Mol Sci.
20:49472019. View Article : Google Scholar
|
|
17
|
Castro-Castro A, Marchesin V, Monteiro P,
Lodillinsky C, Rossé C and Chavrier P: Cellular and molecular
mechanisms of MT1-MMP-dependent cancer cell invasion. Annu Rev Cell
Dev Biol. 32:555–576. 2016. View Article : Google Scholar
|
|
18
|
Shay G, Lynch CC and Fingleton B: Moving
targets: Emerging roles for MMPs in cancer progression and
metastasis. Matrix Biol. 44-46:200–206. 2015. View Article : Google Scholar
|
|
19
|
Gonzalez-Avila G, Sommer B, Mendoza-Posada
DA, Ramos C, Garcia-Hernandez AA and Falfan-Valencia R: Matrix
metalloproteinases participation in the metastatic process and
their diagnostic and therapeutic applications in cancer. Crit Rev
Oncol Hematol. 137:57–83. 2019. View Article : Google Scholar
|
|
20
|
Yousef EM, Tahir MR, St-Pierre Y and
Gaboury LA: MMP-9 expression varies according to molecular subtypes
of breast cancer. BMC Cancer. 14:6092014. View Article : Google Scholar
|
|
21
|
Stuelten CH, DaCosta Byfield S, Arany PR,
Karpova TS, Stetler-Stevenson WG and Roberts AB: Breast cancer
cells induce stromal fibroblasts to express MMP-9 via secretion of
TNF-alpha and TGF-beta. J Cell Sci. 118:2143–2153. 2005. View Article : Google Scholar
|
|
22
|
Sand JM, Larsen L, Hogaboam C, Martinez F,
Han M, Larsen MR, Nawrocki A, Zheng Q, Karsdal MA and Leeming DJ:
MMP mediated degradation of type IV collagen alpha 1 and alpha 3
chains reflects basement membrane remodeling in experimental and
clinical fibrosis - validation of two novel biomarker assays. PLoS
One. 8:e849342013. View Article : Google Scholar
|
|
23
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar
|
|
24
|
Owyong M, Chou J, van den Bijgaart RJ,
Kong N, Efe G, Maynard C, Talmi-Frank D, Solomonov I, Koopman C,
Hadler-Olsen E, et al: MMP9 modulates the metastatic cascade and
immune landscape for breast cancer anti-metastatic therapy. Life
Sci Alliance. 2:e2018002262019. View Article : Google Scholar
|
|
25
|
Li HC, Cao DC, Liu Y, Hou YF, Wu J, Lu JS,
Di GH, Liu G, Li FM, Ou ZL, et al: Prognostic value of matrix
metalloproteinases (MMP-2 and MMP-9) in patients with lymph
node-negative breast carcinoma. Breast Cancer Res Treat. 88:75–85.
2004. View Article : Google Scholar
|
|
26
|
Vizoso FJ, González LO, Corte MD,
Rodríguez JC, Vázquez J, Lamelas ML, Junquera S, Merino AM and
García-Muñiz JL: Study of matrix metalloproteinases and their
inhibitors in breast cancer. Br J Cancer. 96:903–911. 2007.
View Article : Google Scholar
|
|
27
|
Darlix A, Lamy PJ, Lopez-Crapez E,
Braccini AL, Firmin N, Romieu G, Thézenas S and Jacot W: Serum NSE,
MMP-9 and HER2 extracellular domain are associated with brain
metastases in metastatic breast cancer patients: Predictive
biomarkers for brain metastases? Int J Cancer. 139:2299–2311. 2016.
View Article : Google Scholar
|
|
28
|
Ranogajec I, Jakić-Razumović J, Puzović V
and Gabrilovac J: Prognostic value of matrix metalloproteinase-2
(MMP-2), matrix metalloproteinase-9 (MMP-9) and aminopeptidase
N/CD13 in breast cancer patients. Med Oncol. 29:561–569. 2012.
View Article : Google Scholar
|
|
29
|
Li H, Qiu Z, Li F and Wang C: The
relationship between MMP-2 and MMP-9 expression levels with breast
cancer incidence and prognosis. Oncol Lett. 14:5865–5870. 2017.
|
|
30
|
Dhar M, Lam JN, Walser T, Dubinett SM,
Rettig MB and Di Carlo D: Functional profiling of circulating tumor
cells with an integrated vortex capture and single-cell protease
activity assay. Proc Natl Acad Sci USA. 115:9986–9991. 2018.
View Article : Google Scholar
|
|
31
|
Cierna Z, Mego M, Janega P, Karaba M,
Minarik G, Benca J, Sedlácková T, Cingelova S, Gronesova P,
Manasova D, et al: Matrix metalloproteinase 1 and circulating tumor
cells in early breast cancer. BMC Cancer. 14:4722014. View Article : Google Scholar
|
|
32
|
Cardoso F, Kyriakides S, Ohno S,
Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S and Senkus E;
ESMO Guidelines Committee, : Early breast cancer: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 30:16742019. View Article : Google Scholar
|
|
33
|
Allison KH, Brogi E and Ellis IO: WHO
Classification of Tumours Editorial Board. Breast Tumours. 5th
edition. IARC Press; Lyon: 2019
|
|
34
|
van Nes JG, de Kruijf EM, Putter H,
Faratian D, Munro A, Campbell F, Smit VT, Liefers GJ, Kuppen PJ,
van de Velde CJ, et al: Co-expression of SNAIL and TWIST determines
prognosis in estrogen receptor-positive early breast cancer
patients. Breast Cancer Res Treat. 133:49–59. 2012. View Article : Google Scholar
|
|
35
|
Chovanec M, Cierna Z, Miskovska V,
Machalekova K, Svetlovska D, Kalavska K, Rejlekova K, Spanik S,
Kajo K, Babal P, et al: Prognostic role of programmed-death ligand
1 (PD-L1) expressing tumor infiltrating lymphocytes in testicular
germ cell tumors. Oncotarget. 8:21794–21805. 2017. View Article : Google Scholar
|
|
36
|
Chovanec M, Cierna Z, Miskovska V,
Machalekova K, Kalavska K, Rejlekova K, Svetlovska D, Macak D,
Spanik S, Kajo K, et al: β-catenin is a marker of poor clinical
characteristics and suppressed immune infiltration in testicular
germ cell tumors. BMC Cancer. 18:10622018. View Article : Google Scholar
|
|
37
|
Mego M, Karaba M, Minarik G, Benca J,
Sedlácková T, Tothova L, Vlkova B, Cierna Z, Janega P, Luha J, et
al: Relationship between circulating tumor cells, blood
coagulation, and urokinase-plasminogen-activator system in early
breast cancer patients. Breast J. 21:155–160. 2015. View Article : Google Scholar
|
|
38
|
Mego M, Karaba M, Minarik G, Benca J,
Silvia J, Sedlackova T, Manasova D, Kalavska K, Pindak D,
Cristofanilli M, et al: Circulating tumor cells with
epithelial-to-mesenchymal transition phenotypes associated with
inferior outcomes in primary breast cancer. Anticancer Res.
39:1829–1837. 2019. View Article : Google Scholar
|
|
39
|
Mego M, Mani SA, Lee BN, Li C, Evans KW,
Cohen EN, Gao H, Jackson SA, Giordano A, Hortobagyi GN, et al:
Expression of epithelial-mesenchymal transition-inducing
transcription factors in primary breast cancer: The effect of
neoadjuvant therapy. Int J Cancer. 130:808–816. 2012. View Article : Google Scholar
|
|
40
|
Yadav L, Puri N, Rastogi V, Satpute P,
Ahmad R and Kaur G: Matrix metalloproteinases and cancer - roles in
threat and therapy. Asian Pac J Cancer Prev. 15:1085–1091. 2014.
View Article : Google Scholar
|
|
41
|
Westermarck J and Kähäri VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999. View Article : Google Scholar
|
|
42
|
Alaseem A, Alhazzani K, Dondapati P,
Alobid S, Bishayee A and Rathinavelu A: Matrix metalloproteinases:
A challenging paradigm of cancer management. Semin Cancer Biol.
56:100–115. 2019. View Article : Google Scholar
|
|
43
|
González LO, Pidal I, Junquera S, Corte
MD, Vázquez J, Rodríguez JC, Lamelas ML, Merino AM, García-Muñiz JL
and Vizoso FJ: Overexpression of matrix metalloproteinases and
their inhibitors in mononuclear inflammatory cells in breast cancer
correlates with metastasis-relapse. Br J Cancer. 97:957–963. 2007.
View Article : Google Scholar
|
|
44
|
Radisky ES, Raeeszadeh-Sarmazdeh M and
Radisky DC: Therapeutic Potential of Matrix Metalloproteinase
Inhibition in Breast Cancer. J Cell Biochem. 118:3531–3548. 2017.
View Article : Google Scholar
|
|
45
|
Song J, Su H, Zhou YY and Guo LL:
Prognostic value of matrix metalloproteinase 9 expression in breast
cancer patients: A meta-analysis. Asian Pac J Cancer Prev.
14:1615–1621. 2013. View Article : Google Scholar
|
|
46
|
Ren F, Tang R, Zhang X, Madushi WM, Luo D,
Dang Y, Li Z, Wei K and Chen G: Overexpression of MMP family
members functions as prognostic biomarker for breast cancer
patients: A systematic review and meta-analysis. PLoS One.
10:e01355442015. View Article : Google Scholar
|
|
47
|
Pellikainen JM, Ropponen KM, Kataja VV,
Kellokoski JK, Eskelinen MJ and Kosma VM: Expression of matrix
metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special
reference to activator protein-2, HER2, and prognosis. Clin Cancer
Res. 10:7621–7628. 2004. View Article : Google Scholar
|
|
48
|
González LO, Corte MD, Junquera S,
González-Fernández R, del Casar JM, García C, Andicoechea A,
Vázquez J, Pérez-Fernández R and Vizoso FJ: Expression and
prognostic significance of metalloproteases and their inhibitors in
luminal A and basal-like phenotypes of breast carcinoma. Hum
Pathol. 40:1224–1233. 2009. View Article : Google Scholar
|
|
49
|
Zhao S, Ma W, Zhang M, Tang D, Shi Q, Xu
S, Zhang X, Liu Y, Song Y, Liu L, et al: High expression of CD147
and MMP-9 is correlated with poor prognosis of triple-negative
breast cancer (TNBC) patients. Med Oncol. 30:3352013. View Article : Google Scholar
|
|
50
|
Yang J, Min KW, Kim DH, Son BK, Moon KM,
Wi YC, Bang SS, Oh YH, Do SI, Chae SW, et al: High TNFRSF12A level
associated with MMP-9 overexpression is linked to poor prognosis in
breast cancer: Gene set enrichment analysis and validation in
large-scale cohorts. PLoS One. 13:e02021132018. View Article : Google Scholar
|
|
51
|
Radisky ES and Radisky DC: Matrix
metalloproteinase-induced epithelial-mesenchymal transition in
breast cancer. J Mammary Gland Biol Neoplasia. 15:201–212. 2010.
View Article : Google Scholar
|
|
52
|
Foroni C, Broggini M, Generali D and Damia
G: Epithelial-mesenchymal transition and breast cancer: Role,
molecular mechanisms and clinical impact. Cancer Treat Rev.
38:689–697. 2012. View Article : Google Scholar
|
|
53
|
Li W, Li S, Deng L, Yang S, Li M, Long S,
Chen S, Lin F and Xiao L: Decreased MT1-MMP in gastric cancer
suppressed cell migration and invasion via regulating MMPs and EMT.
Tumour Biol. 36:6883–6889. 2015. View Article : Google Scholar
|
|
54
|
Zhong Y, Lu YT, Sun Y, Shi ZH, Li NG, Tang
YP and Duan JA: Recent opportunities in matrix metalloproteinase
inhibitor drug design for cancer. Expert Opin Drug Discov.
13:75–87. 2018. View Article : Google Scholar
|
|
55
|
Arkadash V, Yosef G, Shirian J, Cohen I,
Horev Y, Grossman M, Sagi I, Radisky ES, Shifman JM and Papo N:
Development of high affinity and high specificity inhibitors of
matrix metalloproteinase 14 through computational design and
directed evolution. J Biol Chem. 292:3481–3495. 2017. View Article : Google Scholar
|
|
56
|
Winer A, Adams S and Mignatti P: Matrix
metalloproteinase inhibitors in cancer therapy: Turning past
failures intofuture successes. Mol Cancer Ther. 17:1147–1155. 2018.
View Article : Google Scholar
|
|
57
|
Pozzi A, LeVine WF and Gardner HA: Low
plasma levels of matrix metalloproteinase 9 permit increased tumor
angiogenesis. Oncogene. 21:272–281. 2002. View Article : Google Scholar
|
|
58
|
Montel V, Kleeman J, Agarwal D, Spinella
D, Kawai K and Tarin D: Altered metastatic behavior of human breast
cancer cells after experimental manipulation of matrix
metalloproteinase 8 gene expression. Cancer Res. 64:1687–1694.
2004. View Article : Google Scholar
|
|
59
|
Decock J, Hendrickx W, Thirkettle S,
Gutiérrez-Fernández A, Robinson SD and Edwards DR: Pleiotropic
functions of the tumor- and metastasis-suppressing matrix
metalloproteinase-8 in mammary cancer in MMTV-PyMT transgenic mice.
Breast Cancer Res. 17:382015. View Article : Google Scholar
|
|
60
|
Juurikka K, Butler GS, Salo T, Nyberg P
and Åström P: The role of MMP8 in cancer: A systematic review. Int
J Mol Sci. 20:45062019. View Article : Google Scholar
|