Introduction
Lung cancer is one of the most common tumour types
and remains the leading cause of cancer-related death worldwide,
with a 5-year survival rate of only 16% in 2013 (1). The occurrence of lung cancer is closely
associated with smoking (2). The
incidence of lung adenocarcinoma is the highest among all subtypes
of lung cancer and has surpassed that of squamous cell carcinoma
(3), and the pathology of PTLC often
indicates lung adenocarcinoma. Pneumonia-type lung cancer (PTLC) is
often misdiagnosed as inflammation and its treatment is usually
delayed (4). Therefore, accurate
identification and rapid diagnosis of this disease is essential to
improve patient survival.
Several mechanisms of lung cancer metastasis have
been proposed, including hematogenous spread, lymphatic metastasis
and direct infiltration (5).
However, accumulating studies have demonstrated that lung cancer
can spread through the trachea (6–9). Gaikwad
et al (10) have suggested
that aerogenous spread of lung cancer occurs and serves an
important role in the staging and management of this disease. At
present, the possible mechanism of lung cancer metastasis is
considered to be mediated through the airway (10). This facilitates tumour cell adherent
growth and metastasis to the distal parts of the trachea, which are
located away from the primary site of the malignancy (10).
The current study aimed to investigate the possible
mechanism of the formation of PTLC by studying the CT and
pathological characteristics of pneumonia-type mucinous
adenocarcinoma in order to deepen the understanding of this
disease, reduce the misdiagnosis rate and the detection time, and
improve the survival rate of the patients.
Materials and methods
Patients
A total of 17 patients diagnosed with PTLC between
June 2012 and June 2017 were selected from three tertiary hospitals
in Beijing (The General Hospital of the People's Liberation Army,
The Affiliated Beijing Shijitan Hospital of Capital Medical
University and The Peking Union Medical College Hospital; Beijing,
China) and their clinical, imaging and pathological data were
collected. The present study was approved by the Research Ethics
Committee of Beijing Shijitan Hospital affiliated to Capital
Medical University [Beijing, China; approval no.
sjtkyll-lx-2018(30)]. Since the study was carried out
retrospectively, the Ethics Committees of the three hospitals
decided to waive the patients' informed consent.
Immunohistochemical staining
The paraffin sections of the lesions were sectioned
into ~4-µm slices, which were placed on slides overnight at room
temperature or dried at 60°C for 1 h. The primary anti-thyroid
transcription factor-1 (TTF-1) antibody (dilution, 1:50; cat. no.
12373s; Roche Diagnostics) was incubated at 37°C for 16 min. The
primary antibody and DAB staining solution (Roche Diagnostics) were
added to a reagent tray and placed into the Ventana Benchmark XT
(Roche Diagnostics) fully automatic immunohistochemical instrument,
and the slides were placed directly inside the instrument.
Following dyeing, the slides were removed, cleaned with a mild
detergent to remove the solution on the cover glass and washed
thoroughly with distilled water to remove the residual detergent.
After dehydration and cleaning, the cover glass was sealed with
sealing agent. The sections were observed under a light microscope
with ×400 magnification.
H&E staining
The sections were dewaxed in xylene for 5–10 min and
transferred into the mixture of xylene and pure ethanol (1:1) for
~5 min. Subsequently, the sections were rehydrated in a decreasing
series of ethanol (100, 95, 85 and 70% for 2–5 min each) and
transferred to a dye solution through distilled water for staining
with haematoxylin for 5–15 min. Eosin dye solution (0.1–0.5%) was
added for 1–5 min, and then the sections were dehydrated by 70, 85,
95 and 100% ethanol for 2–3 min. Finally, the sections were sealed
with neutral gum under cover slips. The sections were observed
under a light microscope with ×100 and ×400 magnification.
Imaging and diagnosis
With the exception of three patients who underwent
lobectomy, the remaining 14 patients underwent needle biopsies. A
total of seven patients underwent rapid on-the-spot evaluation
(ROSE) under bronchoscopy. All patients underwent 128-slice CT with
5-mm thickness, 1.5 mm scan and parallel coronal and sagittal
reconstruction. The diagnosis was made by two deputy chief
physicians at the Department of Radiology and was based on the
patient clinical symptoms and previous imaging data.
Results
Clinical characteristics of the
patients
Among the 17 recruited patients, 12 were male and
five were female. Their age ranged between 35 and 75 years, with a
mean age of 60.18 years (Table I).
The Tumor-Node-Metastasis stage of all patients was T4N0M0
(11). The sample included 9 smokers
(Table I). The clinical
manifestations were cough in 16 patients and absence of apparent
symptoms in one patient.
 | Table I.Basic information of the patients
included in the present study. |
Table I.
Basic information of the patients
included in the present study.
| No. | Smoking, years | Clinical
symptoms | CT manifestation | Location | Diagnosis method | Therapeutic
measures | Prognosis/survival
time |
|---|
| 1 | NOT | No cough or
expectoration | Flaky density,
peripheral ground glass density and cavity | Lower lobe of the
left lung | Lobectomy of the left
lower lobe | Lobectomy | >60 months |
| 2 | NOT | Cough | Flaky density,
peripheral ground glass density, air bronchogenic signs | Lower lobe of the
right lung | Lobectomy of the
right lower lobe | Lobectomy | 3 months |
| 3 | NOT | Cough,
expectoration | Flaky density,
peripheral ground glass density | Lower lobe of the
right lung | Lobectomy of the
right lower lobe | Lobectomy | 4 months |
| 4 | NOT | Cough,
expectoration | Flaky density, air
bronchogenic signs | Lower lobe of the
left lung | Ultrasound-guided
lung puncture | Expectant
treatment | 4 months |
| 5 | 40 | Cough,
expectoration | Flaky density,
peripheral ground glass density | Upper lobe of the
right lung | Ultrasound-guided
lung puncture | Chemotherapy | 6 months |
| 6 | 40 | Cough,
expectoration | Flaky density,
peripheral ground glass density | Upper lobe of the
left lung | CT-guided lung
puncture | Chemotherapy | 6 months |
| 7 | NOT | Expectoration | Flaky density, air
bronchogenic signs | Upper lobe of the
right lung | CT-guided lung
puncture | Chemotherapy | 5 months |
| 8 | NOT | Cough,
expectoration | Flaky density,
peripheral ground glass density and cavity | The right lung | CT-guided lung
puncture | Chemotherapy | 8 months |
| 9 | 30 | Cough,
expectoration | Flaky density,
peripheral ground glass density | Bilateral lungs | CT-guided lung
puncture | Chemotherapy | 6 months |
| 10 | 30 | Cough,
expectoration | Flaky density,
peripheral ground glass density and cavity, air bronchogenic
signs | Bilateral lungs | Ultrasound-guided
lung puncture | Chemotherapy | 5 months |
| 11 | 25 | Cough,
expectoration | Flaky density,
peripheral ground glass density | Bilateral
lungs | CT-guided lung
puncture | Chemotherapy | 12 months |
| 12 | NOT | Cough,
expectoration | Flaky density,
peripheral ground glass density | Bilateral
lungs |
Ultrasound-guided | Chemotherapy lung
puncture | >60 months |
| 13 | NOT | Cough,
expectoration | Flaky density,
peripheral ground glass density | Bilateral
lungs | CT-guided lung
puncture | Chemotherapy | 5 months |
| 14 | NOT | Cough,
expectoration | Flaky density,
peripheral ground glass density | Bilateral
lungs | CT-guided lung
puncture | Chemotherapy | 10 months |
| 15 | 30 | Cough,
expectoration | Flaky density, air
bronchogenic signs, tree bud signs | Bilateral
lungs | CT-guided lung
puncture | Chemotherapy | 9 months |
| 16 | 20 | Cough,
expectoration | Flaky density,
peripheral ground glass density | Bilateral
lungs | CT-guided lung
puncture | Chemotherapy | 12 months |
| 17 | 30 | Cough,
expectoration | Flaky density,
peripheral ground glass density | Bilateral
lungs | CT-guided lung
puncture | Chemotherapy | 10 months |
CT scans
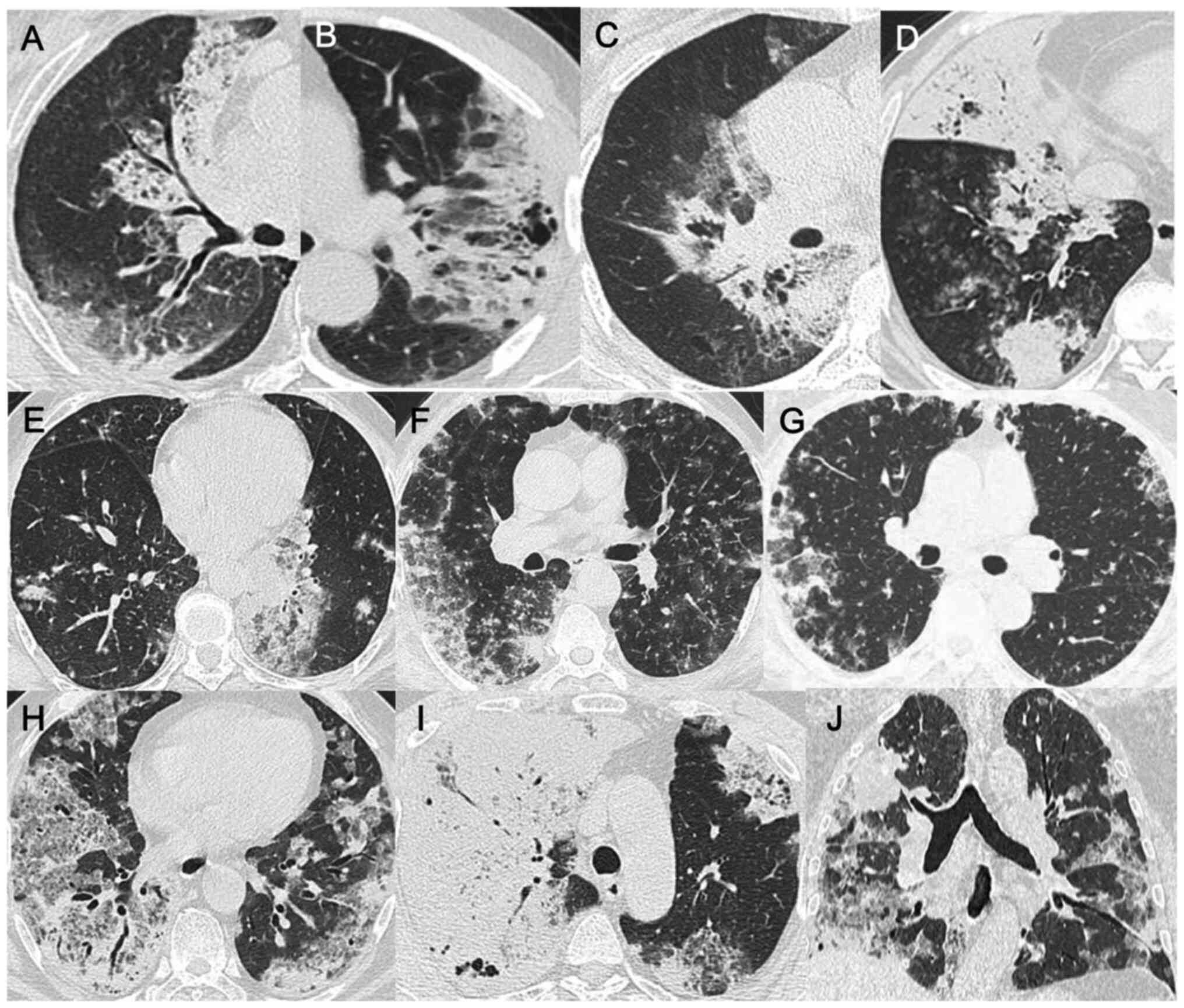
A total of nine cases presented with diffuse lung
disease, of which one was a case of left upper lobe, two of left
lower lobe, two of right upper lobe, two of right lower lobe and
one of right middle and lower lobe disease. All patients exhibited
multiple patchy, flaky densities and peripheral ground glass
density, specifically in the near end of the heart shadow with
visible ground glass opacity (14 cases, 82%), absence of enlarged
lymph nodes and randomly distributed nodule shadows (Fig. 1). In certain patients, air
bronchograms (12 cases, 71%) and vacuoles (10 cases, 59%) were
observed (Fig. 2). According to the
imaging data and clinical symptoms, the disease spread of three
patients was confined to the left/lower right lobe, and no distant
lymph node or hematogenous metastases were noted. Therefore, the
patients were examined with ultrasound/CT guided lung biopsy.
Pathological results
Following surgery, a large amount of mucus was noted
by microscopical evaluation, and the tumour cells were scattered in
the residual alveolar wall (Fig. 3).
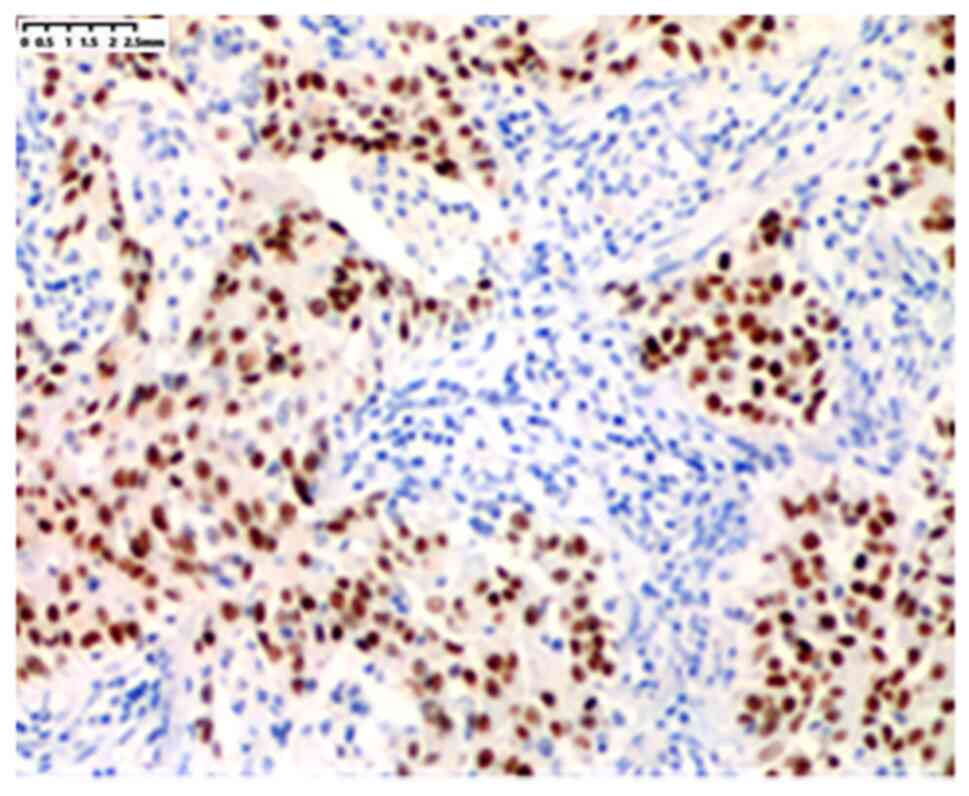
Subsequently, the sections were analysed by immunohistochemistry,
and the results demonstrated that tumour cells secreting mucus were
TTF-1 positive (Fig. 4). The
pathological evaluation indicated mucinous adenocarcinoma, and the
adherent growth was the dominant type of cancer among all patients.
Bronchoscopy was performed in seven cases; a large amount of white
foam-like sputum appeared from the left and right main bronchus in
the form of a spring. A ROSE was also performed, and the results
demonstrated that the full field of view was a tumour (Fig. 5). The cells were examined by multiple
biopsies and H&E staining to confirm that these structures were
tumour cells of mucinous adenocarcinoma origin. Tall-cup-like
tumour cells that grew in the alveolar wall, secreting a large
amount of mucus and leading to the filling of the alveolar cavity,
were identified by microscopic examination. Following coughing of
the patient and scouring of the mucus, the tumour cells were
detached from the wall, and the mucus was spread gradually from the
small airway to other airways, lung segments/leaves or to the
contralateral lung tissue. When the mucus reached the normal lung
tissue, a slightly higher density of ground glass opacity was
formed. The floating tumour cells were planted, and a denser patchy
solid shadow was observed.
Imaging evaluation
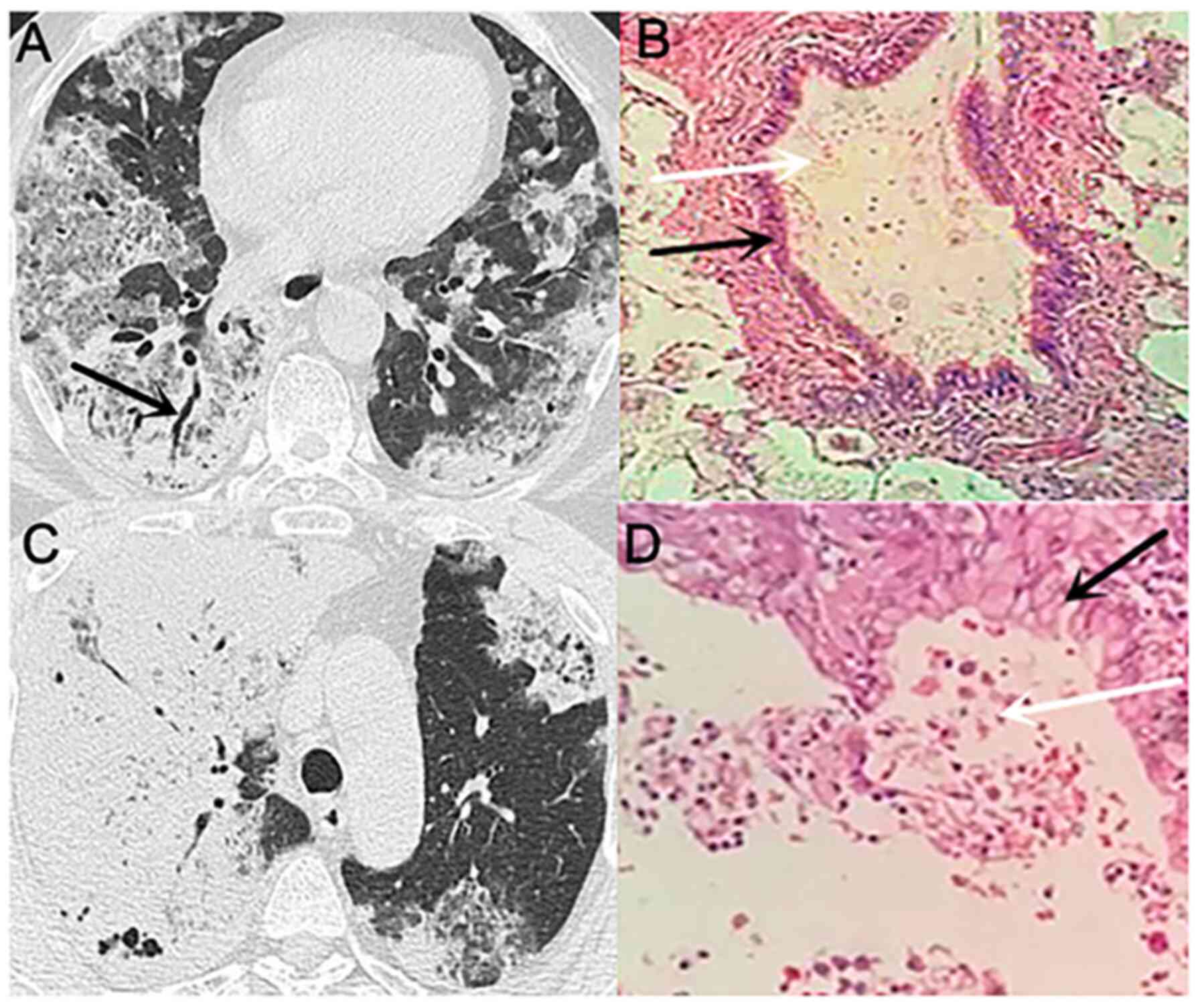
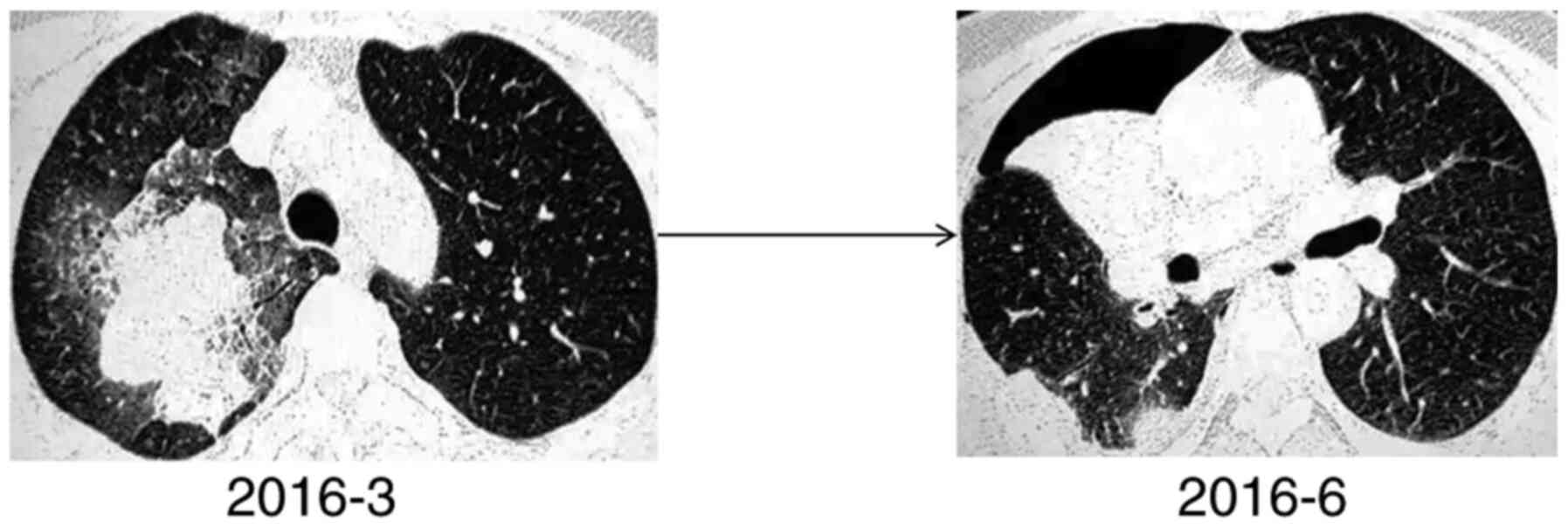
In patient no. 3 (Fig.
6), the CT scan indicated consolidation of the right lower lobe
with an unclear edge and a drenched glass opacity around it. The
ablated bronchus indicated a dry branch-like change with blocked
distal bronchi and slight thickening alterations in the proximal
bronchus wall. An additional small airbag cavity was visible, and
the inner wall of the cavity was smooth. The CT scan of patient no.
8 (Fig. 7) was indicative of a
partial solid tumour in the upper lobe of the right lung. Multiple
grinds were noted in the remaining lungs, and a mild uneven
enhancement was observed in the right upper lung lesion. Therefore,
the CT scans of the two patients suggested that the tumours may be
malignant. Multiple biopsies in patient no. 3 and postoperative
examination in patient no. 8 indicated mucinous adenocarcinoma. In
patient no. 8, a large amount of mucus was noted following
microscopical investigation, corresponding to the unenhanced area
on the CT. Part of this structure was located in the consolidation
zone, where the tumour cells aggregated. The two patients also
underwent MRI of the brain and abdomen, which indicated the absence
of metastatic lesions.
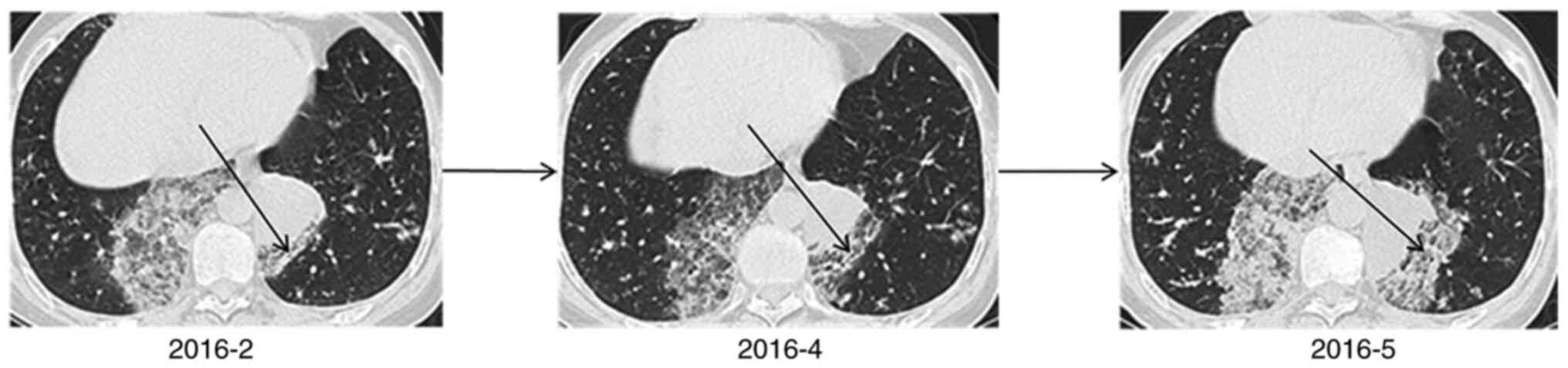
Subsequently, a 3-month follow-up was conducted. In
patient no. 3, the lesion progressed rapidly within 3 months, and
multiple plaques and ground glass opacity were noted in the
contralateral lung. The lesions in the left lower lobe of the lung
were gradually enlarged, whereas those in the right lower lobe did
not change significantly (Fig. 6).
It was subsequently revealed that the patient had been sleeping on
the left side since the onset of the disease. Patient no. 8
received a right upper lung resection after one week of ineffective
anti-inflammatory treatment, and the CT scan 3 months post-surgery
revealed that the shadow had mostly disappeared (Fig. 7).
Follow-up results
Among the patients, one received symptomatic
treatment, three underwent lobectomy, and the remaining 13 received
chemotherapy, which included platinum combined with pemetrexed.
Subsequently, the patients were followed up for 5 years (Table I). Of the 17 patients, two were alive
at the end of the follow-up period. The longest life span among the
patients who succumbed to disease was 12 months.
Discussion
PTLC is a definition proposed for a specific type of
lung cancer that has imaging features comparable to inflammation
(12). PTLC represents the
morphological process of tumour formation; the tumour gradually
develops from single lobe to multiple lobes (13–15).
According to a study by Duruisseaux et al (4), PTLC comprises two main
histopathological types, namely invasive mucinous adenocarcinoma
(40.0%) and invasive adenocarcinoma with adherent growth (31.6%).
However, these classifications do not explain the possible
mechanism of PTLC formation. Gaikwad et al (10) reviewed the aerogenous metastasis of
primary lung adenocarcinoma and suggested that aerogenous spread of
tumor cells may exist and that it may be underrecognized. The
results of the present study were consistent with the
aforementioned study and demonstrated the presence of aerogenous
metastasis as multiple flaky and patchy dense structures were
evident in the CT scans. The surrounding ring was saturated with
ground glass attenuation, especially at the proximal end. The
formation of this sign may be associated with the production of
mucus by tumour cells and their spread through the airway.
Therefore, multiple spots were noted on the CT images.
Lung cancer commonly presents as nodules or masses
in CT scans; other malignant signs, such as lobes, burrs and
cavities may be visible (16–21). By
contrast, PTLC was observed in the present study to be mostly flaky
or patchy on CT scans and did not present as a mass or nodule. The
near-central ground glass opacity, the air bronchus sign and the
vacuole sign are rarely noted in cancerous lymphangitis, and hilar,
mediastinal lymph nodes and random distribution of nodules are
observed (4). In the present study,
the near-central ground glass opacity was the most frequent
observation in the 17 patients. A possible explanation for this may
be that the tumour cells were dispersed with a large amount of
mucus through the airway. However, obstructive pneumonia was
difficult to assess since it usually occurs due to the obstruction
of the airway, leading to distant frosting or solid shadows. These
events were microscopically accompanied by mucus filling and
obstruction of the distal small airway. Tumour cells colonized
other lung tissues and destroyed the original alveolar space. When
the mucus was expelled from the alveoli by coughing or breathing, a
translucent and vesicular area was observed. The high incidence of
these signs also provided a reliable diagnostic value for the
diagnosis of PTLC. Among the patients included in the present
study, chemotherapy (platinum plus pemetrexed) was the main
treatment for those who preferred non-surgical treatment, although
the long-term survival rate of the patients did not appear to
improve. Previous studies have demonstrated that certain drugs
exert cytotoxic side effects on lung cancer cells and can be used
to treat lung cancer. For example, Sani et al (22) have demonstrated that EO,
CH2Cl2 and hexane components exhibit
inhibitory effects on Calu-6 and Mehr-80 cells. Luteolin is the
main compound extracted from ancient shoucao, a plant used in
traditional Chinese medicine, that exhibits considerable
cytotoxicity in Calu-6 and Mehr-80 lung cancer cell lines. Daphedar
and Taranath (23) have
suggested that high concentrations of silver nanoparticles inhibit
the progression of mitotic cells and increase the potential of cell
death caused by chromosomal aberrations. This application may
provide novel insights into the potential treatment of patients
with PTLC.
Chest CT scans have been widely used in the clinical
diagnosis of lung cancer (24,25). In
addition, the use of CT to diagnose PTLC has also been reported
(2–4,11,26–30),
and a limited number of studies have detailed the possible
mechanism of PTLC formation. An important observation of the
present study is that it is impossible to perform a biopsy on all
plaque shadows or ground glass opacities. However, following
multiple means of examination (tracheoscopy, ROSE and pathological
examination), the results indicated that the formation of these
signs may be associated with the spread of tumour cells through the
airway or alveolus. Future studies should combine multiple
examination methods to confirm a possible mechanism of action of
PTLC. However, a notable limitation of the present study was the
low number of cases recruited. In future studies, additional cases
should be recruited to verify the main mechanism of action
underlying PTLC. The secretion of mucus from the tumour cells
requires further investigation based on molecular imaging combined
with immunohistochemical analysis. Additionally, Tasdemir et
al (31) have reported that EGFR
expression levels are upregulated in the majority of patients with
NSCLC, and it is an important target in the treatment of NSCLC. The
mutation rate of EGFR may provide valuable information, which will
be explored in further research.
In conclusion, the results of the present study
suggested that PTLC may be considered a potential differential
diagnosis when multiple plaques and ground glass opacities are
observed around the consolidation area near the heart. These
features may include signs such as air bronchus and vacuole. The
corresponding pathological type of PTLC is mainly adherent
growth-based mucinous adenocarcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Fund Youth Project (grant no. 81700007), the
Research and Innovation Fund of The Ministry of Education (grant
no. 2018A03026), the Beijing Natural Science Foundation (grant no.
2019A10), the ‘Qingmiao’ Plan of the Beijing Municipal Hospital
Administration (grant no. 2018QM4), The Outstanding Top Talent Fund
(grant no. 2019YXBJ1), Capital Health Development Scientific
Research Unit Matching Fund (grant no. 2020-2Z-2086) and the
Excellent Talents in Beijing Youth Top Team Fund (grant no.
2019YXBJ2).
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
JH designed the study, analysed the data and
contributed to the writing and editing of the manuscript. YW
contributed to the experimental design and data analysis. HH
conceived the study and contributed to the first draft of the
manuscript. CW and XX participated in the experimental design,
conducted the experiments, analysed the data and contributed to
drafting the manuscript and revising it for intellectual content.
HD participated in performing the experiments. JG analysed the
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in compliance with
the institutional policy regarding the protection of patient
confidential information and was approved by the Research Ethics
Committee of Beijing Shijitan Hospital affiliated to Capital
Medical University [Beijing, China; approval no.
sjtkyll-lx-2018(30)]. All procedures were performed in accordance
with the approved guidelines of Beijing Shijitan Hospital
affiliated to Capital Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Linnerth-Petrik NM, Walsh SR, Bogner PN,
Morrison C and Wootton SK: Jaagsiekte sheep retrovirus detected in
human lung cancer tissue arrays. BMC Res Notes. 7:1602014.
View Article : Google Scholar
|
|
2
|
Liu J, Shen J, Yang C, He P, Guan Y, Liang
W and He J: High incidence of EGFR mutations in pneumonic-type
non-small cell lung cancer. Medicine (Baltimore). 94:e5402015.
View Article : Google Scholar
|
|
3
|
Casali C, Rossi G, Marchioni A, Sartori G,
Maselli F, Longo L, Tallarico E and Morandi U: A single
institution-based retrospective study of surgically treated
bronchioloalveolar adenocarcinoma of the lung: Clinicopathologic
analysis, molecular features, and possible pitfalls in routine
practice. J Thorac Oncol. 5:830–836. 2010. View Article : Google Scholar
|
|
4
|
Duruisseaux M, Antoine M, Rabbe N, Poulot
V, Fleury-Feith J, Vieira T, Lavolé A, Cadranel J and Wislez M: The
impact of intracytoplasmic mucin in lung adenocarcinoma with
pneumonic radiological presentation. Lung Cancer. 83:334–340. 2014.
View Article : Google Scholar
|
|
5
|
Wislez M, Antoine M, Baudrin L, Poulot V,
Neuville A, Pradere M, Longchampt E, Isaac-Sibille S, Lebitasy MP
and Cadranel J: Non-mucinous and mucinous subtypes of
adenocarcinoma with bronchioloalveolar carcinoma features differ by
biomarker expression and in the response to gefitinib. Lung Cancer.
68:185–191. 2010. View Article : Google Scholar
|
|
6
|
Pelosi G: The new taxonomy of lung
adenocarcinoma stemming from a multidisciplinary integrated
approach: Novel pathology concepts and perspectives. J Thorac
Oncol. 6:241–243. 2011. View Article : Google Scholar
|
|
7
|
Wislez M, Antoine M, Rabbe N, Gounant V,
Poulot V, Lavolé A, Fleury-Feith J and Cadranel J: Neutrophils
promote aerogenous spread of lung adenocarcinoma with
bronchioloalveolar carcinoma features. Clin Cancer Res.
13:3518–3527. 2007. View Article : Google Scholar
|
|
8
|
Seo JB, Im JG, Goo JM, Chung MJ and Kim
MY: Atypical pulmonary metastases: Spectrum of radiologic findings.
Radiographics. 21:403–417. 2001. View Article : Google Scholar
|
|
9
|
Herold CJ, Bankier AA and Fleischmann D:
Lung metastases. Eur Radiol. 6:596–606. 1996. View Article : Google Scholar
|
|
10
|
Gaikwad A, Souza CA, Inacio JR, Gupta A,
Sekhon HS, Seely JM, Dennie C and Gomes MM: Aerogenous metastases:
A potential game changer in the diagnosis and management of primary
lung adenocarcinoma. AJR Am J Roentgenol. 203:W570–W582. 2014.
View Article : Google Scholar
|
|
11
|
Lababede O and Meziane MA: The eighth
edition of TNM staging of lung cancer: Reference chart and
diagrams. Oncologist. 23:844–848. 2018. View Article : Google Scholar
|
|
12
|
Wislez M, Massiani MA, Milleron B, Souidi
A, Carette MF, Antoine M and Cadranel J: Clinical characteristics
of pneumonic-type adenocarcinoma of the lung. Chest. 123:1868–1877.
2003. View Article : Google Scholar
|
|
13
|
Garfield DH, Cadranel JL, Wislez M,
Franklin WA and Hirsch FR: The bronchioloalveolar carcinoma and
peripheral adenocarcinoma spectrum of diseases. J Thorac Oncol.
1:344–359. 2006. View Article : Google Scholar
|
|
14
|
Manson GV and Ma PC: Response to
pemetrexed chemotherapy in lung adenocarcinoma-bronchioloalveolar
carcinoma insensitive to erlotinib. Clin Lung Cancer. 11:57–60.
2010. View Article : Google Scholar
|
|
15
|
Garfield DH, Cadranel J and West HL:
Bronchioloalveolar carcinoma: The case for two diseases. Clin Lung
Cancer. 9:24–29. 2008. View Article : Google Scholar
|
|
16
|
Youlden DR, Cramb SM and Baade PD: The
international epidemiology of lung cancer: Geographical
distribution and secular trends. J Thorac Oncol. 3:819–831. 2008.
View Article : Google Scholar
|
|
17
|
Mavi A, Lakhani P, Zhuang H, Gupta NC and
Alavi A: Fluorodeoxyglucose-PET in characterizing solitary
pulmonary nodules, assessing pleural diseases, and the initial
staging, restaging, therapy planning, and monitoring response of
lung cancer. Radiol Clin North Am. 431–21. (ix)2005. View Article : Google Scholar
|
|
18
|
Acker MR and Burrell SC: Utility of
18F-FDG PET in evaluating cancers of lung. J Nucl Med Technol.
33:69–74; quiz 75-7. 2005.
|
|
19
|
Travis WD, Brambilla E, Nicholson AG,
Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E,
Flieder DB, et al: The 2015 world health organization
classification of lung tumors: Impact of genetic, clinical and
radiologic advances since the 2004 classification. J Thorac Oncol.
10:1243–1260. 2015. View Article : Google Scholar
|
|
20
|
Raz DJ, He B, Rosell R and Jablons DM:
Bronchioloalveolar carcinoma: A review. Clin Lung Cancer.
7:313–322. 2006. View Article : Google Scholar
|
|
21
|
Mornex JF, Thivolet F, De las Heras M and
Leroux C: Pathology of human bronchioloalveolar carcinoma and its
relationship to the ovine disease. Curr Top Microbiol Immunol.
275:225–248. 2003.
|
|
22
|
Sani TA, Mohammadpour E, Mohammadi A,
Memariani T, Yazdi MV, Rezaee R, Calina D, Docea AO, Goumenou M,
Etemad L and Shahsavand S: Cytotoxic and apoptogenic properties of
dracocephalum kotschyi aerial part different fractions on calu-6
and mehr-80 lung cancer cell lines. Farmacia. 65:189–199. 2017.
|
|
23
|
Daphedar A and Taranath TC:
Characterization and cytotoxic effect of biogenic silver
nanoparticles on mitotic chromosomes of Drimia polyantha (Blatt.
& McCann) stearn. Toxicol Rep. 5:910–918. 2018. View Article : Google Scholar
|
|
24
|
Qi Y, Zhang Q, Huang Y and Wang D:
Manifestations and pathological features of solitary thin-walled
cavity lung cancer observed by CT and PET/CT imaging. Oncol Lett.
8:285–290. 2014. View Article : Google Scholar
|
|
25
|
Miyake H, Matsumoto A, Terada A, Yoshida
S, Takaki H and Mori H: Mucin-producing tumor of the lung: CT
findings. J Thorac Imaging Spring. 10:96–98. 1995. View Article : Google Scholar
|
|
26
|
Sato K, Ueda Y, Shikata H and Katsuda S:
Bronchioloalveolar carcinoma of mixed mucinous and nonmucinous
type: Immunohistochemical studies and mutation analysis of the p53
gene. Pathol Res Pract. 202:751–756. 2006. View Article : Google Scholar
|
|
27
|
Isobe K, Hata Y, Iwata M, Ishida F,
Kaburaki K, Gocho K, Kobayashi M, Sakaguchi S, Satou D, Sano G, et
al: An autopsied case of mucinous bronchioloalveolar carcinoma
associated with multiple thin-walled cavities. Nihon Kokyuki Gakkai
Zasshi. 47:512–517. 2009.(In Japanese).
|
|
28
|
Woodring JH: Unusual radiographic
manifestations of lung cancer. Radiol Clin North Am. 28:599–618.
1990.
|
|
29
|
Osoegawa A, Kometani T, Nosaki K, Ondo K,
Hamatake M, Hirai F, Seto T, Sugio K and Ichinose Y: LKB1 mutations
frequently detected in mucinous bronchioloalveolar carcinoma. Jpn J
Clin Oncol. 41:1132–1137. 2011. View Article : Google Scholar
|
|
30
|
Kadota K, Yeh YC, D'Angelo SP, Moreira AL,
Kuk D, Sima CS, Riely GJ, Arcila ME, Kris MG, Rusch VW, et al:
Associations between mutations and histologic patterns of mucin in
lung adenocarcinoma: Invasive mucinous pattern and extracellular
mucin are associated with KRAS mutation. Am J Surg Pathol.
38:1118–1127. 2014. View Article : Google Scholar
|
|
31
|
Tasdemir S, Taheri S, Akalin H, Kontas O,
Onal O and Ozkul Y: Increased EGFR mRNA expression levels in
non-small cell lung cancer. Eurasian J Med. 51:177–185. 2019.
View Article : Google Scholar
|