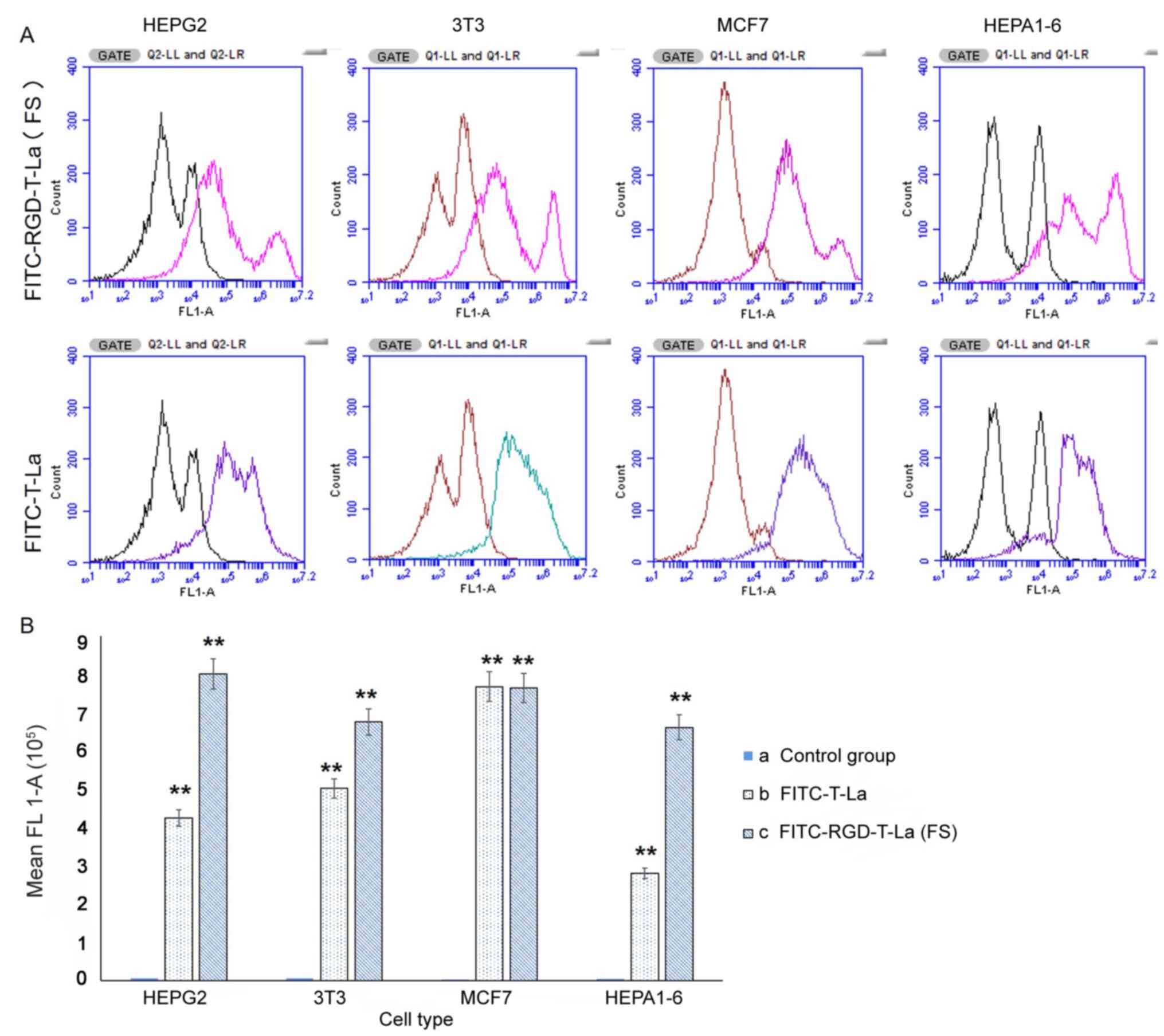
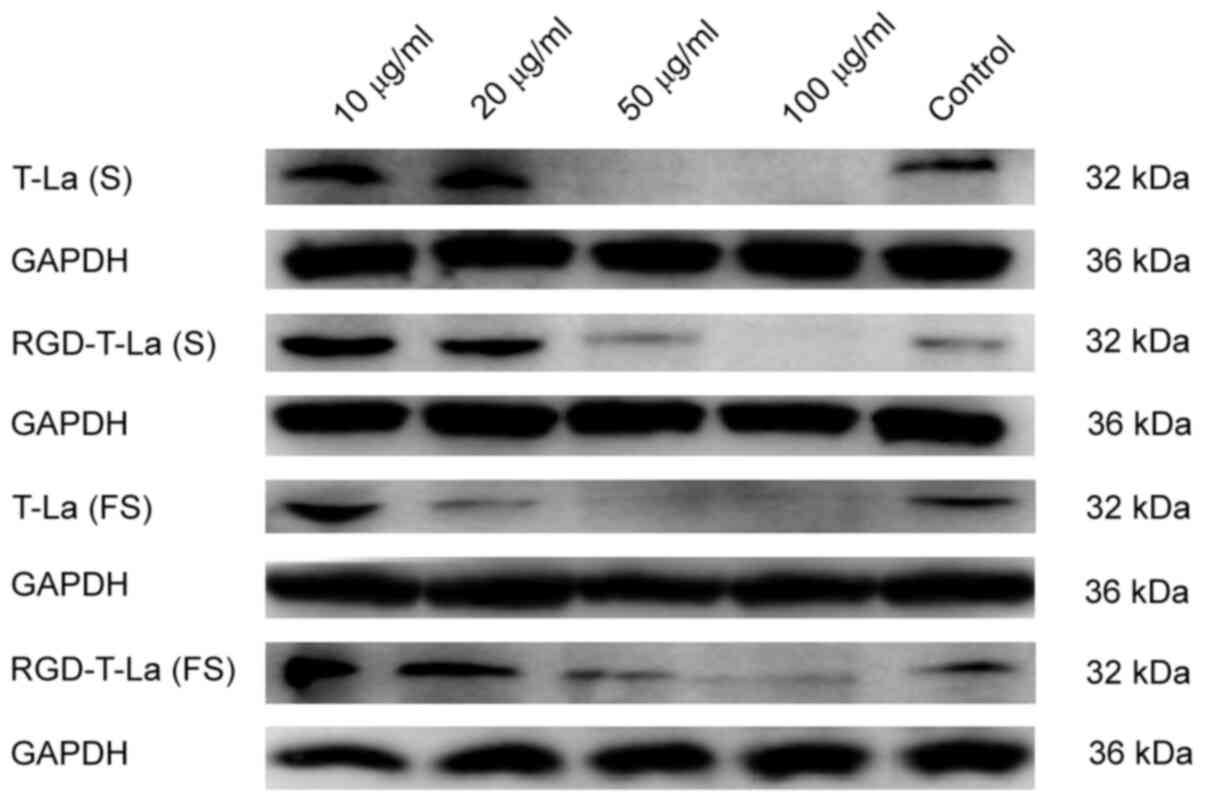
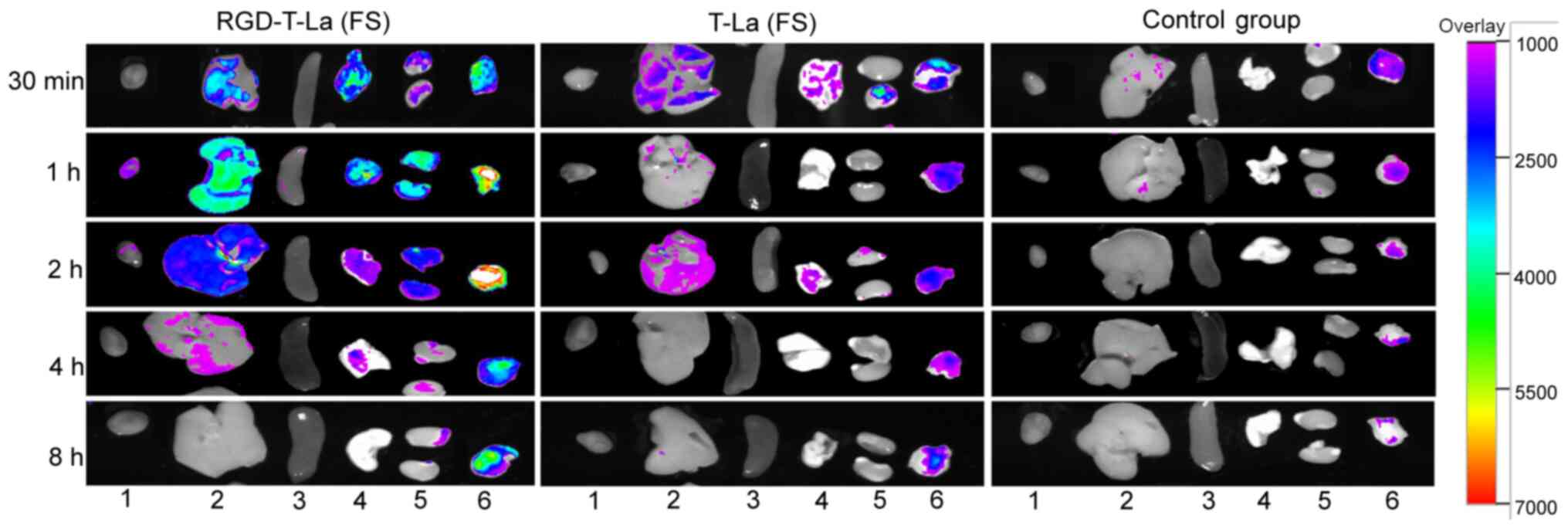
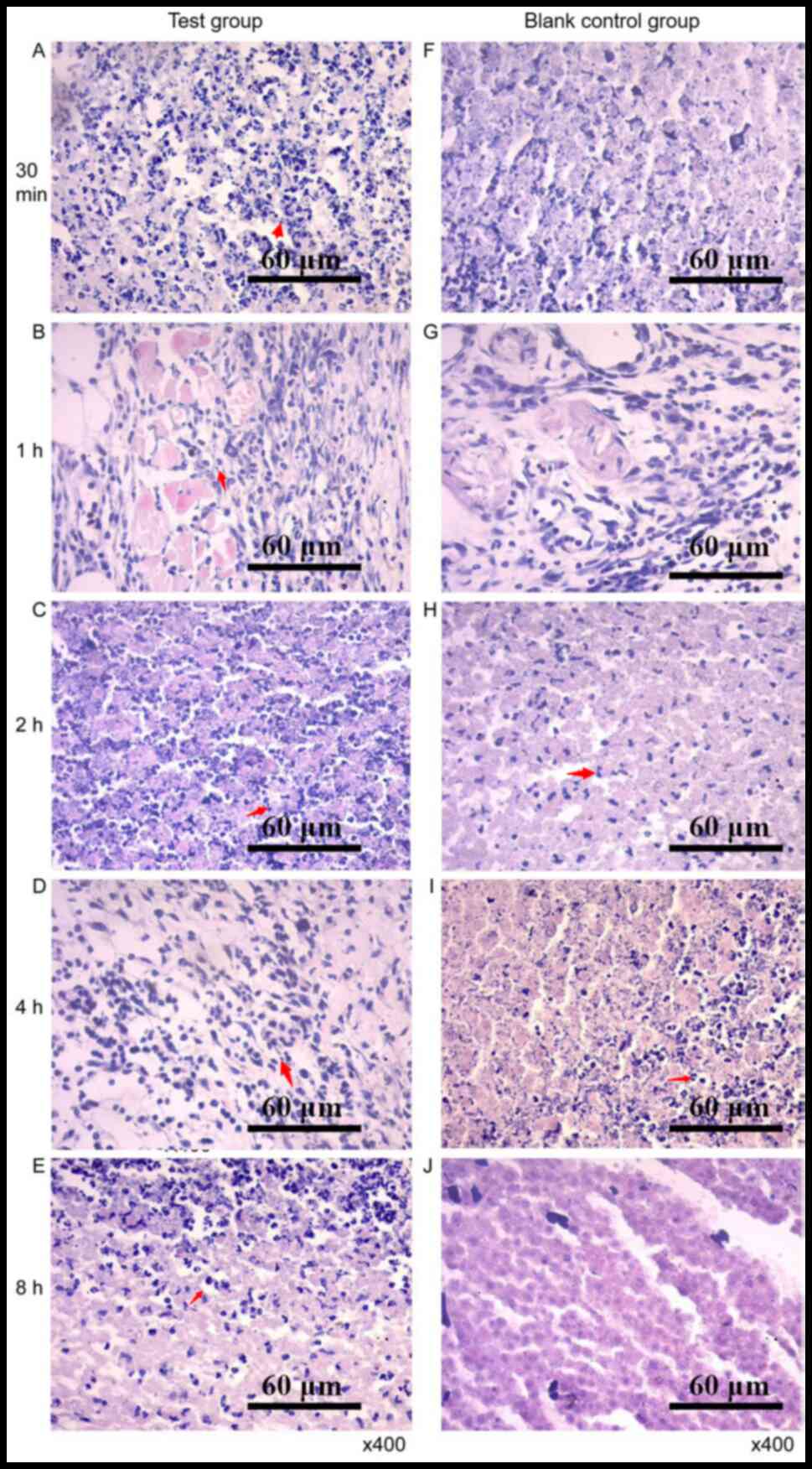
|
1
|
Li S, Hao L, Bao W, Zhang P, Su D, Cheng
Y, Nie L, Wang G, Hou F and Yang Y: A novel short anionic
antibacterial peptide isolated from the skin of Xenopus
laevis with broad antibacterial activity and inhibitory
activity against breast cancer cell. Arch Microbiol. 198:473–482.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang F and Li MS: Antibacterial peptides'
roles in immune defense and anti-tumorogenesis. World Not Antibiot.
38:284–289. 2017.(In Chinese).
|
|
3
|
Gao J, Xie C and Lu WY: Progress in
research of antitumor activity of antimicrobial peptides. World
Clinical Drugs,. 10:122012.
|
|
4
|
Zhu LZR, Liu SS, Ma JF, Jin TM, Liu JF, Hu
Y and Liu MY: Biological activity and in vivo tissue targeting
distribution of catesbeianalctin. Chin J Vet Sci. 36:185–190.
2016.(In Chinese).
|
|
5
|
Diao Y, Han W, Zhao H, Zhu S, Liu X, Feng
X, Gu J, Yao C, Liu S, Sun C, et al: Designed synthetic analogs of
the α-helical peptide temporin-La with improved antitumor
efficacies via charge modification and incorporation of the
integrin αvβ3 homing domain. J Pept Sci. 18:476–486. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma L, Cai ZL, Wang QR and Lei CX: Study on
construction of citrostatin and its bioactivity. Chin J Biochem
Pharm. 31:19–22. 2010.
|
|
7
|
Huang R, Long J and Zhang Y: Synthesise
and activity of dual targeted antineoplastic polypeptide
RGDSY-CTTHWGFTLC. Cancer Res Prev Treat. 41:531–535. 2014.
|
|
8
|
Ma Q, Dong N, Cao Y and Shan A: Rational
design of alpha-helical antimicrobial peptide with Val and Arg
residues. Acta Microbiol Sin. 51:346–451. 2011.
|
|
9
|
Wang XX, Niu B, Chen XJ, Xie J and Zhang
YH: Synthesis and activities of RGD peptide analog. J Chin Pharm
Sci. 43:462–464. 2008.(In Chinese).
|
|
10
|
Liu KY, Li QW and Liu GY: Study progress
of malignant tumor imaging of integrin αvβ3 expression with
radiolabelled RGD peptides. Medical Recapitulate. 12:757–759.
2006.(In Chinese).
|
|
11
|
Zhang CL, Yang M and Wang RF: The
structure-activity relationship of RGD peptides binding to αvβ3
integrin and radiolabeled ligand design. J Oncol. 15:76–81.
2009.
|
|
12
|
Yu YP, Wang Q, Liu YC and Xie Y: Molecular
basis for the targeted binding of RGD-containing peptide to
integrin αvβ3. Biomaterials. 35:1667–1675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ji QZ and Zhang KW: Observation of DFRKN-1
isolate by scanning electron microscope. Anhui Agric Sci. 39:33–34.
2011.
|
|
14
|
Seo GY, Ha Y, Park AH, Kwon OW and Kim YJ:
Leathesia difformis extract inhibits α-MSH-induced
melanogenesis in B16F10 cells via down-regulation of CREB signaling
pathway. Int J Mol Sci. 20:E5362019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang CL, Wang RF and L Z: Design of
~(131)I labeling of a disulfide bridged RGD-peptide targeted to
integrin αvβ3 receptor and its biodistribution and imaging in tumor
bearing mice. J Oncol. 16:276–280. 2010.
|
|
16
|
Dia YW: Design for tumor-targeting
antimicrobial peptide chimera and its anticancer mechanism study
(unpublished PhD thesis). Jilin University; 2012
|
|
17
|
Li ZDLY and Zhen YS: Potentiation of
boanmycin antitumor activity by chemotactic peptide. Chin J Cancer
Res. 17:79–83. 2005.(In Chinese). View Article : Google Scholar
|
|
18
|
Zhao RL, Han JY, Han WY, Liu SS, Ma JF,
Lei LC, Feng X, Zhang AG and Wang X: The antibacterial and
antitumor function of a novel bio-active peptide Catesbeianin-1a of
Rana catesbeiana. Chin J Vet Sci. 33:1407–1411. 2013.(In
Chinese).
|
|
19
|
Wang LZ and Min Y: Advance of” 1” 8F
labeled RGD peptide as integrin α_Vβ_3 receptor imaging agents. J
Isot. 24:68–75. 2011.
|
|
20
|
Jiang Z, Vasil AI, Hale JD, Hancock RE,
Vasil ML and Hodges RS: Effects of net charge and the number of
positively charged residues on the biological activity of
amphipathic α-helical cationic antimicrobial peptides. Biopolymers.
90:369–383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Xiao X, Zhu J, Gao Z, Lai X, Zhu
X and Mao G: Lactoferrin- and RGD-comodified, temozolomide and
vincristine-coloaded nanostructured lipid carriers for gliomatosis
cerebri combination therapy. Int J Nanomedicine. 13:3039–3051.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen T, Chen L, He H, Peng J, Guo X, Zheng
Y and Shao C: Influence of HAnps carrier system with doping Mg and
grafting RGD on endocytosis of MG63 cells. J Cent South Univ.
48:625–634. 2017.
|
|
23
|
Habibi N, Kamaly N, Memic A and Shafiee H:
Self-assembled peptide-based nanostructures: Smart nanomaterials
toward targeted drug delivery. Nano Today. 11:41–60. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lambert E, Fuselier E, Ramont L, Brassart
B, Dukic S, Oudart JB, Dupont-Deshorgue A, Sellier C, Machado C and
Dauchez M: Conformation-dependent binding of a Tetrastatin peptide
to αvβ3 integrin decreases melanoma progression through
FAK/PI3 K/Akt pathway inhibition. Sci Rep. 8:98372018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hou J, Diao Y, Li W, Yang Z, Zhang L, Chen
Z and Wu Y: RGD peptide conjugation results in enhanced antitumor
activity of PD0325901 against glioblastoma by both tumor-targeting
delivery and combination therapy. Int J Pharm. 505:329–340. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen G, Yu F, Sheng G, Wang J and Qu B:
Progress and future direction of vascular mimicry. J Mod Oncol.
1:138–141. 2017.
|
|
27
|
Cao QZ and Lin ZB: Antitumor and
anti-angiogenic activity of Ganoderma lucidum polysaccharides
peptide. Acta Pharmacol Sin. 25:833–838. 2004.PubMed/NCBI
|
|
28
|
Ren HD, Zhang L, Zhao Y, Si XH, Zhang L
and Wang C: Study on inhibitory effect of antimicrobial peptide
Temporin-1CEa and its analogues inhibit metastasis and invasion of
human melanoma A375 cells and reduce angiogenesis. Chin J Biochem
Pharm. 2:19–22. 2016.(In Chinese).
|