Introduction
Thyroid cancer is uncommon, accounting for only ~1%
of all malignancies, but it is the most common malignant endocrine
tumor worldwide. Papillary thyroid carcinoma (PTC) accounts for 85%
of thyroid malignancies and is defined as an indolent malignancy
(1–3). Currently, the treatment for PTC is
surgery, and either thyroid lobectomy or total thyroidectomy may be
considered. Lymph node dissection may be performed in patients with
evidence of nodal disease (4).
Postoperative radioiodine therapy is required for patients at a
high risk of cancer recurrence and persistent disease (5,6). New
therapies that target specific molecular alterations in PTC have
been developed, for example lenvatinib is associated with
significant improvements in progression-free survival and the
response rate among patients with iodine-131-refractory thyroid
cancer (7). In February 2015, the US
FDA approved levatinib for the treatment of advanced thyroid
cancer. Overall prognosis is not as poor in PTC as in several other
human cancer types as the 5-year survival rate of PTC is >95%.
However, the standard therapy including surgery, and adjuvant
radioactive iodine and thyroid-stimulating hormone (TSH)
suppression therapy results in little clinical benefit for patients
with lymph node or distant metastases. Thus, identification of new
markers and potential treatment methods may be highly beneficial
for these patients (3,8).
Lin28 is a highly conserved RNA-binding and microRNA
(miRNA/miR)-regulated protein. Recent studies have shown that Lin28
contributes to multiple biological processes, including
tumorigenesis, cellular differentiation, pluripotency and
reprogramming (9,10). Lin28 expression is dysregulated in
various human epithelial-type neoplasms, such as oral squamous cell
carcinoma (11), colon (12), ovarian (13) and gastric cancer (14), hepatocellular carcinoma (15), and lung (16), breast (17) and esophageal cancer (18). Our previous studies have indicated
that overexpression of Lin28 is correlated with lymph node
metastasis and poor prognosis in breast (17) and gastric cancer (14). In general, Lin28 can selectively
block the biogenesis of mature let-7, which acts as a tumor
suppressor by inhibiting the expression of the oncogenes downstream
of the Lin28/let-7 axis, including RAS, Myc and high mobility group
protein HMGI-C (19). Let-7 miRNAs
are downregulated in numerous types of cancer, such as
hepatocellular carcinoma, gastric adenocarcinoma, pancreatic
ovarian and prostate cancer, Burkitt lymphoma, renal cell carcinoma
and breast cancer, and can suppress the proliferation of
self-renewing tumor-initiating cells and facilitate differentiation
(20). To the best of our knowledge,
only one study has investigated the relationship between Lin28 and
PTC (21). However, the number of
clinical samples used in this previous study was very low, with
only 57 patients after thyroid carcinoma radical surgery and 30
patients with nodular goiters. Moreover, this group investigated
the role of the Lin28a/let-7a/c-Myc pathway in PTC tumorigenesis
and malignancy but did not investigate the relationship between
Lin28 expression and clinicopathological parameters. The objective
of the current study was to analyze the association between the
clinicopathological parameters and prognosis of PTC, whereby new
preventive or therapeutic targets for PTC may be identified.
Materials and methods
Case selection
For the purpose of this study, a total of 237
surgically resected specimens of patients with PTC treated at the
Department of Breast and Thyroid Surgery, Shaoxing People's
Hospital (Shaoxing, China) between January 2016 and December 2018
were collected. The inclusion criteria for the study presented were
as follows: i) Patients who had complete clinical data and had
undergone radical resection of thyroid carcinoma and ii) patients
who were definitively diagnosed with PTC by two experienced
pathologists at Shaoxing People's Hospital. The present study
excluded patients unwilling to abide by the protocol, as well as
those that were legally incapacitated. Patients who had either
received radiotherapy or chemotherapy prior to the surgical
resection procedure were also excluded. In total, 237 patients were
included in the study, including 47 males (19.83%) and 190 females
(80.17%) aged 19–75 years, with an average age of 47.4 years. The
original data (with respect to the specimens) are provided in
Table SI. All patients signed
informed consent under the guidelines of The Ethics Committee of
Shaoxing People's Hospital before the study. The records reviewed
included age, sex, tumor size, lymph node metastasis, bilateral
multifocality, extrathyroidal extension and Lin28 expression level.
Pathological features were classified according to the guidelines
of the International Union against Cancer and the 8th edition of
the American Joint Committee (22).
Ethics approval
The study was approved by The Ethics Committee of
the Shaoxing People's Hospital (Shaoxing, China) before initiation.
Written informed consent or verbal informed consent via phone
(which was recorded) was provided by all participants or their
guardians. These two forms of consent are both acceptable by the
Ethics Committee of The Shaoxing People's Hospital. The consent
forms can be provided upon reasonable request.
Immunohistochemical (IHC)
analysis
IHC analysis was performed to evaluate the
expression levels of the Lin28 protein in PTC tissues. The
specimens were fixed with formaldehyde and embedded in paraffin, as
previously described (18). Paraffin
sections were deparaffinized in xylene and dehydrated with a
gradient ethanol series with 5-min washes at room temperature as
follows: 100, 100, 95, 95, 80, 70 and 50% ethanol, followed by two
washes with ddH2O. To quench the activity of endogenous
peroxidase, the sections were treated with 0.3%
H2O2 in methanol for 5 min. Before starting
IHC analysis, the sections were immersed in 10 mM citrate buffer
(pH=6.1) at 95°C for 15 min for antigen retrieval. Non-specific
antibody binding was blocked by pre-incubating the sections with
10% fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.) in PBS
with 0.01% sodium azide at room temperature for 30 min, and then
the sections were incubated with an antibody against Lin28 (rabbit
polyclonal, H-44, 1:100; Santa Cruz Biotechnology Inc.) overnight
at 4°C. After washing three times with PBS, the sections were
incubated with the EnVision-HRP complex (undiluted; Dako; Agilent
Technologies, Inc.) for 1 h at room temperature. The sections were
then stained with diaminobenzidine at room temperature for 25 sec
and counterstained with hematoxylin at room temperature for 5 min.
PBS, instead of Lin28, was used as a negative control. Gastric
cancer tissue, which is known to exhibit high levels of Lin28
(19), was used as a positive
control.
All the IHC analysis steps were carried out in the
same laboratory. Expression levels were semi-quantitatively
assessed based on the product of the cell staining intensity score
and positive cell percentage score. The staining results were
determined by two independent observers to avoid subjective biases.
Staining intensity, which represented the average intensity of the
stained tumor cells, was scaled as 0 (no staining), 1 (weak
staining), 2 (moderate staining) and 3 (strong staining). Scores of
percent positive cells were as follows: 0 points for positive
rate=0%, 1 point for 0% <positive rate ≤25%, 2 points for 25%
<positive rate ≤50%, 3 points for 50% <positive rate ≤75%,
75% <positive rate ≤100% is 4 points. The two scores were
calculated to obtain a final score, which ranged from 0 to 9. Three
visual fields were randomly selected for each specimen under a
light microscope, and their average values were applied for the
final analysis. Lin28 protein-positive expression was defined as
total score ≥2.
Bioinformatics analysis
Gene expression data and corresponding clinical data
were obtained from the Gene Expression Omnibus (GEO) database
(ncbi.nlm.nih.gov/gds). The dataset
GSE33630 (23) was extracted from
the GEO database was analyzed using a paired student's t-test to
compare the differential expression levels of Lin28 between PTC and
normal thyroid tissues. These databases were searched for data on
Lin28 expression and lymph node metastasis, but no appropriate data
was found. The human gene expression array GPL15207 (24) was downloaded from the GEO database.
Three different groups were included in the study: i)
Non-aggressive tumor (intrathyroidal tumors, with no lymphovascular
invasion) with carcinoma sizes <2 cm (group A, n=3), ii)
patients with minimal invasion of the extrathyroidal soft tissue
(group B, n=3) and iii) patients with distant organ metastasis
(group C, n=3). The age distributions of the three groups were
47.50±7.44, 47.00±16.16, and 55.00±15.63, respectively. The
proportions of women are 75.0, 86.4, and 75.0%. The gene expression
data of Lin28 were extracted to perform one-way ANOVA
followed by Tukey's post hoc test among three groups to explore
whether Lin28 expression influences distant organ metastasis or
invasion of the extrathyroidal soft tissue of patients with
PTC.
Statistical analysis
All the statistical analyses were conducted using
SPSS version 15.0. (SPSS, Inc). Quantitative data is expressed as
mean ± SD. The unpaired Student's t-test and χ2 test
were performed to evaluate the statistical significance of the
differences between two groups and the association between Lin28
expression and clinicopathological parameters, respectively.
One-way ANOVA and Tukey's test was used for multiple comparisons.
The predictive values of the presence of Lin 28 and other factors
for lymph node metastases were assessed using univariate and
multivariate logistic regression models, respectively. All the
variables that were significantly associated with lymph node
metastasis in the univariate model were included in the
multivariate analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression profiling of Lin28 protein
in tissues
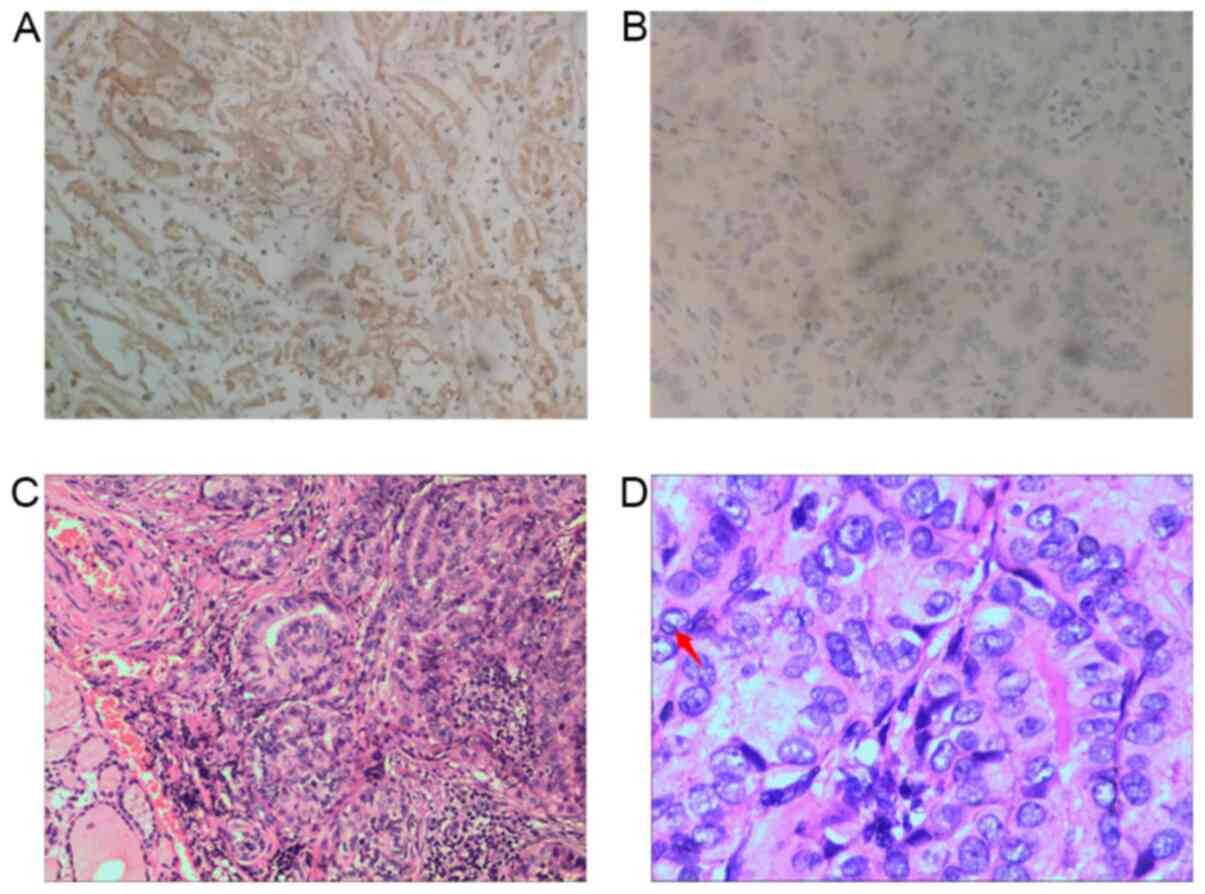
Lin28 protein was expressed in 96/237 (40.5%) PTC
specimens. Lin28 staining was predominantly localized in the
cytoplasm in these tissues (Fig.
1A). A representative PTC specimen stained with hematoxylin and
eosin is shown in Fig. 1C and D. It
shows the nuclear features of papillary thyroid carcinoma,
including crowded and overlapping grooves and
pseudo-inclusions.
Association between Lin28 expression
and clinicopathological features
Table I shows the
association between Lin28 expression levels and clinicopathological
characteristics, including age, sex, tumor size, lymph node
metastasis, bilateral multifocality and extrathyroidal extension.
Statistical analysis results showed that Lin28-positive cases had
significantly larger tumors (P=0.011) and more lymph node
metastases compared Lin28-negative cases (P<0.001). However, no
difference was observed between the two groups with regards to sex,
age, or the presence of bilateral multifocality or extrathyroidal
extension (Table I).
 | Table I.Clinicopathological characteristics of
237 patients with papillary thyroid carcinoma. |
Table I.
Clinicopathological characteristics of
237 patients with papillary thyroid carcinoma.
|
| Lin28 expression, n
(%) |
|
|---|
|
|
|
|
|---|
| Variable | Positive | Negative | P-value |
|---|
| Age, years | 47.54±10.6 | 47.27±11.8 | 0.859 |
| Sex |
|
| 0.990 |
|
Female | 77
(80.2) | 113 (80.1) |
|
|
Male | 19
(19.8) | 28
(19.9) |
|
| Tumor size, cm |
|
| 0.011 |
| ≤2 | 87
(90.6) | 135 (95.7) |
|
| >2
and ≤4 | 6
(6.2) | 5
(3.5) |
|
|
>4 | 3
(3.1) | 1
(0.7) |
|
| Lymph node
metastasis |
|
| <0.001 |
|
Yes | 73
(30.8) | 35
(14.8) |
|
| No | 23 (9.7) | 106 (44.7) |
|
| Multiple foci |
|
| 0.647 |
|
Yes | 21
(21.9) | 19
(13.5) |
|
| No | 75
(78.1) | 122 (86.5) |
|
| Extrathyroidal
extension |
|
| 0.441 |
|
Yes | 6
(6.3) | 12 (8.5) |
|
| No | 90
(93.8) | 129 (91.5) |
|
Multivariate analysis
A logistic regression model was constructed for the
factors associated with lymph node metastasis in PTC (Table II). Sex (odds ratio [OR] 0.44;
P=0.015), tumor size (OR 3.46; P<0.001) and the presence of
Lin28 (OR 9.61; P<0.001) were associated with lymph node
metastasis in the univariate analysis. These parameters were then
analyzed using multivariate analysis. The results showed that sex,
tumor size and the presence of Lin28 were risk factors for lymph
node metastasis in PTC (Table II).
The presence of Lin28 was found to be a risk factor for lymph node
metastasis in PTC.
 | Table II.Results of multivariate analysis for
the risk factors of lymph node metastases in papillary thyroid
carcinoma (n=237). |
Table II.
Results of multivariate analysis for
the risk factors of lymph node metastases in papillary thyroid
carcinoma (n=237).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | OR (95% CI) | P-value | OR (95%CI) | P-value |
|---|
| Lin28 expression,
positive vs. negative | 9.61
(5.25–17.60) | <0.001 | 10.16
(5.25–19.64 | <0.001 |
| Sex, female vs.
male | 0.44
(0.23–0.85) | 0.015 | 0.37
(0.16–0.82) | 0.015 |
| Tumor
sizea, cm | 3.46
(2.02–5.94) | <0.001 | 3.16
(1.73–5.77) | <0.001 |
| Agea, years | 0.99
(0.97–1.01) | 0.288 | – | – |
| Multiple foci,
multifocal vs. unifocal | 1.05
(0.49–2.27) | 0.897 | – | – |
| Extrathyroidal
extension, yes vs. no) | 1.13
(0.77–1.66) | 0.538 | – | – |
RNA expression levels of Lin28 in PTC
according to GEO and TCGA databases
In the GEO database, Lin28A expression was
slightly elevated in PTC tissues compared with the levels in normal
thyroid tissue, but without statistical significance (P=0.2022;
Fig. S1A). In addition, data
referring to PTC patients' lymph node metastasis could not be
obtained from both TCGA and GEO databases. However, data on distant
organ metastasis in the context of PTC were obtained from the GEO
database (Fig. S1B and C). It was
revealed that Lin28 is associated with aggressive
characteristics for patients with PTC, but this was not
significant.
Discussion
As the most common type of thyroid carcinoma, PTC
has received increasing attention because of its increasing
morbidity rate worldwide (1). It is
estimated that there were 567,233 new cases of thyroid cancer
worldwide in 2018 (25). Benefiting
from considerable therapeutic development, PTC prognosis and
overall survival time have been associated with significant
improvements (26). In PTC,
metastasis to lymph nodes is associated with an increased risk of
cancer recurrence but not with survival (3,27,28).
Additionally, extranodal extension of lymph node metastases is
associated with a significantly increased risk of recurrence and
mortality (29,30). However, some patients with PTC have
poor overall survival due to the presence of distant metastases at
diagnosis (31,32). Consequently, it is important to
identify novel biological targets that can be used as prognostic
markers for patients with PTC.
Numerous cancer-related genes are closely correlated
with PTC progression. These genes affect the pathogenesis and
prognosis of PTC. For example, Siraj et al (33) demonstrated a strong relationship
between DNA (cytosine-5)-methyltransferase 3A mutations, and
adverse clinical outcomes in PTC cases. Previous studies have
reported that telomerase reverse transcriptase promoter mutations
have an independent prognostic value in differentiated thyroid
carcinomas and in PTC (32,34). In addition, Collina et al
(35) reported that tyrosine-protein
kinase receptor UFO levels can be used as a predictor of
radioactive iodine refractoriness and as a possible therapeutic
target for radioactive iodine-resistant PTCs. Han et al
(36) demonstrated that miR-215
plays a critical role as a tumor suppressor in PTC and is a
prognostic biomarker influencing proliferation and metastasis by
targeting the ADP ribosylation factor guanine nucleotide-exchange
factor 1. Taken together, these prior studies have highlighted the
importance of the involvement of genes in cancer development.
Lin28 expression has been widely profiled in
multiple cancer types, such as glioblastoma and ovarian, gastric,
prostate and breast cancer, and most studies have shown that Lin28
serves as a marker for poor survival in patients (19,37,38).
Lin28 can promote the proliferation, migration and invasion of
various human epithelial-type neoplasms, and might be a promising
target for therapeutic intervention in the future (19). However, limited investigation has
been conducted on the relationship between PTC and Lin28. The only
such investigation to date, carried out by Huang et al
(21), reported that the
Lin28A/let-7a/c-Myc pathway is involved in tumor growth and
malignancy in PTC and is a potential target for therapeutic
intervention for this disease.
The present study attempted to provide a further
prognostic basis regarding the association between Lin28 expression
and PTC. Therefore, Lin28 expression patterns in PTC specimens were
preliminarily investigated. It was demonstrated that Lin28
expression tends to be associated with more extensive lymph node
metastasis in PTC. To validate the present results, additional data
was retrieved and analyzed from the GEO online database. However,
the results obtained for our clinical samples were not reproduced.
A possible explanation for the partial non-reproducibility of the
results reported in the present study might be due to the limited
data on PTC and Lin28 available in the online databases, especially
considering the presence of lymph node metastasis.
In future research, the molecular mechanisms and
pathways underlying the involvement of Lin28 in PTC should be
investigated. In addition, the present results should be verified
using data retrieved from additional online databases, depending on
availability. It is considered that with a higher number of
datasets, the data will align with what is reported in the present
study. This said, well-designed studies including animal models and
studies with larger ample sizes will also be needed to validate the
present observations.
In summary, Lin28 expression was markedly elevated
in PTC tissues. In addition, the expression of the Lin28 protein
could be used as a novel molecular marker for predicting the
clinical outcome of PTC from specimens. Hence, future
investigations on the precise roles of Lin28 in PTC will enhance
our understanding of this disease and provide new insights into its
diagnosis and treatment.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The study was supported by grants from The National
Natural Science Foundation of China (grant no. 81341135), Zhejiang
Non-profit Technology-Applied Research Projects of China (grant no.
2016C33226) and Shaoxing Non-profit Technology-Applied Research
Projects of China (grant no. 2017B70037 2017QN002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
requests. The datasets generated and/or analyzed during the current
study are available in The Cancer Genome [cancergenome.nih.gov] and Gene Expression Omnibus
repositories [ncbi.nlm.nih.gov/gds].
Authors' contributions
CX, LW and SJ conceived and directed the project. JW
provided the pathological sections and performed the immunochemical
scoring together with SW. CX and SJ collected, analyzed and
interpreted the clinical data. SJ drafted and finalized the
manuscript. All the authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The study protocols were approved by The Ethics
Committee of the Shaoxing People's Hospital (Shaoxing, China;
approval no. 2020-K-Y-079-01). All the participants or their
guardians provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kitahara CM and Sosa JA: The changing
incidence of thyroid cancer. Nat Rev Endocrinol. 12:646–653. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fagin JA and Wells SA Jr: Biologic and
clinical perspectives on thyroid cancer. N Engl J Med.
375:23072016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosai J, Albores Saavedra J, Asioli S, et
al: Papillary thyroid carcinoma. WHO Classification of Tumors of
Endocrine Organs. Lloyd RV, Osamura RY, Klöppel G and Rosai J: 4th
edition. IARC; Lyon: 2017
|
|
4
|
Ito Y, Higashiyama T, Takamura Y, Miya A,
Kobayashi K, Matsuzuka F, Kuma K and Miyauchi A: Risk factors for
recurrence to the lymph node in papillary thyroid carcinoma
patients without preoperatively detectable lateral node metastasis:
Validity of prophylactic modified radical neck dissection. World J
Surg. 31:2085–2091. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooper DS, Doherty GM, Haugen BR, Kloos
RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F,
Schlumberger M, et al: Revised American Thyroid Association
management guidelines for patients with thyroid nodules and
differentiated thyroid cancer. Thyroid. 19:1167–1214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Francis GL, Waguespack SG, Bauer AJ,
Angelos P, Benvenga S, Cerutti JM, Dinauer CA, Hamilton J, Hay ID,
Luster M, et al: Management guidelines for children with thyroid
nodules and differentiated thyroid cancer. Thyroid. 25:716–759.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schlumberger M, Tahara M, Wirth LJ,
Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff
AO, et al: Lenvatinib versus placebo in radioiodine-refractory
thyroid cancer. N Engl J Med. 372:621–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khatami F, Larijani B, Nikfar S, Hasanzad
M, Fendereski K and Tavangar SM: Personalized treatment options for
thyroid cancer: Current perspectives. Pharmgenomics Pers Med.
12:235–245. 2019.PubMed/NCBI
|
|
9
|
Li M, Chen H and Wu T: LIN28: A cancer
stem cell promoter for immunotherapy in head and neck squamous cell
carcinoma. Oral Oncol. 98:92–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Dong N, Li J, Zhao L, Gao L, Zhang
Y and Ruan J: RNA-binding protein Lin28 is associated with injured
dentin-dental pulp complex in Sprague-Dawley rats. Int J Clin Exp
Pathol. 11:4385–4394. 2018.PubMed/NCBI
|
|
11
|
Wu J, Zhao W, Wang Z, Xiang X, Zhang S and
Liu L: Long non-coding RNA SNHG20 promotes the tumorigenesis of
oral squamous cell carcinoma via targeting miR-197/LIN28 axis. J
Cell Mol Med. 23:680–688. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Zong Y, Qiu G, Jia R, Xu X, Wang
F and Wu D: Silencing Lin28 promotes apoptosis in colorectal cancer
cells by upregulating let7c targeting of antiapoptotic BCL2L1. Mol
Med Rep. 17:5143–5149. 2018.PubMed/NCBI
|
|
13
|
He Y, Wang H, Yan M, Yang X, Shen R, Ni X,
Chen X, Yang P, Chen M, Lu X, et al: High LIN28A and PLK4
coexpression is associated with poor prognosis in epithelial
ovarian cancer. Mol Med Rep. 18:5327–5336. 2018.PubMed/NCBI
|
|
14
|
Xu C, Shen J, Xie S, Jiang Z, Huang L and
Wang L: Positive expression of Lin28 is correlated with poor
survival in gastric carcinoma. Med Oncol. 30:3822013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McDaniel K, Hall C, Sato K, Lairmore T,
Marzioni M, Glaser S, Meng F and Alpini G: Lin28 and let-7: Roles
and regulation in liver diseases. Am J Physiol Gastrointest Liver
Physiol. 310:G757–G765. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y, Li H, Liu Y, Chi C, Ni J and Lin
X: MiR-4319 hinders YAP expression to restrain non-small cell lung
cancer growth through regulation of LIN28-mediated RFX5 stability.
Biomed Pharmacother. 115:1089562019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu C, Jin S and Huang L: Expression of
Lin28 is correlated with prognosis and expression of HER-2 and
steroid receptors in breast cancer. Onco Targets Ther.
12:1105–1110. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ling R, Zhou Y, Zhou L, Dai D, Wu D, Mi L,
Mao C and Chen D: Lin28/microRNA-let-7a promotes metastasis under
circumstances of hyperactive Wnt signaling in esophageal squamous
cell carcinoma. Mol Med Rep. 17:5265–5271. 2018.PubMed/NCBI
|
|
19
|
Balzeau J, Menezes MR, Cao S and Hagan JP:
The LIN28/let-7 pathway in cancer. Front Genet. 8:312017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji J and Wang XW: A Yin-Yang balancing act
of the lin28/let-7 link in tumorigenesis. J Hepatol. 53:974–975.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang J, Lin H, Zhong M, Huang J, Sun S,
Lin L and Chen Y: Role of Lin28A/let-7a/c-Myc pathway in growth and
malignant behavior of papillary thyroid carcinoma. Med Sci Monit.
24:8899–8909. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The Eighth edition AJCC Cancer Staging Manual:
Continuing to build a bridge from population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dom G, Tarabichi M, Unger K, Thomas G,
Oczko-Wojciechowska M, Bogdanova T, Jarzab B, Dumont JE, Detours V
and Maenhaut C: A gene expression signature distinguishes normal
tissues of sporadic and radiation-induced papillary thyroid
carcinomas. Br J Cancer. 107:994–1000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akyay OZ, Gov E, Kenar H, Arga KY, Selek
A, Tarkun I, Canturk Z, Cetinarslan B, Gurbuz Y and Sahin B:
Mapping the molecular basis and markers of papillary thyroid
carcinoma progression and metastasis using global transcriptome and
microRNA profiling. OMICS. 24:148–159. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He J, Zhou M, Li X, Gu S, Cao Y, Xing T,
Chen W, Chu C, Gu F, Zhou J, et al: SLC34A2 simultaneously promotes
papillary thyroid carcinoma growth and invasion through distinct
mechanisms. Oncogene. 39:2658–2675. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Loh KC, Greenspan FS, Gee L, Miller TR and
Yeo PP: Pathological tumor-node-metastasis (pTNM) staging for
papillary and follicular thyroid carcinomas: A retrospective
analysis of 700 patients. J Clin Endocrinol Metab. 82:3553–3562.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo K and Wang Z: Risk factors influencing
the recurrence of papillary thyroid carcinoma: A systematic review
and meta--analysis. Int J Clin Exp Pathol. 7:5393–5403.
2014.PubMed/NCBI
|
|
29
|
Veronese N, Luchini C, Nottegar A, Kaneko
T, Sergi G, Manzato E, Solmi M and Scarpa A: Prognostic impact of
extra-nodal extension in thyroid cancer: A meta-analysis. J Surg
Oncol. 112:828–833. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chereau N, Buffet C, Tresallet C, Tissier
F, Leenhardt L and Menegaux F: Recurrence of papillary thyroid
carcinoma with lateral cervical node metastases: Predictive factors
and operative management. Surgery. 159:755–762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su DH, Chang SH and Chang TC: The impact
of locoregional recurrences and distant metastases on the survival
of patients with papillary thyroid carcinoma. Clin Endocrinol
(Oxf). 82:286–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Melo M, Gaspar da Rocha A, Batista R,
Vinagre J, Martins MJ, Costa G, Ribeiro C, Carrilho F, Leite V,
Lobo C, et al: TERT, BRAF, and NRAS in primary thyroid cancer and
metastatic disease. J Clin Endocrinol Metab. 102:1898–1907. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siraj AK, Pratheeshkumar P, Parvathareddy
SK, Bu R, Masoodi T, Iqbal K, Al-Rasheed M, Al-Dayel F, Al-Sobhi
SS, Alzahrani AS, et al: Prognostic significance of DNMT3A
alterations in Middle Eastern papillary thyroid carcinoma. Eur J
Cancer. 117:133–144. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Melo M, da Rocha AG, Vinagre J, Batista R,
Peixoto J, Tavares C, Celestino R, Almeida A, Salgado C, Eloy C, et
al: TERT promoter mutations are a major indicator of poor outcome
in differentiated thyroid carcinomas. J Clin Endocrinol Metab.
99:E754–E765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Collina F, La Sala L, Liotti F, Prevete N,
La Mantia E, Chiofalo MG, Aquino G, Arenare L, Cantile M, Liguori
G, et al: AXL is a novel predictive factor and therapeutic target
for radioactive iodine refractory thyroid cancer. Cancers (Basel).
11:7852019. View Article : Google Scholar
|
|
36
|
Han J, Zhang M, Nie C, Jia J, Wang F, Yu
J, Bi W, Liu B, Sheng R, He G, et al: miR-215 suppresses papillary
thyroid cancer proliferation, migration, and invasion through the
AKT/GSK-3β/Snail signaling by targeting ARFGEF1. Cell Death Dis.
10:1952019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Xu A, Miao C, Yang J, Gu M and
Song N: Prognostic value of Lin28A and Lin28B in various human
malignancies: A systematic review and meta-analysis. Cancer Cell
Int. 19:792019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Viswanathan SR, Powers JT, Einhorn W,
Hoshida Y, Ng TL, Toffanin S, O'Sullivan M, Lu J, Phillips LA,
Lockhart VL, et al: Lin28 promotes transformation and is associated
with advanced human malignancies. Nat Genet. 41:843–848. 2009.
View Article : Google Scholar : PubMed/NCBI
|