Introduction
Hepatocellular carcinoma (HCC) is a common malignant
cancer in humans, and the fourth leading cause of cancer-associated
morality worldwide (1,2). Globally, from 2004 to 2017, the
incidence of hepatocellular carcinoma was 1/100,000 (3), with a low overall 5-year survival rate
of 18% (4). In addition to liver
resection and liver transplantation, targeted therapy,
immunotherapy and transarterial chemoembolization are used to treat
advanced HCC (5–7). However, patients with HCC suffer from a
high prevalence of mortality and recurrence (5,7–11). Thus, identification of novel
effective targets for the treatment of advanced HCC remains
essential.
Ribosomes take part in the synthesis of proteins, as
the site of mRNA translation (12).
Recently, increasing evidence has demonstrated that ribosomes can
regulate cancer progression and drug reactions by ‘alternative
translation’, which enables tumor cells to adapt to their
environment in order to proliferate (13–16).
Methylation of ribosomal (r)RNA is the primary method to control
protein synthesis (17–19).
The nucleolar protein, fibrillarin (FBL) catalyzes
the 2′-O-methylation (2′-O-Me) of rRNAs to manage translation of
mRNAs (20,21), which enables changes in the
combination of rRNAs and specific mRNAs (22–24). FBL
can promote cancer-cell proliferation by regulating mRNA
translation and controlling the methylation of rRNAs, which has
been demonstrated in breast cancer and cancer of the prostate gland
(20,25,26).
Marcel et al (20) reported that p53 acts as a safeguard
of protein synthesis by regulating FBL expression to inhibit tumor
occurrence. In addition, reversible acetylation of FBL regulates
methylation of nucleolar H2AQ104, thereby reinforcing oscillation
of Pol-I transcription during the cell cycle (27). Shubina et al (21) demonstrated the potential roles of FBL
in the regulation of cell proliferation, cancer progression and
aging. Taken together, these results suggest that FBL may function
as an oncogene. However, the function of FBL in HCC and the effect
of FBL expression on HCC cells remain unknown.
To investigate the expression difference of FBL in
tumor tissues and para-tumor tissues of HCC, immunohistochemistry
(IHC) and bioinformatics analyses demonstrated that FBL expression
was upregulated in patients with HCC. Furthermore, high FBL
expression signified an advanced tumor stage and a poor prognosis.
Using publicly available RNA-sequencing data, it was demonstrated
that the methylated modification of FBL was frequent in HCC. Taken
together, the results of the present study suggest that FBL may
regulate the biological behavior of tumor cells by DNA
damage/repair, cell-cell adhesion, the cell cycle, as well as
signaling pathways involving fibroblast growth factor receptors,
epidermal growth factor receptors and nuclear factor-κB
(NF-κB)-inducing kinase/NF-κB.
Materials and methods
Ethical approval of the study
protocol
The present study was approved by the Ethics
Committee of the Affiliated Hospital of North Sichuan Medical
College (Nanchong, China; approval no. 2020ER(A)024; May 29, 2019).
Written informed consent was provided by patients, their authorized
agents or their close relatives prior to the study start for use of
their tissue samples and clinical details.
Study cohort
A total of 139 patients with HCC from the Affiliated
Hospital of North Sichuan Medical College formed the study cohort,
between June 2014 and December 2016. The present study included 114
men and 25 women with HCC, with an average age of 54.22 years (age
range, 36–73 years). HCC was confirmed via histological analysis. A
total of 139 HCC tissues and 81 paired para-tumor tissues were
selected from patients who had not undergone radiotherapy or
chemotherapy prior to surgery. In addition, patients with distant
metastasis, Child-Pugh (28) liver
function of grade C or other types of cancer were excluded from the
present study. The cancer tissues and corresponding adjacent
tissues were applied to produce tissue microarrays (TMAs).
Follow-up
Survival analysis data was obtained via the
telephone, the follow-up visit comprised liver-function tests,
chest radiography and measurement of α-fetoprotein (AFP)
expression. Follow-up was at least every 2 months in the first 6
months following surgery, every 3 months for 6 months-2 years after
surgery, and every 6 months for 2–5 years following surgery. If
necessary, computed tomography or magnetic resonance imaging were
also performed to diagnose tumor recurrence. Postoperative adjuvant
therapy and treatment of tumor recurrence were communicated by our
multidisciplinary team. Surpassing 5 years post-surgery, the
survival of patients with HCC was largely affected by several
clinically unrelated factors, for example household income
(3), thus the survival of all
patients was calculated up to 5 years post-surgery.
IHC analysis
Fresh tissue samples were fixed in 4% formalin for
24 h at room temperature, washed five times with PBS (3 min each)
and subsequently dehydrated with ethanol. Tissue samples were
embedded in paraffin and cut into 4-µm-thick sections (diameter of
2 mm) to produce TMAs. TMAs were deparaffinized in xylene and
rehydrated in a descending ethanol series (100, 95 and 85%) at room
temperature. TMAs were incubated with 0.3%
H2O2 to inhibit endogenous peroxidase
activity, and antigen-retrieval was subsequently performed with
ethylenediamine tetraacetic acid antigen-retrieval solution
(Beyotime Institute of Biotechnology; P0085), using a microwave for
15 min. TMAs were blocked with 10% goat serum (Beijing Solarbio
Science & Technology Co., Ltd.; SL038) for 30 min at room
temperature, and incubated with anti-FBL (1:100; Abcam; cat. no.
ab166630) overnight at 4°C. Following the primary incubation, TMAs
were incubated with a secondary antibody for 1 h at 37°C (1:1;
Dako; Agilent Technologies, Inc; cat. no. K5007). The REAL™
EnVision™ immunohistochemistry kit include the secondary antibody,
and the secondary antibody from the manufacturer has come out of
the working fluid concentration. The TAMs were color rendered using
the REAL™ EnVision™ immunohistochemistry kit (Dako; Agilent
Technologies, Inc; cat. no. K5007), according to the manufacturer's
instructions. To score the expression of FBL in the TMAs, the TMAs
were imaged using an automatic immunohistochemical section scanning
system (Pannorramic SCAN, Hungary).
IHC scoring
Color intensity was divided into four
classifications of staining, as follows: None, score=0; weak,
score=1; moderate, score=2 and intense, score=3. The proportion of
positive cells was ranked into five levels, as follows: <5%,
score=0; 5–25%, score=1; 26–50%, score=2; 51–74%, score=3 and ≥75%,
score=4. Multiplication of the staining score with the score for
the percentage of positive cells provided the final score. The
cut-off point for high FBL expression was based on the median IHC
score −6 (29). Thus, a score <6
was classified as low FBL protein expression, while a score ≥6 was
classified as high FBL protein expression. Each score was
independently evaluated by two experienced pathologists from the
Department of Pathology, The First People's Hospital of Neijiang
(Neijiang, China). In the case of ambiguity, a third experienced
pathologist from the Department of Pathology, The First People's
Hospital of Neijiang (Neijiang, China) judged the final
outcome.
Bioinformatic analysis
The UALCAN database (ualcan.path.uab.edu) was used to analyze FBL
expression in HCC, the methylation level of its promoters and
identify the associated genes, which was downloaded from The Cancer
Genome Atlas (TCGA) RNA-sequencing data (ualcan.path.uab.edu). The statistical tests were
performed via TCGA analysis of UALCAN (Figs. 1A and B, and 3B). FBL expression was assessed in HCC from
50 normal liver tissues and 371 primary tumor tissues. Transcripts
per million was used to measure expression. Functional enrichment
analysis of the associated genes was performed using the Database
for Annotation, Visualization and Integrated Discovery (DAVID 6.7,
David.ncifcrf.gov). The Gene Ontology (GO)
database (David.ncifcrf.gov) was used to assess
186 FBL-related genes (Person-CC≥0.5). The mutation or
amplification of FBL was validated using cBioportal (www.cbioportal.org), which involved four datasets, as
follows: INSERM, Nat Genet 2015, 243 samples (30); AMC, Hepatology 2014, 231 samples
(31); TCGA, Firehorse Legacy, 442
samples (http://gdac.broadinstitute.org/runs/stddata),
Pancancer Atlas, 372 samples (32–41). The
micro (mi)RNAs that bind with the 3′-untranslated region (UTR) of
FBL were predicted using TargetScan 3.1 (www.targetscan.org). The methylation of promoters was
assessed within UALCAN using TCGA RNA-sequencing data. The β-value
indicated the level of DNA methylation ranging from 0
(unmethylated) to 1 (fully methylated).
Statistical analysis
Statistical analysis were performed using SPSS v23
software (IBM Corp.). The χ2 test was for categorical
data, as well as to determine the association between FBL protein
expression and the clinicopathological characteristics of patients
with HCC. Survival analysis was performed using the Kaplan-Meier
method and log-rank test. Univariate and multivariate analyses was
performed using the Cox proportional hazards model to determine the
prognostic values of the risk factors associated with HCC.
P<0.05 was considered to indicate a statistically significant
difference.
Results
FBL expression is increased in
HCC
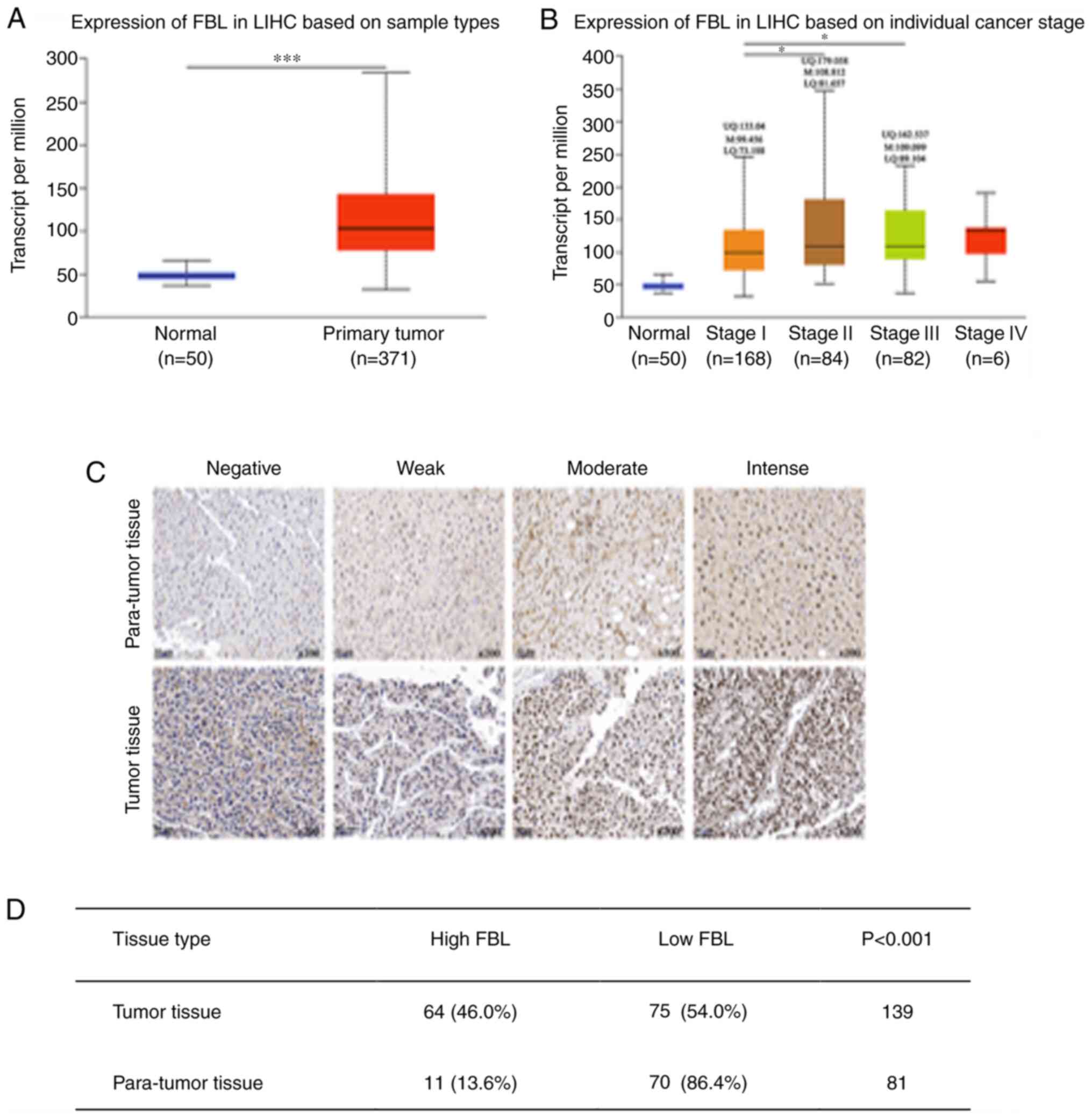
Analyses of the UALCAN database demonstrated that
FBL mRNA expression was higher in HCC tissues compared with normal
liver tissues; 50 normal tissues and 371 primary tumor tissues were
assessed (Fig. 1A). In addition,
high FBL mRNA expression was observed in patients with advanced HCC
than those with early HCC (Fig. 1B).
A total of 139 tumor tissues and 81 paired para-tumor tissues were
used in the present study to assess FBL protein expression. IHC
analysis was undertaken by our research team and the results
demonstrated that FBL protein was predominantly located in the
nuclei, at significantly higher levels in HCC tissues (Fig. 1C; upper panel, para-tumor tissues;
lower panel, tumor tissues). Among the 139 tumor tissues, IHC
analysis revealed 64 patients (46.0%) with high FBL expression.
However, among the 81 para-tumor tissues, 11 patients (13.6%) had
high FBL expression (P<0.001; Fig.
1D).
Association between FBL expression and
clinicopathological characteristics
The study cohort comprised 114 men and 25 women with
HCC, and the average age was 54.22 years (Table I). The χ2 test was used to
assess the association between FBL expression and the
clinicopathological characteristics of patients with HCC. The
results demonstrated that high FBL expression was significantly
associated with larger tumor diameter (P<0.001) and advanced TNM
stage (P=0.003). However, no significant associations were observed
between FBL expression and age, sex, tumor number, tumor
differentiation, infection with the hepatitis-B virus, liver
cirrhosis, AFP level and Child-Pugh grade (Table I).
 | Table I.Association between FBL expression
and the clinicopathological characteristics of patients with
hepatocellular carcinoma (n=139). |
Table I.
Association between FBL expression
and the clinicopathological characteristics of patients with
hepatocellular carcinoma (n=139).
|
Characteristics | No. of
patients | High FBL expression
(n=64) | Low FBL expression
(n=75) | P-value |
|---|
| Age, years | 54.22±10.13 | 53.17±11.20 | 55.12±9.10 |
0.260 |
|
<55 | 73 | 35 (47.9%) | 38 (52.1%) |
0.734 |
|
≥55 | 66 | 29 (43.9%) | 37 (56.1%) |
|
| Sex |
|
|
|
0.658 |
|
Male | 114 | 51 (44.7%) | 63 (55.3%) |
|
|
Female | 25 | 13 (52.0%) | 12 (48.0%) |
|
| AFP, ng/ml |
|
|
|
0.724 |
|
<400 | 88 | 42 (47.7%) | 46 (52.3%) |
|
|
≥400 | 51 | 22 (43.1%) | 29 (56.9%) |
|
| Child-Pugh |
|
|
|
0.845 |
| A | 104 | 47 (45.2%) | 57 (54.8%) |
|
| B | 35 | 17 (48.6%) | 18 (51.4%) |
|
| HBV infection |
|
|
|
0.327 |
|
Negative | 35 | 19 (54.3%) | 16 (45.7%) |
|
|
Positive | 104 | 45 (43.3%) | 59 (56.7%) |
|
| Liver
cirrhosis |
|
|
|
0.214 |
|
Absent | 50 | 27 (54.0%) | 23 (46.0%) |
|
|
Present | 89 | 37 (41.6%) | 52 (58.4%) |
|
| Tumor size, cm | 5.39±3.20 | 4.18±2.11 | 6.81±3.65 | <0.001 |
|
<5 | 69 | 19 (27.5%) | 50 (72.5%) | <0.001 |
| ≥5 | 70 | 45 (64.3%) | 25 (35.7%) |
|
| TNM stage |
|
|
|
0.003 |
| I | 82 | 29 (35.4%) | 53 (64.6%) |
|
|
II–III | 57 | 35 (61.4%) | 22 (38.6%) |
|
| Tumor number |
|
|
|
0.227 |
| 1 | 119 | 52 (43.7%) | 67 (56.3%) |
|
|
2-3 | 20 | 12 (60.0%) | 8 (40.0%) |
|
| Tumor
differentiation |
|
|
|
0.353 |
|
Low/moderate | 99 | 43 (43.4%) | 56 (56.6%) |
|
|
High | 40 | 21 (52.5%) | 19 (47.5%) |
|
| Tumor
encapsulation |
|
|
|
0.863 |
|
Complete | 56 | 25 (44.6%) | 31 (55.4%) |
|
|
Incomplete | 83 | 39 (47.0%) | 44 (53.0%) |
|
| Recurrence |
|
|
|
0.159 |
|
Yes | 107 | 53 (49.5%) | 54 (50.5%) |
|
| No | 32 | 11 (34.4%) | 21 (65.6%) |
|
| Death |
|
|
|
0.305 |
|
Yes | 80 | 40 (50.0%) | 40 (50%) |
|
| No | 59 | 24 (40.7%) | 35 (59.3%) |
|
High FBL expression is associated with
a poor prognosis in patients with HCC
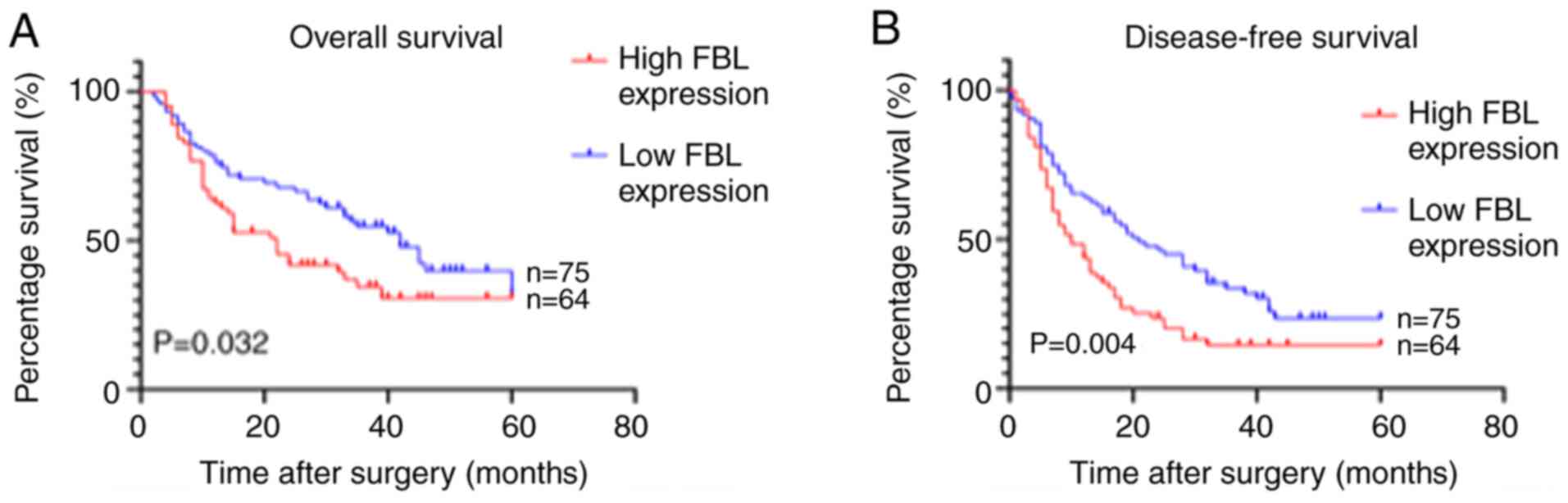
Kaplan-Meier survival analysis was performed to
assess the effect of FBL protein expression on the survival of
patients with HCC. The results demonstrated that overall survival
time (P=0.032) and disease-free survival time (P=0.004) were
significantly shorter in the high FBL expression group compared
with the low FBL expression group (Fig.
2). Cox regression analysis was subsequently performed to
determine the prognostic values of the risk factors in patients
with HCC. As presented in Table II,
univariate analysis demonstrated that high FBL expression
(P=0.036), tumor diameter (P<0.001) and TNM stage (P<0.001)
predicted a shorter overall survival time, while high FBL
expression (P=0.006), tumor diameter (P0.001), TNM stage
(P<0.001) and tumor number (P=0.019) predicted a shorter
disease-free survival time. Notably, multivariate analysis
demonstrated that high FBL expression was not an independent risk
factor for overall survival and disease-free survival time,
suggesting that FBL mainly affects the prognosis of patients with
HCC by promoting tumor progression (Table II).
 | Table II.Univariate and multivariate Cox
regression analyses of the risk factors in hepatocellular
carcinoma. |
Table II.
Univariate and multivariate Cox
regression analyses of the risk factors in hepatocellular
carcinoma.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Clinical
features | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Overall
survival |
| Sex
(male vs. female) | 1.026
(0.565–1.863) |
0.933 | – | – |
| Age,
years (≥55 vs. <55) | 0.999
(0.644–1.549) |
0.997 | – | – |
| HBV
infection (positive vs. negative) | 1.565
(0.904–2.709) |
0.110 | – | – |
| Liver
cirrhosis (present vs. absent) | 1.194
(0.747–1.908) |
0.458 | – | – |
|
Child-Pugh (A vs. B) | 1.217
(0.745–1.989) |
0.433 | – | – |
| Tumor
number (1 ns. 2–3) | 1.520
(0.866–2.668) |
0.145 | – | – |
| Tumor
size, cm (≥5 vs. <5) | 2.799
(1.761–4.447) | <0.001 | 2.232
(1.377–3.610) |
0.001 |
| TNM
stage (II–III vs. I) | 2.890
(1.849–4.519) | <0.001 | 2.315
(1.456–3.676) | <0.001 |
| Tumor
differentiation (III–IV vs. I–II) | 0.786
(0.473–1.305) |
0.352 | – | – |
| AFP,
ng/ml (≥400 vs. <400) | 1.088
(0.690–1.717) |
0.717 | – | – |
| FBL
expression (high vs. low) | 1.610
(1.031–2.514) |
0.036 | 1.044
(0.647–1.684) |
0.860 |
| Disease-free
survival |
| Sex
(male vs. female) | 0.987
(0.594–1.640) |
0.960 | – | – |
| Age,
years (≥55 vs. <55) | 1.107
(0.696–1.487) |
0.929 | – | – |
| HBV
infection (positive vs. negative) | 1.503
(0.940–2.405) |
0.089 | – | – |
| Liver
cirrhosis (present vs. absent) | 1.238
(0.824–1.862) |
0.304 | – | – |
|
Child-Pugh (A vs. B) | 1.111
(0.722–1.710) |
0.633 | – | – |
| Tumor
number (1 vs. 2–3) | 1.821
(1.105–3.002) |
0.019 | 1.110
(0.630–1.955) |
0.718 |
| Tumor
size, cm (≥5 vs. <5) | 2.377
(1.607–3.514) | <0.001 | 2.058
(1.377–3.067) | <0.001 |
| TNM
stage (II–III vs. I) | 2.707
(1.830–4.002) | <0.001 | 2.375
(1.690–3.546) | <0.001 |
| Tumor
differentiation (III–IV vs. I–II) | 1.208
(0.818–1.786) |
0.342 | – | – |
| AFP,
ng/ml (≥400 vs. <400) | 1.165
(0.787–1.724) |
0.447 | – | – |
| FBL
expression (high vs. low) | 1.721
(1.169–2.533) |
0.006 | 1.267
(0.834–1.927) |
0.267 |
Bioinformatics analysis of FBL-related
genes and the potential regulatory mechanism of FBL expression
The UALCAN database was used to identify genes
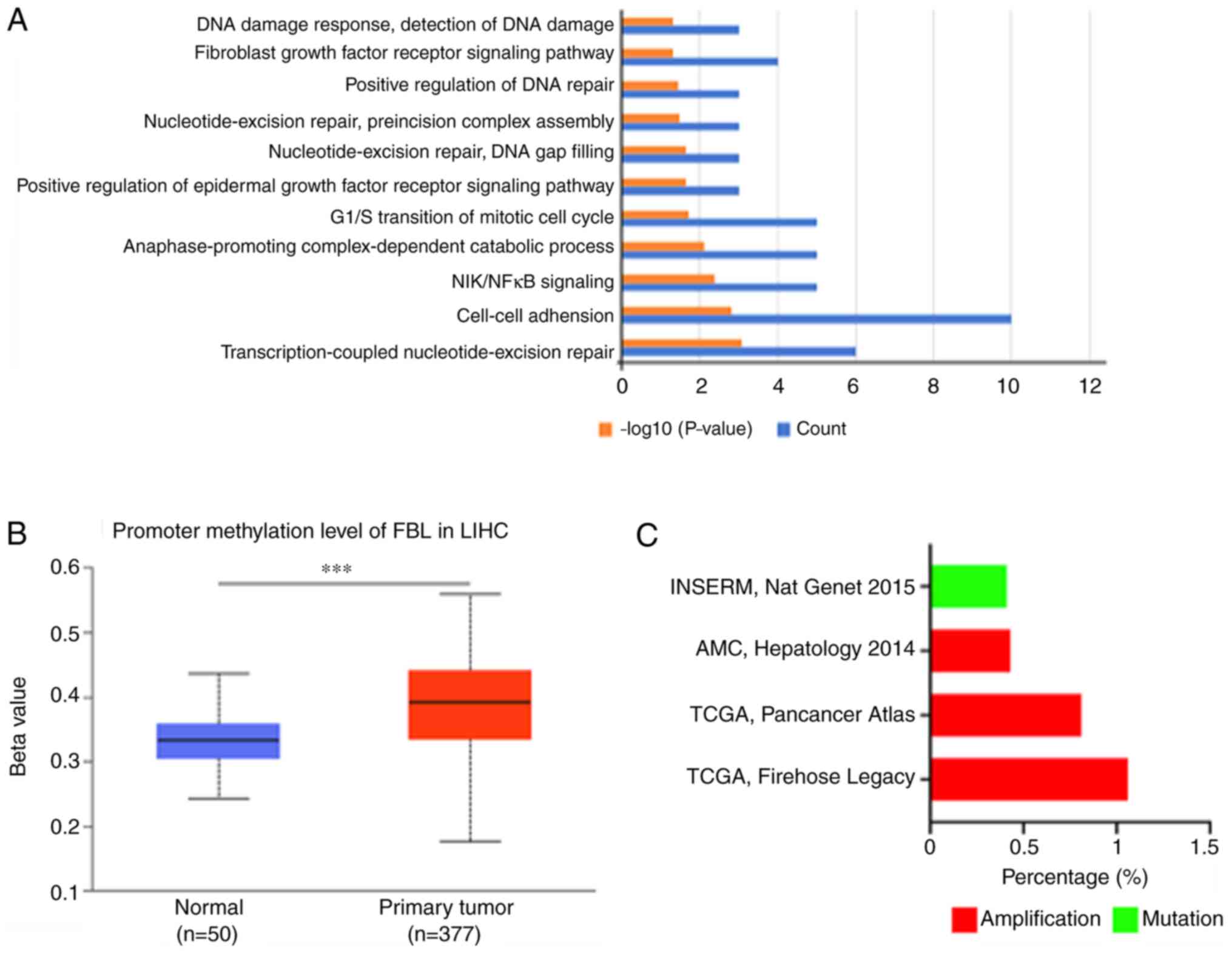
associated with FBL. The results demonstrated that 186 genes
(Person-CC≥0.5) were significantly associated with the FBL gene.
Functional enrichment analysis using the DAVID and GO databases
demonstrated a majority of biological processes and signaling
pathways associated with cancer progression, such as ‘cell-cell
adhesion’, ‘G1/S transition of mitotic cell cycle’ and ‘positive
regulation of epidermal growth factor receptor signaling pathway’.
The notable cancer-associated biological processes are presented in
Fig. 3A. The molecular mechanisms
regulating FBL expression are poorly understood (21,42).
Using publicly available data, it was demonstrated that methylation
of the FBL promoter was high (Fig.
3B); however, the frequency of amplification and mutation was
notably low in HCC (Fig. 3C). The
miRNAs that may bind with the 3′-UTR of the FBL gene were predicted
using TargetScan; however, no conserved miRNAs were identified for
FBL. Taken together, these results suggest that FBL expression is
mainly regulated by methylation.
Discussion
The prognosis of patients with HCC is poor (43–45),
thus understanding the pathogenesis, development, invasion, and
metastasis of HCC cells remains vital. The results of the present
study demonstrated high FBL expression in HCC tissues compared with
Para-tumor tissues. In addition, FBL expression was significantly
associated with the diameter and TNM stage of tumors, and high FBL
expression signified shorter overall survival time and disease-free
survival time in patients with HCC.
The ribosome is a complex “molecular machine”
composed of distinct proteins and nucleic acids, and is responsible
for protein synthesis (46,47). A broader role for dysregulated
ribosome biogenesis has been reported during the development and
progression of most types of malignant cancer (47–49).
Ribosomes can regulate some oncogenes and tumor suppressors by
alternatively translating specific mRNAs, such as p27, p53 and
vascular endothelial growth factor (50–52).
Modifications of rRNA have important roles in
regulating ribosome function (53),
and 2′-O-Me is the most common modification (23,24,54).
rRNA methylation can change the combination of specific mRNAs and
ribosomes to regulate protein expression (18,24). FBL
plays a key role in 2′-O-Me, whereby changes in FBL expression
notably affect the translation process (23). Thus, FBL can affect the course of
some cellular processes (55–57).
Marcel et al (20) reported
that high FBL expression is an independent marker of a poor outcome
in breast cancer, and that FBL can induce proliferation of MCF7
cells by regulating rRNA methylation. In addition, FBL is required
for proliferation, clonogenic survival and appropriate rRNA
accumulation/processing in human prostate cancer cells (25). However, the results of the present
study demonstrated that high FBL expression was a predictive factor
for poor prognosis, advanced tumor stage and large tumor diameter
in HCC.
Koh et al demonstrated that the classical
oncogene, MYC can induce FBL overexpression to accelerate prostate
cancer (25). As a tumor-suppressor
gene, p53 can safeguard protein synthesis by suppressing FBL
expression and regulating the subsequent quality and intrinsic
activity of ribosomes (20). El
Hassouni et al reported that FBL inhibition prior to
interaction with C/D box snoRNA is an ideal target to inhibit
ribosome biogenesis during cancer therapy (58). To further determine the regulatory
mechanism of FBL, the UALCAN, cBioportal and TargetScan databases
were used. The UALCAN database predicted highly frequent
methylation of FBL in HCC. Using publicly available sequencing
data, low frequencies of amplifications and mutations were
demonstrated in HCC. Furthermore, no conserved miRNAs were
identified for FBL according to the TargetScan database. Taken
together, these results suggest that methylation may be the main
cause for FBL overexpression in HCC. In the present study, KEGG
analysis demonstrated that the function of FBL correlated genes was
mainly associated with the occurrence and development of
tumors.
To the best of our knowledge, the present study was
the first to assess the association between FBL expression and the
clinicopathological characteristics of patients with HCC, as well
as determine the prognostic and predictive values of FBL in HCC.
The results demonstrated that FBL was highly expressed in HCC and
may accelerate HCC; however, the regulatory mechanism of FBL
remains unknown. Taken together, the results of the present study
suggest that FBL may be an ideal target for HCC therapy.
Acknowledgements
The authors of the present study would like to thank
Dr Qiang Li and Gang Yang (Department of Hepatobiliary Surgery,
Affiliated Hospital of North Sichuan Medical College), for
supplying the tissue samples and clinical information of
patients.
Funding
Not applicable.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the present
study.
Authors' contribution
FX conceived the present study. JZ drafted the
initial manuscript, and performed immunohistochemistry and
statistical analyses. GY and QL collected the tissue samples and
analyzed patient's clinical information. All authors contributed to
the critical revision of the article for important intellectual
content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of North Sichuan Medical
College (Nanchong, China; approval no. 2020ER(A)024; May 29, 2019).
Written informed consent was provided by patients or their close
relatives prior to the study start for use of their tissue samples
and clinical details.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
FBL
|
fibrillarin
|
|
HCC
|
hepatocellular carcinoma
|
|
IHC
|
immunohistochemical
|
|
TMAs
|
tissue microarrays
|
|
M
|
median
|
|
AFP
|
α-fetoprotein
|
References
|
1
|
McGlynn KA, Petrick JL and London WT:
Global epidemiology of hepatocellular carcinoma: An emphasis on
demographic and regional variability. Clin Liver Dis. 19:223–238.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villanueva A: Hepatocellular Carcinoma.
Reply. N Engl J Med. 381:e22019.Reply. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong RJ, Kim D, Ahmed A and Singal AK:
Patients with hepatocellular carcinoma from more rural and lower
income households have more advanced tumor stage at diagnosis and
significantly higher mortality. Cancer cncr. 33211:2020.
|
|
4
|
Javadian P and Nezhat F: Endometrial
carcinoma and its precursors. Adv Exp Med Biol. 1242:59–72. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Couri T and Pillai A: Goals and targets
for personalized therapy for HCC. Hepatol Int. 13:125–137. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raoul JL, Forner A, Bolondi L, Cheung TT,
Kloeckner R and de Baere T: Updated use of TACE for hepatocellular
carcinoma treatment: How and when to use it based on clinical
evidence. Cancer Treat Rev. 72:28–36. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raza A and Sood GK: Hepatocellular
carcinoma review: Current treatment, and evidence-based medicine.
World J Gastroenterol. 20:4115–4127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fu J and Wang H: Precision diagnosis and
treatment of liver cancer in China. Cancer Lett. 412:283–288. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Llovet JM, Montal R, Sia D and Finn RS:
Molecular therapies and precision medicine for hepatocellular
carcinoma. Nat Rev Clin Oncol. 15:599–616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rashed WM, Kandeil MAM, Mahmoud MO and
Ezzat S: Hepatocellular carcinoma (HCC) in Egypt: A comprehensive
overview. J Egypt Natl Canc Inst. 32:52020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Waidmann O: Recent developments with
immunotherapy for hepatocellular carcinoma. Expert Opin Biol Ther.
18:905–910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mauro VP and Matsuda D: Translation
regulation by ribosomes: Increased complexity and expanded scope.
RNA Biol. 13:748–755. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gentilella A, Kozma SC and Thomas G: A
liaison between mTOR signaling, ribosome biogenesis and cancer.
Biochim Biophys Acta. 1849:812–820. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lawrence MG, Obinata D, Sandhu S, Selth
LA, Wong SQ, Porter LH, Lister N, Pook D, Pezaro CJ, Goode DL, et
al: Patient-derived models of abiraterone- and
enzalutamide-resistant prostate cancer reveal sensitivity to
ribosome-directed therapy. Eur Urol. 74:562–572. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mugridge JS and Gross JD: Decapping
enzymes STOP ‘cancer’ ribosomes in their tracks. EMBO J.
37:e1008012018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sriram A, Bohlen J and Teleman AA:
Translation acrobatics: how cancer cells exploit alternate modes of
translational initiation. EMBO Rep. 19:e459472018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dunn S, Lombardi O and Cowling VH: c-Myc
co-ordinates mRNA cap methylation and ribosomal RNA production.
Biochem J. 474:377–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Monaco PL, Marcel V, Diaz JJ and Catez F:
2′-O-methylation of ribosomal RNA: towards an epitranscriptomic
control of translation? Biomolecules. 8:1062018. View Article : Google Scholar
|
|
19
|
Sergiev PV, Aleksashin NA, Chugunova AA,
Polikanov YS and Dontsova OA: Structural and evolutionary insights
into ribosomal RNA methylation. Nat Chem Biol. 14:226–235. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marcel V, Ghayad SE, Belin S, Therizols G,
Morel AP, Solano-Gonzàlez E, Vendrell JA, Hacot S, Mertani HC,
Albaret MA, et al: p53 acts as a safeguard of translational control
by regulating fibrillarin and rRNA methylation in cancer. Cancer
Cell. 24:318–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shubina MY, Musinova YR and Sheval EV:
Proliferation, cancer, and aging-novel functions of the nucleolar
methyltransferase fibrillarin? Cell Biol Int. 42:1463–1466. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ayadi L, Galvanin A, Pichot F, Marchand V
and Motorin Y: RNA ribose methylation (2′-O-methylation):
Occurrence, biosynthesis and biological functions. Biochim Biophys
Acta Gene Regul Mech. 1862:253–269. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Erales J, Marchand V, Panthu B, Gillot S,
Belin S, Ghayad SE, Garcia M, Laforêts F, Marcel V, Baudin-Baillieu
A, et al: Evidence for rRNA 2′-O-methylation plasticity: Control of
intrinsic translational capabilities of human ribosomes. Proc Natl
Acad Sci USA. 114:12934–12939. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li L, Miao W, Williams P, Guo C and Wang
Y: SLIRP interacts with helicases to facilitate 2′-O-methylation of
rRNA and to promote translation. J Am Chem Soc. 141:10958–10961.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koh CM, Gurel B, Sutcliffe S, Aryee MJ,
Schultz D, Iwata T, Uemura M, Zeller KI, Anele U, Zheng Q, et al:
Alterations in nucleolar structure and gene expression programs in
prostatic neoplasia are driven by the MYC oncogene. Am J Pathol.
178:1824–1834. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su H, Xu T, Ganapathy S, Shadfan M, Long
M, Huang TH, Thompson I and Yuan ZM: Elevated snoRNA biogenesis is
essential in breast cancer. Oncogene. 33:1348–1358. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iyer-Bierhoff A and Grummt I: Stop-and-Go:
Dynamics of nucleolar transcription during the cell cycle. Epigenet
Insights. 12:25168657198490902019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsoris A and Marlar CA: Use Of The Child
Pugh Score In Liver Disease. StatPearls Publishing; Treasure
Island, FL: 2020
|
|
29
|
Jing JS, Li H, Wang SC, Ma JM, Yu LQ and
Zhou H: NDRG3 overexpression is associated with a poor prognosis in
patients with hepatocellular carcinoma. Biosci Rep.
38:BSR201809072018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schulze K, Imbeaud S, Letouzé E,
Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C,
Shinde J, Soysouvanh F, et al: Exome sequencing of hepatocellular
carcinomas identifies new mutational signatures and potential
therapeutic targets. Nat Genet. 47:505–511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ahn SM, Jang SJ, Shim JH, Kim D, Hong SM,
Sung CO, Baek D, Haq F, Ansari AA, Lee SY, et al: Genomic portrait
of resectable hepatocellular carcinomas: Implications of RB1 and
FGF19 aberrations for patient stratification. Hepatology.
60:1972–1982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhandari V, Hoey C, Liu LY, Lalonde E, Ray
J, Livingstone J, Lesurf R, Shiah YJ, Vujcic T, Huang X, et al:
Molecular landmarks of tumor hypoxia across cancer types. Nat
Genet. 51:308–318. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bonneville R, Krook MA, Kautto EA, Miya J,
Wing MR, Chen HZ, Reeser JW, Yu L and Roychowdhury S: Landscape of
microsatellite instability across 39 cancer types. JCO Precis
Oncol. 2017:PO.17.00073. 2017.PubMed/NCBI
|
|
34
|
Ding L, Bailey MH, Porta-Pardo E, Thorsson
V, Colaprico A, Bertrand D, Gibbs DL, Weerasinghe A, Huang KL,
Tokheim C, et al Cancer Genome Atlas Research Network, :
Perspective on oncogenic processes at the end of the beginning of
cancer genomics. Cell. 173:305–320.e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ellrott K, Bailey MH, Saksena G, Covington
KR, Kandoth C, Stewart C, Hess J, Ma S, Chiotti KE, McLellan M, et
al MC3 Working Group; Cancer Genome Atlas Research Network, :
Scalable open science approach for mutation calling of tumor exomes
using multiple genomic pipelines. Cell Syst. 6:271–281.e7. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao Q, Liang WW, Foltz SM, Mutharasu G,
Jayasinghe RG, Cao S, Liao WW, Reynolds SM, Wyczalkowski MA, Yao L,
et al Fusion Analysis Working Group; Cancer Genome Atlas Research
Network, : Driver fusions and their implications in the development
and treatment of human cancers. Cell Rep. 23:227–238.e3. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hoadley KA, Yau C, Hinoue T, Wolf DM,
Lazar AJ, Drill E, Shen R, Taylor AM, Cherniack AD, Thorsson V, et
al Cancer Genome Atlas Network, : Cell-of-origin patterns dominate
the molecular classification of 10,000 tumors from 33 types of
cancer. Cell. 173:291–304.e6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu J, Lichtenberg T, Hoadley KA, Poisson
LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee
AV, et al Cancer Genome Atlas Research Network, : An integrated
TCGA pan-cancer clinical data resource to drive high-quality
survival outcome analytics. Cell. 173:400–416.e11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Poore GD, Kopylova E, Zhu Q, Carpenter C,
Fraraccio S, Wandro S, Kosciolek T, Janssen S, Metcalf J, Song SJ,
et al: Microbiome analyses of blood and tissues suggest cancer
diagnostic approach. Nature. 579:567–574. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sanchez-Vega F, Mina M, Armenia J, Chatila
WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia
S, et al Cancer Genome Atlas Research Network, : Oncogenic
signaling pathways in The Cancer Genome Atlas. Cell.
173:321–337.e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Taylor AM, Shih J, Ha G, Gao GF, Zhang X,
Berger AC, Schumacher SE, Wang C, Hu H, Liu J, et al Cancer Genome
Atlas Research Network, : Genomic and functional approaches to
understanding cancer aneuploidy. Cancer Cell. 33:676–689.e3. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rodriguez-Corona U, Sobol M,
Rodriguez-Zapata LC, Hozak P and Castano E: Fibrillarin from
Archaea to human. Biol Cell. 107:159–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ascione A, Fontanella L, Imparato M,
Rinaldi L and De Luca M: Mortality from cirrhosis and
hepatocellular carcinoma in Western Europe over the last 40 years.
Liver Int. 37:1193–1201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Beal EW, Tumin D, Kabir A, Moris D, Zhang
XF, Chakedis J, Washburn K, Black S, Schmidt CM and Pawlik TM:
Trends in the mortality of hepatocellular carcinoma in the United
States. J Gastrointest Surg. 21:2033–2038. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weaver AJ, Stafford R, Hale J, Denning D
and Sanabria JR; GBD Collaborators, : Geographical and temporal
variation in the incidence and mortality of
hepato-pancreato-biliary primary malignancies: 1990–2017. J Surg
Res. 245:89–98. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brandman O and Hegde RS:
Ribosome-associated protein quality control. Nat Struct Mol Biol.
23:7–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
van Riggelen J, Yetil A and Felsher DW:
MYC as a regulator of ribosome biogenesis and protein synthesis.
Nat Rev Cancer. 10:301–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Arthurs C, Murtaza BN, Thomson C, Dickens
K, Henrique R, Patel HRH, Beltran M, Millar M, Thrasivoulou C and
Ahmed A: Expression of ribosomal proteins in normal and cancerous
human prostate tissue. PLoS One. 12:e01860472017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sulima SO, Kampen KR, Vereecke S, Pepe D,
Fancello L, Verbeeck J, Dinman JD and De Keersmaecker K: Ribosomal
lesions promote oncogenic mutagenesis. Cancer Res. 79:320–327.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bellodi C, Kopmar N and Ruggero D:
Deregulation of oncogene-induced senescence and p53 translational
control in X-linked dyskeratosis congenita. EMBO J. 29:1865–1876.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bellodi C, Krasnykh O, Haynes N,
Theodoropoulou M, Peng G, Montanaro L and Ruggero D: Loss of
function of the tumor suppressor DKC1 perturbs p27 translation
control and contributes to pituitary tumorigenesis. Cancer Res.
70:6026–6035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Rocchi L, Pacilli A, Sethi R, Penzo M,
Schneider RJ, Treré D, Brigotti M and Montanaro L: Dyskerin
depletion increases VEGF mRNA internal ribosome entry site-mediated
translation. Nucleic Acids Res. 41:8308–8318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sloan KE, Warda AS, Sharma S, Entian KD,
Lafontaine DLJ and Bohnsack MT: Tuning the ribosome: The influence
of rRNA modification on eukaryotic ribosome biogenesis and
function. RNA Biol. 14:1138–1152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nachmani D, Bothmer AH, Grisendi S, Mele
A, Bothmer D, Lee JD, Monteleone E, Cheng K, Zhang Y, Bester AC, et
al: Germline NPM1 mutations lead to altered rRNA 2′-O-methylation
and cause dyskeratosis congenita. Nat Genet. 51:1518–1529. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Amin MA, Matsunaga S, Ma N, Takata H,
Yokoyama M, Uchiyama S and Fukui K: Fibrillarin, a nucleolar
protein, is required for normal nuclear morphology and cellular
growth in HeLa cells. Biochem Biophys Res Commun. 360:320–326.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bouffard S, Dambroise E, Brombin A,
Lempereur S, Hatin I, Simion M, Corre R, Bourrat F, Joly JS and
Jamen F: Fibrillarin is essential for S-phase progression and
neuronal differentiation in zebrafish dorsal midbrain and retina.
Dev Biol. 437:1–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Newton K, Petfalski E, Tollervey D and
Cáceres JF: Fibrillarin is essential for early development and
required for accumulation of an intron-encoded small nucleolar RNA
in the mouse. Mol Cell Biol. 23:8519–8527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
El Hassouni B, Sarkisjan D, Vos JC,
Giovannetti E and Peters GJ: Targeting the Ribosome Biogenesis Key
Molecule Fibrillarin to Avoid Chemoresistance. Curr Med Chem.
26:6020–6032. 2019. View Article : Google Scholar : PubMed/NCBI
|