Introduction
Acute promyelocytic leukaemia (APL), a distinct
subtype of acute myeloid leukaemia (AML), responds well to
differentiation therapy (1).
All-trans retinoic acid (ATRA) has been used successfully to treat
APL as a differentiation therapy (1). Therapeutic concentrations of ATRA
induce a conformational change in the promyelocytic
leukemia-retinoic acid α receptor (RARα) molecular complex, the
leukaemia-generating fusion protein which leads to the development
of APL (2). When the corepressors
are released from the complex, normal RARα-responsive gene
regulation occurs, which induces terminal APL cell differentiation
(2,3). However, previous studies have
demonstrated that ring finger protein 8 is responsible for ATRA
resistance in variant APL with general transcription factor
IIi/RARα fusion, and inhibition of the ubiquitin-proteasome
signalling pathway contributes to the reversion of ATRA resistance
(4). A previous study demonstrated
that RARα and its fusion proteins are degraded by the
ubiquitin/proteasome signalling pathway, which may limit
ATRA-mediated myeloid cell differentiation (5). A small proportion of patients do not
respond to induction therapy, which makes variant APL a challenging
disease to treat (1).
Bortezomib, a synthetic, biologically active boronic
acid dipeptide, is commercially available, and is the first
proteasome inhibitor approved by the US Food and Drug
Administration (6). This novel agent
has been approved for the treatment of multiple myeloma (MM)
(7) and mantel cell lymphoma
(8), as well as other types of
haematological malignancies (6),
even in relapsed or refractory patients (9). Furthermore, it exhibits antitumor
properties in certain types of solid tumour cells and in murine
xenograft models (10,11). The anticancer activity of this drug
is exerted via several different mechanisms, including cell
proliferation inhibition, apoptosis and autophagy induction, as
well as anti-angiogenesis (12).
Therefore, bortezomib may serve as a potent therapeutic to overcome
resistance to conventional chemotherapy.
Autophagy is an orchestrated process in which
damaged organelles and misfolded proteins are engulfed by
autophagosomes, which then fuse with lysosomes for degradation
(13). Generally, autophagy is
critical for maintaining cellular homeostasis during stress and
adverse conditions (14). A previous
study suggested that autophagy may represent a potential
therapeutic target for cancer treatment (15). Bortezomib-induced autophagy has been
demonstrated in certain types of cancer cells, such as non-small
cell lung cancer cells, glioblastoma cells and B-cell acute
lymphoblastic leukaemia cells (16–18). It
has been previously reported that bortezomib, combined with arsenic
trioxide, exhibits a beneficial effect in APL therapy (19). However, the exact molecular
mechanisms by which bortezomib exerts its effects on APL cells
remain unknown.
In the present study, bortezomib treatment reduced
the viability of NB4 cells in a time- and dose-dependent manner,
and the effects of bortezomib treatment on apoptosis and autophagy
were investigated in NB4 cells. Furthermore, inhibition of
autophagy increased cell apoptosis induced by bortezomib in NB4
cells, highlighting a potential novel treatment strategy for APL in
addition to differentiation therapy.
Materials and methods
Reagents
Antibodies against Bax (cat. no. 2774), Bcl-2 (cat.
no. 15071), Beclin-1 (cat. no. 3738), caspase-3 (cat. no. 9662),
poly(ADP-ribose) polymerase (PARP; cat. no. 9532) and sequestosome
1/p62 (cat. no. 88588) were purchased from Cell Signaling
Technology, Inc.. Antibodies against β-actin (cat. no. 60008-1)
were purchased from ProteinTech Group, Inc.. Antibodies against
LC3B (cat. no. L7543) were purchased from Sigma-Aldrich; Merck
KGaA. Bortezomib (cat. no. DELS800) was purchased from BSP
Pharmaceuticals S.p.A., dissolved in 0.9% normal saline solution
and aliquoted at a stock concentration of 2 mM, and stored at
−20°C.
Cell culture
The human APL NB4 and HL-60 cell lines were obtained
from The Cell Bank of Type Culture Collection of The Chinese
Academy of Sciences. NB4 and HL-60 cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% heat-inactivated FBS (HyClone; Cytiva) and 50 µg/ml penicillin
and 50 µg/ml streptomycin (Beijing Solarbio Science &
Technology Co., Ltd.) in a humidified incubator at 37°C with 5%
CO2.
Water-soluble tetrazolium salts
(WST)-8 assay
The cytotoxicity of bortezomib in NB4 cells was
determined using a WST-8 assay. Briefly, exponentially growing NB4
cells (5×103 cells/well) were cultured in 96-well plates
for 24 h and then treated with various concentrations of bortezomib
(0, 1.25, 2.5, 5, 10, 20 and 40 nM) for 24–48 h at 37°C.
Subsequently, cell viability was evaluated by incubating with WST-8
(Dojindo Molecular Technologies, Inc.) for 1 h at 37°C according to
the manufacturer's protocol in triplicate. The absorbance of
samples was measured at 450 nm using an EnSpire Multilabel Plate
Reader (PerkinElmer, Inc.).
Flow cytometry analysis of cell
apoptosis
The apoptotic effect of bortezomib was detected
using an Annexin V-FITC/propidium iodide (PI) Apoptosis Detection
kit (cat. no. KGA107; Nanjing KeyGen Biotech Co., Ltd.). Briefly,
cells (2×105 cells/well) were cultured in 6-well plates
and treated with various concentrations of bortezomib for 24–48 h
at 37°C. Subsequently, cells were washed twice in PBS and then
stained with Annexin V-FITC and PI for 15 min at room temperature.
Cell apoptosis was detected using a flow cytometer (FACSCalibur; BD
Biosciences) and analysed using CellQuest analysis version 5.0
software (BD Biosciences).
Monodansylcadaverine (MDC)
staining
The autophagic vacuoles in NB4 and HL-60 cells were
visualized using MDC staining. Briefly, cells (2×105
cells/well) were cultured in 6-well plates and treated with
bortezomib (20 nM) for 24 h at 37°C. Subsequently, the incubated
cells were treated with MDC (cat. no. 30432; Sigma-Aldrich; Merck
KGaA) at a concentration of 0.05 mM in PBS at 37°C for 1 h.
Subsequently, the cells were centrifuged onto coverslips in 1,500 ×
g for 15 min at room temperature, in which the coverslips were
pre-coated with poly-L-lysine (cat. no. P8920; Sigma-Aldrich; Merck
KGaA) at 4°C for 1 h. The cells were fixed with 4% paraformaldehyde
(cat. no. 158127; Sigma-Aldrich; Merck KGaA) at 4°C for 10 min and
then the coverslips containing the fixed cells were washed twice
with PBS. The MDC-stained cells were analysed using a fluorescence
microscope at a magnification of 10×40 (eye lens × objective lens;
excitation, 380 nm; emission, 525 nm; DP73; Olympus
Corporation).
Lentiviral transfection
Lentiviral particles containing short hairpin RNA
(shRNA/sh) Beclin-1 (cat. no. 131209BZ;
5′-CCGACTTGTTCCTTACGGAAA-3′) and shRNA negative control (shCTRL;
cat. no. 131127CZ; 5′-TTCTCCGAACGTGTCACGTTTC-3′) expression vectors
were obtained from Shanghai GenePharma Co., Ltd., and were then
cloned into pGLV3/H1/GFP-Puro vector (Shanghai GenePharma Co.,
Ltd.). High titre production of lentivector was achieved by
transiently co-transfecting 293T cells (The Cell Bank of Type
Culture Collection of The Chinese Academy of Sciences) with
lentiviral packaging plasmids, 3 µg pGag/Pol, 1 µg pRev, 1 µg
pVSV-G (Shanghai GenePharma Co., Ltd.) and 3 µg shBeclin-1
expressing constructs via Lipofectamine® 2000 (cat. no.
11668-500; Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for
6 h, followed by a further 72-h culture period with replaced
medium. The lentivirus particles were collected by centrifuging the
cell supernatant at 1,500 × g for 4 min at 4°C. Subsequently, the
supernatant in the centrifuge tube was filtered using a 0.45-µm
filter and centrifuged at 40,000 × g for 2 h at 4°C. Exponentially
growing NB4 or HL-60 cells were seeded and cultured in 6-well
plates, and exposed to the lentivirus particles (shBeclin-1 or
shCTRL) at 37°C for 2 h at a multiplicity of infection of
1×108 TU/ml, followed by a further 48-h culture period
before subsequent experiments. Subsequently, cells stably
expressing shBeclin-1 and shCTRL vectors were selected using
puromycin as a selection pressure (2 µg/ml).
Detection of autophagosomes by
transmission electron microscopy
NB4 or HL-60 cells (2×105 cells/well)
were cultured in 6-well plates and treated with bortezomib (20 nM)
for 24 h at 37°C. The collected cells were then fixed with 2.5%
glutaraldehyde for 2 h at 4°C, washed with PBS three times and
fixed with 1% osmium tetroxide for 1 h at 4°C. Following gradient
dehydration with ethanol-acetone, the cells were embedded in epoxy
for 4–6 h at 4°C for sectioning. The thin sections (50–70-nm-thick)
were doubly stained with 1% (w/v) uranyl acetate for 30 min at room
temperature and lead citrate for 20 min at room temperature, and
then observed using a JEM 1011CX transmission electron microscope
(JEOL, Ltd.).
Western blotting
The treated NB4 or HL-60 cells were washed with cold
PBS twice and then lysed in RIPA lysis buffer [Tris (50 mM) pH 8.0,
sodium chloride (150 mM), 0.5% sodium deoxycholate, 0.1% SDS and 1%
NP-40] supplemented with protease inhibitors
[Na3VO4 (1 mM), leupeptin (1 µg/ml) and PMSF
(1 mM)] on ice for 30 min. Protein concentration was determined
using a BCA assay and protein samples (20 µg/lane) were separated
by SDS-PAGE (8–12%) and then transferred onto a nitrocellulose
membrane (EMD Millipore). Subsequently, membranes were blocked
using 5% (w/v) skimmed milk at room temperature for 1 h, and then
washed with TBS with 0.1% Tween-20 three times. Subsequently, the
membranes were incubated with primary antibodies (1:1,000) for 12 h
at 4°C. Following incubation with anti-mouse (1:5,000; cat. no.
ab97040; Abcam) or anti-rabbit (1:5,000; cat. no. ab7090; Abcam)
HRP-conjugated secondary antibodies at room temperature for 1 h,
signals were visualized using an enhanced chemiluminescence western
blotting kit (cat. no. K-12045-D50; Advansta, Inc.) and analysed
using Image Lab version 4.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. GraphPad Prism 5 (GraphPad
Software, Inc.) was used for statistical analysis, and unpaired
Student's t-test (comparison of two groups) or one-way ANOVA
followed by Sidak's multiple comparisons post hoc test (comparison
of more than two groups) was used to determine the significance of
differences among groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Bortezomib reduces cell viability and
induces cell apoptosis in NB4 cells
To determine the effects of bortezomib on cell
viability, NB4 cells were treated with a range of concentrations of
bortezomib for 24 and 48 h. The WST-8 assay revealed that
bortezomib treatment resulted in a time- and dose-dependent
inhibition of cell viability in NB4 cells (Fig. 1A). Reduction of cell viability
reached a plateau with 20 nM bortezomib treatment and this
concentration was selected for all subsequent experiments. The
apoptotic effect of bortezomib was determined by flow cytometry,
the most convenient technique to study apoptotic cell death, and
western blotting, a widely used technique for qualitative or
semi-quantitative analysis of apoptosis (20). NB4 and HL-60 cells were treated with
20 nM bortezomib for 0, 24 and 48 h, and apoptosis was determined
using Annexin V/PI staining. The results indicated that bortezomib
treatment significantly decreased live cells compared with the
control cells (0 h; Figs. 1B and C
and S1A and B), indicating that
apoptosis was increased. Bortezomib-induced apoptosis was further
demonstrated by increased levels of cleaved caspase-3, cleaved PARP
and Bax, and decreased expression levels of Bcl-2 (Figs. 1D and S1C).
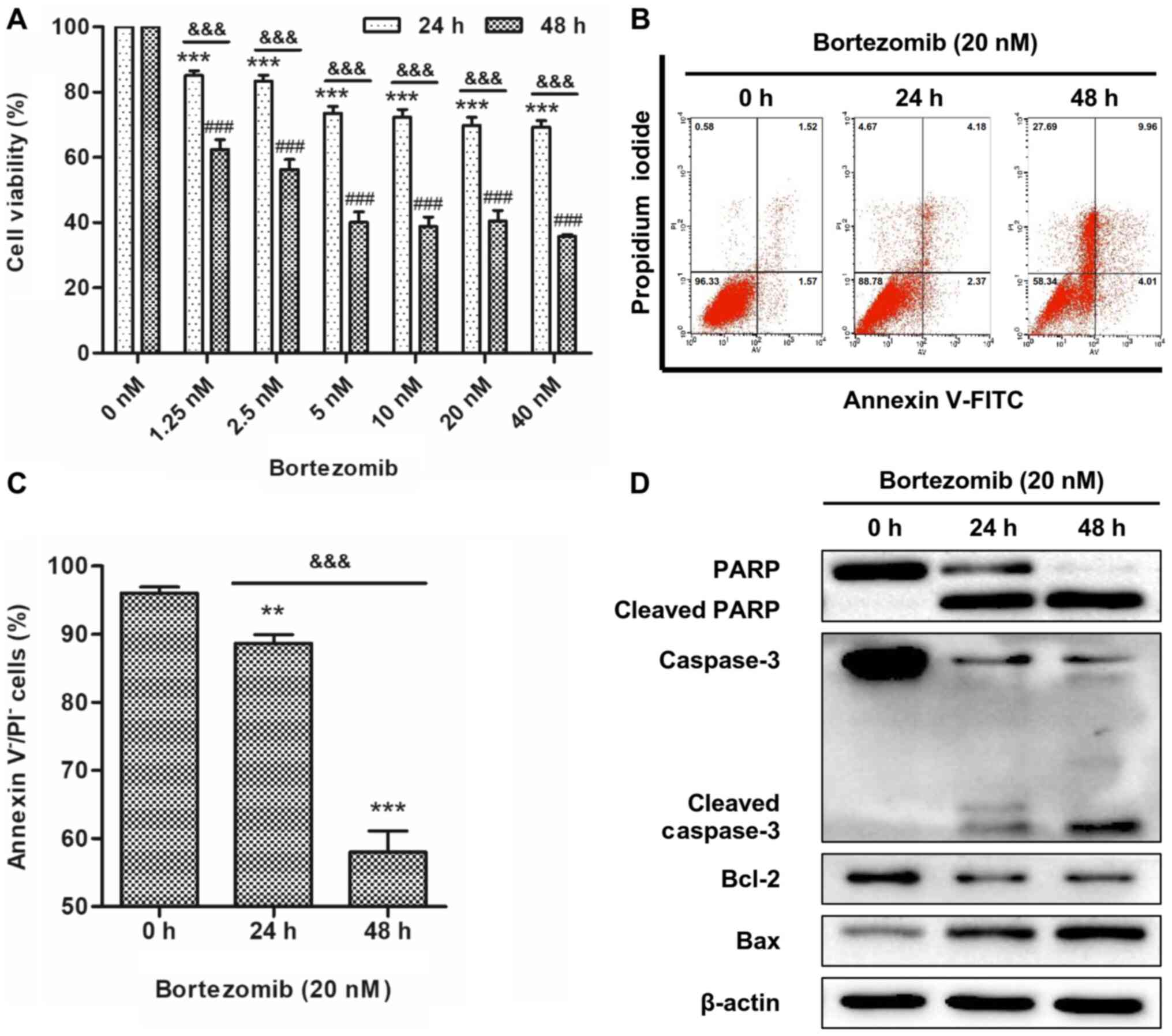 | Figure 1.Bortezomib reduces cell viability and
induces cell apoptosis in NB4 cells. (A) Cells were treated with
bortezomib (0, 1.25, 2.5, 5, 10, 20 and 40 nM) for 24 or 48 h, and
cell viability was assessed using a water-soluble tetrazolium
salts-8 assay. Error bars represent the standard error of the mean
of three independent repeats. ***P<0.001 vs. 0 nM 24 h.
###P<0.001 vs. 0 nM 48 h.
&&&P<0.001 24 vs. 48 h. (B) Cells were
treated with bortezomib (20 nM) for 0, 24 and 48 h, and then cells
were stained with Annexin V and PI. The percentage of apoptotic
cells was assessed using FACS analysis. (C) Bar graph showing the
percentage of cells negative for Annexin V and PI staining. Data
are presented as the mean ± standard deviation of three independent
repeats. **P<0.01 and ***P<0.001 vs. control (0 h).
&&&P<0.001 24 vs. 48 h. (D) Cells were
treated with bortezomib (20 nM) for 0, 24 and 48 h, and the
expression levels of cleaved PARP, cleaved caspase-3, Bcl-2 and Bax
were assessed by western blotting. PARP, poly(ADP-ribose)
polymerase; PI, propidium iodide. |
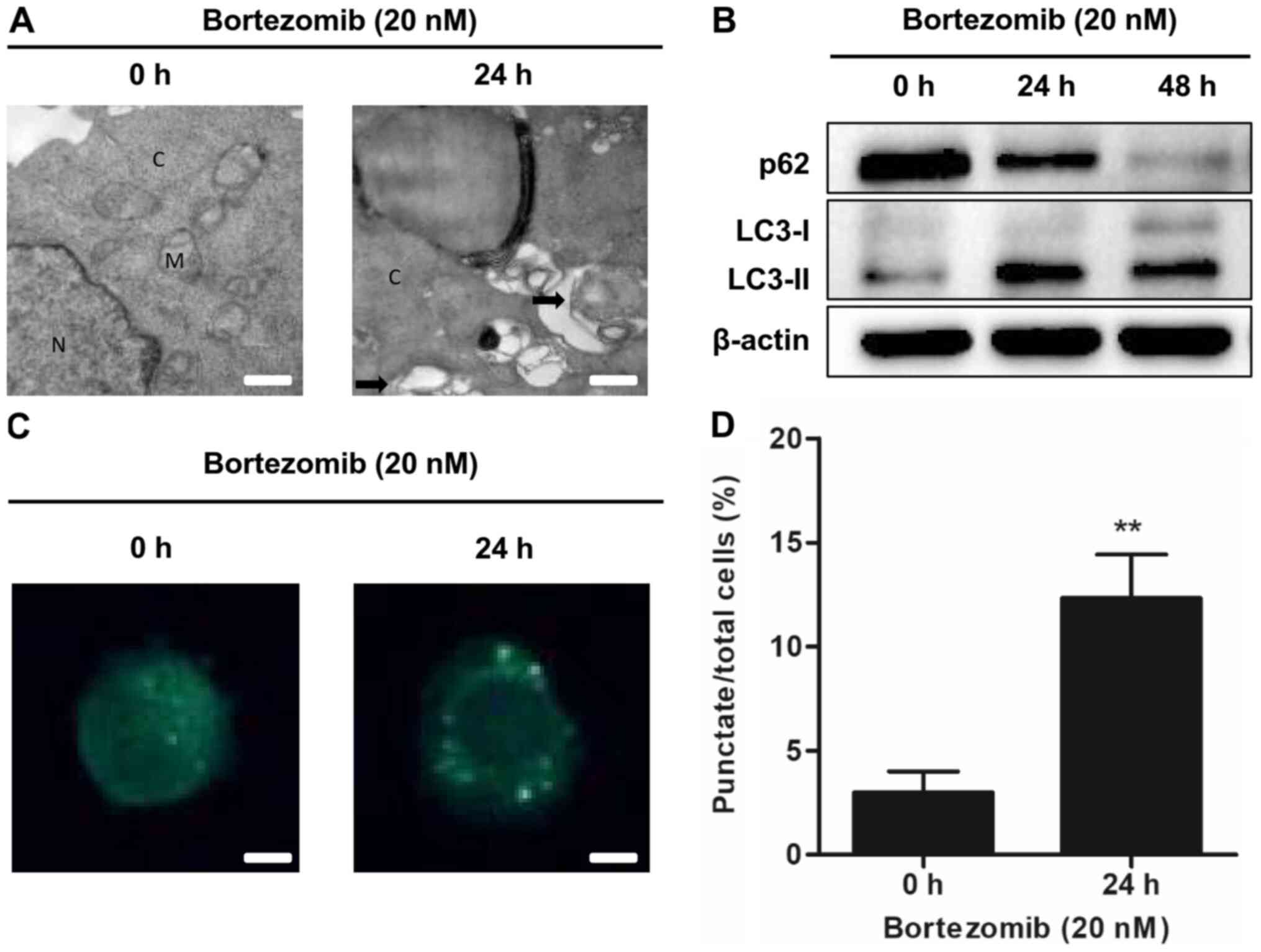
Bortezomib induces autophagy in NB4
cells
To determine whether bortezomib could induce
autophagy in NB4 and HL-60 cells, transmission electron microscopy
was used to detect autophagosome formation following treatment with
bortezomib. The presence of autophagosomes was increased in the
bortezomib-treated cells compared with the control cells (0 h;
Figs. 2A and S2A). Bortezomib treatment increased the
expression levels of the autophagy-related protein LC3-II and
decreased the levels of the autophagic marker p62 in a
time-dependent manner (Figs. 2B and
S2B). The presence of MDC staining
was used as a marker of autophagic vacuoles (21). Bortezomib significantly increased the
accumulation of MDC in treated cells compared with the control
cells (0 h; Figs. 2C and D and
S2C and D). These results
demonstrated that bortezomib treatment induced autophagy in NB4 and
HL-60 cells.
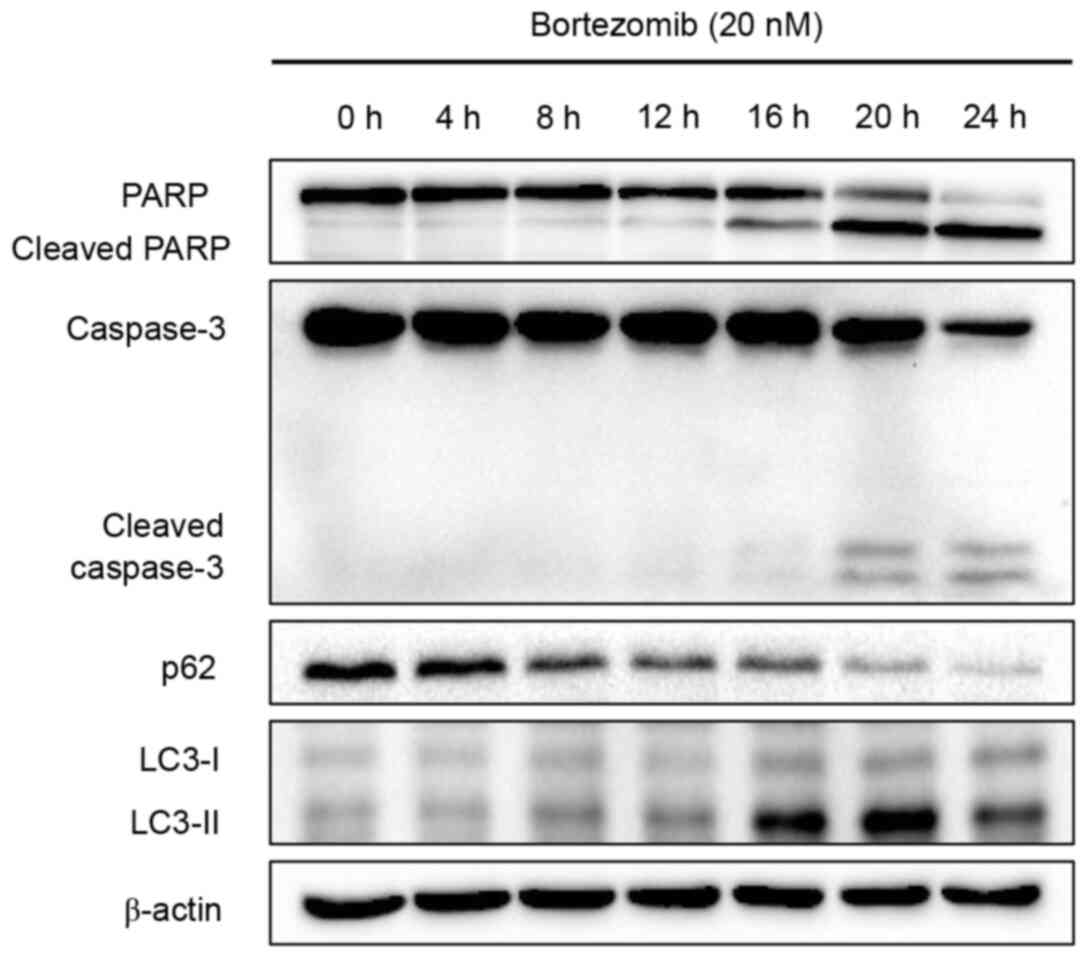
Autophagy occurs before apoptosis
following treatment with bortezomib
The aforementioned results demonstrated that
bortezomib induced apoptosis and autophagy in NB4 cells. A
time-course study was performed to determine when apoptosis and
autophagy were initiated following treatment with bortezomib.
Apoptosis, as determined by PARP and caspase-3 cleavage levels, was
detected at 16 h (cleaved PARP) or 20 h (cleaved caspase-3) after
bortezomib treatment, whereas autophagy, as indicated by p62
degradation and LC3 conversion, was detected at an earlier time
point (8 h; Fig. 3). Overall, these
results indicated that bortezomib induced autophagy prior to
apoptosis in NB4 cells.
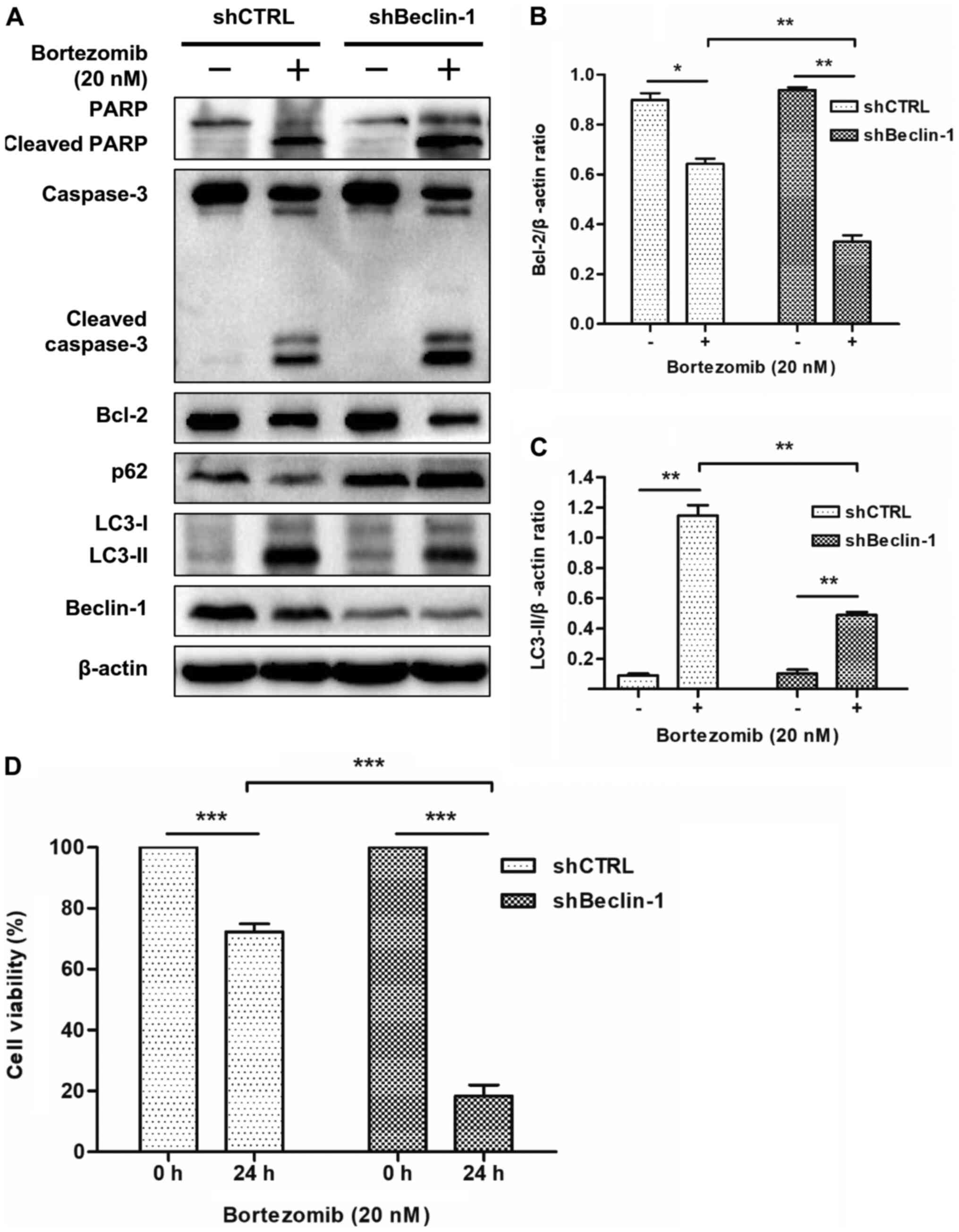
Inhibition of autophagy using shRNA
enhances cell apoptosis and reduces cell viability induced by
bortezomib in NB4 cells
The role of autophagy in bortezomib-mediated
apoptosis was further investigated by knocking down Beclin-1
expression using shRNA. As shown in Fig.
4A, the expression levels of Beclin-1 were markedly suppressed
in NB4 cells transfected with the shRNA-Beclin-1 lentivirus, but
not in those transfected with the non-targeting shRNA.
Subsequently, shBeclin-1 was used to inhibit autophagy in order to
determine the role of autophagy in apoptosis induced by bortezomib.
Western blot analysis demonstrated that bortezomib treatment
significantly decreased the expression levels of Bcl-2 (Fig. 4A and B) and LC3-II (Fig. 4A and C), and increased the expression
levels of p62, cleaved PARP and cleaved caspase-3 (Fig. 4A) in shBeclin-1 NB4 cells compared
with in shCTRL cells. The WST-8 assay revealed that bortezomib
treatment significantly decreased cell viability in shBeclin-1 NB4
cells compared with in shCTRL cells (Fig. 4D). These results indicated that
inhibition of autophagy enhanced apoptosis in NB4 cells and HL-60
cells (Figs. 4A-C and S3) and decreased cell viability induced by
bortezomib in NB4 cells (Fig.
4D).
Discussion
Autophagy is an evolutionarily conserved process in
which intracellular membrane structures sequester misfolded
proteins and damaged organelles into autophagosomes for
degradation, after which the contents are recycled (22). Generally, autophagy functions to
maintain cellular homeostasis in response to stress and starvation
(23). Increasing evidence has
demonstrated that autophagy may protect cancer cells from
anticancer drug treatments, and that suppression of autophagy
enhances cancer cell death (22,24). Our
previous study demonstrated that, in haematological malignant
cells, inhibition of autophagy by chloroquine, 3-methyladenine or a
combination of these two agents enhanced the apoptosis induced by
chemotherapy (15). In the present
study, it was demonstrated that bortezomib treatment inhibited cell
viability, and induced autophagy and apoptosis in NB4 cells.
Additionally, it was demonstrated that inhibition of autophagy
enhanced apoptosis induced by bortezomib in NB4 cells.
After revealing that bortezomib induced apoptosis
and autophagy in NB4 cells, the sequence of these two events was
determined. Therefore, a time-dependent study was performed and the
results revealed that autophagy occurred before apoptosis, as
demonstrated by the upregulation of apoptosis and autophagy
markers. p62 degradation and LC-I/LC-II conversion occurred after 8
h, whereas cleaved caspase-3 and cleaved PARP were detected after
16 h. Subsequently, to further clarify the role of autophagy in
bortezomib-induced cell death, shBeclin-1 was used to knockdown
Beclin-1 expression, and the role of autophagy was evaluated more
directly. Notably, bortezomib alone had an apoptosis- and
autophagy-inducing effect; however, following knockdown using
shBeclin-1, autophagy was blocked, as demonstrated by the increased
p62 expression and decreased conversion of LC-II. Bortezomib
treatment in the knockdown cells increased the levels of cleaved
caspase-3 and cleaved PARP, and decreased the expression levels of
Bcl-2, indicating that autophagy inhibition in combination with
proteasome suppression resulted in increased apoptosis. Therefore,
it was hypothesized that when tumour cells encounter
chemotherapeutic drugs or stress, autophagy occurs first to protect
the tumour cells from harm. If stress persists, the cells will then
undergo apoptosis, indicating that autophagy may serve a role in
protecting tumour cells. A previous study has demonstrated that
autophagy serves a protective role in apoptotic cell death under
conditions of stress and starvation, as well as contributing to
drug resistance (25). It has been
reported that inhibition of autophagy augments 5-fluorouracil
chemotherapy in colon cancer cells (26) and overcomes glucocorticoid resistance
in lymphoid malignant cells (15).
Conversely, studies have also demonstrated that autophagy
contributes to tumour cell death (27,28). A
possible explanation for the contradictory results stemming from
the same process may lie in the stimulus type, organism
development, nutrient availability and the signalling pathways
involved (29). Previous studies
have demonstrated that the PI3K-AKT-mTOR signalling pathway, which
is activated in several types of cancer, is involved in autophagy
regulation (21,30). 3-Methyladenine inhibits class III
PI3K, thus blocking autophagic sequestration at the initial stage,
which has been widely used in several studies (21,30).
Therefore, further investigations are required to clarify the
signalling pathway involved in bortezomib-treated APL.
Bortezomib is a proteasome inhibitor that has been
used to treat MM and mantel cell lymphoma for decades (8,9). In AML,
constitutive activity of NF-κB has been detected in 40% of cases
and its aberrant activity enables leukaemia cells to evade
apoptosis and stimulate proliferation (31,32).
Bortezomib, which is also an NF-κB inhibitor, is widely used in the
treatment of AML as a therapeutic strategy in combination with
other chemotherapeutic agents (33,34).
Other possible mechanisms involved in bortezomib-treated AML cells
are as follows: i) Riccioni et al (35) demonstrated that bortezomib mediates
apoptosis by activating caspase-8 and caspase-3 in M4 and M5 cells;
ii) Yan et al (19) reported
that protein kinase Cδ serves an important role in bortezomib- and
arsenic trioxide-induced apoptosis, and that the combined regimen
may serve as a potential therapeutic remedy for the treatment of
leukaemia; and iii) Ying et al (36) revealed that bortezomib sensitizes AML
cells to ATRA-induced differentiation by modifying the RARα/STAT1
axis. Therefore, further studies focusing on the mechanisms of
combination of bortezomib and autophagy inhibition may provide
novel insights into APL treatment. Given the hypothesis of
autophagy inhibition as an anticancer strategy, more data in in
vivo models should be accumulated in future studies.
In conclusion, the present study demonstrated that
bortezomib inhibited cell viability and induced autophagy, which
occurred prior to apoptosis. Furthermore, it was demonstrated that
inhibition of autophagy using shBeclin-1 enhanced the anticancer
effects of bortezomib. The present study improved the understanding
of the combined effects of bortezomib on autophagy inhibition in
haematological malignancy chemotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Anhui Higher Education Institutions (grant
no. KJ2017A833; to LJ) and the Backbone of Scientific Research (to
LJ).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LJ and MZY designed the study. LJ performed all
experiments. YMZ analyzed the results. LJ wrote, reviewed and
edited the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Raffoux E, Rousselot P, Poupon J, Daniel
MT, Cassinat B, Delarue R, Taksin AL, Réa D, Buzyn A, Tibi A, et
al: Combined treatment with arsenic trioxide and all-trans-retinoic
acid in patients with relapsed acute promyelocytic leukemia. J Clin
Oncol. 21:2326–2334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nitto T and Sawaki K: Molecular mechanisms
of the antileukemia activities of retinoid and arsenic. J Pharmacol
Sci. 126:179–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jing Y and Waxman S: The design of
selective and non-selective combination therapy for acute
promyelocytic leukemia. Curr Top Microbiol Immunol. 313:245–269.
2007.PubMed/NCBI
|
|
4
|
Yan W, Li J, Zhang Y, Yin Y, Cheng Z, Wang
J, Hu G, Liu S, Wang Y, Xu Y, et al: RNF8 is responsible for ATRA
resistance in variant acute promyelocytic leukemia with GTF2I/RARA
fusion, and inhibition of the ubiquitin-proteasome pathway
contributes to the reversion of ATRA resistance. Cancer Cell Int.
19:842019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kopf E, Plassat JL, Vivat V, de The H,
Chambon P and Rochette-Egly C: Dimerization with retinoid X
receptors and phosphorylation modulate the retinoic acid-induced
degradation of retinoic acid receptors alpha and gamma through the
ubiquitin-proteasome pathway. J Biol Chem. 275:33280–33288. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robak P and Robak T: Bortezomib for the
treatment of hematologic malignancies: 15 Years later. Drugs R D.
19:73–92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scott K, Hayden PJ, Will A, Wheatley K and
Coyne I: Bortezomib for the treatment of multiple myeloma. Cochrane
Database Syst Rev. 4:CD0108162016.PubMed/NCBI
|
|
8
|
Yazbeck V, Shafer D, Perkins EB, Coppola
D, Sokol L, Richards KL, Shea T, Ruan J, Parekh S, Strair R, et al:
A phase II trial of bortezomib and vorinostat in mantle cell
lymphoma and diffuse large B-cell lymphoma. Clin Lymphoma Myeloma
Leuk. 18:569–575.e1. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Richardson PG, Oriol A, Beksac M, Liberati
AM, Galli M, Schjesvold F, Lindsay J, Weisel K, White D, Facon T,
et al: Pomalidomide, bortezomib, and dexamethasone for patients
with relapsed or refractory multiple myeloma previously treated
with lenalidomide (OPTIMISMM): A randomised, open-label, phase 3
trial. Lancet Oncol. 20:781–794. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caravita T, de Fabritiis P, Palumbo A,
Amadori S and Boccadoro M: Bortezomib: Efficacy comparisons in
solid tumors and hematologic malignancies. Nat Clin Pract Oncol.
3:374–387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nencioni A, Grunebach F, Patrone F,
Ballestrero A and Brossart P: Proteasome inhibitors: Antitumor
effects and beyond. Leukemia. 21:30–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roccaro AM, Vacca A and Ribatti D:
Bortezomib in the treatment of cancer. Recent Pat Anticancer Drug
Discov. 1:397–403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang L, Xu L, Xie J, Li S, Guan Y, Zhang
Y, Hou Z, Guo T, Shu X, Wang C, et al: Inhibition of autophagy
overcomes glucocorticoid resistance in lymphoid malignant cells.
Cancer Biol Ther. 16:466–476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu G, Li H, Ji Z, Jiang X, Lei Y and Sun
M: Inhibition of autophagy by autophagic inhibitors enhances
apoptosis induced by bortezomib in non-small cell lung cancer
cells. Biotechnol Lett. 36:1171–1178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Li W, Wang C, Leng X, Lian S,
Feng J, Li J and Wang H: Inhibition of autophagy enhances apoptosis
induced by proteasome inhibitor bortezomib in human glioblastoma
U87 and U251 cells. Mol Cell Biochem. 385:265–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Zhu S, Zhang G and Liu S:
Inhibition of autophagy enhances the anticancer activity of
bortezomib in B-cell acute lymphoblastic leukemia cells. Am J
Cancer Res. 5:639–650. 2015.PubMed/NCBI
|
|
19
|
Yan H, Wang YC, Li D, Wang Y, Liu W, Wu YL
and Chen GQ: Arsenic trioxide and proteasome inhibitor bortezomib
synergistically induce apoptosis in leukemic cells: The role of
protein kinase Cdelta. Leukemia. 21:1488–1495. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galluzzi L, Aaronson SA, Abrams J, Alnemri
ES, Andrews DW, Baehrecke EH, Bazan NG, Blagosklonny MV, Blomgren
K, Borner C, et al: Guidelines for the use and interpretation of
assays for monitoring cell death in higher eukaryotes. Cell Death
Differ. 16:1093–1107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Folkerts H, Hilgendorf S, Vellenga E,
Bremer E and Wiersma VR: The multifaceted role of autophagy in
cancer and the microenvironment. Med Res Rev. 39:517–560. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
White E: The role for autophagy in cancer.
J Clin Invest. 125:42–46. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Levy JMM, Towers CG and Thorburn A:
Targeting autophagy in cancer. Nat Rev Cancer. 17:528–542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36:522017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Hou N, Faried A, Tsutsumi S and
Kuwano H: Inhibition of autophagy augments 5-fluorouracil
chemotherapy in human colon cancer in vitro and in vivo model. Eur
J Cancer. 46:1900–1909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Apel A, Zentgraf H, Buchler MW and Herr I:
Autophagy-A double-edged sword in oncology. Int J Cancer.
125:991–995. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eisenberg-Lerner A and Kimchi A: The
paradox of autophagy and its implication in cancer etiology and
therapy. Apoptosis. 14:376–391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dalby KN, Tekedereli I, Lopez-Berestein G
and Ozpolat B: Targeting the prodeath and prosurvival functions of
autophagy as novel therapeutic strategies in cancer. Autophagy.
6:322–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Napetschnig J and Wu H: Molecular basis of
NF-κB signaling. Annu Rev Biophys. 42:443–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perkins ND: The diverse and complex roles
of NF-κB subunits in cancer. Nat Rev Cancer. 12:121–132. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dai Y, Chen S, Wang L, Pei XY, Kramer LB,
Dent P and Grant S: Bortezomib interacts synergistically with
belinostat in human acute myeloid leukaemia and acute lymphoblastic
leukaemia cells in association with perturbations in NF-κB and Bim.
Br J Haematol. 153:222–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fang J, Rhyasen G, Bolanos L, Rasch C,
Varney M, Wunderlich M, Goyama S, Jansen G, Cloos J, Rigolino C, et
al: Cytotoxic effects of bortezomib in myelodysplastic
syndrome/acute myeloid leukemia depend on autophagy-mediated
lysosomal degradation of TRAF6 and repression of PSMA1. Blood.
120:858–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Riccioni R, Senese M, Diverio D, Riti V,
Buffolino S, Mariani G, Boe A, Cedrone M, Lo-Coco F, Foà R, et al:
M4 and M5 acute myeloid leukaemias display a high sensitivity to
Bortezomib-mediated apoptosis. Br J Haematol. 139:194–205. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ying M, Zhou X, Zhong L, Lin N, Jing H,
Luo P, Yang X, Song H, Yang B and He Q: Bortezomib sensitizes human
acute myeloid leukemia cells to all-trans-retinoic acid-induced
differentiation by modifying the RARα/STAT1 axis. Mol Cancer Ther.
12:195–206. 2013. View Article : Google Scholar : PubMed/NCBI
|