Introduction
In 2018, breast cancer was one of the leading causes
of death for women worldwide, apart from other types of cancer,
such as lung and colorectal cancer (1). Of the most resourceful organizations
that have updated breast cancer data are the World Health
Organization's International Agency for Research on Cancer (IARC)
and the Centers for Disease Control and Prevention (CDC). In 2018,
IARC compiled a brief study known as GLOBOCAN 2018 (1) that highlighted the prevalence,
mortality and incidence rates of different cancer forms. According
to the aforementioned study, breast cancer has the highest
incidence (24.2%), mortality (15.0%), and prevalence (30.1%) rates
among female patients worldwide (1).
GLOBOCAN 2018 also reported that Asia has the most significant
number of breast cancer cases, followed by Europe and North America
(1). According to a report released
by the CDC in 2019, there has been no change in the incidence of
breast cancer acquisition over the last decade (2). In spite this, the trend has risen for
black, Asian and Pacific Islander women (2).
Breast cancer occurs when healthy breast cells begin
to develop rapidly without the completion of their regular cell
cycle (3). Much of the initial
cancer growth occurs within the breast lobules and ducts (2) However, the progression of cancer
development can cause cancer to spread beyond the breast (4,5). Such an
occurrence is called metastasis (6)
Metastasis is caused by the dissociation of cancer cells from the
primary tumor site, accompanied by the breakdown of the
extracellular membrane allowing the cancer cells to enter the
bloodstream or lymphatic vessels, creating a secondary tumor in
other parts of the body which includes primary organs, such as
lungs and liver (6,7).
The presence of voltage-gated sodium channels
(VGSCs) has been identified over the last decade to increase the
progression of metastases (8,9). The
functional overexpression of VGSCs has been documented in different
types of carcinomas (cancers of epithelial origin), such as breast,
ovarian and cervical cancers (10–17). The
VGSC structure consists of one α-subunit (pore-forming) and smaller
β-subunits (10). There is a total
of 9 separate subunits of α (Nav1.1-Nav1.9) and four β subunits
(β1-β4) (10). The neonatal
alternative splice variant of Nav1.5 (nNav1.5) has been widely
recognized as one of the culprits in cancerous breast cells that
metastasize (10–12). Nav1.5 is encoded by the SCN5A
gene and generally suppresses the action mechanism of most sodium
channels within the VGSC family (18). The Nav1.5 channel typically carries
an inward sodium ion current which depolarizes the membrane
potential during a heart attack (19).
The neonatal isoform of Nav1.5 has developed as a
result of epigenetic dysregulation through the VGSCα alternative
splicing at D1:S3 (20). The
upregulation of nNav1.5 in breast cancer is suggestive of
onco-foetal gene expression since nNav1.5 would typically be
expressed during the foetal stage of human development (21,22). The
distinction between Nav1.5 and nNav1.5 in terms of molecular
characteristics are the distinguishable 7 amino acid substitutions
in the extracellular region and the alternatively spliced exons
(D1:S3 5′denotes neonatal Nav1.5 while D1:S3 3′denotes adult
Nav1.5) that allow the recognition of these two isoforms (20,23).
The link between Nav1.5 and nNav1.5 with metastasis
in breast cancer has been highlighted in several high-quality
publications (10–12). The aforementioned studies
demonstrated that by enhancing metastasis, functional upregulation
of these proteins in breast cancer can contribute to breast cancer
progression (10–12). Numerous in vitro studies have
suggested that overexpression of these proteins can be observed in
highly metastatic breast cancer cells, such as MDA-MB-231 compared
with less metastatic breast cancer cell lines, such as MCF-7
(11,23–25). An
extensive in vivo study performed by Nelson et al
(26) provided a model in 2015,
which further solidified that Nav1.5 serves a significant role in
fostering breast cancer metastasis. The aforementioned study
demonstrated that the downregulation of Nav1.5 expression achieved
using lentiviral shRNA led to significantly reduced tumor
development, decreased local invasion of the surrounding tissue and
mitigated the progression of tumor metastasis to the liver, lungs
and spleen in an orthotopic breast tumor mouse model (26). In a clinical study by Fraser et
al (12), the expression of
nNav1.5 in human breast cancer biopsy sections was significantly
upregulated compared with healthy breast tissue. In addition, in
the aforementioned study it was demonstrated that the expression of
nNav1.5 tested in these tissues of breast cancer was closely
associated with metastasis to the lymph nodes (12).
Since metastasis requires blood circulation and
lymphatic systems as conventional pathways for progression, it was
hypothesized in the present study that the immune system may target
the presence of nNav1.5 antigen on the circulating metastasizing
cancer cells in the blood system and produce antibodies against it.
The evasion of the immune system was identified as one of the
‘Hallmarks of Cancer’ in 2011 (27),
making the investigation of the immune system essential for
identification of the existence of antibodies against nNav1.5 and
reducing the development of breast cancer metastasis in the present
study. To the best of our knowledge, the present study is the first
study that aimed to detect the presence of naturally produced
antinNav1.5-Ab in the serum of patients with breast cancer. In
addition, the present study also elucidated the effect of breast
cancer therapy on the expression of antinNav1.5-Ab to provide
insight into its role as an immune-surveillance marker in
monitoring treatment outcomes. In addition, the expression of T
regulatory (Treg) cells and metastasis-related cytokines, such as
interleukin (IL)-6 and vascular endothelial growth factor (VEGF)
were investigated in the present study to support and validate the
association of antinNav1.5-Ab with breast cancer metastasis.
Materials and methods
Recruitment of patients
The present study was conducted at the Hospital
Universiti Sains Malaysia (HUSM) in collaboration with the USM
Breast Cancer Awareness and Research Unit (BestARi) (Kubang Kerian,
Malaysia). Ethical approval was granted by the Human Research
Ethics Committee of USM (JEPeM) (approval no. USM/JEPeM/18100518).
A total of 96 participants were recruited in the present study from
March 2019-March 2020. Of these, 32 participants were healthy
females (controls), while the remaining 64 participants were
patients with breast cancer. These breast cancer patients were
classified into two groups (n=32 each) based on their treatment
status: Pretreatment (treatments were not performed) and
ongoing-treatment (multiple forms of treatment were performed, such
as chemotherapy, radiotherapy and surgery). The age range for the
subjects in the present study was 23–70 years old. The mean ± SEM
of age for the breast cancer group was 49.160±2.243 [95% confidence
interval (CI), 44.58–53.73] whereas for the control group it was
36.59±1.377 (95% CI, 33.78–39.40). The inclusion criteria for
patients with breast cancer were as follows: i) Early-invasive or
advanced stage breast cancer; ii) no past history of other types of
cancer, iii) having received treatments; and iv) no chronic
diseases, such as immune disorders, severe diabetes and chronic
hypertension. The inclusion criteria for healthy participants were
as follows: i) No history of breast cancer, ii) absence of other
types of cancer; and no iii) chronic diseases such as immune
disorders, severe diabetes and chronic hypertension. Written
informed consent was obtained from all subjects prior to blood
collection.
Serum collection
In total ~3 ml of blood was collected from the
participants with the assistance of certified nurses or medical
officers. Serum samples were extracted from whole blood by
centrifugation at 1,800 × g for 15 min and stored at −80°C.
In-house indirect enzyme-linked
immunosorbent assay (ELISA)
To the best of our knowledge, there is no commercial
ELISA kit available at present for the detection of antinNav1.5-Ab,
an optimized in-house indirect ELISA was performed to detect the
presence of antibodies produced against nNav1.5 antigen found in
the serum. The Nunc Maxisorp ELISA plate (Thermo Fisher Scientific
Inc.) was coated with 100 µl of nNav1.5 peptide (GenScript Biotech
Corp.) working solution (5 µg/ml) and was left to rest at 4°C
overnight. Following overnight incubation, the plate was washed
thrice with PBS. Next, the plate was blocked at 4°C with 200 µl of
5% skim milk for 2 h. Following brief washing 3 times, 100 µl of
serum samples were added at a dilution of 1:400 and the plates were
left to rest at 4°C overnight. On the third day, the same washing
process was repeated with PBS-TWEEN 20 (0.05%), and the plate was
then incubated with 100 µl of rabbit anti-human IgG, HRP conjugated
secondary antibody (1:5,000; cat. no. CSB-PA00120F1Rb; Cusabio
Technology LLC) at 4°C for 2 h. Following incubation, the plate was
washed thrice with PBS-TWEEN 20. Subsequently, 100 µl of
3,3′,5,5′-Tetramethylbenzidine (TMB) substrate (Sigma-Aldrich;
Merck KgaA) was added to each well for 30 min at room temperature
Sulfuric acid was added to stop the reaction and the absorbances
were read spectrophotometrically at 450 nm. The optical density of
each sample was scanned using the Varioskan Flash spectral scanning
multimode reader (Thermo Fisher Scientific Inc.). Pooled positive
and negative sera were used as positive and negative controls,
respectively. There was no standard reagent (standard value)
available since to the best of our knowledge this is the first time
such an assay was developed. All the values were normalized using
the blank value.
CD25 sandwich ELISA
A commercial Human Interleukin 2 Receptor α
(IL-2Rα/CD25) kit (Elabscience) was used according to the
manufacturer's instructions. In total, ~100 µl of standard and
samples were added to the precoated ELISA plate. The plate was
precoated with an antibody specific to CD25. In total, ~100 µl of
biotinylated detection antibodies specific to human CD25 and 100 µl
of avidin-horseradish peroxidase (HRP) conjugates which were part
of the kit were successively added to each microplate well and
incubated for 90 min respectively at 37°C. Next, the unbound
components were washed away, and the substrate was added. The
enzyme-substrate reaction was stopped by the addition of 50 µl of
stop solution. The optical densities were measured
spectrophotometrically at a wavelength of 450 nm using the
Varioskan Flash spectral scanning multimode reader (Thermo Fisher
Scientific Inc.). The CD25 concentration was calculated using the
standard curve.
Magnetic luminex assay
An additional method to indicate the presence of
metastases in the serum of the participants was included in the
study. A custom-made magnetic Luminex assay kit, Human Magnetic
Luminex Screening Assay-10 PLEX (R&D Systems, Inc.) was
produced based on a selection of 10 cytokines. However, only 6
cytokines were selected: Pro-inflammatory cytokines, such as IL-8,
IL-6, TNF-α, VEGF, chemokine (C-C motif) ligand 2 (CCL2) and
antiinflammatory cytokines, such as IL-10. The other four cytokines
depicted low expressions below the detection range, thus were not
reported here. About 20 serum samples were utilized to represent
the control group whereas for pretreatment and ongoing-treatment
groups, 10 serum samples were utilized for each group.
The Luminex assay was performed according to the
manufacturer's instructions. In brief, ~50 µl of standard or sample
was pipetted into the respective wells followed by 50 µl of diluted
microparticle cocktail. Incubation took place in a shaker at 72 × g
for 2 h at room temperature. After washing, 50 µl of the diluted
biotin antibody cocktail was added to each well (plate was covered)
for 1 h at room temperature on the shaker at 72 × g. The same
washing process was repeated before adding 50 µl of diluted
streptavidin-PE substrate to each well for 30 min at room
temperature on the shaker at 72 × g. The washing process was
repeated, followed by additional washing, which involved adding 100
µl of wash buffer to each well at room temperature at 72 × g for 2
min. The plate was read within 1.5 h using a Luminex®
analyzer (Luminex Corporation).
Statistical analysis
GraphPad Prism version 8 (GraphPad Inc.) was used
for all the statistical analyses. For all the tests, duplicate
biological replicates were performed. Multiple comparisons that
were parametric were analyzed using one-way ANOVA followed by the
post hoc Tukey's whereas Kruskal-Wallis test was used for
non-parametric multiple comparisons followed by the post hoc Dunn's
multiple comparison test. Spearman correlation was used for the
correlation analysis between cytokines and antinNav1.5-Ab
expression. Error bars represent either standard error of mean
(SEM) or interquartile range (IQR). The strength of the r-value
correlation was interpreted based on a previous study (28): Poor-correlation (r≤0.25);
fair-correlation (r=0.26–0.50); good correlation (r=0.51–0.75) and
excellent correlation (r=0.76–1.00). Normality of the data
(skewness and kurtosis) was determined based on the guidelines by
George and Mallery (29) and
Schmider et al (30).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Optical density of antinNav1.5-Ab in
the serum samples
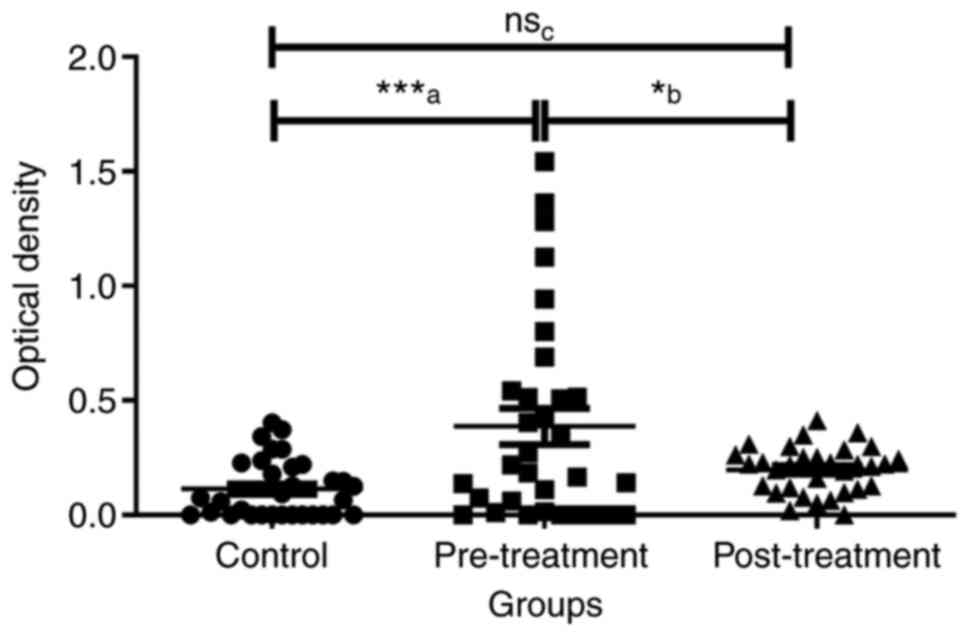
AntinNav1.5-Ab expression in the serum of control,
pretreatment and ongoing-treatment groups were compared.
Considering the mean expression of antinNav1.5-Ab expression of the
control group as a baseline, it was demonstrated that the
expression of antinNav1.5-Ab were upregulated in patients with
breast cancer regardless of their treatment status. When examining
the effect of breast cancer treatment on the antibodies, the
expression of antinNav1.5-Ab was higher in the pretreatment group
compared with the ongoing-treatment group (Fig. 1). The mean ± SEM for control,
pretreatment and ongoing-treatment groups were 0.1139±0.0225,
0.3869±0.0787 and 0.1973±0.0180, respectively (Fig. 1). The optical densities of
antinNav1.5-Ab in the serum of control, pretreatment and
ongoing-treatment groups were compared using one-way ANOVA test and
the difference between the means of the three groups were
significant (P=0.0005). Further analysis using the post hoc Tukey's
test demonstrated that the mean differences between two pairs:
Control vs. pretreatment and pretreatment vs. ongoing-treatment
were significant (Fig. 1). However,
the mean difference between control and ongoing-treatment groups
was not significant (Fig. 1).
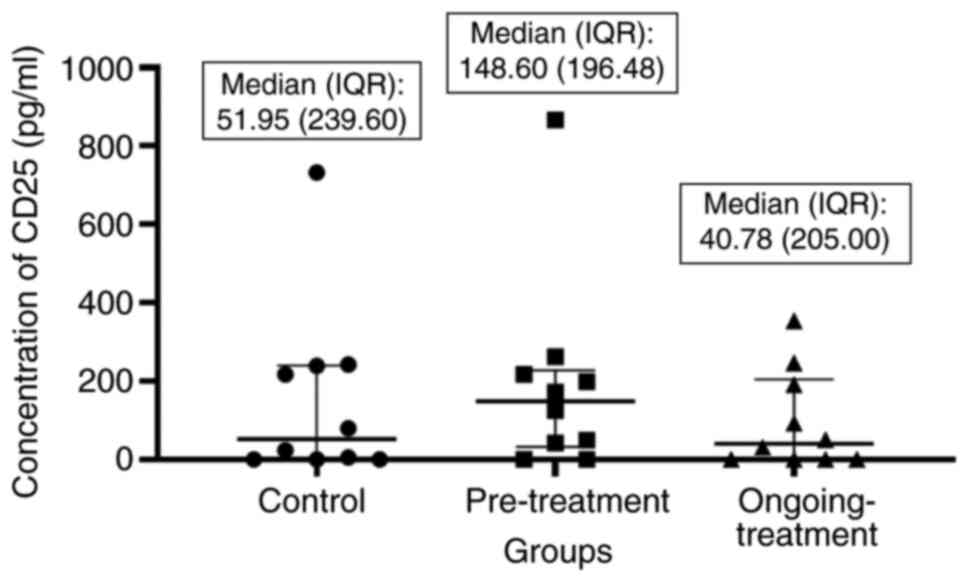
Concentration of CD25 a Treg cell
marker in the serum samples
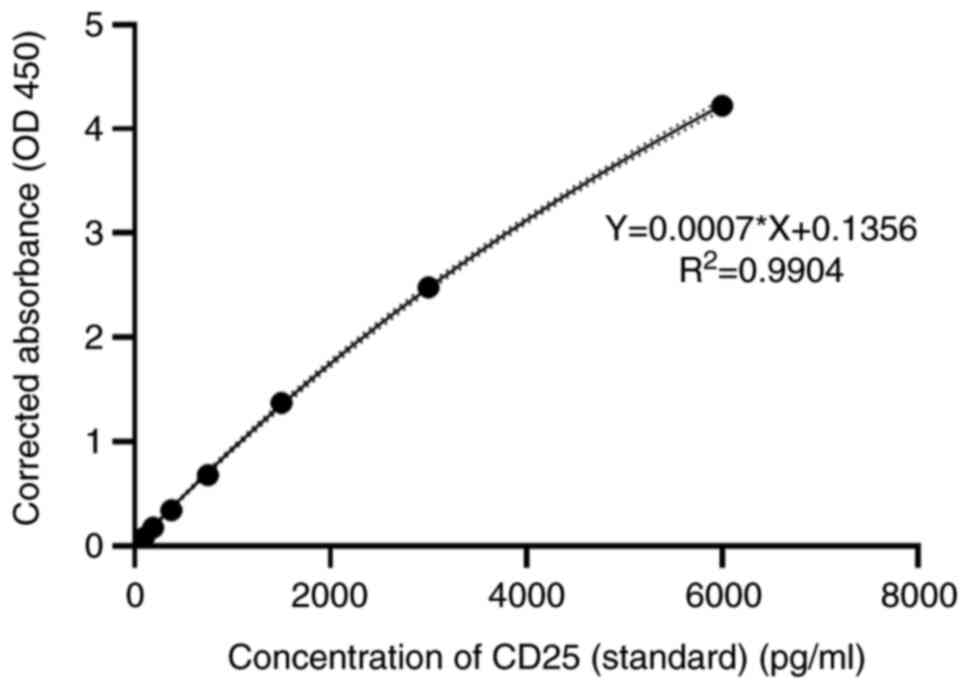
A commercial sandwich ELISA kit was used to detect
the concentration of CD25 in the serum samples of control,
pre-treatment and ongoing-treatment groups. Concentrations of
unknown samples were interpreted from the standard curve plotted
based on the known concentrations of the standards (Fig. 2). The standard curve presented was
used to calculate the actual concentration of CD25 by inserting the
optical densities into the equation. The concentrations of CD25 in
the serum of control, pretreatment, and ongoing-treatment groups
were compared using the Kruskal Wallis test and the median
differences between these groups were not significant (P=0.6390).
However, based on the median comparison of the CD25 concentrations
between the three groups, it was evident that the expression of T
reg cells was increased in the pretreatment group and reduced in
the ongoing-treatment group (Fig.
3).
Expression of cytokines associated
with breast cancer metastasis
Upregulation and downregulation of cytokines in the
pretreatment and ongoing-treatment groups were determined by
considering the cytokine profile of the control as a baseline. The
pretreatment group exhibited the highest concentration of IL-8
compared with the other two groups (Table I). There were minute differences in
the concentration levels of VEGF and CCL2 cytokines among the three
groups (Table I). The control group
exhibited the highest concentration of VEGF and CCL2 followed by
the pretreatment group. As anticipated, the ongoing-treatment group
had the lowest concentrations of VEGF and CCL2 compared with the
other two groups (Table I).
 | Table I.Concentration of IL-8, VEGF and CCL2
in control (n=20), pretreatment and ongoing-treatment groups (n=10
each). |
Table I.
Concentration of IL-8, VEGF and CCL2
in control (n=20), pretreatment and ongoing-treatment groups (n=10
each).
| Cytokines | Control | Pretreatment |
Ongoing-treatment | P-value |
|---|
| IL-8 | 4.074±0.392 | 8.557±2.269 | 5.159±0.594 | 0.0490a |
| VEGF | 70.33±12.07 | 64.59±18.65 | 60.75±13.89 | 0.9250 |
| CCL2 | 269.8±30.08 | 249.6±34.53 | 240.7±31.13 | 0.8020 |
Comparison of net median fluorescence
intensity (MFI) values of IL-6, IL-10 and TNF-α cytokines in the
serum samples
MFI values were used to analyze the trend between
three study groups, as the concentration of the analytes in the
samples were relatively low (Table
II). A similar method has also been published, which supports
the use of MFI as valuable data for statistical analysis (31). The serum samples from patients with
breast cancer in the pretreatment group had a higher intensity of
IL-6 and TNF-α compared with the other groups. However, the
downregulation of IL-6 and TNF-α in the ongoing-treatment group was
notable as the serum samples from this group exhibited the lowest
expression of IL-6, IL-10 and TNF-α compared with the other groups.
In contrast, the intensity of IL-10 was the lowest in the serum of
the pretreatment group compared with the other two groups (Table II).
 | Table II.Expression (net MFI values) of IL-6,
IL-10 and TNF-α in control (n=20), pretreatment and
ongoing-treatment groups (n=10 each). |
Table II.
Expression (net MFI values) of IL-6,
IL-10 and TNF-α in control (n=20), pretreatment and
ongoing-treatment groups (n=10 each).
| Cytokines | Control | Pretreatment |
Ongoing-treatment | P-value |
|---|
| IL-6 | 5.313±0.656 | 16.13±7.905 | 5.025±0.797 | 0.3570 |
| IL-10 | 4.163±0.361 | 3.875±0.582 | 3.900±0.404 | 0.8080 |
| TNF-α | 9.300±0.609 | 9.525±1.570 | 7.075±0.822 | 0.1340 |
Correlation between the expression
levels of cytokines and antinNav1.5-Ab in the serum samples
Correlation between the expression levels of
antinNav1.5-Ab and the six cytokines among the three groups were
analyzed using the Spearman correlation (Fig. 4). There was a significant positive
correlation between the expression of antinNav1.5-Ab and IL-6 in
the pre-treatment group. The other cytokines however, only showed
poor to fair correlations when paired with antinNav1.5-Ab in the
pretreatment group. In the ongoing-treatment group, the correlation
between VEGF and the expression of antinNav1.5-Ab had a
statistically significant inverse relationship. The other 5
cytokines, IL-8, IL-6, CCL2, IL-10 and TNF-α demonstrated good to
poor correlations when paired with antinNav1.5-Ab in the
ongoing-treatment group. However, these correlations were not
significant. In the control group, all the cytokines exhibited poor
to fair non-significant correlations with antinNav1.5-Ab.
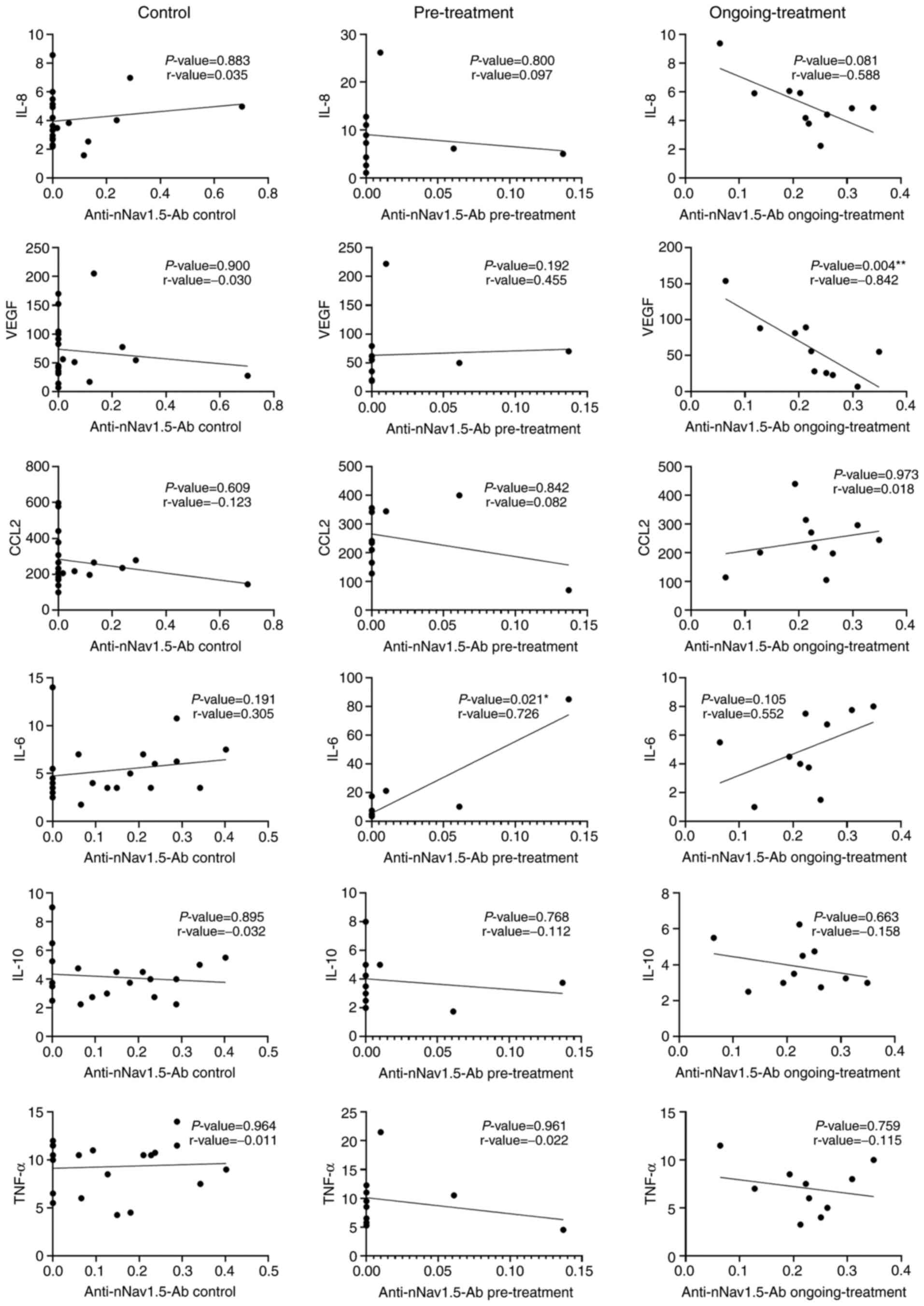 | Figure 4.Correlation between cytokines and
antinNav1.5-Ab expression. Correlations between antinNav1.5-Ab
expression and cytokine levels of IL-8, VEGF, CCL2, IL-6, IL-10 and
TNF-α among the three groups, control (n=20), pre-treatment (n=10)
and ongoing-treatment (n=10). *P=0.01–0.049, **P≤0.01. IL,
interleukin; VEGF, vascular endothelial growth factor; CCL-2, C-C
motif chemokine ligand 2; antineonatal Nav1.5 antibodies
(antinNav1.5-Ab). |
Discussion
The metastatic potential of breast cancer cells
increases with the degree of cell aggressiveness (32). Cells that originate from metastatic
tumors are known to have unique genetic alterations to maintain
their malignant characteristics, such as the ability to invade and
metastasize (32). These genetic
alterations may lead to the development of unique markers or
antigens that contribute to the progression of metastases (32). The involvement of VGSCs was
attributed to the metastatic capacity of breast cancer cells
(23). Studies have demonstrated
that the aberrant expression of a member of the VGSC family, Nav1.5
and its neonatal splice variant, nNav1.5, has established a
mechanism to support the advancement of the disease (10–12).
nNav1.5 has demonstrated onco-foetal upregulation in highly
metastatic breast cancer cells as established in various in
vitro (23,24) and in vivo (26) studies. However, clinical studies
involving nNav1.5 expression are only limited to breast tissues and
biopsy samples (12,21). Hence, the present study used serum
samples collected from patients with breast cancer to discover the
presence of antinNav1.5-Ab that were naturally present in the
samples. The assessment of antinNav1.5-Ab in the present study
reflected the expression level of nNav1.5 antigen carried by the
circulating cancer cells present within the body systems of the
participants.
As predicted, in the current study the serum samples
of the breast cancer pre-treatment group exhibited the highest
antinNav1.5-Ab expression compared with the other two groups
tested. The pretreatment group did not receive any prior treatment,
which contributed to the high level of antibodies present in the
samples which may be due to the uninterrupted expression of nNav1.5
antigen carried by the metastatic or circulating breast cancer
cells. The presence of antinNav1.5-Ab detected in the serum in the
present study indicated the vulnerability and immunogenicity of the
protein towards the actions of the immune system. The expression of
antinNav1.5-Ab in the controls in the present study was somewhat
surprising and indicated the natural presence of antinNav1.5-Ab in
the serum of healthy female participants. Yamaci et al
(21) demonstrated that some healthy
epithelial breast ductal cells showed nNav1.5 immunoreactivity to a
certain degree compared with breast cancer biopsies. In addition,
in the aforementioned study immunoreactivity-staining was performed
and the findings indicated that nNav1.5 expression appeared diffuse
and less intense in healthy breast tissues compared with breast
cancer tissues.
Metastatic breast disease can be treated or
controlled by implementing various treatment strategies to achieve
long-lasting remission and potentially cure (33). Breast cancer patients are often
treated with chemotherapy (34,35),
radiation therapy (36), hormonal
therapy (37), molecular therapy
(38), immunotherapy (39) and surgery, such as mastectomy
(40). In the present study, the
expression of antinNav1.5-Ab in the serum of the ongoing-treatment
group was much lower compared with the pre-treatment group. The
lower expression of antinNav1.5-Ab in the ongoing treatment group
in the present study was consistent with the declined state of
metastasis due to treatment. Hence, it can be postulated that
patients receiving treatment have a more stable disease control
with respect to the downregulation of metastases compared with
those who have not received any treatment, which may reflect the
reduced state of nNav1.5 expression that favors metastasis.
However, there is another perspective that suggests that breast
cancer treatment may reduce the expression of antibodies produced
against nNav1.5, indicating the side effects of therapy that
require further investigation. A study by Evans et al
(41) found that the combination of
two forms of treatment, which includes chemotherapy, radiotherapy
or hormonal therapy tends to reduce the expression of
autoantibodies produced against tumor-associated antigens in
patients with breast cancer. The difference in the pattern of
antinNav1.5-Ab expression demonstrated between the three groups in
the present study highlights the capacity of this antibody to act
as an immunosurveillance marker during breast cancer treatment that
can be used to monitor the efficacy of breast cancer treatment in
eliminating breast cancer metastasis.
CD25 is the α-chain of the heterotrimeric IL-2
receptor complex (42). According to
Lundin et al (43), in
comparison to the γ-chain, considerable amounts of the α-chain can
be found in serum. Hence, serum samples are reliable biological
sources that can be used to measure the concentration of CD25
(44). CD25 is a well-established T
cell activation marker that is widely used to measure Treg cell
expression levels (44). Established
immune evasion has been identified as one of the critical factors
for tumor development and cancer progression (27). Over time, Treg cells have been
studied in association with tumor progression. Studies have
demonstrated that Treg cells promote the initiation and progression
of tumors as well as the induce neo-angiogenesis (45–47). In
the case of cancer, Treg cells compromise the antitumor response by
reducing the efficiency of T cells. For instance in a study by
Shang et al (48), there was
increased Treg tumor infiltration and a low T-effector cell ratio
was associated with poor prognosis in cases of solid tumors
including cervical, renal, melanomas, and breast cancers. CD25 was
the first surface marker identified to distinguish Treg cells
(49) prior to the discovery of
their master regulator known as Fork-head box P3 (Foxp3). C-C
chemokine receptor 4 on Treg cells allow the recruitment of cells
to the microenvironment of the tumor after the release of CCL22 by
tumor associated macrophages (50).
Studies have found that Treg cells impart immunosuppression by
blocking the production of interferon-gamma (IFN-γ) and IL-2 cells
by T-effector cells (51,52). In the present study as predicted, the
CD25 concentration was the highest in the pretreatment group
followed by the control and ongoing treatment groups. The elevated
presence of metastases in the pretreatment group indicated by
antinNav1.5-Ab expression appeared to be validated by the high
concentration of CD25, representing the Treg population. This
finding is consistent with a study by Nishikawa et al
(53) in which it was assumed that
the promotion of metastases could result from the immunosuppressive
effects of Treg cells. However, in the present study the CD25
concentration in the serum of the ongoing-treatment group was lower
compared with the pretreatment and control groups, highlighting the
effects of breast cancer treatment on metastatic breast cancer by
indirectly lowering the expression of Treg cells. Treg cells have
been found to have been reduced following a single intravenous
infusion of daclizumab in patients with metastatic breast cancer
(54). In 2011, an in vivo
mouse mesothelioma model study found that the repopulation of tumor
cells between cycles of chemotherapy was inhibited by the depletion
of Treg cells (55). Lissoni et
al (56) reported that the
induction of chemotherapy (irrespective of the regimen) led to a
decrease in number of Treg cells in patients with stable disease or
tumor regression as compared with those with progressive disease
which suggested that metastatic cancer disease is characterized by
an upregulation in the Treg cell count.
The presence of metastases may interfere with the
expression levels of the immune system's vital components, such as
cytokines (57). The assessment of
the levels of pro-inflammatory and antiinflammatory cytokines
associated with breast cancer metastases provides a picture of the
progression of cancer in the body system (57). Cytokines are secreted proteins that
may be induced to mediate intercellular communication within the
immune system (57). Cytokines may
be grouped into several categories, such as TNF, IL, IFN,
chemokines and colony-stimulating factors (57). The cytokine levels of IL-8, VEGF,
CCL2, IL-6, IL-10 and TNF-α were examined in the present study on
the basis of their association with metastases for breast
cancer.
In the present study, the pre-treatment group had
the highest expression levels of IL-8, IL-6 and TNF-α compared to
the control and ongoing-treatment groups. These findings indicated
the presence of metastases among pre-treatment patients with breast
cancer as these 3 cytokines are well-known contributors to the
progression of metastases. IL-8 is generally associated with lymph
node metastases (58) and it
promotes breast cancer stem cell activity via its cognate
receptors, chemokine receptors (CXCR)1/2 (59) whereas IL-6 promotes metastases by
favoring signal transducer and activator of transcription 3 (STAT3)
pathway activation (60). The
ligation of IL-6 to its receptor stimulates Janus kinase (JAK)
tyrosine kinases leads to the phosphorylation of STAT3 (61). The homodimerization and entrance of
STAT3 in cancer cells modulates the proliferation, survival and
transformation (61). Increased
level of IL-6 is often associated with poor survival and prognosis
in patients with breast cancer (62,63). The
positive correlation between the expression of IL-6 and
antinNav1.5-Ab in the pre-treatment group in the present study
further validates that the provocation of the immune system to
produce antibodies against nNav1.5 happens in parallel with the
progression of metastases reflected by the upregulation of IL-6. On
the other hand, TNF-α enhances the process of metastases by
promoting the inflammatory microenvironment and enhances the
expressions of matrix metalloproteinases (64), IL-8 (65) and CXCR (66). In the present study, the pretreatment
group conversely had the lowest expression of IL-10. IL-10 is a
pleiotropic immunoregulatory cytokine that exhibits both pro- and
antitumor activities (67). In spite
of the contrasting opinions regarding the IL-10 expression in
breast cancer (67), the findings of
the present study are in concert of those of Li et al
(68). Li et al (68) discovered that the low expression of
IL-10 leads to poor survival outcome in patients with breast cancer
and is also associated with disease-free survival (recovered from
breast cancer) which explains why the pre-treatment group in the
present study had the lowest expression of IL-10. In summary, the
imbalance between metastasis-favoring (IL-8, IL-6 and TNF-α) and
metastasis-opposing cytokines (IL-10) is beneficial to the
progression of metastases. Based on the findings of the present
study, antinNav1.5-Ab expression and its association with
metastasis-favoring cytokines in the pretreatment group,
antinNav1.5-Ab may be a metastasis marker with immune-surveillance
properties capable of monitoring the progression of metastasis
among patients with breast cancer.
The ongoing-treatment group in the present study had
the lowest expression levels of CCL2, VEGF, IL-6 and TNF-α compared
with the other tested groups. The low expressions of these
cytokines indicated that the breast cancer therapies conducted had
productively overcome metastases to a certain extent and may be
able to provide a good treatment prognosis.
CCL2, also known as monocyte chemoattractant
protein-1 (MCP-1) is implicated in the progression of cancer where
it enhances cell invasion via the modulation of the MAP kinase
pathway (MCP-1-CCR2 axis) (69).
CCL2 binds to its cognate receptor, CCR2 and promotes multiple
pro-tumorigenic roles which include intravasation, extravasation
and angiogenesis (70) and formation
of metastasis foci (71). The
upregulation transcription factor, Twist1 in human mammary cells
favors angiogenesis via the induction of CCL2 (72). Conversely, the downregulation of CCL2
leads to an improved outcome and lower metastasis in patients with
breast cancer as suggested by Qian et al (73).
VEGF is a pro-angiogenic protein that triggers the
‘angiogenic switch’ to promote the formation of
endothelial-mediated blood vessels (57). Tumor development is often associated
with a decrease in the oxygen tension which is mainly due to poor
vascularization (57). To overcome
this, the process of tumor angiogenesis is stimulated to provide
nutrients and oxygen for the tumor sites that have reached a state
of hypoxia (57). Nav1.5, the adult
isoform of nNav1.5 was discovered as one of the significant VGSCα
isoforms (91% of total VGSCα) present in HUVEC cells (74). In 2011, Andrikopoulos et al
(74), found that Nav1.5 potentiates
angiogenesis via VEGF-induced ERK1/2 activation through the protein
kinase C α-B-RAF signaling axis. In addition, the aforementioned
study demonstrated that the potentiation takes place through the
modulation of VEGF-induced HUVEC depolarization and by altering the
kinetics of calcium ions (74). The
involvement of calcium ions in different phases of angiogenesis and
the modulation of VEGF was highlighted in another study (75). The inflow of calcium ions via the
reverse mode sodium-calcium exchanger is necessary for the
activation of PKC, VEGF-induced ERK1/2 phosphorylation and the
downstream role of endothelial cells in angiogenesis (76). The findings of the current study may
indicate that the neonatal isoform also has the capacity to
initiate VEGF-induced endothelial angiogenesis as the increased
production of antibodies targeting nNav1.5 was parallel to the
decrease in the level of VEGF.
As a metastatic preferring cytokine, it was
anticipated that IL-8 will be reduced after treatment in the
present study. Surprisingly, IL-8 was upregulated in the serum
samples of the ongoing treatment group in the present study. This
is in concert with the findings of Ginestier et al (77). The aforementioned study postulated
that chemotherapy-induced cell injury may lead to the upregulation
of IL-8 which could stimulate cancer stem cells to replicate to
resume the progression of cancer (77). Another study also demonstrated the
production of IL-8 by injured cancer cells, which reinitiates
cancer progression (78). The
findings of this study were in agreement with those of Ginestier
et al (77).
The limitations of the present study included the
absence of a positive control to validate the antinNav1.5-Ab
indirect ELISA assay and the absence of a standard curve to
evaluate the concentration of antinNav1.5-Ab in the serum of
patients with breast cancer. In the upcoming studies, the
availability of these positive controls and standards will further
validate the efficiency of the in-house assay.
In conclusion, the detection of antinNav1.5-Ab in
the serum of the present study clearly indicated the crosstalk
between breast cancer metastasis and immune cells, adding another
layer of complexity to the understanding of metastasis formation
while opening new therapeutic opportunities for patients with
breast cancer. The consistency of antinNav1.5-Ab expression with
the manifestation of other immune system components such as Tregs
and cytokines in the present study provides a promising opportunity
to promote the function of nNav1.5 as a metastasis marker as well
as an immune-surveillance tool in breast cancer therapy as such
therapy does affect the expression of antinNav1.5-Ab.
Acknowledgements
The authors would like to thank the medical
officers, Dr Norshahida Mohamed Noordin, Dr Nur'atikah Adnan, Dr
Lee Lih Shin and nurses, Sister Roslaini Che Romli and Siti Eshah
Harun from the Breast Cancer Awareness and Research Unit (BestARi)
under Hospital Universiti Sains Malaysia (HUSM) for their
corporation during patient recruitment. The authors would also like
to thank Dr Wong Weng Kin from the School of Health Sciences for
his guidance on planning the in-house indirect ELISA.
Funding
The present study was funded by the Research
University Individual (RUI) grant (grant no. 1001/PPSK/8012275) and
the Science Fund, Ministry of Science, Technology, Innovation,
Malaysia (MOSTI) (grant no. 06-01-05-SF0844).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HR made substantial contributions to the conception
and design, acquisition, analysis and interpretation of data and in
writing the manuscript. NSR was involved in the interpretation of
the data and the statistical analyses. TADAA was involved in the
acquisition of data and recruitment of breast cancer patients. NFM
contributed to the idea for the conception and design,
interpretation of data, writing the manuscript, revising it
critically for important intellectual content and obtained funding
for the peptide solution. MMY and WZWZ were extensively involved in
the patient recruitment, blood withdrawal, patient diagnosis,
interpretation of the data, revising and editing the manuscript
critically for intellectual content. NAA was involved in both
conception and idea generation, drafting the manuscript, revising
it critically for important intellectual content and involved in
analysis and interpretation of data. WEMF was involved in
conception and idea generation, ethical defense, acquisition of
data, interpretation of data, drafting the manuscript, revising it
critically for important intellectual content, giving final
approval of the version to be published and overall funding for the
study.
Ethics approval and consent to
participate
Ethical approval for this study was granted by the
Human Research Ethics Committee of USM (JEPeM; Kubang Kerian,
Malaysia) (approval no. USM/JEPeM/18100518). Written informed
consents were obtained from all subjects prior to blood
collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
International Agency for Research on
Cancer (IARC). GLOBOCAN report. 2018..Retrieved from. simplehttps://www.iarc.fr/
|
|
2
|
Centers for Disease Control and Prevention
(CDC), Division of Cancer Prevention and Control. Breast Cancer
Statistics. 2019.Retrieved from. simplehttps://www.cdc.gov/cancer/breast/basic_info/index.htm
|
|
3
|
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z,
Zhang L, Ji X, Liu W, Huang B, Luo W, et al: Breast cancer
development and progression: Risk factors, cancer stem cells,
signaling pathways, genomics, and molecular pathogenesis. Genes
Dis. 5:77–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma GN, Dave R, Sanadya J, Sharma P and
Sharma KK: Various types and management of breast cancer: An
overview. J Adv Pharm Technol Res. 1:109–126. 2010.PubMed/NCBI
|
|
5
|
Akram M, Iqbal M, Daniyal M and Khan AU:
Awareness and current knowledge of breast cancer. Biol Res. 50:33.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Zijl F, Krupitza G and Mikulits W:
Initial steps of metastasis: Cell invasion and endothelial
transmigration. Mutat Res. 728:23–34. 2011. View Article : Google Scholar
|
|
7
|
Seyfried TN and Huysentruyt LC: On the
origin of cancer metastasis. Crit Rev Oncog. 18:43–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Djamgoz MB, Coombes RC and Schwab A: Ion
transport and cancer: From initiation to metastasis. Philos Trans R
Soc Lond B Biol Sci. 369:201300922014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koltai T: Cancer: Fundamentals behind pH
targeting and the double-edged approach. Onco Targets Ther.
9:6343–6360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brackenbury WJ: Voltage-gated sodium
channels and metastatic disease. Channels. 6:352–361. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roger S, Besson P and Le Guennec JY:
Involvement of a novel fast inward sodium current in the invasion
capacity of a breast cancer cell line. Biochim Biophys Acta.
1616:107–111. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fraser SP, Diss JK, Chioni AM, Mycielska
ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, et
al: Voltage-gated sodium channel expression and potentiation of
human breast cancer metastasis. Clin Cancer Res. 11:5381–5389.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Onganer PU, Seckl MJ and Djamgoz MB:
Neuronal characteristics of small-cell lung cancer. Br J Cancer.
93:1197–1201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Diaz D, Delgadillo DM, Hernández-Gallegos
E, Ramírez-Domínguez ME, Hinojosa LM, Ortiz CS, Berumen J, Camacho
J and Gomora JC: Functional expression of voltage-gated sodium
channels in primary cultures of human cervical cancer. J Cell
Physiol. 210:469–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao R, Shen Y, Cai J, Lei M and Wang Z:
Expression of voltage-gated sodium channel α subunit in human
ovarian cancer. Oncol Rep. 23:1293–1299. 2010.PubMed/NCBI
|
|
16
|
Guzel RM, Ogmen K, Ilieva KM, Fraser SP
and Djamgoz MBA: Colorectal cancer invasiveness in vitro:
Predominant contribution of neonatal Nav1.5 under normoxia and
hypoxia. J Cell Physiol. 234:6582–6593. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
House CD, Vaske CJ, Schwartz AM, Obias V,
Frank B, Luu T, Sarvazyan N, Irby R, Strausberg RL, Hales TG, et
al: Voltage-gated Na+ channel SCN5A is a key
regulator of a gene transcriptional network that controls colon
cancer invasion. Cancer Res. 70:6957–6967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma RSY, Kayani K, Whyte-Oshodi D,
Whyte-Oshodi A, Nachiappan N, Gnanarajah S and Mohammed R: Voltage
gated sodium channels as therapeutic targets for chronic pain. J
Pain Res. 12:2709–2722. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Utrilla RG, Nieto-Marín P, Alfayate S,
Tinaquero D, Matamoros M, Pérez-Hernández M, Sacristán S, Ondo L,
de Andrés R, Díez-Guerra FJ, et al: Kir2. 1-Nav1. 5 channel
complexes are differently regulated than Kir2. 1 and Nav1. 5
channels alone. Front Physiol. 8:9032017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Onkal R, Mattis JH, Fraser SP, Diss JK,
Shao D, Okuse K and Djamgoz MB: Alternative splicing of Nav1.5: An
electrophysiological comparison of ‘neonatal’ and ‘adult’ isoforms
and critical involvement of a lysine residue. J Cell Physiol.
216:716–726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamaci RF, Fraser SP, Battaloglu E, Kaya
H, Erguler K, Foster CS and Djamgoz MBA: Neonatal Nav1.5 protein
expression in normal adult human tissues and breast cancer. Pathol
Res Pract. 213:900–907. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ben-Porath I, Thomson MW, Carey VJ, Ge R,
Bell GW, Regev A and Weinberg RA: An embryonic stem cell-like gene
expression signature in poorly differentiated aggressive human
tumors. Nat Genet. 40:499–507. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brackenbury WJ, Chioni AM, Diss JK and
Djamgoz MB: The neonatal splice variant of Nav1.5 potentiates in
vitro invasive behaviour of MDA-MB-231 human breast cancer cells.
Breast Cancer Res Treat. 101:149–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Isbilen B, Fraser SP and Djamgoz MB:
Docosahexaenoic acid (omega-3) blocks voltage-gated sodium channel
activity and migration of MDA-MB-231 human breast cancer cells. Int
J Biochem Cell Biol. 38:2173–2182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Erdogan MA and Ozpolat B: Targeting of
Voltage-gated sodium channel NaV1. 5 inhibits cell
proliferation and colony formation in breast and ovarian cancer
cells. Cancer Res. 73 (8 Suppl):2013.doi:
10.1158/1538-7445.AM2013-514.
|
|
26
|
Nelson M, Yang M, Millican-Slater R and
Brackenbury WJ: Nav1.5 regulates breast tumor growth and metastatic
dissemination in vivo. Oncotarget. 6:32914–32929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar
|
|
28
|
Norsa'adah B: Univariable Analyses Using
IBM SPSS statistics version 20.0, Universiti Sains Malaysia,
Malaysia. 2013.
|
|
29
|
George D and Mallery P: Chapter 7:
Descriptive Statistics. SPSS for Windows Step by Step: a Simple
Study Guide and Reference 17.0 Update. 10th edition. Pearson;
Boston, MA: 2010
|
|
30
|
Schmider E, Ziegler M, Danay E, Beyer L
and Bühner M: Is it really robust? Reinvestigating the robustness
of ANOVA against violations of the normal distribution.
Methodology. 6:147–151. 2010. View Article : Google Scholar
|
|
31
|
Breen EJ, Tan W and Khan A: The
statistical value of raw fluorescence signal in Luminex xMAP Based
Multiplex Immunoassays. Sci Rep. 6:26996. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rhana P, Trivelato RRJ, Beirao PSL, Cruz
JS and Rodrigues ALP: Is there a role for voltage-gated
Na+ channels in the aggressiveness of breast cancer?
Braz J Med Biol Res. 50:e60112017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pagani O, Senkus E, Wood W, Colleoni M,
Cufer T, Kyriakides S, Costa A, Winer EP and Cardoso F; ESO-MBC
Task Force, : International guidelines for management of metastatic
breast cancer: Can metastatic breast cancer be cured? J Natl Cancer
Inst. 102:456–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujii T, Le Du F, Xiao L, Kogawa T,
Barcenas CH, Alvarez RH, Valero V, Shen Y and Ueno NT:
Effectiveness of an adjuvant chemotherapy regimen for early-stage
breast cancer: A systematic review and network meta-analysis. JAMA
Oncol. 1:1311–1318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Krug D: Adjuvant radiotherapy for breast
cancer: More than meets the eye. Breast Care (Basel). 15:109–111.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tremont A, Lu J and Cole JT: Endocrine
therapy for early breast cancer: Updated review. Ochsner J.
17:405–411. 2017.PubMed/NCBI
|
|
38
|
Munagala R, Aqil F and Gupta RC: Promising
molecular targeted therapies in breast cancer. Indian J Pharmacol.
43:236–245. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
U.S Food and Drug Administration (FDA), .
FDA approves Atezolizumab for PD-L1 positive unresectable locally
advanced or metastatic triple-negative breast cancer. Retrieved
from. simplehttps://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-atezolizumab-pd-l1-positive-unresectable-locally-advanced-or-metastatic-triple-negative
|
|
40
|
Zurrida S, Bassi F, Arnone P, Martella S,
Del Castillo A, Ribeiro Martini R, Semenkiw ME and Caldarella P:
The changing face of mastectomy (from Mutilation to Aid to Breast
Reconstruction). Int J Surg Oncol. 2011:9801582011.PubMed/NCBI
|
|
41
|
Evans RL, Pottala JV, Nagata S and Egland
KA: Longitudinal autoantibody responses against tumor-associated
antigens decrease in breast cancer patients according to treatment
modality. BMC Cancer. 18:1192018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Witkowska AM: On the role of sIL-2R
measurements in rheumatoid arthritis and cancers. Mediators
Inflamm. 2005:121–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lundin K, Tuukkanen AM, Jansson C,
Nordström T and Lindqvist C: No soluble common cytokine receptor
gamma chain (gamma(c)) in activated human lymphocyte
cultures-comparison with soluble IL-2Ralpha. Immunol Lett.
82:235–240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gotoh Y, Okamoto Y, Uemura O, Mori N,
Tanaka S, Ando T and Nishida M: Determination of age-related
changes in human soluble interleukin 2 receptor in body fluids of
normal subjects as a control value against disease states. Clin
Chim Acta. 289:89–97. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mougiakakos D, Choudhury A, Lladser A,
Kiessling R and Johansson CC: Regulatory T cells in cancer. Adv
Cancer Res. 107:57–117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dwarakanath BS, Farooque A and Gupta S:
Targeting regulatory T cells for improving cancer therapy:
Challenges and prospects. Cancer Rep (Hoboken). 1:e211052018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Halvorsen EC, Hamilton MJ, Young A,
Wadsworth BJ, LePard NE, Lee HN, Firmino N, Collier JL and
Bennewith KL: Maraviroc decreases CCL8-mediated migration of
CCR5+ regulatory T cells and reduces metastatic tumor
growth in the lungs. Oncoimmunology. 5:e11503982016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shang B and Liu Y, Jiang SJ and Liu Y:
Prognostic value of tumor-infiltrating FoxP3+ regulatory
T cells in cancers: A systematic review and meta-analysis. Sci Rep.
5:151792015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sakaguchi S, Sakaguchi N, Asano M, Itoh M
and Toda M: Immunologic self-tolerance maintained by activated T
cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a
single mechanism of self-tolerance causes various autoimmune
diseases. J Immunol. 155:1151–1164. 1995.PubMed/NCBI
|
|
50
|
Wei S, Kryczek I, Edwards RP, Zou L,
Szeliga W, Banerjee M, Cost M, Cheng P, Chang A, Redman B, et al:
Interleukin-2 administration alters the
CD4+FOXP3+ T-cell pool and tumor trafficking
in patients with ovarian carcinoma. Cancer Res. 67:7487–7494. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu S and Sun X, Luo J, Zhu H, Yang X, Guo
Q, Song Y and Sun X: Effects of radiation on T regulatory cells in
normal states and cancer: Mechanisms and clinical implications. Am
J Cancer Res. 5:3276–3285. 2015.PubMed/NCBI
|
|
52
|
Yu P, Lee Y, Liu W, Krausz T, Chong A,
Schreiber H and Fu YX: Intratumor depletion of CD4+
cells unmasks tumor immunogenicity leading to the rejection of
late-stage tumors. J Exp Med. 201:779–791. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nishikawa H, Kato T, Tanida K, Hiasa A,
Tawara I, Ikeda H, Ikarashi Y, Wakasugi H, Kronenberg M, Nakayama
T, et al: CD4+ CD25+ T cells responding to
serologically defined autoantigens suppress antitumor immune
responses. Proc Natl Acad Sci USA. 100:10902–10906. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rech AJ and Vonderheide RH: Clinical use
of Anti-CD25 antibody daclizumab to enhance immune responses to
tumor antigen vaccination by targeting regulatory T cells. Ann N Y
Acad Sci. 1174:99–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wu L, Yun Z, Tagawa T, Rey-McIntyre K,
Anraku M and Perrot M: Tumor cell repopulation between cycles of
chemotherapy is inhibited by regulatory T-cell depletion in a
murine mesothelioma model. J Thorac Oncol. 6:1578–1586. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lissoni P, Brivio F, Fumagalli L, Messina
G, Meregalli S, Porro G, Rovelli F, Vigorè L, Tisi E and D'Amico G:
Effects of the conventional antitumor therapies surgery,
chemotherapy, radiotherapy and immunotherapy on regulatory T
lymphocytes in cancer patients. Anticancer Res. 29:1847–1852.
2009.PubMed/NCBI
|
|
57
|
Esquivel-Velázquez M, Ostoa-Saloma P,
Palacios-Arreola MI, Nava-Castro KE, Castro JI and Morales-Montor
J: The role of cytokines in breast cancer development and
progression. J Interferon Cytokine Res. 35:1–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Benoy IH, Salgado R, Van Dam P, Geboers K,
Van Marck E, Scharpé S, Vermeulen PB and Dirix LY: Increased serum
interleukin-8 in patients with early and metastatic breast cancer
correlates with early dissemination and survival. Clin Cancer Res.
10:71572004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Singh JK, Farnie G, Bundred NJ, Simões BM,
Shergill A, Landberg G, Howell SJ and Clarke RB: Targeting CXCR1/2
significantly reduces breast cancer stem cell activity and
increases the efficacy of inhibiting HER2 via HER2-dependent and
-independent mechanisms. Clin Cancer Res. 19:643–656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chang Q, Bournazou E, Sansone P, Berishaj
M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, et al:
The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and
metastasis. Neoplasia. 15:848–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Masjedi A, Hashemi V, Hojjat-Farsangi M,
Ghalamfarsa G, Azizi G, Yousefi M and Jadidi-Niaragh F: The
significant role of interleukin-6 and its signaling pathway in the
immunopathogenesis and treatment of breast cancer. Biomed
Pharmacother. 108:1415–1424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lin S, Gan Z, Han K, Yao Y and Min D:
Interleukin-6 as a prognostic marker for breast cancer: A
meta-analysis. Tumori. 101:535–541. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shibayama O, Yoshiuchi K, Inagaki M,
Matsuoka Y, Yoshikawa E, Sugawara Y, Akechi T, Wada N, Imoto S,
Murakami K, et al: Association between adjuvant regional
radiotherapy and cognitive function in breast cancer patients
treated with conservation therapy. Cancer Med. 3:702–709. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wolczyk D, Zaremba-Czogalla M,
Hryniewicz-Jankowska A, Tabola R, Grabowski K, Sikorski AF and
Augoff K: TNF-α promotes breast cancer cell migration and enhances
the concentration of membrane-associated proteases in lipid rafts.
Cell Oncol (Dordr). 39:353–363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Osawa Y, Nagaki M, Banno Y, Brenner DA,
Asano T, Nozawa Y, Moriwaki H and Nakashima S: Tumor necrosis
factor alpha-induced interleukin-8 production via NF-kappaB and
phosphatidylinositol 3-kinase/Akt pathways inhibits cell apoptosis
in human hepatocytes. Infect Immun. 70:6294–6301. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kulbe H, Hagemann T, Szlosarek PW,
Balkwill FR and Wilson JL: The inflammatory cytokine tumor necrosis
factor-alpha regulates chemokine receptor expression on ovarian
cancer cells. Cancer Res. 65:10355–10362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sheikhpour E, Noorbakhsh P, Foroughi E,
Farahnak S, Nasiri R and Neamatzadeh H: A survey on the role of
interleukin-10 in breast cancer: A narrative. Rep Biochem Mol Biol.
7:30–37. 2018.PubMed/NCBI
|
|
68
|
Li Y, Gao P, Yang J, Yu H, Zhu Y and Si W:
Relationship between IL-10 expression and prognosis in patients
with primary breast cancer. Tumour Biol. 35:11533–11540. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dutta P, Sarkissyan M, Paico K, Wu Y and
Vadgama JV: MCP-1 is overexpressed in triple-negative breast
cancers and drives cancer invasiveness and metastasis. Breast
Cancer Res Treat. 170:477–486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lim SY, Yuzhalin AE, Gordon-Weeks AN and
Muschel RJ: Targeting the CCL2-CCR2 signaling axis in cancer
metastasis. Oncotarget. 7:28697–28710. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kitamura T, Qian BZ, Soong D, Cassetta L,
Noy R, Sugano G, Kato Y, Li J and Pollard JW: CCL2-induced
chemokine cascade promotes breast cancer metastasis by enhancing
retention of metastasis-associated macrophages. J Exp Med.
212:1043–1059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Low-Marchelli JM, Ardi VC, Vizcarra EA,
van Rooijen N, Quigley JP and Yang J: Twist1 induces CCL2 and
recruits macrophages to promote angiogenesis. Cancer Res.
73:662–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumor metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Andrikopoulos P, Fraser SP, Patterson L,
Ahmad Z, Burcu H, Ottaviani D, Diss JK, Box C, Eccles SA and
Djamgoz MB: Angiogenic functions of voltage-gated Na+
channels in human endothelial cells: Modulation of vascular
endothelial growth factor (VEGF) signaling. J Biol Chem.
286:16846–16860. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fiorio Pla A and Munaron L: Functional
properties of ion channels and transporters in tumor
vascularization. Philos Trans R Soc Lond B Biol Sci.
369:201301032014. View Article : Google Scholar
|
|
76
|
Andrikopoulos P, Baba A, Matsuda T,
Djamgoz MBA, Yaqoob MM and Eccles SA: Ca2+ influx
through reverse mode Na+/Ca2+ exchange is
critical for vascular endothelial growth factor-mediated
extracellular signal-regulated kinase (ERK) 1/2 activation and
angiogenic functions of human endothelial cells. J Biol Chem.
286:37919–37931. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ginestier C, Liu S, Diebel ME, Korkaya H,
Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum
D, Guan JL, et al: CXCR1 blockade selectively targets human breast
cancer stem cells in vitro and in xenografts. J Clin Invest.
120:485–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Todorović-Raković N and Milovanović J:
Interleukin-8 in breast cancer progression. J Interferon Cytokine
Res. 33:563–570. 2013. View Article : Google Scholar : PubMed/NCBI
|