Introduction
The sentinel lymph node biopsy (SLNB) method has
been widely used to evaluate axillary lymphatic status. Compared
with SLNB, the incidence and severity of postoperative
complications, including lymphedema, swelling of the arm and
sensory loss, caused by axillary lymph node dissection are higher
(1). Since Krag et al
(2) first reported the use of
radioisotopes (RI) in 1993 and Giuliano et al (3) reported using blue dye (BD) in 1994, the
combination of BD and RI has been used as a standard technique to
increase the detection rate of sentinel lymph nodes (4). Certain clinical limitations of BD and
RI, including allergies and radioactivity, have prompted the
development of other tracer technologies (4). Indocyanine green (ICG)-guided SLNB has
been employed for the staging of the axillary lymphatic status
since 2005 (4) and numerous clinical
trials and cohort studies have shown that ICG is a promising
technology in patients with early stage breast cancer (4,5). Though
the majority of these data supported the conclusion that using ICG
was not worse in SLNB compared with other tracers, some studies
reported that ICG is a less effective tracer. A systematic review
performed in 2014 stated that ICG was significantly better than BD
with regard to improving sentinel lymph node identification
(5). However, a more recent
systematic review reported that the ICG fluorescence method
demonstrated improved axillary staging compared with the RI method
(6). Therefore, it is difficult to
draw a clear conclusion, because the results for comparing ICG with
traditional tracers are usually contradictory. To the best of our
knowledge, whether ICG can be applied clinically as a valid tracer
and replace traditional standard techniques has not yet been
established.
Considering the lack of conclusions regarding the
clinical utility of ICG, the present study collected data from
relevant randomized controlled trials and cohort studies and
compared the tracer ability of ICG with BD and RI, both
individually and in combination. The aim of the present study was
to confirm whether ICG can act as a better tracer agent compared
with conventional techniques.
Materials and methods
Search strategy
The present study was performed according to the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
statement (7). To assess the level
of sensitivity, the main international electronic data sources,
including PubMed (https://pubmed.ncbi.nlm.nih.gov), EMBASE (http://www.embase.com) and the Cochrane Library
(https://www.cochranelibrary.com), were
searched simultaneously.
The terms ‘breast cancer’, ‘sentinel lymph node
biopsy’, ‘blue dye’, ‘indocyanine green’, ‘radioisotope’ and
similar terms were cross-searched using the following search
algorithms: ((((breast cancer OR breast neoplasms OR breast
carcinoma)) AND (SLNB OR sentinel lymph node biopsy)) AND
(indocyanine green OR ICG OR radioisotope OR RI OR blue dye OR
BD))). All relevant studies were published between May 2009 and
March 2017.
Selection criteria
The current meta-analysis included all studies
meeting the following criteria: i) Patients: Patients with clinical
axillary lymph node-negative early breast cancer; ii) research
methods: SLNB using ICG-guided near-infrared fluorescence imaging,
using ≥ two tracers and using the patient as the self control; iii)
study type: Cohort study or randomized clinical trial; and iv)
language: English.
The following exclusion criteria was used: i)
Meeting abstracts and studies that did not contain comparisons of
ICG with other tracers and articles with neoadjuvant therapy; ii)
study sample sizes <10; iii) studies that performed axillary
reverse mapping; and iv) studies that did not use the patients as
their own controls.
All eligible studies were categorized into three
groups: i) ICG vs. BD; ii) ICG vs. RI; and iii) ICG vs. BD and RI.
The outcomes considered included studies that comprised the
identification rate (IR) of the patients, the IR of the sentinel
lymph nodes (SLNs) and the IR of the positive SLNs and false
negative rate (FNR).
Data extraction
In the present study, RY and XZ assessed and
screened the literature independently. Titles and abstracts were
first inspected, then full texts of potentially relevant
publications were obtained and screened. Any discrepancy was
resolved by discussion between the reviewers. Disagreements were
solved by full discussion until consensus was reached.
The characteristics of the cohort and randomized
clinical studies, including first author, year of publication,
number of cases and controls, device, dose of tracers and detection
outcomes for each study are presented in Table I. Different equipment, including
PhotoDynamic Eye (PDE), Mini-fluorescence-assisted resection and
exploration (Mini-FLARE) and a charge-coupled camera (CCD) were
used.
 | Table I.Characteristics and technical details
in each group of the selected studies in the current
meta-analysis. |
Table I.
Characteristics and technical details
in each group of the selected studies in the current
meta-analysis.
| A, ICG vs. BD |
|---|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
| IR of patients | IR of SLNs | IR of positive
SLNs | FNR |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Author, year | Type | No. of tracers | Device | Concentration,
mg/ml | Volume, ml | Dose, mg | Procedures | SLNs | Positive SLNs | Positive SLN
patients | ICG | BD | ICG | BD | ICG | BD | ICG | BD | (Refs.) |
|---|
| Hirano et
al, 2012 | Cohort | 2 | PDE | N/A | 2.5 | N/A | 108 | N/A | N/A | 16 | 107/108 | 100/108 | N/A | N/A | N/A | N/A | 1/16 | 5/16 | (19) |
| Wishart et
al, 2012 | Cohort | 3 | PDE | 0.39 | 2 | 0.78 | 104 | 201 | 25 | 18 | 104/104 | 101/104 | 201/201 | 191/201 | 25/25 | 25/25 | 0/18 | 0/18 | (17) |
| van der Vorst,
2012 | RCT | 3 | Mini-FLARE | 0.39 | 1.6 | 0.62 | 12 | 19 | N/A | N/A | 12/12 | 12/12 | 19/19 | 16/19 | N/A | N/A | N/A | N/A | (20) |
| Jung et al,
2014 | RCT | 3 | ICG-F | 0.6 | 1 | 0.6 | 43 | N/A | N/A | 9 | 43/43 | 39/43 | N/A | N/A | N/A | N/A | N/A | N/A | (21) |
| Hojo et al,
2010 | Cohort | 3 | PDE | N/A | 2 | N/A | 113 | N/A | N/A | 31 | 113/113 | 105/113 | N/A | N/A | N/A | N/A | 0/31 | 5/31 | (22) |
| Sugie et al,
2013 | Cohort | 2 | PDE | 0.39 | 0.5-1.0 | N/A | 99 | 340 | N/A | 20 | 98/99 | 77/99 | 281/340 | 121/340 | N/A | N/A | 0/20 | 6/20 | (18) |
| Pitsinis et
al, 2015 | Cohort | 2 | PDE | 0.39 | 5 | 1.95 | 50 | 87 | 18 | 10 | 50/50 | 48/50 | 87/87 | 84/87 | 18/18 | 18/18 | 0/10 | 0/10 | (26) |
| Guo et al,
2014 | Cohort | 2 | PDE | 1.25 | 1 | 1.25 | 86 | 291 | N/A | 25 | 80/86 | 70/86 | 281/291 | 255/291 | N/A | N/A | 3/25 | 4/25 | (28) |
| Schaafsma et
al, 2013 | Cohort | 3 | Mini-FLARE | 0.125/0.25 | N/A | N/A | 32 | 48 | N/A | 13 | N/A | N/A | 48/48 | 42/48 | N/A | N/A | N/A | N/A | (9) |
| Liu et al,
2017 | Cohort | 2 | PDE | 1 | 1 | 1 | 60 | 177 | N/A | 12 | 60/60 | 53/60 | 177/177 | 106/177 | N/A | N/A | N/A | N/A | (10) |
| Tong et al,
2014 | Cohort | 2 | PDE | 5 | 2 | 10 | 96 | N/A | N/A | 28 | 93/96 | 83/96 | N/A | N/A | N/A | N/A | 1/29 | 4/29 | (16) |
| Ji et al,
2017 | RCT | 2 | PDE | 0.39 | 1 | 0.39 | 65 | 243 | 31 | 20 | N/A | N/A | 232/243 | 198/243 | 29/31 | 26/31 | N/A | N/A | (14) |
| Hutteman et
al, 2011 | RCT | 3 | Mini-FLARE | 0.39 | 1.6 | 0.62 | 10 | 14 | N/A | N/A | N/A | N/A | 14/14 | 10/14 | N/A | N/A | N/A | N/A | (23) |
| Verbeek et
al, 2014 | Cohort | 3 | Mini-FLARE | 0.39 | 1.6 | 0.62 | 27 | 40 | 5 | 3 | N/A | N/A | 40/40 | 31/40 | 5/5 | 3/5 | 0/5 | 2/5 | (24) |
|
| B, ICG vs.
RI |
|
|
|
|
|
|
|
|
|
|
|
|
| IR of
patients | IR of
SLNs | IR of positive
SLNs | FNR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Author,
year | Type | No. of
tracers | Device | Concentration,
mg/ml | Volume,
ml | Dose,
mg |
Procedures | SLNs | Positive
SLNs | Positive SLN
patients | ICG | BD | ICG | BD | ICG | BD | ICG | BD | (Refs.) |
|
| Ballardini et
al, 2013 | Cohort | 2 | PDE | 0.39 | 1 | 0.39 | 134 | 246 | N/A | N/A | 134/134 | 133/134 | 245/246 | 231/246 | N/A | N/A | N/A | N/A | (25) |
| Samorani et
al, 2015 | Cohort | 2 | PDE | 5 | 0.4-1.2 | 2.0-6.0 | 301 | 589 | 70 | 46 | 297/301 | 287/301 | 583/589 | 452/589 | 70/70 | 55/70 | 0/46 | 6/46 | (12) |
| Wishart et
al, 2012 | Cohort | 2 | PDE | 0.39 | 2 | 0.78 | 104 | 201 | 25 | 18 | 104/104 | 93/104 | 201/201 | 156/201 | 25/25 | 25/25 | 0/18 | 0/18 | (17) |
| van der Vorst et
al, 2012 | RCT | 3 | Mini-FLARE | 0.39 | 1.6 | 0.62 | 24 | 37 | N/A | N/A | 23/24 | 23/24 | 37/37 | 35/37 | N/A | N/A | N/A | N/A | (20) |
| Polom et al,
2012 | Cohort | 2 | PDE | 0.1/10 | 1 | 0.1/10 | 28 | 68 | N/A | 3 | 28/28 | 27/28 | 68/68 | 58/68 | N/A | N/A | 0/3 | 0/3 | (27) |
| Jung et al,
2014 | RCT | 3 | ICG-F | 0.6 | 1 | 0.6 | 43 | N/A | N/A | 9 | 43/43 | 43/43 | N/A | N/A | N/A | N/A | N/A | N/A | (21) |
| Hojo et al,
2010 | Cohort | 3 | PDE | N//A | 2 | N/A | 29 | N/A | N/A | N/A | 29/29 | 27/29 | N/A | N/A | N/A | N/A | N/A | N/A | (22) |
| Sugie et al,
2016 | Cohort | 2 | PDE | 0.39 | 1 | 0.39 | 821 | N/A | N/A | 180 | 798/821 | 796/821 | N/A | N/A | N/A | N/A | 12/180 | 18/180 | (11) |
| Schaafsma et
al, 2013 | Cohort | 3 | Mini-FLARE | 160/320uM | N/A | N/A | 32 | 48 | N/A | 13 | 32/32 | 32/32 | 48/48 | 48/48 | N/A | N/A | N/A | N/A | (9) |
| Murawa et
al, 2009 | Cohort | 2 | IC-View | 5-15 | 1-3 | N/A | 20 | N/A | N/A | 13 | 20/20 | 17/20 | N/A | N/A | 12/13 | 10/13 | 1/13 | 3/13 | (29) |
| Stoffels et
al, 2015 | Cohort | 2 | CCD | 0.5 | N/A | N/A | 80 | 147 | 27 | 24 | 78/80 | 80/80 | 147/147 | 141/147 | 27/27 | 27/27 | 0/24 | 0/24 | (13) |
| Grischke et
al, 2015 | Cohort | 2 | CCD | 2 | 5 | 10 | 105 | 162 | N/A | 27 | 93/105 | 103/105 | 138/162 | 157/162 | N/A | N/A | N/A | N/A | (15) |
| Hutteman et
al, 2011 | RCT | 3 | Mini-FLARE | 0.39 | 1.6 | 0.62 | 10 | 14 | N/A | 0 | 10/10 | 10/10 | 14/14 | 14/14 | N/A | N/A | N/A | N/A | (23) |
| Verbeek et
al, 2014 | Cohort | 3 | Mini-FLARE | 0.39 | 1.6 | 0.62 | 95 | 177 | 22 | N/A | N/A | N/A | 177/177 | 155/177 | 22/22 | 20/22 | N/A | N/A | (24) |
|
| C, ICG vs. BD
and RI |
|
|
|
|
|
|
|
|
|
|
|
|
| IR of
patients | IR of
SLNs | IR of positive
SLNs | FNR |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Author,
year | Type | No. of
tracers | Device | Concentration,
mg/ml | Volume,
ml | Dose,
mg |
Procedures | SLNs | Positive
SLNs | Positive SLN
patients | ICG | BD | ICG | BD | ICG | BD | ICG | BD | (Refs.) |
|
| Wishart et
al, 2012 | Cohort | 3 | PDE | 0.39 | 2 | 0.78 | 104 | 201 | 25 | 18 | 104/104 | 102/104 | N/A | N/A | 25/25 | 25/25 | N/A | N/A | (17) |
| van der Vorst et
al, 2012 | RCT | 3 | Mini-FLARE | 0.39 | 1.6 | 0.62 | 12 | 19 | N/A | 6 | 12/12 | 12/12 | 19/19 | 19/19 | N/A | N/A | N/A | N/A | (20) |
| Jung et al,
2014 | RCT | 3 | ICG-F | 0.6 | 1 | 0.6 | 43 | N/A | N/A | N/A | 43/43 | 43/43 | N/A | N/A | N/A | N/A | N/A | N/A | (21) |
| Hutteman et
al, 2011 | RCT | 3 | Mini-FLARE | 0.39 | 1.6 | 0.62 | 10 | 14 | N/A | N/A | 10/10 | 10/10 | 14/14 | 10/14 | N/A | N/A | N/A | N/A | (23) |
Quality assessment
The quality and bias risk of the selected papers
were critically appraised separately by RY and LD. A quality
assessment was performed for each of the eligible studies using the
validated Newcastle-Ottawa Quality Assessment Scale (NOS) (8). This scale is composed of eight items
that assess patient selection, study comparability and outcome with
scores ranging 0–9. In the present meta-analysis, studies with a
score of ≥6 were graded as high quality. The quality of the
included studies assessed by NOS are presented in Table II. Disagreements were discussed
until a consensus was reached.
 | Table II.The quality of the included studies
as assessed by the Newcastle-Ottawa Quality Assessment Scale. |
Table II.
The quality of the included studies
as assessed by the Newcastle-Ottawa Quality Assessment Scale.
| Author, year | Selection | Compa-rability | Outcome | Score | (Refs.) |
|---|
| Hirano et
al, 2012 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 9 | (19) |
| Wishart et
al, 2012 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (17) |
| van der Vorst et
al, 2012 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (20) |
| Jung et al,
2014 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| 8 | (21) |
| Hojo et al,
2010 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (22) |
| Sugie et al,
2013 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| 8 | (18) |
| Pitsinis et
al, 2015 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| 8 | (26) |
| Guo et al,
2014 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (28) |
| Schaafsma et
al, 2013 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (9) |
| Liu et al,
2017 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 9 | (10) |
| Tong et al,
2014 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (16) |
| Ji et al,
2017 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
| 8 | (14) |
| Hutteman et
al, 2011 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (23) |
| Verbeek et
al, 2014 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (24) |
| Ballardini et
al, 2013 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (25) |
| Samorani et
al, 2015 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (12) |
| Polom et al,
2012 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (27) |
| Sugie et al,
2016 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (11) |
| Murawa et
al, 2009 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (29) |
| Grischke et
al, 2015 | ☆ | ☆ | ☆ | ☆ | ☆ |
| ☆ |
|
| 6 | (15) |
| Stoffels et
al, 2015 | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ |
|
| 7 | (13) |
Statistical analysis
Dichotomous results were summarized as pooled odds
ratios (ORs) and 95% CIs around the point estimates. OR was
abstracted or calculated to quantitatively evaluate the association
between ICG and the other tracers. The overall pooled effect was
assessed using the z-statistic with P≤0.05 indicating a
statistically significantly difference. Heterogeneity between
studies was assessed by the ‘I2’ value. When
I2≥50% or the P-value for the I2 statistic
was <0.05, which indicated significant heterogeneity across the
studies, the pooled estimate was calculated using a random-effects
model. If the data were contrary, a fixed-effect model was adopted.
Statistical heterogeneity was explored using the χ2 and
Τau2 statistical tests. Subgroup analysis was based on
the ICG dose, and studies were divided into ‘standard dose of
reference’, ‘more than standard dose of reference’ and ‘less than
standard dose of reference’. All statistical analyses were
performed using RevMan software (version 5.3; The Nordic Cochrane
Centre) and Stata software (version 15.1; StataCorp LLC). Forest
plot and receiver operating characteristic plot were obtained to
evaluate the sensitivity and specificity of subgroup. Funnel plots
with Egger's test were used to identify publication bias. All
analyses were based on previous published studies; therefore, no
ethical approval or patient consent was required.
Results
Characteristics of eligible
studies
A total of 262 articles were retrieved from PubMed,
EMBASE and The Cochrane Library and, ultimately, 21 studies were
selected with a total of 2,499 patients for detailed assessment
(9–29). A flow diagram of the selection
process is presented in Fig. 1. All
the selected studies were scored ≥6 according to NOS (Table II).
Meta-analysis results
Comparison of SLNB using BD, RI and ICG was
performed in 21 studies and analyzed using four outcome variables.
ICG was compared with BD alone, RI alone and RI with BD separately.
The results of the meta-analysis are presented in Table III.
 | Table III.Results of the meta-analysis. |
Table III.
Results of the meta-analysis.
|
| Pooled
estimates | Heterogeneity |
|---|
|
|
|
|
|---|
| Variable | OR | 95% CI | I2,
% | P-value |
|---|
| IR of patients |
|
|
|
|
| ICG vs.
BD | 7.17 | 3.98-12.94 | 0 | 0.68 |
| ICG vs.
RI | 1.63 | 0.65-4.10 | 55 | 0.02 |
|
Standard dose of
reference | 1.77 | 1.09-2.85 | 47 | 0.11 |
| More
than standard dose | 0.86 | 0.41-1.83 | 71 | 0.02 |
| Less
than standard dose | N/A | N/A | N/A | N/A |
| ICG vs.
BD and RI |
|
|
|
|
| IR of SLNs |
|
|
|
|
| ICG vs.
BD | 8.84 | 6.71-11.66 | 37 | 0.11 |
| ICG vs.
RI | 12.05 | 1.57-92.74 | 90 | <0.05 |
|
Standard dose of
reference | 21.62 | 5.23-89.43 | 0 | 0.53 |
| More
than standard dose | 2.50 | 1.56-3.99 | 95 | <0.05 |
| Less
than standard dose | N/A | N/A | N/A | N/A |
| IR of positive
SLNs |
|
|
|
|
| ICG vs.
BD | 3.54 | 0.78-16.06 | 0 | 0.59 |
| ICG vs.
RI | 21.32 | 2.85-160.14 | 0 | 0.34 |
| ICG vs.
BD and RI | N/A | N/A | N/A | N/A |
| FNR |
|
|
|
|
| ICG vs.
BD | 0.20 | 0.08-0.48 | 0 | 0.59 |
| ICG vs.
RI | 0.46 | 0.23-0.91 | 23 | 0.27 |
| ICG vs.
BD and RI | N/A | N/A | N/A | N/A |
ICG vs. RI
IR of patients
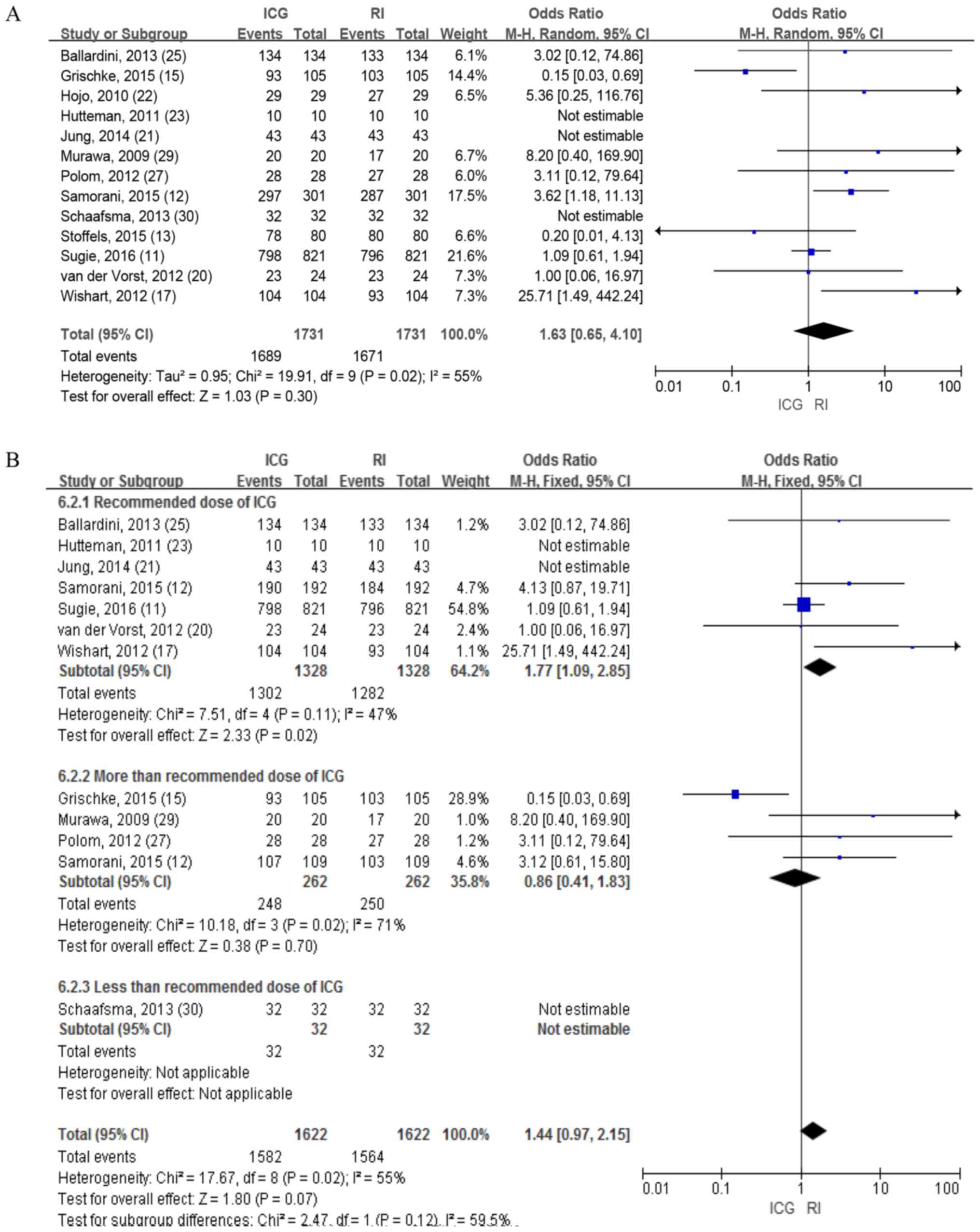
A total of 13 studies involving 1,731 patients
reported the identification rate for patients using ICG and RI,
which revealed no differences in the random-effects model (OR=1.63;
95% CI, 0.65-4.10; P=0.30; Fig. 2A).
The heterogeneities of the detection rate of these patients were
high (I2=55%; P=0.02).
Taking the high heterogeneities into account, the
concentration of ICG was variable and ranged from 0.1-10 mg/ml.
Therefore, a subgroup analysis was performed based on a previous
study by Mieog et al (30),
which concluded that the optimal dose of ICG was 400–800 µM. The
fixed-effects model was used to calculate the results and
demonstrated that the detection rate of patients with ICG was
significantly higher compared with that of RI and the heterogeneity
was decreased in the standard dose subgroup (OR=1.77; 95% CI,
1.09-2.85; P=0.02; Fig. 2B).
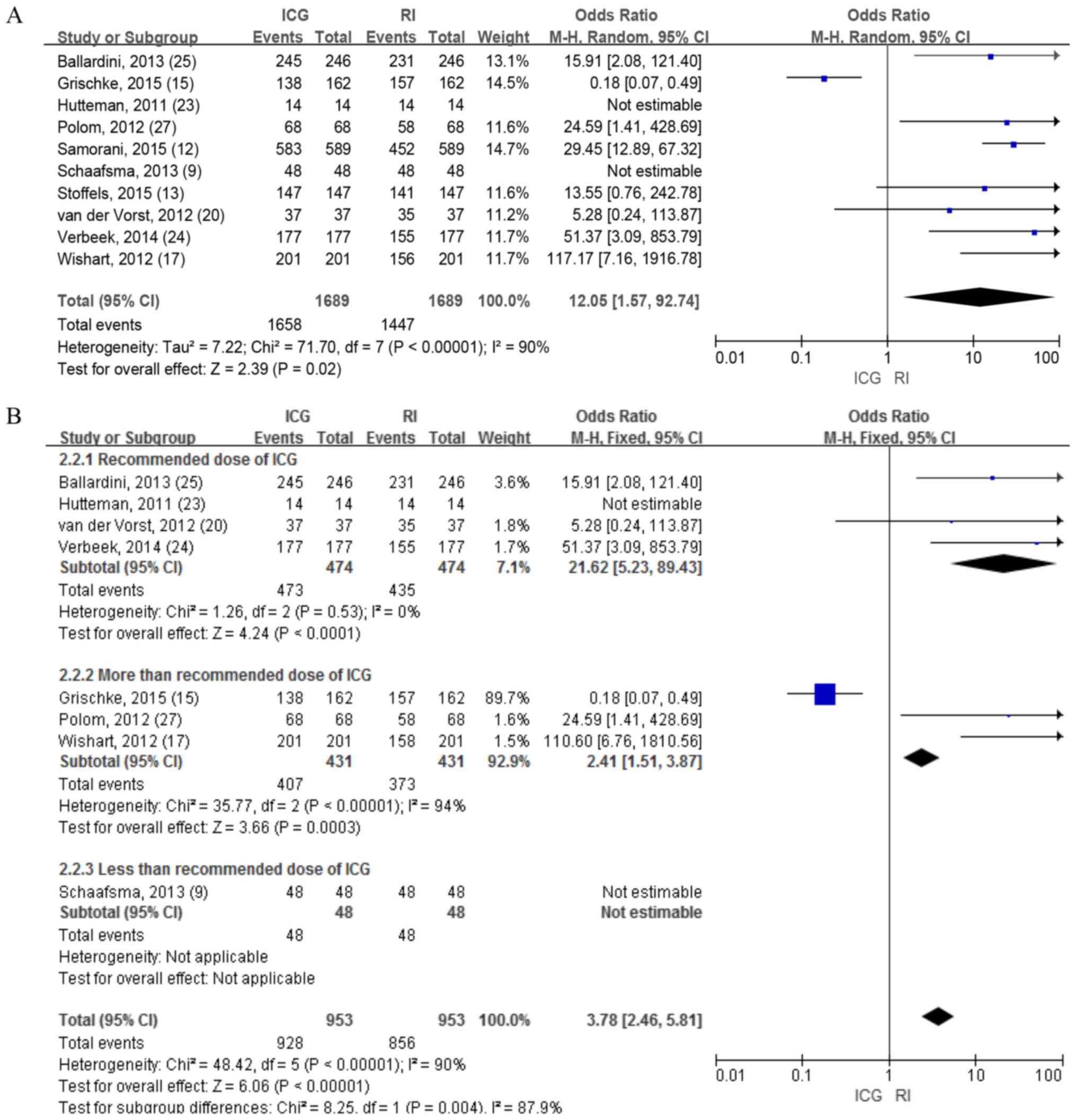
IR of SLNs
A total of 10 studies with 881 patients investigated
the detection rate for SLNs between ICG and RI, and revealed
significant differences in the random-effects model (OR=12.05; 95%
CI, 1.57-92.74; P=0.02; Fig. 3A).
Considering the significant heterogeneity (I2=90%;
P<0.01), a subgroup analysis was performed according to the dose
of ICG, and the results demonstrated that the SLN detection rate of
ICG was significantly higher compared with that of the standard
dose of RI (OR=21.62; 95% CI, 5.23-89.43; P<0.0001; Fig. 3B) and I2 dropped from 90
to 0%, which indicated that the heterogeneity comes from the high
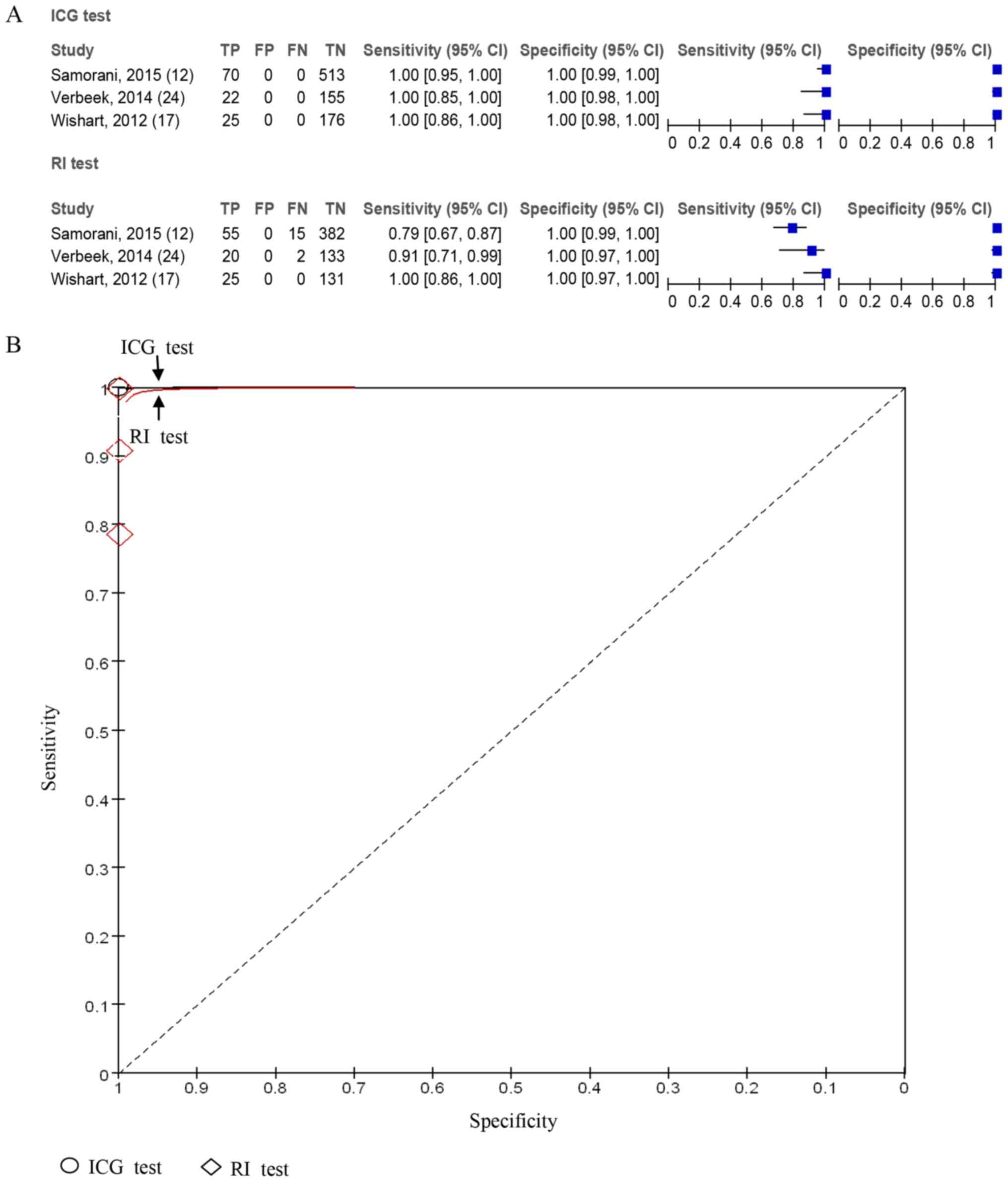
dose group. Furthermore, a sensitivity and specificity analysis of
ICG and RI lymph node detection was performed in the recommended
dose group. A total of three studies including 961 lymph nodes
reported the accuracy of ICG in lymph node detection was higher
compared with RI (Fig. 4).
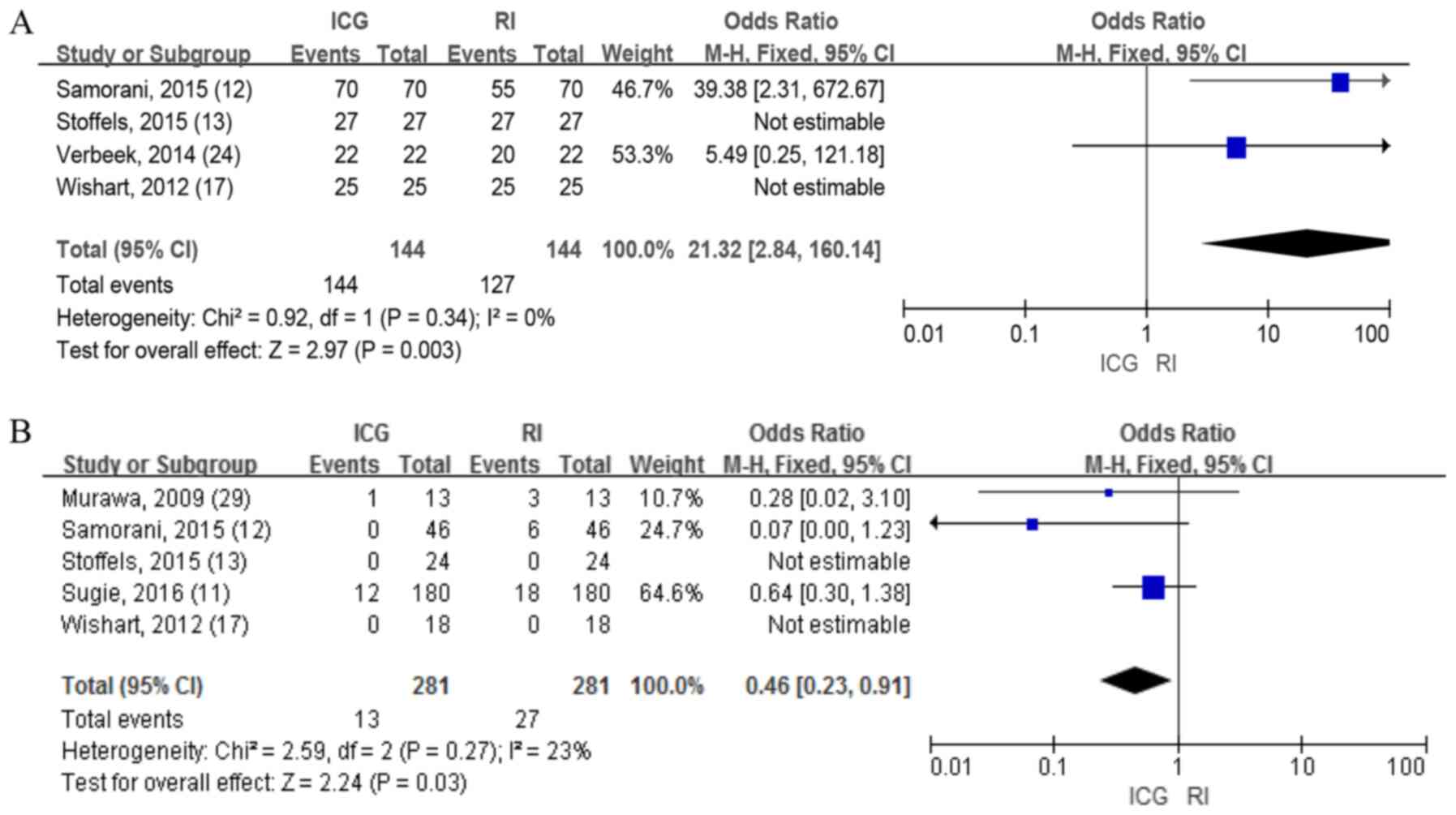
IR of positive SLNs
A total of four studies consisting of 744 patients
reported the detection rate of positive SLNs between ICG and RI,
which revealed significant differences in the fixed-effects model
(OR=21.32; 95% CI, 2.84-160.14; P=0.003; Fig. 5A), indicating that ICG had an
improved identification rate of positive SLNs compared with RI.
FNR
A total of five studies with 1,326 patients reported
the FNR between ICG and RI, which revealed significant differences
in the fixed-effects model (OR=0.46; 95% CI 0.23-0.91; P=0.03;
Fig. 5B).
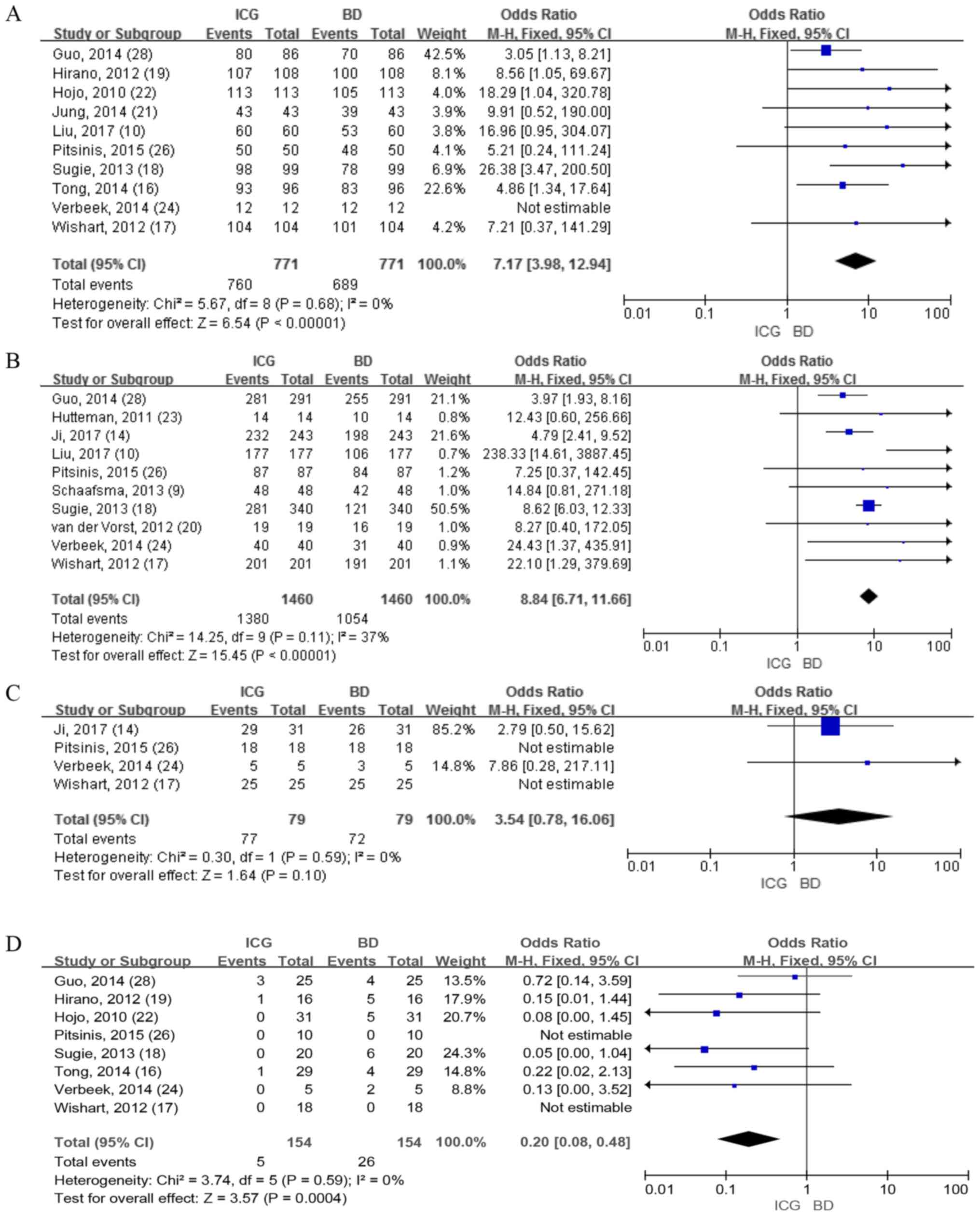
ICG vs. BD
IR of patients
A total of 10 studies that included 771 patients
reported the detection rate of patients using ICG and BD. The
results demonstrated a significant difference in the fixed-effects
model (OR=7.17; 95% CI, 3.98-12.94; P<0.00001; Fig. 6A), indicating that the detection rate
of ICG was higher compared with BD. There was no heterogeneity
(I2=0%. P=0.68; Fig.
6A).
IR of SLNs
A total of 10 studies involving 449 patients
reported the detection rate for SLNs between ICG and BD. The
results identified 1,460 SLNs and the mean number of SLNs (the
number of lymph nodes detected/total number of patients) retrieved
using ICG was 3.07, which was higher compared with the number of
SLNs retrieved using BD (2.35). The fixed-effects model (OR=8.84;
95% CI, 6.71-11.66; P<0.00001; Fig.
6B) was used and significant differences between ICG and BD
were observed, demonstrating that ICG had a statistically higher
detection rate of SLNs. The heterogeneity was low with
I2=37% and P=0.11 (Fig.
6B).
IR of positive SLNs
A total of four studies involving 246 patients
reported the detection rate for positive SLNs between ICG and BD.
The results revealed no differences in the fixed-effects model
(OR=3.54; 95% CI, 0.78-16.06; P=0.10; Fig. 6C). However, the overall detection
rate of positive SLNs (the number of positive lymph nodes
detected/total positive lymph nodes) using ICG was 97.5% and the
detection rate of using BD was 91.1%. No heterogeneity was observed
at I2=0% and P=0.59.
FNR
A total of eight studies including 683 patients
reported the FNR between ICG and BD, a total of 154 positive SLNs
were identified and the overall FNR (the number of positive lymph
nodes not detected/total positive lymph nodes) using ICG was 3.25%.
This was lower than the FNR of using BD, which was 16.88%. Using
the fixed-effects model, the results revealed significant
differences (OR=0.20; 95% CI, 0.08-0.48; P=0.0004; Fig. 6D), which demonstrated that ICG had a
lower FNR compared with BD. There was no heterogeneity at
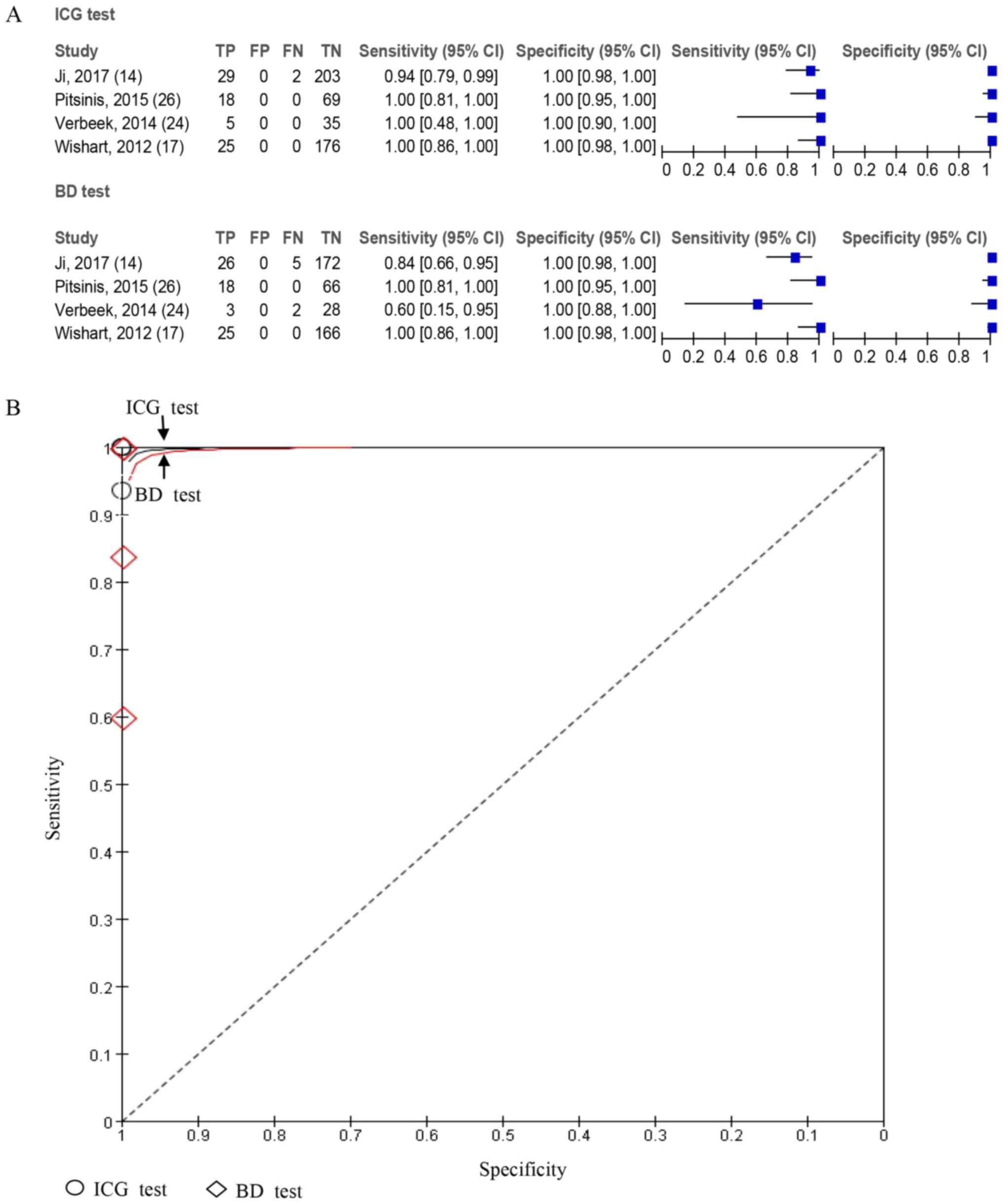
I2=0% and P=0.59. For further analysis, sensitivity and
specificity analyses on this group of studies were performed and
the results demonstrated that the accuracy of ICG was higher
compared with that of BD in lymph node detection (Fig. 7).
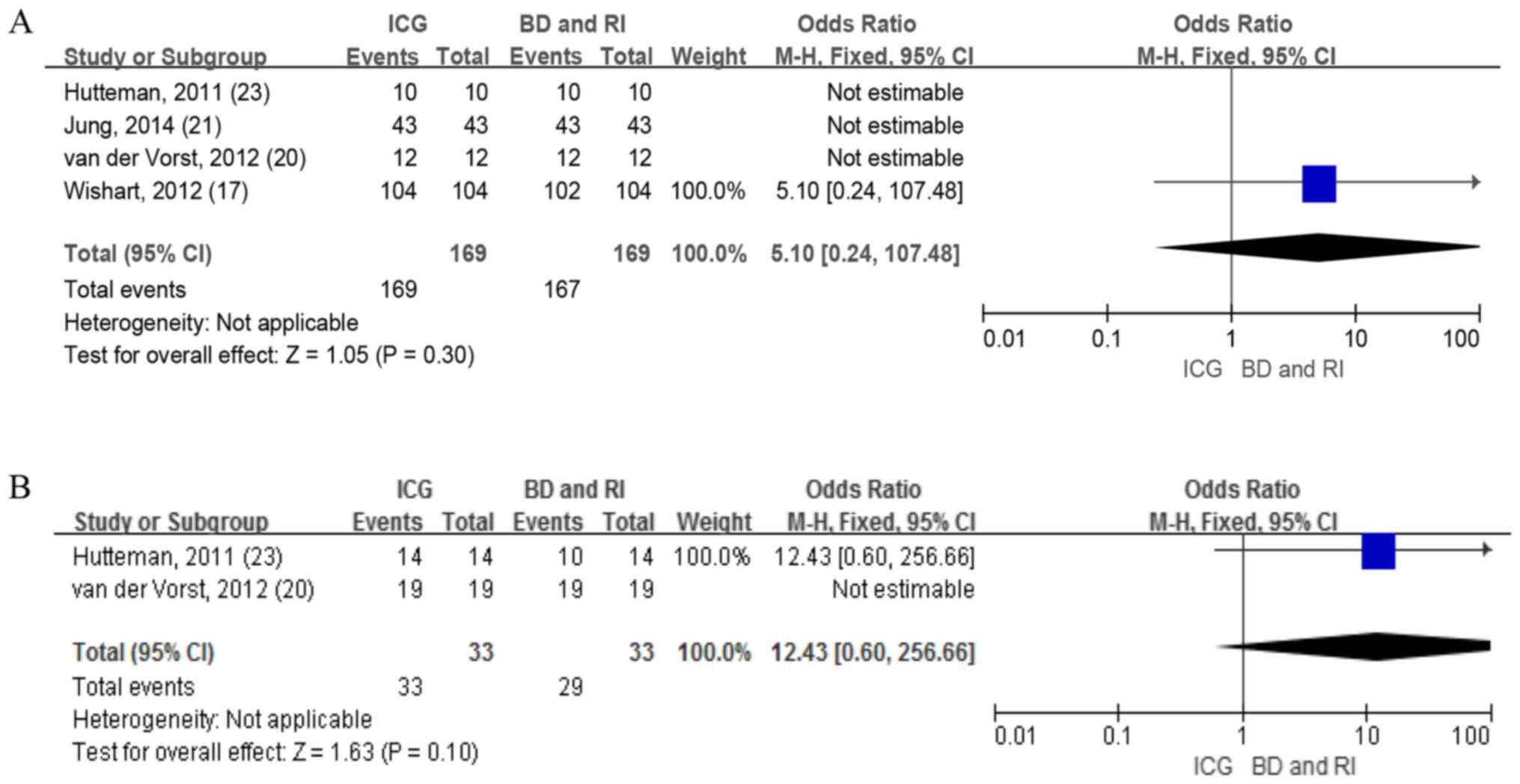
ICG vs BD and RI
A total of four studies including 169 patients
reported the detection rate of patients with ICG and BD combined
with RI. The identification rate using the ICG method was 100%,
while the identification rate of patients using the BD combined
with the RI method ranged from 98–100%. No difference was reported
in the fixed-effects model (OR=5.10; 95% CI, 0.24-107.48; Fig. 8A). Therefore, heterogeneity was not
applicable.
Only two studies with 22 patients provided data on
the detection rate of SLNs with ICG and BD combined with RI. The
mean number of SLNs (the number of lymph nodes detected/total
number of patients) retrieved using ICG was 1.50, which was more
compared with the number detected using BD combined with RI at
1.32. No difference was revealed in the fixed-effects model
(OR=12.43; 95% CI, 0.60-256.66; Fig.
8B), with heterogeneity not being applicable.
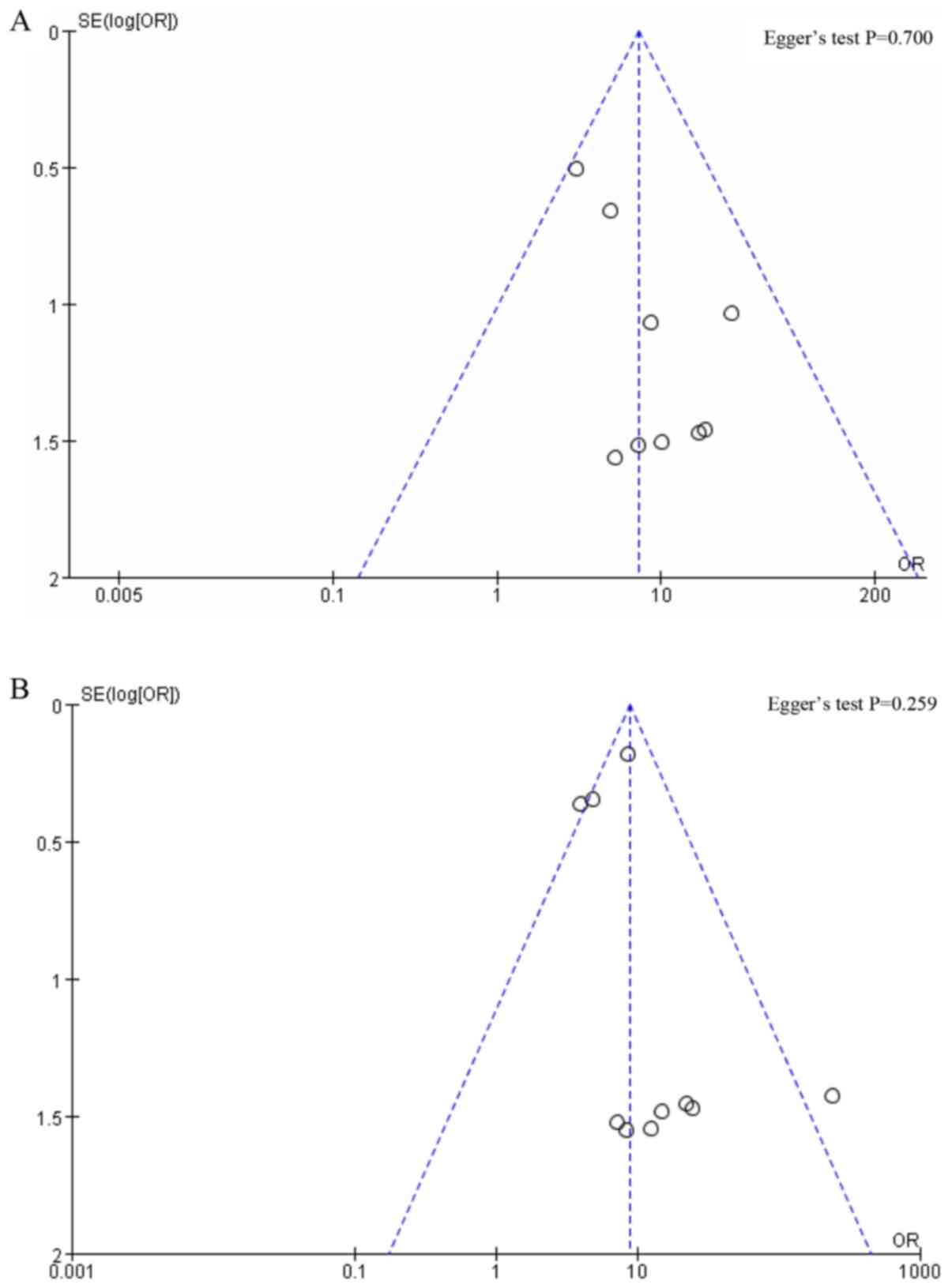
For groups with <10 studies, the publication bias
was not assessed. The Egger's test was performed using the software
Stata. No obvious publication bias was found since P=0.700
(Fig. 9A) and 0.259 (Fig. 9B) for IR of patients and IR of SLNs,
respectively.
Discussion
The number of lymph nodes obtained from SLNB is used
as a guide to decide whether or not to continue subsequent axillary
lymph node dissection according to the latest National
Comprehensive Cancer Network guidelines (10). Although the range of the armpit area
subjected to surgery has been a decreasing, the accuracy of lymph
node biopsies is becoming increasingly important and obtaining
effective and convenient tracers are particularly important for
surgeons (11).
The near-infrared fluorescence released by the ICG
after being excited by infrared light can be imaged using an in
vitro device, revealing the shape of the lymphatic vessel and
positioning the breast SLN (6). It
is a sufficient in vivo imaging tool due to its soft tissue
penetration and is less disturbed by natural light (10).
The use of BD has the advantages of being
inexpensive and easy to prepare intraoperatively (12); however, the surgeon needs to rely on
vision to locate lymph nodes during tracing. Therefore, the
detection rate is more dependent on the surgeon's experience and
requires a longer learning curve. Additionally, the low detection
rate of SLNs and high FNR render it an unsuitable tracer agent
(10). RI can aid in positioning
SLNs by detecting γ rays from lymph nodes on the body surface
(13). It is superior to BD in
detection rate; however, it has an increased surgery cost and
complex operation as radioactive tracers need to be injected into
the patient the day prior to surgery (10). Furthermore, the subsequent processing
of radionuclides limits their use in high-volume centers (6,12).
Although the combined use of BD and RI increases the
detection rate of SLNs, the advantages of an inexpensive procedure
and rapid localization of lymph nodes do not apply. ICG has the
clinical advantages of both BD and RI (14). ICG is a real-time navigation system
that can guide the surgical procedure with visible lymphatic
drainage using near-infrared fluorescence devices (12). Furthermore, ICG has the advantages of
low cost and convenient preparation prior to surgery compared with
BD (15). Additionally, ICG has the
potential to improve operating room efficiency considering that
patients are not required to visit the nuclear medicine department
prior to surgery, which may contribute to improved patients
experience (12). Although the
required dose of RI carries safety concerns for healthcare
professionals, using a non-radiative substance can certainly be an
advantage (12).
A meta-analysis by Ahmed et al (5) demonstrated that ICG was superior to BD
in with regard to the IR of SLNs. Furthermore, Sugie et al
(6) reported that the ICG
fluorescence method was better at determining axillary staging
compared with the RI method. However, this previous meta-analysis
(5) did not agree with the results
of the present study, which indicated that ICG was superior to RI
in the detection rate of SLNs and the positive SLN detection rate.
Additionally, the current meta-analysis indicated that when the
dose was limited to the standard dose, there was still a
significant difference in the combined OR.
The effect of the ICG dose on the tracer effect has
been controversial. Mitsuo et al (31) hypothesized that the concentration and
total dose of ICG injection varies depending on the application.
For instance, 2.5 mg/ml concentration and 0.5-1.0 ml ICG are
generally used for breast cancer SLN navigation surgery. However,
there is certain evidence of improved SLN detection rate at a
concentration of 0.5 mg/ml compared with 2.5 mg/ml concentration.
The present study analyzed the traceability of ICG by dose. The
grouping of ICG doses was based on a study by Mieog et al
(30), which concluded that the
optimal dose of ICG was between 400 and 800 µM by assigning
patients to different ICG concentration groups of 50–1,000 µM. As
per the study by Mieog et al (30), the grouping of ICG doses is
reasonable. The results of the current study demonstrated that
using the recommended dose of ICG obtained an improved detection
result and the use of a higher dose may cause difficulties in
detection due to leakage of fluorescent tracer in lymphatic vessels
(14).
The present study has the following differences
compared with previous studies. Firstly, the condition of patients
being their own controls was incorporated into the inclusion
criteria, which avoids bias due to population differences.
Secondly, the patient detection rate was differentiated from the
SLN detection rate, making the definition ‘sentinel lymph node
detection rate’ more objective. Lastly, different doses of ICG
ultimately have different effects on lymph node detection, which
provided guidance for the future use of ICG doses.
The present meta-analysis compared ICG with BD
combined with RI in breast cancer. The results demonstrated that
ICG alone is not significantly different to BD combined with RI in
terms of the detection rate of patients and the detection rate of
SLNs, indicating that ICG alone is not worse compared with BD
combined with RI. Although there was no statistical difference
observed in the overall lymph node detection rate using ICG at
100%, this was higher than the 87.88% of BD combined with RI.
Several limitations in the current meta-analysis
should be noted. Firstly, the ICG fluorescence imaging equipment
used by the previous studies was not uniform; therefore, data was
extracted from studies that used different equipment, including
PDE, Mini-FLARE and CCD. Among these, PDE was the most commonly
used. However, other equipment have also been used clinically and
were able to achieve a high detection rate of SLNs. Secondly, the
definition of SLNs varies in different trials. Wishart et al
(17) demonstrated that all tracers
(including ICG, BD and RI) detected lymph nodes as SLNs, while
Sugie et al (18) concluded
that intraoperatively palpable lymph nodes, termed para-SLN, should
also be classified as SLNs. Other studies (19–21) did
not reach the same conclusion. Therefore, altering the intended
definition of SLN may lead to certain differences in the detection
rate of SLN. Thirdly, the criteria for enrollment for each trial
were different, particularly in regard to previous axillary surgery
history. Although all patients were clinically node-negative, there
were studies that did not specify the exclusion criteria, making
the patients heterogeneous in the present meta-analysis. Lastly,
the authors may have missed certain unpublished investigations,
considering studies with positive results are usually more prone to
being published.
In conclusion, the present comprehensive
meta-analysis indicated that using ICG alone is a better tracer
agent compared with using BD or RI alone, and is not worse compared
with BD combined with RI. A suitable dose of ICG can increase the
detectability and accuracy, and decrease the heterogeneity.
Considering the clinical convenience of ICG, it may be used as a
suitable alternative to traditional tracers to detect SLNs in
patients with breast cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
RY conceived the current study and collected data.
XZ put forward the concept of ICG as a new tracer superior to
traditional tracers and helped design the article and gave final
approval of the version to be published, participated in the
revision of the article, checked the data, provided the final
conclusion for the version to be published, provided support for
obtaining some documents in the article, provided administrative
support and gave final approval of the version to be published. LD,
QW, YZ and GT collected, analyzed and interpreted the data. RY
drafted and revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SLNB
|
sentinel lymph node biopsy
|
|
RI
|
radioisotopes
|
|
BD
|
blue dye
|
|
ICG
|
indocyanine green
|
|
IR
|
identification rate
|
|
SLN
|
sentinel lymph nodes
|
|
FNR
|
false negative rate
|
References
|
1
|
Mansel RE, Fallowfield L, Kissin M, Goyal
A, Newcombe RG, Dixon JM, Yiangou C, Horgan K, Bundred N, Monypenny
I, et al: Randomized multicenter trial of sentinel node biopsy
versus standard axillary treatment in operable breast cancer: The
ALMANAC trial. J Natl Cancer Inst. 98:599–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krag DN, Weaver DL, Alex JC and Fairbank
JT: Surgical resection and radiolocalization of the sentinel lymph
node in breast cancer using a gamma probe. Surg Oncol. 2:335–340.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giuliano AE, Kirgan DM, Guenther JM and
Morton DL: Lymphatic mapping and sentinel lymphadenectomy for
breast cancer. Ann Surg. 220:391–401. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kitai T, Inomoto T, Miwa M and Shikayama
T: Fluorescence navigation with indocyanine green for detecting
sentinel lymph nodes in breast cancer. Breast Cancer. 12:211–215.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahmed M, Purushotham AD and Douek M: Novel
techniques for sentinel lymph node biopsy in breast cancer: A
systematic review. Lancet Oncol. 15:e351–e362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugie T, Ikeda T, Kawaguchi A, Shimizu A
and Toi M: Sentinel lymph node biopsy using indocyanine green
fluorescence in early-stage breast cancer: A meta-analysis. Int J
Clin Oncol. 22:11–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA statement. Ann Intern Med.
151:264–269, W64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schaafsma BE, Verbeek FP, Rietbergen DD,
van der Hiel B, van der Vorst JR, Liefers GJ, Frangioni JV, van de
Velde CJ, van Leeuwen FW and Vahrmeijer AL: Clinical trial of
combined radio and fluorescence guided sentinel lymph node biopsy
in breast cancer. Br J Surg. 100:1037–1044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Huang L, Wang N and Chen P:
Indocyanine green detects sentinel lymph nodes in early breast
cancer. J Int Med Res. 45:514–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugie T, Kinoshita T, Masuda N, Sawada T,
Yamauchi A, Kuroi K, Taguchi T, Bando H, Yamashiro H, Lee T, et al:
Evaluation of the clinical utility of the ICG fluorescence method
compared with the radioisotope method for sentinel lymph node
biopsy in breast cancer. Ann Surg Oncol. 23:44–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Samorani D, Fogacci T, Panzini I, Frisoni
G, Accardi FG, Ricci M, Fabbri E, Nicoletti S, Flenghi L, Tamburini
E, et al: The use of indocyanine green to detect sentinel nodes in
breast cancer: A prospective study. Eur J Surg Oncol. 41:64–70.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stoffels I, Dissemond J, Pöppel T,
Schadendorf D and Klode J: Intraoperative fluorescence imaging for
sentinel lymph node detection: Prospective clinical trial to
compare the usefulness of indocyanine green vs technetium Tc 99m
for identification of sentinel lymph nodes. JAMA Surg. 150:617–623.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji Y, Luo N, Jiang Y, Li Q, Wei W, Yang H
and Liu J: Clinical utility of the additional use of blue dye for
indocyanine green for sentinel node biopsy in breast cancer. J Surg
Res. 215:88–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grischke EM, Röhm C, Hahn M, Helms G,
Brucker S and Wallwiener D: ICG fluorescence technique for the
detection of sentinel lymph nodes in breast cancer: Results of a
prospective open-label clinical trial. Geburtshilfe Frauenheilk.
75:935–940. 2015. View Article : Google Scholar
|
|
16
|
Tong M, Guo W and Gao W: Use of
fluorescence imaging in combination with patent blue dye versus
patent blue dye alone in sentinel lymph node biopsy in breast
cancer. J Breast Cancer. 17:250–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wishart GC, Loh SW, Jones L and Benson JR:
A feasibility study (ICG-10) of indocyanine green (ICG)
fluorescence mapping for sentinel lymph node detection in early
breast cancer. Eur J Surg Oncol. 38:651–656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sugie T, Sawada T, Tagaya N, Kinoshita T,
Yamagami K, Suwa H, Ikeda T, Yoshimura K, Niimi M, Shimizu A and
Toi M: Comparison of the indocyanine green fluorescence and blue
dye methods in detection of sentinel lymph nodes in early-stage
breast cancer. Ann Surg Oncol. 20:2213–2218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hirano A, Kamimura M, Ogura K, Kim N,
Hattori A, Setoguchi Y, Okubo F, Inoue H, Miyamoto R, Kinoshita J,
et al: A comparison of indocyanine green fluorescence imaging plus
blue dye and blue dye alone for sentinel node navigation surgery in
breast cancer patients. Ann Surg Oncol. 19:4112–4116. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van der Vorst JR, Schaafsma BE, Verbeek
FP, Hutteman M, Mieog JS, Lowik CW, Liefers GJ, Frangioni JV, van
de Velde CJ and Vahrmeijer AL: Randomized comparison of
near-infrared fluorescence imaging using indocyanine green and
99(m) technetium with or without patent blue for the sentinel lymph
node procedure in breast cancer patients. Ann Surg Oncol.
19:4104–4111. 2012. View Article : Google Scholar
|
|
21
|
Jung SY, Kim SK, Kim SW, Kwon Y, Lee ES,
Kang HS, Ko KL, Shin KH, Lee KS, Park IH, et al: Comparison of
sentinel lymph node biopsy guided by the multimodal method of
indocyanine green fluorescence, radioisotope, and blue dye versus
the radioisotope method in breast cancer: A randomized controlled
trial. Ann Surg Oncol. 21:1254–1259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hojo T, Nagao T, Kikuyama M, Akashi S and
Kinoshita T: Evaluation of sentinel node biopsy by combined
fluorescent and dye method and lymph flow for breast cancer.
Breast. 19:210–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hutteman M, Mieog JS, van der Vorst JR,
Liefers GJ, Putter H, Löwik CW, Frangioni JV, van de Velde CJ and
Vahrmeijer AL: Randomized, double-blind comparison of indocyanine
green with or without albumin premixing for near-infrared
fluorescence imaging of sentinel lymph nodes in breast cancer
patients. Breast Cancer Res Treat. 127:163–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Verbeek FP, Troyan SL, Mieog JS, Liefers
GJ, Moffitt LA, Rosenberg M, Hirshfield-Bartek J, Gioux S, van de
Velde CJ, Vahrmeijer AL and Frangioni JV: Near-infrared
fluorescence sentinel lymph node mapping in breast cancer: A
multicenter experience. Breast Cancer Res Treat. 143:333–342. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ballardini B, Santoro L, Sangalli C,
Gentilini O, Renne G, Lissidini G, Pagani GM, Toesca A, Blundo C,
del Castillo A, et al: The indocyanine green method is equivalent
to the 99mTc-labeled radiotracer method for identifying the
sentinel node in breast cancer: A concordance and validation study.
Eur J Surg Oncol. 39:1332–1336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pitsinis V, Provenzano E, Kaklamanis L,
Wishart GC and Benson JR: Indocyanine green fluorescence mapping
for sentinel lymph node biopsy in early breast cancer. Surg Oncol.
24:375–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Polom K, Murawa D, Nowaczyk P, Rho YS and
Murawa P: Breast cancer sentinel lymph node mapping using near
infrared guided indocyanine green and indocyanine green-human serum
albumin in comparison with gamma emitting radioactive colloid
tracer. Eur J Surg Oncol. 38:137–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo W, Zhang L, Ji J, Gao W, Liu J and
Tong M: Evaluation of the benefit of using blue dye in addition to
indocyanine green fluorescence for sentinel lymph node biopsy in
patients with breast cancer. World J Surg Oncol. 12:2902014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Murawa D, Hirche C, Dresel S and Hünerbein
M: Sentinel lymph node biopsy in breast cancer guided by
indocyanine green fluorescence. Br J Surg. 96:1289–1294. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mieog JS, Troyan SL, Hutteman M, Donohoe
KJ, Van Der Vorst JR, Stockdale A, Liefers GJ, Choi HS,
Gibbs-Strauss SL, Putter H, et al: Toward optimization of imaging
system and lymphatic tracer for near-infrared fluorescent sentinel
lymph node mapping in breast cancer. Ann Surg Oncol. 18:2483–2491.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kusano M, Kokudo N, Toi M and Kaibori M:
ICG fluorescence imaging and navigation surgery. Springer; Japan:
2016, View Article : Google Scholar
|